Abstract
The infiltration and accumulation of pro- and anti-inflammatory leukocytes within the intimal layer of the arterial wall is a hallmark of developing and progressing atherosclerosis. While traditionally perceived as macrophage- and foam cell-dominated disease, it is now established that atherosclerosis is a partial autoimmune disease that involves the recognition of peptides from ApoB (apolipoprotein B), the core protein of LDL (low-density lipoprotein) cholesterol particles, by CD4+ T-helper cells and autoantibodies against LDL and ApoB. Autoimmunity in the atherosclerotic plaque has long been understood as a pathogenic T-helper type-1 driven response with proinflammatory cytokine secretion. Recent developments in high-parametric cell immunophenotyping by mass cytometry, single-cell RNA-sequencing, and in tools exploring antigen-specificity have established the existence of several unforeseen layers of T-cell diversity with mixed TH1 and T regulatory cells transcriptional programs and unpredicted fates. These findings suggest that pathogenic ApoB-reactive T cells evolve from atheroprotective and immunosuppressive CD4+ T regulatory cells that lose their protective properties over time. Here, we discuss T-cell heterogeneity in atherosclerosis with a focus on plasticity, antigen-specificity, exhaustion, maturation, tissue residency, and its potential use in clinical prediction.
Keywords: atherosclerosis, autoantibodies, autoimmunity, immunophenotyping, peptide
Highlights.
T cells represent the largest population of leukocytes in the atherosclerotic plaque.
High-parametric immunophenotyping of plaque T cells by single-cell RNA sequencing and cytometry by time of flight shows an unexpected phenotypic diversity with previously unknown immature T cells and unconventional, mixed T helper types.
Lesional CD4+ T cells with mixed phenotypes seem to originate from initially protective T-regulatory cells that lost their immunosuppressive properties and switched into pathogenic counterparts over time.
The phenotypic heterogeneity of plaque CD4+ T cells is driven by states of activation, exhaustion, development, and inflammation rather than by traditional types of T-helper cells.
Appearance, transcriptomes, and proteomes of specific CD4+ T-cell populations in the atherosclerotic lesion correlate with clinical disease severity in humans.
Please see www.ahajournals.org/atvb/atvb-focus for all articles published in this series.
T cells are regulators of adaptive immunity that are defined by surface expression of CD3 and the T-cell receptor (TCR) chains-α/β+ or TCR-γ/δ+. Classical functions of α/β+ T cells include protection from intracellular pathogens, larger parasites, elimination of tumor cells, and protection from autoimmune diseases. The role of γ/δ+ T cells, which are outnumbered by α/β+ T cells in tissues and circulation, will not be covered in this review. T cells develop in the thymus, where bone-marrow derived progenitors gradually develop into CD4−CD8− (double-negative [DN]) cells. At this stage, T cells divide into either α/β+ or γ/δ+ T cells. α/β+ T cells transit into double-positive (DP) CD4+CD8+ cells and eventually into naive CD4+CD8− T-helper cells or cytotoxic CD8+CD4− T cells.1 CD4+ T cells recognize antigen-derived peptides presented on MHC (major histocompatibility complex)-II, whereas CD8+ T cells recognize peptides presented on MHC-I. The maturation of T cells is accompanied by recombinase activating genes-1 and -2 dependent rearrangement of TCR genes and the generation of a random, unique TCR on each T cell. This arrangement also results in autoreactive T cells that express a high-affinity TCR recognizing endogenously expressed self-peptides. Most of these self-reactive T cells are eliminated through a process termed negative selection, but this process does not eliminate all autoreactive CD4+ T cells.2 Increasing evidence argues for the presence of CD4+ T cells recognizing peptides from ApoB (apolipoprotein B)-100, an established self-antigen in atherosclerosis.3–5 The presentation of cognate peptides by antigen-presenting cells (APCs) on surface-expressed MHC-II induces the activation and differentiation of naive CD4+ T cells into functionally and phenotypically distinct T-helper (TH) types that express exclusive sets of transcription factors (TFs) and cytokines.6,7 TH type 1 (TH1) CD4+ T cells are proatherogenic and express the TF T-bet and the lead-cytokine IFN-γ (interferon-gamma).8 In experimental mouse models, CD4+ T cells from atherosclerosis-prone apolipoprotein-deficient (Apoe−/−) mice home to the atherosclerotic aorta and secrete IFN-γ.9,10 The genetic deletion of T-bet or IFN-γ reduces atherosclerosis markedly.11,12 TH2 cells express GATA3 (GATA-binding protein 3) and IL (interleukin)-4, -5, -10, and -1313 and may support atheroprogression or -protection.14,15 TH17 cells express the lineage-defining TF RORγt (RAR-related orphan receptor) and the cytokines IL-17A, -17F, and -21. Their role in atherosclerosis is controversial.16–19 TH9 cells are characterized by IL-9 secretion; treatment of Apoe−/− mice with IL-9 increases atherosclerosis,20 but the overall role of TH9 cells in atherosclerosis remains unknown. Follicular T helper cells (TFH) express the transcription factor Bcl-6, the costimulatory molecule inducible T-cell costimulator and CXCR5 (C-X-C chemokine receptor type 5).21 TFH are mostly found in germinal centers in secondary lymphoid organs where they provide help to B cells for immunoglobulin class switching. Emerging data suggest that TFH contribute to the proinflammatory response in atherosclerosis.22,23 T regulatory cells (Treg), defined by expression of FoxP3 and IL-2 receptor (CD25), are immunosuppressive24,25 and prevent from atherosclerosis by multiple mechanisms, including secretion of IL-10 and TGF-β (transforming growth factor-β).26,27
The regulation of Tregs in mouse and human atherosclerosis and the relationship to pathogenic TH-types has been controversial: fractions of FoxP3+ Tregs decrease in murine atherosclerotic aortas and blood but increase in the spleen of the same animals.28 A proportion of Tregs in the aorta and in lymph nodes of mice seems to obtain a TH1-like phenotype and expresses IFN-γ.29 While numbers of Tregs decrease during acute coronary syndromes in humans,30–32 frequencies of blood Tregs are higher in patients with angiographically documented atherosclerosis compared with healthy individuals.5 Other studies have failed to establish that frequencies of blood Tregs predict future myocardial infarction.33 Traditional TF staining in mouse atherosclerotic aortas by intracellular flow cytometry (fluorescence-activated cell sorting) and a canonical gating strategy has suggested a mix of FoxP3+ (Treg), RORγT+ (TH17), and T-bet+ (TH1) CD4+ T cells,5,29,34–36 while the quantification of TH-associated surface-expressed chemokine-receptor patterns in humans suggests a predominant TH1 and TH2 phenotype.8,37 These partially conflicting findings have sparked several fundamental questions: are patterns of cytokines, chemokine receptors, and transcription factors sufficient to define functionally relevant phenotypes of lesional and circulating T-helper cells? Do transcriptional programs in TH more precisely define TH cell diversity than single key TFs or cytokines? How are TH-cells regulated in different stages of disease and may these predict clinical complications?
Recent Developments in High-Parameter Immunophenotyping
Fluorescence-activated cell sorting is considered the gold standard for immunophenotyping of aortic leukocytes since 2006.38 Clinical routine fluorescence-activated cell sorting-workflows allow the simultaneous quantification of up to 18 parameters (marker expression, size, and granularity) on single cells by fluorescently tagged antibodies, albeit up to 40 markers are technically possible. Several reports have used either pan-leukocyte antibody panels to cover major principal hematopoietic lineages, that is, myeloid, T-, and B cells, or lineage-specific sets of antibodies to obtain phenotypic insight in particular lesional leukocyte lineages.38–40 These approaches, however, cover cellular heterogeneity only incompletely. Two newer technologies have demonstrated the ability to overcome this limitation. Cytometry by Time of Flight (CyTOF) uses rare metals conjugated to antibodies, which expands the number of simultaneously detectable extra- and intracellular protein targets up to 40 to 50. To date, CyTOF has been used to study leukocytes in atherosclerotic plaques in 3 studies.37,39,41 Single-cell RNA sequencing (scRNA-seq), on the other hand, provides transcriptomes of single cells with an average of 1300 to 8000 genes per cell, which enables the detection of transcriptionally defined cell populations. The first application of scRNA-seq in atherosclerosis used a microfluidics-based platform for 96-well sorting that allowed to study only limited numbers of cells.29 Today, technologies encapsulating up to 10 000 individual cells into oil droplets are commercially available (drop-sequencing). These have successfully been applied to atherosclerotic plaques from mice and humans.39,40,42–52 A newer strategy enables the combination of single cell transcriptomes and cell surface expression of proteins detected by RNA-barcoded antibodies (cellular indexing of transcriptomes and epitopes by sequencing).37,53 In scRNA-seq based approaches, genes encoding for Cd3d or Cd3e can be used to identify T cells. In contrast to Cd8, Cd4-transcripts are only weakly expressed. The segregation of T-cell lineages by scRNA-seq is challenging as the sensitivity to detect genes with low expression differs across technical pipelines: for example, the sensitivity of Smart-Seq2 is 2.5× higher compared with droplet-based sequencing by 10× Genomics 3’ v.2.54 Other technical challenges include gene dropouts caused by low RNA-expression, inefficient mRNA capture, and the stochastic, pulsatile nature of gene expression on a single-cell level.55 Accordingly, cells positive for TF-coding and other genes at low expression are often sparse in scRNA-seq datasets. Even in the meta-analysis of several mouse datasets, expression of transcripts coding for T-bet and FoxP3 was rare and did not allow the identification of distinct TH1 or Treg-clusters based on TFs alone.47
Of note, all high-parametric tools require the mechanical disruption of tissue-resident cells by enzymatic digestion, which may lead to an underestimation of fragile cellular subsets, such as macrophages that populate up to 50% of the atherosclerotic plaque area based on imaging- or histology-based detection.51,56 Other technical requirements and limitations of CyTOF and scRNA-seq have been reviewed recently.57 An ongoing challenge remains to combine and integrate different datasets into one cellular atlas of atherosclerotic lesions with a unified nomenclature—an attempt recently undertaken in the first meta-analysis of mouse plaque-resident leukocytes.47 Ultimately, novel immunophenotyping workflows may be combined with reagents to detect antigen-specific TH cells recognizing particular antigenic peptide laden MHC multimers labeled with fluorochromes.58 Recently, we have demonstrated the existence of human and mouse ApoB-specific TH cells on a single-cell level by MHC-II tetramers3,5 and linked these to single-cell transcriptomes.5 These technical developments helped to describe several novel layers of T-cell diversity in atherosclerosis with respect to spatial, temporal, phenotypic, antigen-specific, and transcriptional resolution that fundamentally differs from traditional discrimination and nomenclature of CD4+ T cells.
Quantities and Locations of Lesional T Cells
The presence of T cells in atherosclerotic plaques and the surrounding adventitial tissue has been first observed >30 years ago. In immunohistochemistry, CD4+ and CD8+ T cells accumulate in the shoulder region, the fibrous cap, and the intima of human atherosclerotic plaques as well as in adventitial tissue and account for up to ≈54% of all leukocytes in the shoulder region and ≈14% in the necrotic core.59,60 Contrastingly, (dying) macrophages dominate the necrotic core of plaques while B cells can only be found in relevant numbers in adventitial tissue.39,61 Recent immunophenotyping has validated the frequency of T cells in human atherosclerotic tissue, where T cells accounted for up to 65% of all leukocytes in CyTOF37 and ≈52% in scRNA-seq of human atherosclerotic plaques.45 In mice, relative frequencies of T cells seem to be lower: in 2 studies, myeloid cells, T cells, B cells, and other smaller leukocyte subsets accounted each for ≈25% of all leukocytes in atherosclerotic aortas of Apoe−/− and Ldlr−/− mice,39,46 while another study found ≈6% of all leukocytes to be CD3+TCR-β+ T cells by CyTOF of atherosclerotic mouse aortas.41 Notably, the approximate frequencies of parental leukocyte lineages seem to be consistent between conventional flow cytometry, CyTOF, and scRNA-seq in direct comparison.37,39 Besides potential differences related to the species, experimental protocols, and tested tissues (whole aorta versus plaque), the loss of particular cell types during tissue digestion may explain these inconsistent findings.39,46,49 T cells are already detectable in adventitial tissue before the initiation of atherosclerosis: scRNA-seq of adventitial tissue demonstrated the existence of several T-cell subsets already in wild-type animals,52 including 2 CD4+ subsets (clusters 12, 13 in the original dataset) and one CD8+ population (cluster 2 in the original dataset).52 CD8+, but not CD4+, T cells were also found in the healthy arterial vessel of Apoe−/− mice,39 whereas the highest numbers of CD4+ and CD8+ T cells were detected in atherosclerotic aortas. Notably, frequencies of T cells do not substantially differ between Apoe−/− and Ldlr−/− mice.39,46 In aggravated hypercholesterolemia, T-cell fractions in Apoe−/− and Ldlr−/− mice decrease, while total numbers increase,39,46 suggesting that the relative contribution of myeloid cells is increasing in advanced atherosclerosis. It is noteworthy that adventitial CD4+ T cells increase up to 10-fold in the proximity of atherosclerotic plaques.62,63 These may be partially located in artery tertiary lymphoid organs, structures that harbor significant numbers of CD4+ T cells, B cells, and other lymphocytes in aged mice.61 It is therefore tempting to speculate how the spatial organization of T cells impacts on lesion development: while CD4+ T cells in atherosclerotic lesions could instruct and interact with other tissue-resident cells, specialized TFH cells in the adventitia could interact with highly concentrated B cells in artery tertiary lymphoid organs to induce immunoglobulin switching. Such side-specific cellular functions will have to be determined in future work, which could take advantage of the recent development of spatial single-cell transcriptomics that provide simultaneous gene expression and spatial information.64
How circulating T cells correspond to T cells in the plaque has not been investigated in-depth, but a recent report applying cellular indexing of transcriptomes and epitopes by sequencing indicated that CD4+ and CD8+ effector-memory T cells37 and T cells with a clonal restriction of the TCR37,65 are overrepresented in atherosclerotic plaques in comparison with circulating T cells. These findings raise the question whether activated T cells primarily home to the plaque as recently suggested9,66,67 or are generated in situ by local proliferation as a result of their interaction with local APCs.9 Interestingly, 2 scRNA-seq reports have detected clusters of proliferating leukocytes that include T cells,39,47 arguing for in situ proliferation. Consistently, earlier evidence has suggested that DNA-synthesis, which typically occurs in proliferating cells, colocalizes with T cells.68 It is therefore likely that both mechanisms—migration and in situ proliferation—contribute to the pool of lesional T cells.
Distinction of T-Helper Cell Phenotypes in the Plaque
Traditional immunophenotyping by fluorescence-activated cell sorting is per se limited by the usage of predefined sets of antibodies: in most studies, staining for CD62L and CD44, which discriminates between naive, effector-memory T cells, and central-memory T cells in the mouse, has been combined with intracellular expression of TH-defining TFs or cytokines for canonical gating and identification strategies.2,3,5,28,69 Contrastingly, in most available scRNA-seq studies, TH-cell phenotypes were inferred from dimensionality reduction algorithms to detect clusters of cells with similar transcriptome without the bias of preselecting markers. Several T-cell populations that partially overlap have been identified by these screening approaches: 4 in Apoe−/−39 and in Ldlr−/− mice,46 3 populations in adventitial cell preparations from Apoe−/− and wild-type mice,52 and 5 clusters in an integrative analysis of published datasets.47 The latter meta-analysis expands over the original studies by identifying one discrete population of Tregs, which did not form a separate cluster in the original datasets. Some datasets contained a cluster with a proliferation and T-cell gene signature, albeit it is not clear whether this cluster contains other cell types as well.39 The most consistent unique T-cell phenotypes across these studies include: a multilineage-committed (Treg-TH1-TH17-TH2) Cxcr6-expressing T cell (1), a naive T cell (2), a thymocyte-like T cell (3), and a Cd8+ cytotoxic T cell (4). Phenotypes, frequencies, and proposed functions of these populations will be discussed in detail (Figure 1) in the following.
Figure 1.
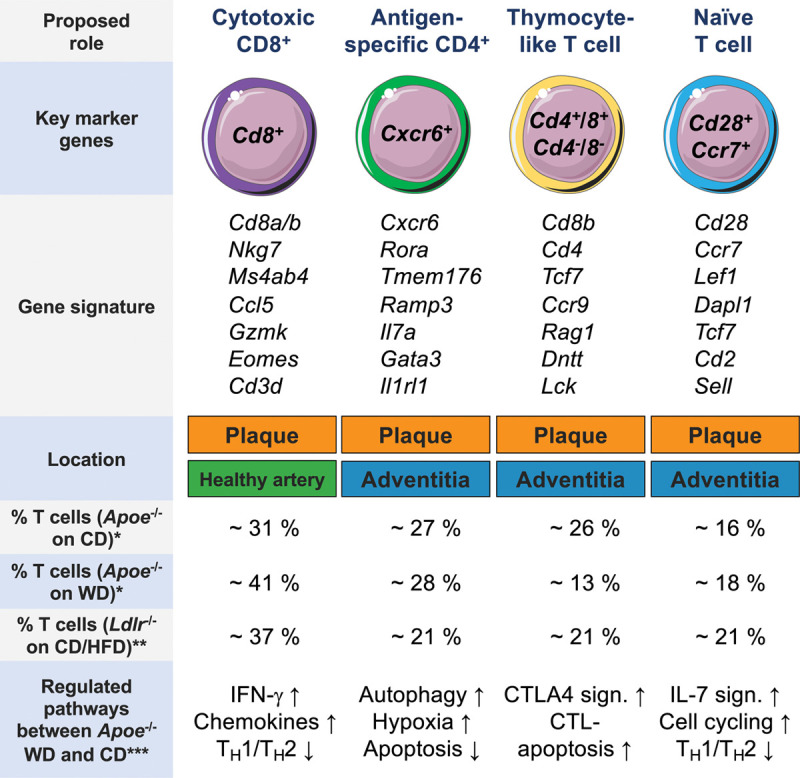
Distinct vascular T-cell phenotypes in the healthy and atherosclerotic mouse aortas. Overview of the 4 major T-cell phenotypes identified by single-cell RNA-sequencing with key gene expression, frequencies, and regulated pathways during atherosclerosis progression. Gene signatures were retrieved by differentially expressed genes among all leukocytes across several studies. Frequencies of clusters were retrieved by an analysis of the original data set (*),39 or estimations based on the original publication (**).46 CD indicates chow diet–consuming mice; CTL, cytotoxic T-cell–mediated apoptosis; HFD, high-fat diet–consuming mice; IFN-γ, interferon-gamma; and WD, Western diet–consuming mice. ***Regulated pathways between CD and WD-fed mice were retrieved from study by Winkels et al.39 Schematics adapted from “Smart Servier Medical Art.”
Cytotoxic CD8+ T Cells
One cluster of CD8+ (Cd8b+) cytotoxic T cells has been detected by scRNA-seq in atherosclerotic and healthy aortas but not in the adventitia,39,46,52 suggesting CD8+ T cells are already present in the healthy intima or media of arteries. Genes differentially expressed by CD8+ T cells include Cd8a, Cd8b, Nkg7 (coding for natural killer cell granule protein 7), Ms4a4b, the CD20 homologue in T cells, Ccl5 (coding for C-C motif chemokine-ligand 5), Eomes (coding for the TF eomesodermin), and Gzmk (coding for Granzyme K). In scRNA-seq, 31% to 41% of lesional T cells were identified as cytotoxic T cells depending on tested genotypes and diets.39,46 The overall frequency of CD8+ T cells was confirmed by 2 CyTOF-studies of mouse aortas (≈30% of T cells).39,41 In humans, ≈30% of all leukocytes (and ≈50% of T cells) in plaques are CD8+ T cells.37 In histology, the accumulation of CD8+ T cells seems to be particularly dense in the fibrous cap region,70 where they outnumber CD4+ T cells.70,71 The frequency of some CD8+ T-cell subsets found only in CyTOF correlates to TCR-clonality in plaques,37 suggesting their antigen-specific expansion. However, cognate antigens for CD8+ T cells remain to be determined, although HSP60 (heat shock protein 60)72 and ApoB73,74 are among the prime candidates. Generally, functions of CD8+ T cells include the removal of virus-infected and cancer cells.75 The function of CD8+ T cells in atherosclerosis is ambivalent and stage dependent: antibody-mediated depletion of CD8+ T cells prevented atherosclerosis76–78 by attenuating proinflammatory cytokine levels and myelopoiesis.78 In established atherosclerosis, the depletion of CD8+ T cells was proatherogenic and reduced plaque stability, which was accompanied by elevated macrophage and T cell reponses.77,79 Vaccination studies with HSP60 and ApoB peptides evidenced antiatherogenic effects by CD8+ T cells,72–74 although no effect on atherosclerosis was observed in a humanized HLA mouse model.80 Vaccination-induced atheroprotection was discussed to be a consequence of lower dendritic cell numbers in aortas,74 enhanced macrophage death, and a blockade of TH17 cell formation.81 Atheroprotective properties of CD8+ T cells have been linked to CD8+ Tregs,82 a known subpopulation with immunosuppressive propensities.83 It remains unclear how this functional dichotomy of CD8+ T cells corresponds to distinct subsets found in recent CyTOF studies.39,41 It is well possible, that some CD8+ T cells with distinct features occur uniquely in atherosclerotic lesions similar to recently discovered subsets of noncytotoxic CD8+ T cells in the tumor microenvironment.84
Naive T Cells
A Cd28+Ccr7+ cluster is detectable in the atherosclerotic aorta and the adventitia across several scRNA-seq data sets.39,46,47,52 The consensus genes for this cluster include Lef1, Dapl1, Tcf7, Sell, and Cd2 (Figure 1). The chemokine receptor CCR7 (gene Ccr7) is expressed by naive and central memory cells and instrumental for lymph node homing.85 The costimulatory molecule CD28 (gene Cd28) is required for effective T-cell co-stimulation by APCs and predominantly expressed by naive CD4+ T cells.86 Although CD28 costimulation is not necessary for a memory recall-response, its therapeutic blockade reduces proatherogenic TH1 responses.87 Cd28+Ccr7+ T cells account for ≈21% to 27% of all T cells.39,46 They become rarer and engage cell cycling genes and increase IL-7 signaling,39 which supports survival, differentiation, and proliferation of T cells,88 in more advanced atherosclerosis. This indicates that naive T cells either leave the plaque or transform into activated cell types represented by one of the other clusters. Because the core genes of this cluster are expressed by CD4+ and CD8+ cells,89 this cluster likely contains naive CD4+, CD8+, and immature-like T cells. Notably, the naive (versus activated) gene signature seems to excel transcriptional differences between principal lineages (CD4+ versus CD8+). In CyTOF of human plaques, naive T cells did surprisingly not form a distinct cluster but were located within principal lineage clusters.37 Numerically, naive T cells were relatively sparse in carotid plaques compared with blood,37 corroborating the predominant activation of lesional T cells.
The CXCR6+ Multilineage Committed T-Helper Cell—an exTreg?
The most discrete cluster among non-Cd8+ T cells39,46,47,52 expresses the transcripts Cxcr6 coding for chemokine receptor CXCR6 (C-X-C chemokine receptor type 6) and Rora coding for the TF ROR-α, which is expressed by TH17 cells and by a subsets of Tregs.90 Other core genes include Tmem176, Ramp3, Il7a, and Il1rl1 (Figure 1). This cluster was termed Il17+Cxcr6+ in a recent meta-analysis.47 It accounts for ≈21% of T cells in Ldlr−/− mice (cluster 8 in the original dataset46) and ≈28 % in Apoe−/− mice (cluster 6 in the original dataset39). CXCR6 is expressed by CD4+ T cells and natural killer T cells and guides T-cell homing. Global deficiency of CXCR6 reduces atherosclerosis and T-cell accumulation,67 particularly of IL-17A-producing CD4+ T cells,91 which is consistent with the concomitant expression of Rora in this subset. Accordingly, Tmem176a and Tmem176b are known to be overexpressed in TH17 cells.92 Yet, the gene signature of this cluster does not seem to be entirely consistent over different studies and comprises a strong TH1746 and/or an additional TH2 gene signature.39,52 A selective analysis of scRNA-seq of filtered Cd4+Cd8− transcriptomes in the aorta showed the highest expression of Cxcr6 in clusters with a mixed TH1/TH2/Treg and an TH1/TH17 transcriptome.5 These findings argue for a more complex phenotype and TH-type of this cluster.
Traditionally, differentiation into TH-types is thought to occur unidirectional with only minimal overlap between distinct lineages. This concept was challenged by the discovery of CD4+ T cells co-expressing Foxp3, T-bet, and/or RORγT in various (auto-) immune pathologies.3,10,29,93–95 Expression of >1 TF may argue for an enhanced plasticity of cells that are in transition into another TH-type. On the other hand, in the intestine, RORγt+ Tregs96 and TH1-like Tregs97 represent unique lineages that control mucosal immunity. Depending on the condition, these mixed phenotypes result in pro- and/or anti-inflammatory properties.2,3,10,29,93–95 Based on flow cytometry with canonical gating strategies and immunohistochemistry 22% to 30% of all CD4+ T cells are pathogenic TH1 cells.29,35 Tregs are less abundant in the aorta (≈5% to 30%).98,99 It should be noted that aortic Tregs numbers decline in atherosclerotic mice over time.28 Despite the contrasting role of TH1 and Treg in atherosclerosis, 2 newer studies reported TH1-like Tregs co-expressing FoxP3, T-bet, IFN-γ, and CCR5 in lymph nodes and atherosclerotic aortas of Apoe−/− mice.6,10,29 Fate mapping experiments with adoptive transfers established that these TH1/Tregs evolve from Tregs.5,29 Consistently, in early scRNA-seq experiments, about 40% of all CD4+ T cells expressed Treg and TH1 lineage genes.29 In bulk RNA-seq, TH1/Tregs were transcriptionally closer to effector T cells than to Tregs.10 Functionally, these cells failed to fully suppress Teffs and did not protect from atherosclerosis—despite the expression of FoxP3.10,29 Recent evidence has validated that 50% of all lesional CD4+ T cells express Cxcr6 and TH1/TH17/Treg/TFH genes simultaneously.5 The coexpression of TH1- and TH17-associated genes has also been shown in CD4+ T cells isolated from human carotid plaques.37
The loss of the Treg-defining TF FoxP3, particularly in antigen-specific TH cells, in the context of hypercholesterolemia and intracellular cholesterol accumulation seems to contribute to the switch of a Treg into an exTreg.5,22 In fact, in FoxP3-lineage-tracer mice, about 15% to 26% of all lesional CD4+ T cells stem from initial Tregs.5,22 The exact molecular or cellular stimuli that cause a loss or an inactivity of FoxP3 in the setting of atherosclerosis are largely unknown. A solid line of evidence has identified inflammatory cues as crucial drivers of such Treg plasticity: Certain thymus-derived Tregs undergo a reprogramming to effector-like T cells that depends on the downregulation of the transcription factor EOS encoded by Izkf4, an important co-repressor of Foxp3,100 at sites of inflammation. This process seems to require IL-6, a proinflammatory cytokine that is elevated in atherosclerotic plaques.101 IFN-γ, another proatherogenic key cytokine secreted by proinflammatory TH1 cells102 has the ability to promote Treg destabilization.103 Under chronic cellular hypoxia as detected in atherosclerotic plaques,104 Foxp3 becomes polyubiquinated and degraded in lysosomes. A key transcription factor induced by hypoxia is HIF-1α (hypoxia-inducible factor 1-alpha), which can bind to Foxp3 and directly cause its degradation while simultaneously stabilizing TH17 gene expression.105 In addition, it has been established that other proinflammatory cytokines or lipopolysaccharide promote Foxp3 degradation.106 Likewise, several signaling pathways such as these involving the TF NFκB (nuclear factor κ-light-chain-enhancer), IRF (interferon regulatory factor)-4, Runx (Runt-related transcription factor), FoxP (Forkhead box protein P)-1, the nuclear protein DBC (deleted in breast cancer)-1, and serine/threonine-Pak (protein kinase)-2 (reviewed in study by Korn and Muschaweckh107) as well as self-peptide induced TCR-signaling108 have been linked to the plasticity of Tregs. Altogether, these findings demonstrate a strong association of inflammation and Treg-instability, which sparks the yet unproven hypothesis that the multitude of factors that initiate and maintain atherosclerosis, such as local and systemic inflammation, LDL (low-density lipoprotein), vascular integrity and injury, immune cell accumulation, and antigen-recognition by Tregs, favor a stage- and context-specific conversion of an initial protective into a pathogenic immune response (Figure 2). This process is likely gradual. Yet, it remains to be determined, which of the known signaling pathways and upstream mediators have a dominant role specifically in the context of atherosclerosis.
Figure 2.
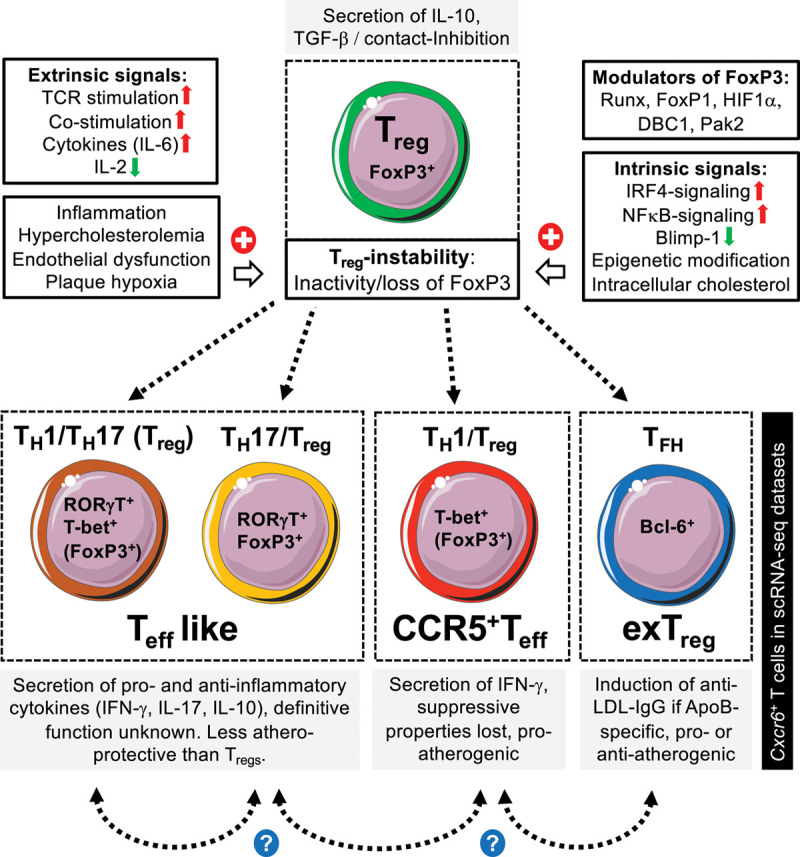
Proposed fate of T regulatory cells in atherosclerosis. In the course of atherosclerosis, regulatory T cells (Treg) with an immunosuppressive and atheroprotective function lose their protective properties. In atherosclerotic plaques or lymph nodes, former Tregs are characterized by an entire loss (exTreg) or an inactivity of FoxP3 (Teff-like, CCR5+ Teff). We propose that several classes of signals may induce such inactivity or loss of FoxP3. These include extrinsic signals (hypercholesterolemia, chronic low-grade inflammation, endothelial dysfunction, and plaque hypoxia) and overwhelming immune-signaling caused by repetitive (auto-) antigen presentation and costimulation. Intrinsic proinflammatory signaling events and epigenetic modifications may partially be promoted by such extrinsic stimuli and/or intracellular cholesterol accumulation. In addition, several modulators of FoxP3 have been identified. Destabilized Tregs can develop into distinct phenotypes, which can express several opposing TFs and canonical T-cell cytokines simultaneously: (1) TH1/TH17 Tregs, (2) TH17 Tregs, (3) TH1 Tregs, and (4) follicular T helper cells (TFH). Because of the composite TH1/TH17/TH2/TFH phenotype and the expression of CXCR6 (C-X-C chemokine receptor type 6), it has been speculated that ApoB (apolipoprotein B)-specific T cells (ApoB+) correspond to the Cxcr6+ T-cell subset in single-cell RNA sequencing studies. However, it is not clear if an ApoB+ cell evolves from pure a FoxP3+ Treg (shown in green) or only exists in the reported composite phenotype. Likewise, the relationship of the 4 switched Treg phenotypes and the existence of more intermediate phenotypes remains unclear. Schematics adapted from “Smart Servier Medical Art.” Transcription factors displayed in brackets indicates only a low level of residual gene or protein expression. Bcl-6 indicates B-cell lymphoma protein-6; DBC1, deleted in breast cancer-1; FoxP, Forkhead box protein P; IFN-γ, interferon-gamma; IL, interleukin; IRF4, interferon regulatory factor-4; LDL, low-density lipoprotein; NFκB, nuclear factor κ-light-chain-enhancer; Pak, protein kinase; Runx, Runt-related transcription factor; TCR, T-cell receptor; and TGF-β, transforming growth factor-β.
The definitive function of exTregs is controversial: while the mixed Treg/TH17 phenotype may represent an intermediate stage toward a TH1-dominated composite phenotype with a residual atheroprotective function,5 a mixed TH1-Treg aggravated atherosclerosis in a direct T-cell transfer in mice.10,29 On the other hand, Tregs have the ability to acquire a TFH-like phenotype with enhanced IFN-γ secretion: a deletion of the TFH-transcription factor BCL (B-cell lymphoma protein)-6 seems to be atheroprotective, suggesting that the conversion of Tregs into TFH may be proinflammatory.22 However, in a transgenic mouse model, LDL and LDL-reactive CD4+ T cells converted into TFH cells, which protected from atherosclerosis though secretion of LDL-depleting IgG antibodies.109 Thus, TFH cells could be pro- and anti-inflammatory, and this effect may likely dependent on antigen-specificity and tissue localization. Depending on the development of cluster algorithms in dimensionality reduction, it is foreseeable that even more distinct clusters with mixed phenotypes will be discovered. Conclusively, it should be assumed that the plasticity of Tregs represent a gradual shift in phenotypes and effector functions triggered by a multitude of spatial and temporal events rather than a singular event that switches a cell from one to another definitive phenotype (Figure 2).
The ApoB-Specific TH Cell
The existence of ApoB-specific TH cells has been indirectly inferred from a restricted (oligoclonal) TCR repertoire in the plaque37 and of ApoB-specific T cells in lymph nodes,5 cloning of oxLDL (oxidized low-density lipoprotein)-reactive CD4+ T cells from human and mouse atherosclerotic plaques4,110 as well as from inflammatory cytokine secretion by CD4+ T cells restimulated with ApoB or LDL.5,65 Several fundamental characteristics of the autoreactive T-helper cell response have remained unanswered because tools to detect single ApoB-specific T cells (ApoB+) were not available. To overcome these limitations, we have recently developed recombinant MHC-II multimers loaded with ApoB-peptides for the direct fluorochrome-based detection of single ApoB-reactive CD4+ T cells in mice and humans.3,5 These studies helped to define fractions, phenotypes, and dynamics of ApoB-specific cells in the atherosclerotic plaque and systemically: even in the absence of relevant atherosclerosis, we detected small fractions of ApoB+ cells in mice and humans that transcriptionally and phenotypically resembled Tregs.3,5 In disease, ApoB+ cells gradually acquired a proinflammatory phenotype close to that of TH1/TH17/TFH cells (Figure 2). In single cell gene set enrichment analysis, about 50% of aortic CD4+ T cells (Cd4+Cd8−) had a gene signature similar to that of ApoB+ T cells. ApoB+ but not ApoBneg T cells in lymph nodes expressed moderate to high levels of Cxcr6 in bulk RNA-seq,5 suggesting that CXCR6 may serve as the homing receptor of antigen-specific CD4+ T cells. Although integrative analysis of this cellular subset and aortic T cells is not available, the gene signature strongly suggests that Cxcr6+ T cells in the plaque may represent T cells specific for an atherosclerosis-relevant antigen.
While the function of the heterogeneous population of ApoB+ CD4+ T cells in atherogenesis has not been clarified completely, the distinctive function of individual TH-lineages has been validated extensively in the last decades.6,7 Notably, scRNA-seq of ApoB+ T cells has revealed several distinct clusters with mixed Treg, TH17, and TH1 phenotypes in mice. In some of these clusters, a supposedly protective while in others a pathogenic gene expression profile dominated.5 Flow cytometry of human ApoB+ T cells has revealed similar phenotypic patterns.3,5 In another study, LDL-reactive T cells from a mouse expressing a transgenic TCR directed against LDL/ApoB developed into TFH. These were atheroprotective by inducing antibodies that cleared LDL.109 It is therefore to assume that ApoB+ T cells likely exist in several flavors and functions that range from immunosuppressive to pathogenic phenotypes. It should also be noted that in several pathologies, antigen-specific Tregs are prone to mechanisms of plasticity as outlined above.108 In general, effector functions of T-helper cells in atherosclerosis are manifold: they have the ability to interact with other immune and stromal cells and thereby instruct other leukocytes to promote or inhibit plaque regression,50 and to secrete pro- or anti-inflammatory cytokines, myeloid cell growth factors, or matrix-destabilizing proteases that can induce an instable plaque phenotype. In addition, they may act immunosuppressive by direct cellular contact inhibition or cytotoxic by granzyme-induced cell death.111 The exact mechanisms by which ApoB+ T cells potentially modulate atherosclerosis, however, are currently unknown.
Evidence for Immature Thymocyte-Like T Cells in the Plaque?
Several studies employing CyTOF, scRNA-seq, or CITE-seq have demonstrated the existence of aortic and plaque-resident T cells that do not express protein or gene transcripts coding for CD4 and CD8 (DNs) or that express both (DPs).37,39 It has been speculated that aortic/plaque DP and DN may represent immature T cells (thymocyte-like),47 but this hypothesis is controversial: In early scRNA-seq studies and a recent meta-analysis, one cluster had a high cluster-wide expression for Cd4, Cd8, and Cd3g/Cd3d which was termed mixed, possibly because of a mix of single-positive Cd4+ and Cd8+ T cells in the same cluster. This cluster accounts for ≈13% to 26% of aortic T cells in mice39,46 and expresses high levels of the thymocyte genes coding for TCF1 (Tcf7), Rag-1 (Rag1), and C-C motif chemokine receptor 9 (Ccr9) (Figure 1). The existence of DPs (Cd4+Cd8+) on a single cell level, however, was not directly proven in these data sets because of the technical limitations of scRNA-seq described above. In addition, immature DP-thymocytes express only extremely low levels of extracellular TCR-β/CD3-complexes. CyTOF of human lesional T cells, however, detected a cell cluster with surface expression of CD3, CD4, and CD8,37 which is arguing against a real thymocyte-like phenotype. Interestingly, mature CD3+CD4+CD8+ T cells that do not express thymic-stage markers have been identified in cancer tissues112,113 and blood of patients with rheumatoid arthritis.114 CD4+CD8+ DP aortic T cells may in fact be closer to these peripheral DP cells than to immature (thymic) DP cells.
Other clusters in one CyTOF dataset expressed TCR39 (in mice) or CD337,39 (in humans) at a low level, but not CD4 or, which is suggestive of thymocyte DN-like cells or a chronically activated T cell that has downregulated CD4 or CD8, respectively.115,116 In one study, some of these CD4−CD8−CD3+ cells additionally expressed the NK cell marker CD56, and CD45RA, which may argue for natural killer T cells117 rather than for DN T cells. CD4+CD8+ and CD4−CD8− cells could therefore contain several subsets (DP-like, DN-like) with distinct functionality and origin. While some expression patterns argue for immature cells, their exact phenotypes, transcriptomes, and functions are not resolved yet. The most intriguing and controversial question will have to be addressed in future work: Can extrathymic T-cell maturation occur in atherosclerotic plaques?
Evidence for Tissue-Residency and Exhaustion in Atherosclerosis?
In contrast to scRNA-seq, which allows screening of the entire transcriptome at the cost of low-expressed genes, high-parametric protein immunophenotyping by CyTOF helps to interrogate phenotypic heterogeneity based on known surface markers. CyTOF was particularly powerful to subdivide human lesional T cells into distinct states of activation, tissue residency, and exhaustion. The predominant differentiation of lesional T cells into activated (antigen-experienced) effector-memory T cells and central-memory cells (CCR7lowCD45RAhighCD69+) was observed and suggested the existence of tissue-resident memory T cells (TRM) in the atherosclerotic plaque.37 TRMs are a unique lineage of T cells that reside in tissues but do not recirculate and thereby provide local tissue protection in infection. CD69 and CD103 are key markers for TRMs, although not all TRMs express these markers.37,69 In atherosclerotic plaques, a subcluster of CD8+ T cells coexpressed CD69+ and CD103+0.37 While the exact role of this T-cell subpopulation remains to be defined experimentally, it is intriguing to speculate whether TRMs in atherosclerotic lesions are antigen specific or not: a large body of work has suggested that chronic and acute infection caused by cytomegalovirus, HIV, influenza virus, Chlamydia pneumoniae,118 and severe acute respiratory syndrome coronavirus 2119 may directly interfere with the development and progression of atherosclerosis. The in-depth evaluation of TRMs may help to better understand potential interactions between pathogens, the healthy vessel wall, and atherosclerotic disease.
Several populations of plaque-resident T cells were detected by CyTOF37 that express the coinhibitory molecule PD-1 (programmed cell death protein 1), which marks T-cell exhaustion,120 a state of terminal differentiation, dysfunctionality, and hyporesponsiveness induced by chronic antigen-specific stimulation.120 PD-1+ cells coexpressed other known markers of T-cell exhaustion: CD57, which indicates terminal differentiation and senescence in T cells,121 EOMES (eomesodermin), and Lag-3 (lymphocyte-activation gene 3). In mice, the ectonucleotidase CD39 inhibits expression of the effector cytokines TNF-α and IFN-γ in a subset of CD8+ T cells in atherosclerotic aortas.122 Physiologically, T-cell exhaustion seems to arise from a chronic exposure to autoantigens. Whether this state of dysfunctionality is driving or protecting from atherosclerosis is not entirely clear yet. A genetic deficiency of PD-1 or antibody-mediated blockade of PD-1 increased atherosclerotic lesion size,123,124 which is consistent with the clinical observation that cancer patients receiving checkpoint inhibition therapies that block PD-1 and, thus, reverse exhaustion are at an increased risk of atherosclerotic disease.125
Autoimmunity—a Translatable Target for Human Disease?
Preventive concepts for patients with clinical atherosclerosis are currently limited to a modulation of classical risk factors and lipid-lowering strategies, although an increasing body of evidence argues for a role of the immune system and inflammation.6 High parameter immunophenotyping has proven its ability to identify novel and unexpected candidate markers and mediators of atherosclerosis. For instance, scRNA-seq from human carotid atherosclerosis has surprisingly suggested a predominance of proinflammatory IFN-γ-, IL-12-, and TNF-signaling across different cell types in asymptomatic patients—a finding that contradicts the traditional view that proinflammatory pathways trigger plaque instability and cardiovascular complications.126 Likewise, pathways associated with IL-1β, a validated therapeutic target in coronary atherosclerosis in the CANTOS trial (Canakinumab Antiinflammatory Thrombosis Outcome Study),127 were less engaged in T cells from rupture-prone symptomatic carotid plaques. Although these findings may be explained by fundamental differences in carotid versus coronary atherosclerosis or an only partially proatherosclerotic role of IL-1β,128 more extensive research is required to understand the clinical impact of T-cell diversity in humans.
The translation of murine T-cell immunology remains an ongoing challenge. Basic concepts of T-cell heterogeneity have largely been defined in the mouse with an increasing number of single cell studies in mouse atherosclerosis defined to either describe leukocytes39,40,46–52 or stromal cells.42–44 In contrast, only a limited number of studies exploring single cell phenotypes of T cells in human atherosclerosis is available yet.37,45 The latter have established that—in contrast to mice—phenotypic differences in humans are driven mostly by transcriptional programs of activation, exhaustion, and inflammation and not by traditional TH-types. How phenotypes in both species overlap is currently unknown. In addition, human plaques seem to be dominated by T cells, although atherosclerotic plaques from mice contain relatively more macrophages. Beyond differences in fundamental T-cell phenotypes, mechanisms and dynamics of immune cells in mice and humans differ considerably and render the direct comparison difficult. First, T cells in the mouse are generated throughout life, while the production of new T cells in humans ceases over time. This, together with the lack of repetitive infections and a mature microbiome in sterile-housed mice, partially explains the higher degree of exhaustion and activation in human T cells.129,130 Second, inbred mouse strains favor certain types of immune responses, which is exemplified by C57BL/6J mice that have a bias toward TH1 and BALB/C mice that are more likely to elicit TH2 responses. The genetic background itself has a direct impact on atherosclerosis as recently demonstrated by the hybrid mouse diversity panel.131 Third, mice represent an ideal model organism to explore peptide specific T-cell responses: common laboratory mouse strains express only one MHC-II variant (eg, I-Ab in C57BL/6J mice), which allows direct and feasible in silico screening strategies for potential self-peptides. On the contrary, humans exhibit a considerable variability with over 200 MHC-II alleles that form over 10 000 different functional MHC-II complexes. Because the susceptibility of an autoimmune response against self-peptides is determined by the capacity of peptides to bind to the MHC-II complex, antigen-specific responses in humans are far more difficult to predict. Only a few studies have validated the existence of ApoB-specific T cells in humans,3,5,110 and it remains elusive whether the sequences and MHC-II affinities of different self-peptides predispose for distinct phenotypes and outcomes in humans. It is now acknowledged that a more fine-grained analysis of dynamics, phenotypes, and specificities of atherosclerosis-relevant or ApoB-specific T cells is instrumental to establish novel immunomodulatory strategies for human atherosclerosis in the future.
Concluding Remarks
High-parametric leukocyte analysis of atherosclerotic aortas and plaques has led us to a deeper understanding of leukocyte diversity and activation. Several studies have applied transcriptional, proteomic, and immunologic approaches, often in a combination of these techniques, to describe yet unknown layers of T-cell heterogeneity in mouse and human atherosclerosis. Together, these studies pinpoint T cells as powerful modulators of plaque associated inflammation: they represent the largest population of viable leukocytes in the plaque, in particular in humans, with a great and largely unexpected phenotypic diversity. Several novel concepts have arisen from these first studies: first, traditional immunologic concepts that are based on TH-lineages fail to describe the actual heterogeneity of T cells in the atherosclerotic plaque that is rather driven by states of activation, exhaustion, development, and inflammation. Second, a substantial proportion of pathogenic T cells stems from regulatory T cells that are highly plastic and have the ability to pass through different TH-lineages. Third, a relevant fraction of CD4+ T cells in the plaque can be considered antigen-specific and recognizes autoantigens such as LDL/ApoB. Ongoing antigen-recognition and TCR-signaling events, and the proinflammatory microenvironment in the plaque, are the likely causes for a unique T-cell phenotype that combines highly flexible pro- and anti-inflammatory attributes at the same time. Fourth, appearance and gene expression of leukocyte populations in the plaque and in the circulation seem to correlate with disease progression. At the same time, the field of high parameter immunophenotyping has just been evolved and many important questions have to be addressed in future work. It is critical to understand which exact stimuli drive the destabilization of Tregs and the appearance of pathogenic cell clusters and whether these can be targeted by medical therapies. Therefore, it is required to more precisely link phenotypes to clinical outcomes. The in silico enumeration of functional T-cell clusters in peripheral blood or tissues (liquid biopsies, virtual flow cytometry)132 holds great promise to establish high-throughput prediction tools.39 Future immunophenotyping will also have to account for disease-specific tissue gene signatures and the identification of antigen-specific T cells by MHC-II multimers or TCR-sequencing (Figure 3). Only the detailed understanding of underlying mediators of the inflammatory and immune response will allow us to develop tailored immunomodulatory strategies as atheroprotective vaccination in the future.
Figure 3.
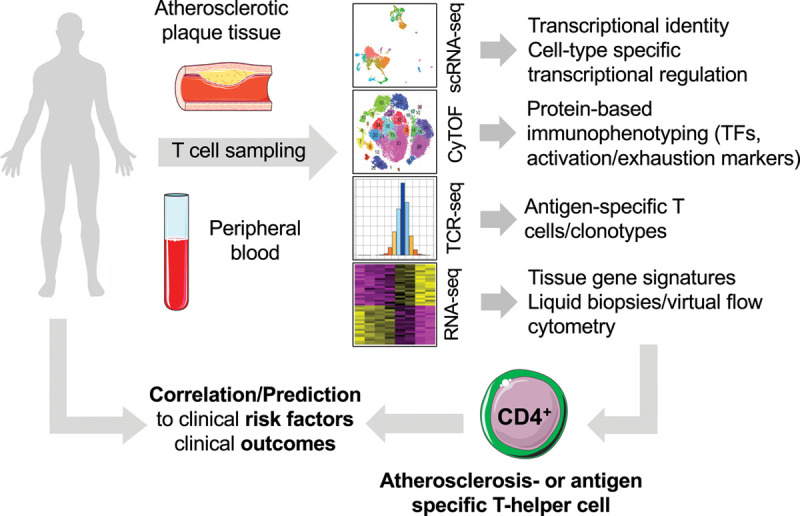
Novel high-parametric and functional immunophenotyping to develop future risk prediction tools. In humans, T cells can be routinely sampled from surgically excised carotid, peripheral arterial atherosclerotic plaques, and peripheral blood. Mass cytometry (cytometry by time of flight [CyTOF]), single cell RNA-sequencing (scRNA-seq), or a combination of antibody-staining and scRNA-seq in cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) have the potential to uncover unique, even rare, cell populations with distinct identities and potentially specialized roles in atherosclerosis (atherosclerosis-specific T cell). The addition of functional layers by staining with tetramers of MHC-II (major histocompatibility complex II) loaded with peptides from autoantigens and with T-cell receptor (TCR) sequencing in a combination with CyTOF or scRNA-seq integrates the cell surface proteome and transcriptome with antigen-specificity. Such integration generates cell type-specific gene signature, which—vice versa—are detectable in silico in bulk RNA-sequencing data sets, an approach referred to as RNA-deconvolution, liquid biopsy, or virtual flow cytometry. The correlation with clinical outcome data will be helpful to discover clinically relevant cell types. Their transcriptomes or frequencies in blood or tissue may predict the clinical outcomes or identify novel targets for future immunomodulatory cell therapy. TF indicates transcription factor. Schematics adapted from “Smart Servier Medical Art.”
Sources of Funding
This work was supported by a fellowship to D. Wolf from the Berta-Ottenstein-Program for Advanced Clinician Scientists at the Faculty of Medicine, University of Freiburg. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 853425). H. Winkels was supported by the Deutsche Forschungsgemeinschaft Sonderforschungsbereich/Transregio 259/1 and the Neven-DuMont Foundation.
Disclosures
None.
Nonstandard Abbreviations and Acronyms
- ApoB
- apolipoprotein B
- CXCR5
- C-X-C chemokine receptor type 5
- CXCR6
- C-X-C chemokine receptor type 6
- CyTOF
- cytometry by time of flight
- DBC
- deleted in breast cancer
- DN
- double-negative
- DP
- double-positive
- EOMES
- eomesodermin
- FoxP
- Forkhead box protein P
- IL
- interleukin
- IRF
- interferon regulatory factor
- Lag-3
- lymphocyte-activation gene 3
- LDL
- low-density lipoprotein
- MHC
- major histocompatibility complex
- NFκB
- nuclear factor κ-light-chain-enhancer
- Pak
- protein kinase
- PD-1
- programmed cell death protein 1
- Runx
- Runt-related transcription factor
- scRNA-seq
- single-cell RNA sequencing
- TCR
- T-cell receptor
- TF
- transcription factor
- TFH
- follicular T helper cell
- TGF-β
- transforming growth factor-β
- TH
- T-helper type
- Treg
- T regulatory cell
For Sources of Funding and Disclosures, see page 560.
References
- 1.Taniuchi I. CD4 helper and CD8 cytotoxic T cell differentiation. Annu Rev Immunol. 2018;36:579–601. doi: 10.1146/annurev-immunol-042617-053411 [DOI] [PubMed] [Google Scholar]
- 2.DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 2016;16:149–163. doi: 10.1038/nri.2015.18 [DOI] [PubMed] [Google Scholar]
- 3.Kimura T, Kobiyama K, Winkels H, Tse K, Miller J, Vassallo M, Wolf D, Ryden C, Orecchioni M, Dileepan T, et al. Regulatory CD4+ T cells recognize major histocompatibility complex class II molecule-restricted peptide epitopes of apolipoprotein B. Circulation. 2018;138:1130–1143. doi: 10.1161/CIRCULATIONAHA.117.031420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermansson A, Ketelhuth DF, Strodthoff D, Wurm M, Hansson EM, Nicoletti A, Paulsson-Berne G, Hansson GK. Inhibition of T cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf D, Gerhardt T, Winkels H, Michel NA, Pramod AB, Ghosheh Y, Brunel S, Buscher K, Miller J, McArdle S, et al. Pathogenic autoimmunity in atherosclerosis evolves from initially protective apolipoprotein B100-reactive CD4+ T-regulatory cells. Circulation. 2020;142:1279–1293. doi: 10.1161/CIRCULATIONAHA.119.042863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124:315–327. doi: 10.1161/CIRCRESAHA.118.313591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17:387–401. doi: 10.1038/s41569-020-0352-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8 [DOI] [PubMed] [Google Scholar]
- 9.Koltsova EK, Garcia Z, Chodaczek G, Landau M, McArdle S, Scott SR, von Vietinghoff S, Galkina E, Miller YI, Acton ST, et al. Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, McArdle S, Gholami A, Kimura T, Wolf D, Gerhardt T, Miller J, Weber C, Ley K. CCR5+T-bet+FoxP3+ effector CD4 T cells drive atherosclerosis. Circ Res. 2016;118:1540–1552. doi: 10.1161/CIRCRESAHA.116.308648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Pablo AM, Jiang Xc, Wang N, Tall AR, Schindler C. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. J Immunol. 2005;175:5–14. [PubMed] [Google Scholar]
- 14.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor-/- mice. Arterioscler Thromb Vasc Biol. 2002;22:456–461. doi: 10.1161/hq0302.104905 [DOI] [PubMed] [Google Scholar]
- 15.Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, Wagner O, Stangl H, Soehnlein O, Binder CJ. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med. 2012;4:1072–1086. doi: 10.1002/emmm.201201374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Böckler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126 [DOI] [PubMed] [Google Scholar]
- 17.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, et al. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gisterå A, Robertson AK, Andersson J, Ketelhuth DF, Ovchinnikova O, Nilsson SK, Lundberg AM, Li MO, Flavell RA, Hansson GK. Transforming growth factor-β signaling in T cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013;5:196ra100 doi: 10.1126/scitranslmed.3006133 [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Tang T, Nie D, Wen S, Jia C, Zhu Z, Xia N, Nie S, Zhou S, Jiao J, et al. IL-9 aggravates the development of atherosclerosis in ApoE-/- mice. Cardiovasc Res. 2015;106:453–464. doi: 10.1093/cvr/cvv110 [DOI] [PubMed] [Google Scholar]
- 21.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50:1132–1148. doi: 10.1016/j.immuni.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, McNamara CA, Kronenberg M, Crotty S, Thomas MJ, et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun. 2018;9:1095 doi: 10.1038/s41467-018-03493-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobrian AD, Hatcher MA, Brotman JJ, Galkina EV, Taghavie-Moghadam P, Pei H, Haynes BA, Nadler JL. STAT4 contributes to adipose tissue inflammation and atherosclerosis. J Endocrinol. 2015;227:13–24. doi: 10.1530/JOE-15-0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490 [PubMed] [Google Scholar]
- 25.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- 26.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343 [DOI] [PubMed] [Google Scholar]
- 27.Klingenberg R, Gerdes N, Badeau RM, Gisterå A, Strodthoff D, Ketelhuth DF, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, et al. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maganto-García E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic changes in regulatory T cells are linked to levels of diet-induced hypercholesterolemia. Circulation. 2011;124:185–195. doi: 10.1161/CIRCULATIONAHA.110.006411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butcher MJ, Filipowicz AR, Waseem TC, McGary CM, Crow KJ, Magilnick N, Boldin M, Lundberg PS, Galkina EV. Atherosclerosis-driven treg plasticity results in formation of a dysfunctional subset of plastic IFNγ+ Th1/Tregs. Circ Res. 2016;119:1190–1203. doi: 10.1161/CIRCRESAHA.116.309764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hasib L, Lundberg AK, Zachrisson H, Ernerudh J, Jonasson L. Functional and homeostatic defects of regulatory T cells in patients with coronary artery disease. J Intern Med. 2016;279:63–77. doi: 10.1111/joim.12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. Eur Heart J. 2006;27:2530–2537. doi: 10.1093/eurheartj/ehl222 [DOI] [PubMed] [Google Scholar]
- 32.Ma Y, Yuan X, Deng L, Xu W, Zheng Y, Yue C, Zhang G, Xie F, Yang YH, Gantier MP, et al. Imbalanced frequencies of Th17 and Treg cells in acute coronary syndromes are mediated by IL-6-STAT3 signaling. PLoS One. 2013;8:e72804 doi: 10.1371/journal.pone.0072804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olson NC, Sitlani CM, Doyle MF, Huber SA, Landay AL, Tracy RP, Psaty BM, Delaney JA. Innate and adaptive immune cell subsets as risk factors for coronary heart disease in two population-based cohorts. Atherosclerosis. 2020;300:47–53. doi: 10.1016/j.atherosclerosis.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ, Hansson GK. Hypercholesterolemia induces differentiation of regulatory T cells in the liver. Circ Res. 2017;120:1740–1753. doi: 10.1161/CIRCRESAHA.116.310054 [DOI] [PubMed] [Google Scholar]
- 35.Gao Q, Jiang Y, Ma T, Zhu F, Gao F, Zhang P, Guo C, Wang Q, Wang X, Ma C, et al. A critical function of Th17 proinflammatory cells in the development of atherosclerotic plaque in mice. J Immunol. 2010;185:5820–5827. doi: 10.4049/jimmunol.1000116 [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, Paulsson G, Stemme S, Hansson GK. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J Clin Invest. 1998;101:1717–1725. doi: 10.1172/JCI1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez DM, Rahman AH, Fernandez NF, Chudnovskiy A, Amir ED, Amadori L, Khan NS, Wong CK, Shamailova R, Hill CA, et al. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25:1576–1588. doi: 10.1038/s41591-019-0590-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winkels H, Ehinger E, Vassallo M, Buscher K, Dinh HQ, Kobiyama K, Hamers AAJ, Cochain C, Vafadarnejad E, Saliba AE, et al. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circ Res. 2018;122:1675–1688. doi: 10.1161/CIRCRESAHA.117.312513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McArdle S, Buscher K, Ghosheh Y, Pramod AB, Miller J, Winkels H, Wolf D, Ley K. Migratory and dancing macrophage subsets in atherosclerotic lesions. Circ Res. 2019;125:1038–1051. doi: 10.1161/CIRCRESAHA.119.315175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole JE, Park I, Ahern DJ, Kassiteridi C, Danso Abeam D, Goddard ME, Green P, Maffia P, Monaco C. Immune cell census in murine atherosclerosis: cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc Res. 2018;114:1360–1371. doi: 10.1093/cvr/cvy109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alencar GF, Owsiany KM, Karnewar S, Sukhavasi K, Mocci G, Nguyen AT, Williams CM, Shamsuzzaman S, Mokry M, Henderson CA, et al. The stem cell pluripotency genes klf4 and oct4 regulate complex smc phenotypic changes critical in late-stage atherosclerotic lesion pathogenesis. Circulation. 2020;142:2045–2059. doi: 10.1161/CIRCULATIONAHA.120.046672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan H, Xue C, Auerbach BJ, Fan J, Bashore AC, Cui J, Yang DY, Trignano SB, Liu W, Shi J, et al. Single-cell genomics reveals a novel cell state during smooth muscle cell phenotypic switching and potential therapeutic targets for atherosclerosis in mouse and human. Circulation. 2020;142:2060–2075. doi: 10.1161/CIRCULATIONAHA.120.048378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dobnikar L, Taylor AL, Chappell J, Oldach P, Harman JL, Oerton E, Dzierzak E, Bennett MR, Spivakov M, Jørgensen HF. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat Commun. 2018;9:4567 doi: 10.1038/s41467-018-06891-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Depuydt MAC, Prange KHM, Slenders L, Örd T, Elbersen D, Boltjes A, de Jager SCA, Asselbergs FW, de Borst GJ, Aavik E, et al. Microanatomy of the human atherosclerotic plaque by single-cell transcriptomics. Circ Res. 2020;127:1437–1455. doi: 10.1161/CIRCRESAHA.120.316770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cochain C, Vafadarnejad E, Arampatzi P, Jaroslav P, Winkels H, Ley K, Wolf D, Saliba AE, Zernecke A. Single-cell rna-seq reveals the transcriptional landscape and heterogeneity of aortic macrophages in murine atherosclerosis. Circ Res. 2018;122:1661–1674. doi: 10.1161/CIRCRESAHA.117.312509 [DOI] [PubMed] [Google Scholar]
- 47.Zernecke A, Winkels H, Cochain C, Williams JW, Wolf D, Soehnlein O, Robbins CS, Monaco C, Park I, McNamara CA, et al. Meta-analysis of leukocyte diversity in atherosclerotic mouse aortas. Circ Res. 2020;127:402–426. doi: 10.1161/CIRCRESAHA.120.316903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin JD, Nishi H, Poles J, Niu X, McCauley C, Rahman K, Brown EJ, Yeung ST, Vozhilla N, Weinstock A, et al. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4:e124574 doi: 10.1172/jci.insight.124574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, Jang MY, Seok Jang H, Yun TJ, Lee SH, et al. Transcriptome analysis reveals nonfoamy rather than foamy plaque macrophages are proinflammatory in atherosclerotic murine models. Circ Res. 2018;123:1127–1142. doi: 10.1161/CIRCRESAHA.118.312804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma M, Schlegel MP, Afonso MS, Brown EJ, Rahman K, Weinstock A, Sansbury BE, Corr EM, van Solingen C, Koelwyn GJ, et al. Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression. Circ Res. 2020;127:335–353. doi: 10.1161/CIRCRESAHA.119.316461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams JW, Zaitsev K, Kim KW, Ivanov S, Saunders BT, Schrank PR, Kim K, Elvington A, Kim SH, Tucker CG, et al. Limited proliferation capacity of aortic intima resident macrophages requires monocyte recruitment for atherosclerotic plaque progression. Nat Immunol. 2020;21:1194–1204. doi: 10.1038/s41590-020-0768-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu W, Ni Z, Tan YQ, Deng J, Zhang SJ, Lv ZC, Wang XJ, Chen T, Zhang Z, Hu Y, et al. Adventitial cell atlas of wt (wild type) and ApoE (apolipoprotein E)-deficient mice defined by single-cell RNA sequencing. Arterioscler Thromb Vasc Biol. 2019;39:1055–1071. doi: 10.1161/ATVBAHA.119.312399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, Satija R, Smibert P. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods. 2017;14:865–868. doi: 10.1038/nmeth.4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patil VS, Madrigal A, Schmiedel BJ, Clarke J, O’Rourke P, de Silva AD, Harris E, Peters B, Seumois G, Weiskopf D, et al. Precursors of human cd4(+) cytotoxic t lymphocytes identified by single-cell transcriptome analysis. Sci Immunol. 2018;3:eaan8664 doi: 10.1126/sciimmunol.aan8664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiu P. Embracing the dropouts in single-cell RNA-seq analysis. Nat Commun. 2020;11:1169 doi: 10.1038/s41467-020-14976-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19:1166–1172. doi: 10.1038/nm.3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams JW, Winkels H, Durant CP, Zaitsev K, Ghosheh Y, Ley K. Single cell RNA sequencing in atherosclerosis research. Circ Res. 2020;126:1112–1126. doi: 10.1161/CIRCRESAHA.119.315940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kotov DI, Jenkins MK. Peptide:MHCII tetramer-based cell enrichment for the study of epitope-specific CD4+ T cells. Curr Protoc Immunol. 2019;125:e75 doi: 10.1002/cpim.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansson GK, Jonasson L. The discovery of cellular immunity in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2009;29:1714–1717. doi: 10.1161/ATVBAHA.108.179713 [DOI] [PubMed] [Google Scholar]
- 60.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131 [DOI] [PubMed] [Google Scholar]
- 61.Hu D, Mohanta SK, Yin C, Peng L, Ma Z, Srikakulapu P, Grassia G, MacRitchie N, Dever G, Gordon P, et al. Artery tertiary lymphoid organs control aorta immunity and protect against atherosclerosis via vascular smooth muscle cell lymphotoxin β receptors. Immunity. 2015;42:1100–1115. doi: 10.1016/j.immuni.2015.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Houtkamp MA, de Boer OJ, van der Loos CM, van der Wal AC, Becker AE. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J Pathol. 2001;193:263–269. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH774>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- 63.van Dijk RA, Duinisveld AJ, Schaapherder AF, Mulder-Stapel A, Hamming JF, Kuiper J, de Boer OJ, van der Wal AC, Kolodgie FD, Virmani R, et al. A change in inflammatory footprint precedes plaque instability: a systematic evaluation of cellular aspects of the adaptive immune response in human atherosclerosis. J Am Heart Assoc. 2015;4:e001403 doi: 10.1161/JAHA.114.001403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burgess DJ. Spatial transcriptomics coming of age. Nat Rev Genet. 2019;20:317 doi: 10.1038/s41576-019-0129-z [DOI] [PubMed] [Google Scholar]
- 65.Paulsson G, Zhou X, Törnquist E, Hansson GK. Oligoclonal T cell expansions in atherosclerotic lesions of apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:10–17. doi: 10.1161/01.atv.20.1.10 [DOI] [PubMed] [Google Scholar]
- 66.Mailer RKW, Gisterå A, Polyzos KA, Ketelhuth DFJ, Hansson GK. Hypercholesterolemia enhances T cell receptor signaling and increases the regulatory T cell population. Sci Rep. 2017;7:15655 doi: 10.1038/s41598-017-15546-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galkina E, Harry BL, Ludwig A, Liehn EA, Sanders JM, Bruce A, Weber C, Ley K. CXCR6 promotes atherosclerosis by supporting T-cell homing, interferon-gamma production, and macrophage accumulation in the aortic wall. Circulation. 2007;116:1801–1811. doi: 10.1161/CIRCULATIONAHA.106.678474 [DOI] [PubMed] [Google Scholar]
- 68.Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res. 1999;41:473–479. doi: 10.1016/s0008-6363(98)00311-3 [DOI] [PubMed] [Google Scholar]
- 69.Jameson SC, Masopust D. Understanding subset diversity in T cell memory. Immunity. 2018;48:214–226. doi: 10.1016/j.immuni.2018.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paul VS, Paul CM, Kuruvilla S. Quantification of various inflammatory cells in advanced atherosclerotic plaques. J Clin Diagn Res. 2016;10:EC35–EC38. doi: 10.7860/JCDR/2016/19354.7879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM, Becker AE. Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest. 1989;61:166–170. [PubMed] [Google Scholar]
- 72.Rossmann A, Henderson B, Heidecker B, Seiler R, Fraedrich G, Singh M, Parson W, Keller M, Grubeck-Loebenstein B, Wick G. T-cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T-cell receptor repertoire. Exp Gerontol. 2008;43:229–237. doi: 10.1016/j.exger.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 73.Dimayuga PC, Zhao X, Yano J, Lio WM, Zhou J, Mihailovic PM, Cercek B, Shah PK, Chyu KY. Identification of apob-100 peptide-specific cd8+ t cells in atherosclerosis. J Am Heart Assoc. 2017;6:e005318 doi: 10.1161/JAHA.116.005318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chyu KY, Zhao X, Dimayuga PC, Zhou J, Li X, Yano J, Lio WM, Chan LF, Kirzner J, Trinidad P, et al. CD8+ T cells mediate the athero-protective effect of immunization with an ApoB-100 peptide. PLoS One. 2012;7:e30780 doi: 10.1371/journal.pone.0030780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kyaw T, Winship A, Tay C, Kanellakis P, Hosseini H, Cao A, Li P, Tipping P, Bobik A, Toh BH. Cytotoxic and proinflammatory CD8+ T lymphocytes promote development of vulnerable atherosclerotic plaques in apoE-deficient mice. Circulation. 2013;127:1028–1039. doi: 10.1161/CIRCULATIONAHA.112.001347 [DOI] [PubMed] [Google Scholar]
- 77.Seijkens TTP, Poels K, Meiler S, van Tiel CM, Kusters PJH, Reiche M, Atzler D, Winkels H, Tjwa M, Poelman H, et al. Deficiency of the T cell regulator Casitas B-cell lymphoma-B aggravates atherosclerosis by inducing CD8+ T cell-mediated macrophage death. Eur Heart J. 2019;40:372–382. doi: 10.1093/eurheartj/ehy714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cochain C, Koch M, Chaudhari SM, Busch M, Pelisek J, Boon L, Zernecke A. CD8+ T cells regulate monopoiesis and circulating Ly6C-high monocyte levels in atherosclerosis in mice. Circ Res. 2015;117:244–253. doi: 10.1161/CIRCRESAHA.117.304611 [DOI] [PubMed] [Google Scholar]
- 79.van Duijn J, Kritikou E, Benne N, van der Heijden T, van Puijvelde GH, Kröner MJ, Schaftenaar FH, Foks AC, Wezel A, Smeets H, et al. CD8+ T-cells contribute to lesion stabilization in advanced atherosclerosis by limiting macrophage content and CD4+ T-cell responses. Cardiovasc Res. 2019;115:729–738. doi: 10.1093/cvr/cvy261 [DOI] [PubMed] [Google Scholar]
- 80.Schaftenaar FH, Amersfoort J, Douna H, Kröner MJ, Foks AC, Bot I, Slütter BA, van Puijvelde GHM, Drijfhout JW, Kuiper J. Induction of HLA-A2 restricted CD8 T cell responses against ApoB100 peptides does not affect atherosclerosis in a humanized mouse model. Sci Rep. 2019;9:17391 doi: 10.1038/s41598-019-53642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Honjo T, Chyu KY, Dimayuga PC, Lio WM, Yano J, Trinidad P, Zhao X, Zhou J, Cercek B, Shah PK. Immunization with an ApoB-100 related peptide vaccine attenuates Angiotensin-II induced hypertension and renal fibrosis in mice. PLoS One. 2015;10:e0131731 doi: 10.1371/journal.pone.0131731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clement M, Guedj K, Andreata F, Morvan M, Bey L, Khallou-Laschet J, Gaston AT, Delbosc S, Alsac JM, Bruneval P, et al. Control of the T follicular helper-germinal center B-cell axis by CD8+ regulatory T cells limits atherosclerosis and tertiary lymphoid organ development. Circulation. 2015;131:560–570. doi: 10.1161/CIRCULATIONAHA.114.010988 [DOI] [PubMed] [Google Scholar]
- 83.Zhang S, Wu M, Wang F. Immune regulation by CD8+ Treg cells: novel possibilities for anticancer immunotherapy. Cell Mol Immunol. 2018;15:805–807. doi: 10.1038/cmi.2018.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.St Paul M, Ohashi PS. The roles of CD8+ T cell subsets in antitumor immunity. Trends Cell Biol. 2020;30:695–704. doi: 10.1016/j.tcb.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 85.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593 [DOI] [PubMed] [Google Scholar]
- 86.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399 [DOI] [PubMed] [Google Scholar]
- 87.Langenhorst D, Haack S, Göb S, Uri A, Lühder F, Vanhove B, Hünig T, Beyersdorf N. CD28 costimulation of T helper 1 cells enhances cytokine release in vivo. Front Immunol. 2018;9:1060 doi: 10.3389/fimmu.2018.01060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Immunological Genome Project. Immgen at 15. Nat Immunol. 2020;21:700–703. doi: 10.1038/s41590-020-0687-4 [DOI] [PubMed] [Google Scholar]
- 90.Dempsey LA. RORα in Treg cells. Nat Immunol. 2018;19:510 doi: 10.1038/s41590-018-0099-x [DOI] [PubMed] [Google Scholar]
- 91.Butcher MJ, Wu CI, Waseem T, Galkina EV. CXCR6 regulates the recruitment of pro-inflammatory IL-17A-producing T cells into atherosclerotic aortas. Int Immunol. 2016;28:255–261. doi: 10.1093/intimm/dxv068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drujont L, Lemoine A, Moreau A, Bienvenu G, Lancien M, Cens T, Guillot F, Bériou G, Bouchet-Delbos L, Fehling HJ, et al. RORγt+ cells selectively express redundant cation channels linked to the Golgi apparatus. Sci Rep. 2016;6:23682 doi: 10.1038/srep23682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tan TG, Mathis D, Benoist C. Singular role for T-BET+CXCR3+ regulatory T cells in protection from autoimmune diabetes. Proc Natl Acad Sci U S A. 2016;113:14103–14108. doi: 10.1073/pnas.1616710113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tartar DM, VanMorlan AM, Wan X, Guloglu FB, Jain R, Haymaker CL, Ellis JS, Hoeman CM, Cascio JA, Dhakal M, et al. FoxP3+RORgammat+ T helper intermediates display suppressive function against autoimmune diabetes. J Immunol. 2010;184:3377–3385. doi: 10.4049/jimmunol.0903324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rizzo A, Di Giovangiulio M, Stolfi C, Franzè E, Fehling HJ, Carsetti R, Giorda E, Colantoni A, Ortenzi A, Rugge M, et al. RORγt-expressing tregs drive the growth of colitis-associated colorectal cancer by controlling IL6 in dendritic cells. Cancer Immunol Res. 2018;6:1082–1092. doi: 10.1158/2326-6066.CIR-17-0698 [DOI] [PubMed] [Google Scholar]
- 96.Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, et al. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORγt+ T cells. Science. 2015;349:989–993. doi: 10.1126/science.aac4263 [DOI] [PubMed] [Google Scholar]
- 97.Di Giovangiulio M, Rizzo A, Franzè E, Caprioli F, Facciotti F, Onali S, Favale A, Stolfi C, Fehling HJ, Monteleone G, et al. Tbet expression in regulatory T cells is required to initiate Th1-mediated colitis. Front Immunol. 2019;10:2158 doi: 10.3389/fimmu.2019.02158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng HY, Gaddis DE, Wu R, McSkimming C, Haynes LD, Taylor AM, McNamara CA, Sorci-Thomas M, Hedrick CC. Loss of ABCG1 influences regulatory T cell differentiation and atherosclerosis. J Clin Invest. 2016;126:3236–3246. doi: 10.1172/JCI83136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Winkels H, Meiler S, Lievens D, Engel D, Spitz C, Bürger C, Beckers L, Dandl A, Reim S, Ahmadsei M, et al. CD27 co-stimulation increases the abundance of regulatory T cells and reduces atherosclerosis in hyperlipidaemic mice. Eur Heart J. 2017;38:3590–3599. doi: 10.1093/eurheartj/ehx517 [DOI] [PubMed] [Google Scholar]
- 100.Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, Pan F, Blazar BR, Pardoll DM, Mellor AL, et al. An inherently bifunctional subset of Foxp3+ T helper cells is controlled by the transcription factor eos. Immunity. 2013;38:998–1012. doi: 10.1016/j.immuni.2013.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schieffer B, Selle T, Hilfiker A, Hilfiker-Kleiner D, Grote K, Tietge UJ, Trautwein C, Luchtefeld M, Schmittkamp C, Heeneman S, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110:3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97 [DOI] [PubMed] [Google Scholar]
- 102.Voloshyna I, Littlefield MJ, Reiss AB. Atherosclerosis and interferon-γ: new insights and therapeutic targets. Trends Cardiovasc Med. 2014;24:45–51. doi: 10.1016/j.tcm.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, et al. Interferon-γ drives treg fragility to promote anti-tumor immunity. Cell. 2017;169:1130–1141.e11. doi: 10.1016/j.cell.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marsch E, Sluimer JC, Daemen MJ. Hypoxia in atherosclerosis and inflammation. Curr Opin Lipidol. 2013;24:393–400. doi: 10.1097/MOL.0b013e32836484a4 [DOI] [PubMed] [Google Scholar]
- 105.Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Z, Barbi J, Bu S, Yang HY, Li Z, Gao Y, Jinasena D, Fu J, Lin F, Chen C, et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity. 2013;39:272–285. doi: 10.1016/j.immuni.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Korn T, Muschaweckh A. Stability and maintenance of Foxp3+ treg cells in non-lymphoid microenvironments. Front Immunol. 2019;10:2634 doi: 10.3389/fimmu.2019.02634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, Bluestone JA. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39:949–962. doi: 10.1016/j.immuni.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gisterå A, Klement ML, Polyzos KA, Mailer RKW, Duhlin A, Karlsson MCI, Ketelhuth DFJ, Hansson GK. Low-density lipoprotein-reactive T cells regulate plasma cholesterol levels and development of atherosclerosis in humanized hypercholesterolemic mice. Circulation. 2018;138:2513–2526. doi: 10.1161/CIRCULATIONAHA.118.034076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Overgaard NH, Jung JW, Steptoe RJ, Wells JW. CD4+/CD8+ double-positive T cells: more than just a developmental stage? J Leukoc Biol. 2015;97:31–38. doi: 10.1189/jlb.1RU0814-382 [DOI] [PubMed] [Google Scholar]
- 113.Bohner P, Chevalier MF, Cesson V, Rodrigues-Dias SC, Dartiguenave F, Burruni R, Tawadros T, Valerio M, Lucca I, Nardelli-Haefliger D, et al. Double positive CD4+CD8+ T cells are enriched in urological cancers and favor T helper-2 polarization. Front Immunol. 2019;10:622 doi: 10.3389/fimmu.2019.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Quandt D, Rothe K, Scholz R, Baerwald CW, Wagner U. Peripheral CD4CD8 double positive T cells with a distinct helper cytokine profile are increased in rheumatoid arthritis. PLoS One. 2014;9:e93293 doi: 10.1371/journal.pone.0093293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiao Z, Mescher MF, Jameson SC. Detuning CD8 T cells: down-regulation of CD8 expression, tetramer binding, and response during CTL activation. J Exp Med. 2007;204:2667–2677. doi: 10.1084/jem.20062376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grishkan IV, Ntranos A, Calabresi PA, Gocke AR. Helper T cells down-regulate CD4 expression upon chronic stimulation giving rise to double-negative T cells. Cell Immunol. 2013;284:68–74. doi: 10.1016/j.cellimm.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Crosby CM, Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol. 2018;18:559–574. doi: 10.1038/s41577-018-0034-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pothineni NVK, Subramany S, Kuriakose K, Shirazi LF, Romeo F, Shah PK, Mehta JL. Infections, atherosclerosis, and coronary heart disease. Eur Heart J. 2017;38:3195–3201. doi: 10.1093/eurheartj/ehx362 [DOI] [PubMed] [Google Scholar]
- 119.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- 120.Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. 2019;19:665–674. doi: 10.1038/s41577-019-0221-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–2720. doi: 10.1182/blood-2002-07-2103 [DOI] [PubMed] [Google Scholar]
- 122.van Duijn J, van Elsas M, Benne N, Depuydt M, Wezel A, Smeets H, Bot I, Jiskoot W, Kuiper J, Slütter B. CD39 identifies a microenvironment-specific anti-inflammatory CD8+ T-cell population in atherosclerotic lesions. Atherosclerosis. 2019;285:71–78. doi: 10.1016/j.atherosclerosis.2019.04.217 [DOI] [PubMed] [Google Scholar]
- 123.Bu DX, Tarrio M, Maganto-Garcia E, Stavrakis G, Tajima G, Lederer J, Jarolim P, Freeman GJ, Sharpe AH, Lichtman AH. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1100–1107. doi: 10.1161/ATVBAHA.111.224709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cochain C, Chaudhari SM, Koch M, Wiendl H, Eckstein HH, Zernecke A. Programmed cell death-1 deficiency exacerbates T cell activation and atherogenesis despite expansion of regulatory T cells in atherosclerosis-prone mice. PLoS One. 2014;9:e93280 doi: 10.1371/journal.pone.0093280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lutgens E, Seijkens TTP. Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. J Immunother Cancer. 2020;8:e00300 doi: 10.1136/jitc-2019-000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Libby P, Sasiela W. Plaque stabilization: can we turn theory into evidence? Am J Cardiol. 2006;98:26P–33P. doi: 10.1016/j.amjcard.2006.09.017 [DOI] [PubMed] [Google Scholar]
- 127.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 128.Gomez D, Baylis RA, Durgin BG, Newman AAC, Alencar GF, Mahan S, St Hilaire C, Müller W, Waisman A, Francis SE, et al. Interleukin-1β has atheroprotective effects in advanced atherosclerotic lesions of mice. Nat Med. 2018;24:1418–1429. doi: 10.1038/s41591-018-0124-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516. doi: 10.1038/nature17655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rosshart SP, Herz J, Vassallo BG, Hunter A, Wall MK, Badger JH, McCulloch JA, Anastasakis DG, Sarshad AA, Leonardi I, et al. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science. 2019;365:eaaw4361 doi: 10.1126/science.aaw4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lusis AJ, Seldin MM, Allayee H, Bennett BJ, Civelek M, Davis RC, Eskin E, Farber CR, Hui S, Mehrabian M, et al. The hybrid mouse diversity panel: a resource for systems genetics analyses of metabolic and cardiovascular traits. J Lipid Res. 2016;57:925–942. doi: 10.1194/jlr.R066944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337 [DOI] [PMC free article] [PubMed] [Google Scholar]


