Supplemental Digital Content is available in the text.
Keywords: cytokines, extracellular traps, fibrinolysis, histone, immunothrombosis, Sars-Cov2, thrombosis
Objective:
The full spectrum of coronavirus disease 2019 (COVID-19) infection ranges from asymptomatic to acute respiratory distress syndrome, characterized by hyperinflammation and thrombotic microangiopathy. The pathogenic mechanisms are poorly understood, but emerging evidence suggest that excessive neutrophil extracellular trap (NET) formation plays a key role in COVID-19 disease progression. Here, we evaluate if circulating markers of NETs are associated with COVID-19 disease severity and clinical outcome, as well as to markers of inflammation and in vivo coagulation and fibrinolysis.
Approach and Results:
One hundred six patients with COVID-19 with moderate to severe disease were enrolled shortly after hospital admission and followed for 4 months. Acute and convalescent plasma samples as well as plasma samples from 30 healthy individuals were assessed for markers of NET formation: citrullinated histone H3, cell-free DNA, NE (neutrophil elastase). We found that all plasma levels of NET markers were elevated in patients with COVID-19 relative to healthy controls, that they were associated with respiratory support requirement and short-term mortality, and declined to those found in healthy individuals 4 months post-infection. The levels of the NET markers also correlated with white blood cells, neutrophils, inflammatory cytokines, and C-reactive protein, as well as to markers of in vivo coagulation, fibrinolysis, and endothelial damage.
Conclusions:
Our findings suggest a role of NETs in COVID-19 disease progression, implicating their contribution to an immunothrombotic state. Further, we observed an association between circulating markers of NET formation and clinical outcome, demonstrating a potential role of NET markers in clinical decision-making, as well as for NETs as targets for novel therapeutic interventions in COVID-19.
Highlights.
Elevated circulating markers of neutrophil extracellular traps (NETs) are prognostic indicators for respiratory support requirements and survival in patients with coronavirus disease 2019 (COVID-19).
Circulating NET markers correlate with markers of inflammation and endothelial damage in COVID-19.
Targeting NETosis to reduce the immunothrombosis in COVID-19 pathogenesis forms a promising therapeutic strategy.
See accompanying editorial on page 995
The novel coronavirus (SARS-CoV-2; severe acute respiratory syndrome coronavirus 2) infection, causing the coronavirus disease 2019 (COVID-19), is rapidly spreading and has taken pandemic proportions. Although the majority of infected individuals display mild symptoms, the full spectrum of the disease ranges from asymptomatic to severe acute respiratory distress syndrome requiring hospitalization, and is in many cases fatal. Patients with COVID-19-associated acute respiratory distress syndrome exhibit increased pulmonary inflammation, extensive lung damage, and microthrombosis driven by an exacerbated and poorly understood host response.1,2 In response to pathogens, neutrophils can form neutrophil extracellular traps (NETs) by releasing decondensed chromosomal DNA decorated by granular proteins. Although first described to combat invading microorganisms,3 excessive NET formation has been shown to induce an immunothrombotic state observed in sepsis, acute respiratory distress syndrome, and cancer.4 Emerging data now suggest a role of NETs also in COVID-19 pathophysiology: (1) neutrophilia and neutrophil-to-lymphocyte ratio are linked to COVID-19 disease severity,5,6 (2) NETs have been demonstrated in pulmonary microvessels of patients with COVID-19,7–11 (3) neutrophils from patients with COVID-19 are prone to spontaneously form NETs,7,8,10 (4) plasma from patients with COVID-19 induce the release of NETs by healthy neutrophils,7,12,13 and (5) circulating markers of NET formation are correlated to COVID-19 disease severity.7,8,13,14 Here, we show that circulating markers of NET formation are prognostic in a well-phenotyped, prospective study of 106 patients, and that they are associated with an inflammatory and prothrombotic state, suggesting the implication of NETs in COVID-19-related immunothrombosis.
Methods
Study Population
One hundred six patients with COVID-19 admitted to Danderyd Hospital, Stockholm, Sweden, between April 15, 2020 and June 8, 2020 were included in the study. Inclusion criterion was COVID-19 diagnosis based on reverse-transcriptase polymerase chain reaction viral RNA detection of nasopharyngeal or oropharyngeal swabs or clinical presentation. The only exclusion criterium was age <18 years. Blood samples were collected within 7 days of admission. Demographics, medications, routine laboratory (C-reactive protein, white blood cell count, lymphocyte count, and neutrophil count) and clinical data including respiratory support (RS) were obtained from medical records. All patients were invited for a follow-up within the study protocol, and follow-up blood samples 4 months (median 122 [109–132] days) postinfection onset were obtained from 55 patients. Patients who did not come to follow-up were either deceased or did not answer on repeated invitations. There was no difference in RS during hospital stay between survivors that came for follow-up and survivors that did not come for follow-up (P=0.196). Plasma samples were prepared by centrifugation at 2000g for 20 minutes in room temperature and stored at −80 °C. Plasma samples from 30 healthy individuals were obtained from a previous study (2015/1533-31/1). These samples were prepared by the same research nurse as in the current study, following the same protocol. The study was approved by the Swedish Ethical Review Authority (2020-01653), and informed consent was obtained from all study participants or, in the case of incapacity, their next-of-kin.
Laboratory Analyses
Plasma levels of nucleosomal citrullinated histone H3 (H3Cit-DNA) were quantified using an in-house and validated ELISA as previously described.15 Briefly, a monoclonal anti-histone H3 (citrulline R8) antibody (Abcam) was used for capture and a monoclonal anti-DNA antibody (Cell Death ELISAPLUS, Roche) was used for detection. Semisynthetic nucleosomes containing citrulline in place of arginine at histone H3, arginine residues 2, 8, and 17 (H3R2,8,17Cit) (EpiCypher) were used as calibration standards. Cell-free DNA (cfDNA), NE (neutrophil elastase), TNFα (tumor necrosis factor alpha), and IL-6 (interleukin-6) were quantified using commercially available kits according to manufacturer (Quant-iT PicoGreen dsDNA assay, Invitrogen, Human PMN Elastase ELISA Kit, Abcam, Human TNF-alpha DuoSet and IL-6 DuoSet ELISA Kits, R&D Systems, respectively). More details are provided in the supplementary material. Plasma levels of markers of in vivo coagulation and fibrinolysis (TAT [thrombin-antithrombin complex], D-dimer, and PAP [plasmin-antiplasmin]), and endothelial activation/damage (VWF [von Willebrand factor], ADAMTS13 [a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13] activity) were obtained from a separate study investigating their relation to COVID-19 disease severity/outcome,16 and quantified as previously described.17
Statistical Analyses
Patients were divided into 2 groups: low level of RS; ≤5 L oxygen on cannula, and high level of RS; >5 L oxygen on cannula, noninvasive respiratory support or intubation. Short-term mortality was defined as mortality <30 days from admission. Comparisons of continuous variables were performed using Mann-Whitney U test and categorical variables with Fisher exact test. Comparisons of >2 groups were performed using Kruskal-Wallis 1-way ANOVA followed by Dunn multiple comparison test. Paired samples were compared using Wilcoxon test. The relationship between NET markers and markers of inflammation, coagulation, fibrinolysis, and endothelial activation/injury was evaluated using the Spearman rho. Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc, San Diego, CA). All tests were 2-sided, and P<0.05 was considered statistically significant.
Results
Patient Characteristics
Demographics and clinical characteristics for the patients with COVID-19 (n=106) and healthy controls (n=30) are presented in Table 1. The median patient age was 60 (50–69) years (64.2% male) and did not differ from that in the healthy control group of 62 (27–71) years (66.7% male). No significant differences were observed in age, sex, or comorbidities between patients with low and high levels of RS. Anticoagulant treatment was given to 90/106 patients, and corticosteroids was given to 14/106 patients.
Table 1.
Demographic Data, Clinical Characteristics, and Laboratory Data of Study Participants

Measurement of Circulating Markers of Neutrophil Activation and NETs in Patients With COVID-19
Plasma levels of H3Cit-DNA, cfDNA, and NE were all elevated in patients with COVID-19 compared with healthy individuals (Figure 1A). As expected, plasma levels of H3Cit-DNA correlated with cfDNA and NE (Table 2), indicating NET formation. Notably, 4 months postinfection onset all markers declined to levels found in healthy individuals (Figure 1B).
Figure 1.
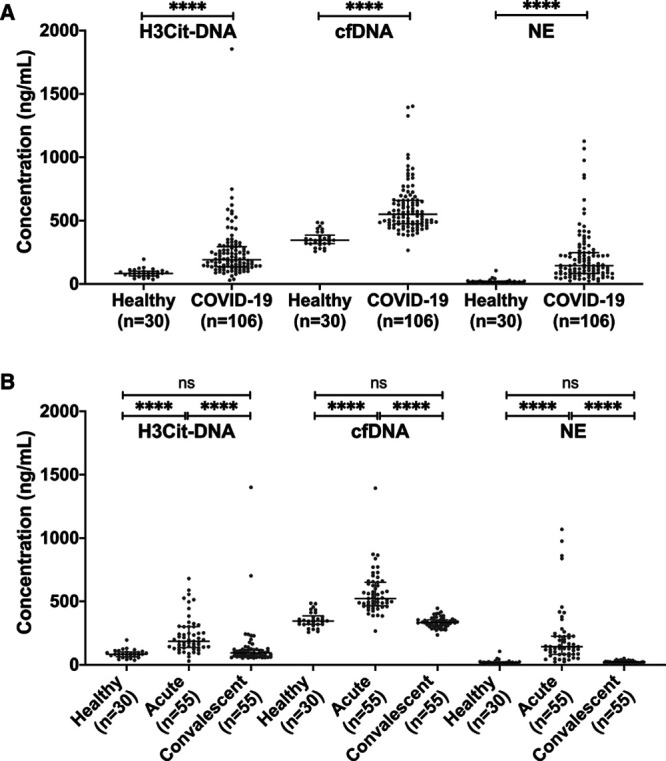
Coronavirus disease 2019 (COVID-19) disease causes alteration in the circulating markers of neutrophil extracellular trap formation. A, Plasma levels of nucleosomal citrullinated histone H3 (H3Cit-DNA), cell-free DNA (cfDNA), and NE (neutrophil elastase) in patients with COVID-19 are increased compared with healthy individuals. B, Four months postinfection onset, plasma levels of H3Cit-DNA, cfDNA, and NE declined to those found in healthy individuals. Data are presented as median with interquartile range.
Table 2.
Circulating Markers of NET Formation Correlate With Markers of Inflammation, Dysregulated Hemostasis, and Endothelial Injury

When the patient group was analyzed based on their need for respiratory support, it was clear that H3Cit-DNA, cfDNA, and NE all increased with increasing RS (Figure 2A). In addition, high levels at the time of admission were associated with poor clinical outcome defined as admission to ICU and/or short-term mortality (P=0.0002, P<0.0001, P=0.0058, respectively), as well as with short-term mortality alone (Figure 2B). This indicates a prognostic value of these markers in COVID-19.
Figure 2.
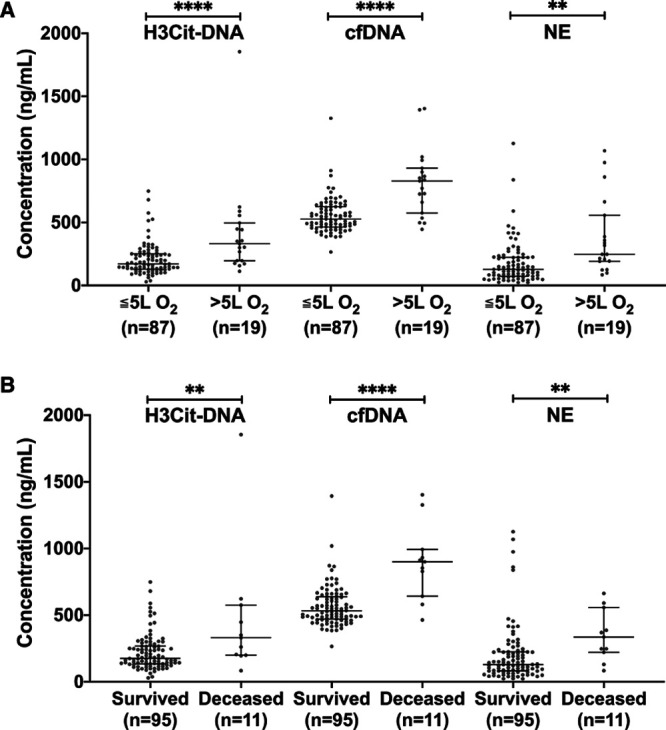
Circulating markers of neutrophil extracellular trap formation are associated to disease severity and clinical outcome in patients with coronavirus disease 2019 (COVID-19). A, Plasma levels of nucleosomal citrullinated histone H3 (H3Cit-DNA), cell-free DNA (cfDNA), and NE (neutrophil elastase) in patients with COVID-19 increase with increased need for respiratory support. B, Plasma levels of H3Cit-DNA, cfDNA, and NE were all lower in survivors compared with nonsurvivors. Data are presented as median with interquartile range.
Circulating Markers of NET Formation Are Associated With Markers of Inflammation, a Prothrombotic State, and Endothelial Activation/Damage
H3Cit-DNA, cfDNA, and NE all correlated with white blood cell count, neutrophil count, neutrophil-to-lymphocyte ratio, and CRP. The link between NETs and an inflammatory state was further corroborated by correlations between all NET markers and TNFα, and between H3Cit-DNA, NE, and IL-6. All NET markers also correlated with D-dimer and PAP, and cfDNA and NE correlated with TAT, indicating a NET-associated prothrombotic state. Moreover, H3Cit-DNA, cfDNA, and NE correlated with the endothelial activation/damage marker VWF, while H3Cit-DNA and NE, but not cfDNA, correlated inversely with the VWF-cleaving protease ADAMTS13 (Table 2), suggesting a NET-associated endothelial activation and injury. Taken together, these findings support a link between COVID-19-associated NET formation and immunothrombosis.
Discussion
Our findings expand upon prior work by showing a link between NETs and immunothrombosis as well as an association between circulating markers of NETs and clinical outcome in a large and unselected cohort of patients with moderate to severe COVID-19.
NETs are released by neutrophils in response to a variety of stimuli, including respiratory viruses18,19 and inflammatory cytokines.20 Given the key feature of virus-induced cytokine storms in severe COVID-19,21 emanating excessive NET formation seems plausible, and is supported by our findings of correlations between NET markers and inflammatory cytokines. COVID-19-associated NET formation may, however, involve other, and perhaps overlapping, mechanisms, such as a direct infection of neutrophils by the SARS-CoV-2 virus,10 and further experimental models are needed to fully understand the link between COVID-19 and NET formation.
Importantly, and regardless of mechanism of formation, an excessive NET formation may pose therapeutic targets in the combat of COVID-19. NETs are key elements in immunothrombosis, where they provide a stimulus and scaffold for thrombus formation, endothelial damage, and organ dysfunction.4,22 Although we did not investigate whether NET formation is a driver or a consequence of the disease, we corroborate a link between NETs and immunothrombosis in COVID-19 by revealing an association between circulating NET markers and inflammation, dysregulated hemostasis, and endothelial injury. Endogenous deoxyribonucleases (DNases) dismantle NETs and regulate NET-driven thrombosis,23 and removal of NETs via recombinant DNase infusion improve microvascular perfusion in mouse models of sepsis.24 Notably, aerosolized DNases are currently being evaluated in trials in COVID-19 (URL: https://www.clinicaltrials.gov/; unique identifiers: NCT04402944, NCT04541979, NCT04445285, NCT04432987, NCT04402970). Further investigations on the mechanisms behind NET formation in COVID-19 may also pave the way for other treatment options, such as agents that neutralize extracellular histones25,26 or inhibit NET formation.27,28 It will, however, be crucial to avoid disrupting protective effects of NET formation and the release of potentially damaging NET degradation products in tailoring novel therapeutic interventions targeting NETs. Circulating markers of NETs may thereby not only be useful in clinical prognostics but also in monitoring response to treatment.
Taken together, our findings support a link between COVID-19-associated NET formation and immunothrombosis and show that circulating markers of NETs are related to both disease severity and clinical outcome in a large cohort of patients with COVID-19. How to further translate the role of NETs in COVID-19 into clinical practice should be a priority for future research. Notably, the assays quantifying circulating H3Cit-DNA, cfDNA, and NE are validated in human plasma samples and easily accessible, allowing for a potential clinical implementation. Our findings now argue for further studies determining if these markers are useful in clinical decision-making.
Acknowledgments
We are grateful to Lena Gabrielsson, Ann-Christin Salomonson, Nina Greilert, and Eva Isaksson at Danderyd Hospital for administration and blood sampling and to Jelle Adelmeijer at University Medical Center Groningen for laboratory hemostasis studies.
Sources of Funding
This study was supported by the Knut and Alice Wallenberg foundation, SciLifeLab, Region Stockholm, the Swedish Cancer Society, and the Swedish Research Council.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ADAMTS13
- a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13
- cfDNA
- cell-free DNA
- COVID-19
- coronavirus disease 2019
- H3Cit-DNA
- nucleosomal citrullinated histone H3
- IL-6
- interleukin-6
- NE
- neutrophil elastase
- NET
- neutrophil extracellular trap
- PAP
- plasmin-antiplasmin
- RS
- respiratory support
- TAT
- thrombin-antithrombin complex
- TNFα
- tumor necrosis factor alpha
- VWF
- von Willebrand factor
This article was sent to William C. Sessa, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
These authors shared senior authorship.
For Sources of Funding and Disclosures, see page 993.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.120.315267.
Contributor Information
Henry Ng, Email: Henry.Ng.5366@student.uu.se.
Sebastian Havervall, Email: sebastian.havervall@sll.se.
Axel Rosell, Email: axel.rosell@sll.se.
Katherina Aguilera, Email: katherina.aguilera-gatica@sll.se.
Kristel Parv, Email: kristel.parv@mcb.uu.se.
Fien A. von Meijenfeldt, Email: f.a.von.meijenfeldt@umcg.nl.
Ton Lisman, Email: j.a.lisman@umcg.nl.
Nigel Mackman, Email: nigel_mackman@med.unc.edu.
Charlotte Thålin, Email: charlotte.thalin@sll.se.
References
- 1.Mackman N, Antoniak S, Wolberg AS, Kasthuri R, Key NS. Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. 2020;40:2033–2044. doi: 10.1161/ATVBAHA.120.314514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 4.Thålin C, Hisada Y, Lundström S, Mackman N, Wallén H. Neutrophil extracellular traps: villains and targets in arterial, venous, and cancer-associated thrombosis. Arterioscler Thromb Vasc Biol. 2019;39:1724–1738. doi: 10.1161/ATVBAHA.119.312463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, Luo M, Chen L, Zhao Y. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81:e6–e12. doi: 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, Mostyka M, Baxter-Stoltzfus A, Borczuk AC, Loda M, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leppkes M, Knopf J, Naschberger E, Lindemann A, Singh J, Herrmann I, Stürzl M, Staats L, Mahajan A, Schauer C, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58:102925 doi: 10.1016/j.ebiom.2020.102925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d’Emal C, Vanwinge C, Cataldo D, Oury C, Delvenne P, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med. 2020;217:e20201012 doi: 10.1084/jem.20201012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, Schneider AH, Caetite D, Tavares LA, Paiva IM, et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. The J Exp Med. 2020;217:e20201129 doi: 10.1084/jem.20201129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skendros P, Mitsios A, Chrysanthopoulou A, Mastellos DC, Metallidis S, Rafailidis P, Ntinopoulou M, Sertaridou E, Tsironidou V, Tsigalou C, et al. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130:6151–6157. doi: 10.1172/JCI141374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5:e138999 doi: 10.1172/jci.insight.138999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busch MH, Timmermans SAMEG, Nagy M, Visser M, Huckriede J, Aendekerk JP, de Vries F, Potjewijd J, Jallah B, Ysermans R, et al. Neutrophils and contact activation of coagulation as potential drivers of Covid-19. Circulation. 2020;142:1787–1790. doi: 10.1161/CIRCULATIONAHA.120.050656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thålin C, Aguilera K, Hall NW, Marunde MR, Burg JM, Rosell A, Daleskog M, Månsson M, Hisada V, Meiners MJ, et al. Quantification of citrullinated histones: Development of an improved assay to reliably quantify nucleosomal H3Cit in human plasma. J Thromb Haemost. 2020;18:2732–2743. doi: 10.1111/jth.15003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Meijenfeldt FA, Havervall S, Adelmeijer J, Lundström A, Rudberg A, Magnusson M, Mackman N, Thålin C, Lisman T. Prothrombotic changes in COVID-19 patients are associated with disease severity and mortality [published online December 6, 2020]. Res Pract Thromb Haemost. doi: 10.1002/rth2.12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blasi A, von Meijenfeldt FA, Adelmeijer J, Calvo A, Ibanez C, Perdomo J, Carlos Reverter J, Lisman T. In vitro hypercoagulability and ongoing in vivo activation of coagulation and fibrinolysis in COVID-19 patients on anticoagulation. J Thromb Haemost. 2020;18:2646–2653. doi: 10.1111/jth.15043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muraro SP, De Souza GF, Gallo SW, Da Silva BK, De Oliveira SD, Vinolo MAR, Saraiva EM, Porto BN. Respiratory syncytial virus induces the classical ROS-dependent NETosis through PAD-4 and necroptosis pathways activation. Sci Rep. 2018;8:14166 doi: 10.1038/s41598-018-32576-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schönrich G, Raftery MJ. Neu iral. Front Immunol. 2016;7:366 doi: 10.3389/fimmu.2016.00366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keshari RS, Jyoti A, Dubey M, Kothari N, Kohli M, Bogra J, Barthwal MK, Dikshit M. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS One. 2012;7:e48111 doi: 10.1371/journal.pone.0048111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565 [DOI] [PubMed] [Google Scholar]
- 23.Jiménez-Alcázar M, Rangaswamy C, Panda R, Bitterling J, Simsek YJ, Long AT, Bilyy R, Krenn V, Renné C, Renné T, et al. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science. 2017;358:1202–1206. doi: 10.1126/science.aam8897 [DOI] [PubMed] [Google Scholar]
- 24.McDonald B, Davis RP, Kim SJ, Tse M, Esmon CT, Kolaczkowska E, Jenne CN. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129:1357–1367. doi: 10.1182/blood-2016-09-741298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silk E, Zhao H, Weng H, Ma D. The role of extracellular histone in organ injury. Cell Death Dis. 2017;8:e2812 doi: 10.1038/cddis.2017.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng Q, Pan B, Alam HB, Liang Y, Wu Z, Liu B, Mor-Vaknin N, Duan X, Williams AM, Tian Y, et al. Citrullinated hstone H3 as a Therapeutic target for endotoxic shock in mice. Front Immunol. 2020;10:2957 doi: 10.3389/fimmu.2019.02957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chirivi RGS, van Rosmalen JWG, van der Linden M, Euler M, Schmets G, Bogatkevich G, Kambas K, Hahn J, Braster Q, Soehnlein O, et al. Therapeutic ACPA inhibits NET formation: a potential therapy for neutrophil-mediated inflammatory diseases. Cell Mol Immunol. 2020. doi: 10.1038/s41423-020-0381-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang Y, Pan B, Alam HB, Deng Q, Wang Y, Chen E, Liu B, Tian Y, Williams AM, Duan X, et al. Inhibition of peptidylarginine deiminase alleviates LPS-induced pulmonary dysfunction and improves survival in a mouse model of lethal endotoxemia. Eur J Pharmacol. 2018;833:432–440. doi: 10.1016/j.ejphar.2018.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


