Abstract
PURPOSE
Piriformis syndrome is a common pain condition affecting the buttock and posterior hip with or without radiation to the leg, and management of the condition involves many treatments. In this study, we hypothesize that a CT-guided injection with botulinum toxin is more effective in providing pain relief than a CT-guided injection without Botox.
METHODS
Overall, 97 consecutive patients with piriformis syndrome presented for a CT-guided injection of the piriformis muscle and perineural injection of the sciatic nerve. After the injection, the patients received a visual analog scale pain log to record their pain level until the follow-up appointment. Values of p < 0.2 were considered as confounder and adjusted by inverse probability of treatment weighting (IPTW) via propensity score. The effect of botulinum toxin on 48-hour response and duration of response was tested using weighted chi-square test and weighted Kaplan-Meier analysis.
RESULTS
There was a total of 97 patients in the study, and 111 injections, as some patients had bilateral injections. Patients in the Botox group had more 48-hour response than patients in the non-botulinum toxin group (p < 0.001 with IPTW, p = 0.005 without IPTW). Median pain-free survival was 30 days for Botox group and 1 day for non-Botox group (p = 0.059 with IPTW, p = 0.10 without IPTW).
CONCLUSION
CT-guided injections with botulinum toxin for patients with piriformis syndrome are more likely to lead to a positive response and a longer duration of response than patients who receive a CT-guided injection without botulinum toxin. We hope that this study facilitates future prospective randomized blind trials for patients with suspected piriformis syndrome.
Piriformis syndrome is a common pain condition affecting the buttock and posterior hip with or without radiation to the leg. It is thought to be caused by prolonged contraction (spasm) or hypertrophy of the piriformis muscle, and it can account for up to 6% of sciatica-like symptoms (1, 2). The most common presentation of piriformis syndrome is buttock pain overlying the wallet area that increases with sitting, which is frequently unilateral or less commonly bilateral (3). This pain can significantly impact a patient’s quality of life. Suggested pathophysiology includes anatomical variations of the piriformis with or without hypertrophy or spasm, trauma, or pinching of the sciatic nerve caused by intramuscular course through the piriformis muscle or adjacent fibrous bands / accessory muscle slips (4, 5).
Piriformis syndrome is diagnosed on the basis of clinical findings of buttock pain, sciatica symptoms and wallet area anesthesia and/or tenderness. The findings can be confirmed with cross-sectional techniques, particularly magnetic resonance imaging (6, 7). Piriformis syndrome is also diagnosed presumptively after workup has revealed no other sources of pain in the buttock, hip, and back. Magnetic resonance neurography (MRN) of the lumbosacral plexus and pelvis has become an important tool for the diagnosis and evaluation of sciatic neuralgia and in guiding management with image-guided nerve blocks and muscle injections (8). Recent studies using MRN for chronic lumbosacral and pelvic pain have shown impact of MRN in diagnostic thinking, management, and outcomes of such patients (9, 10). In piriformis syndrome, the common findings on MRN include hypertrophied or atrophied piriformis muscles, accessory muscle slips, split sciatic nerve, and increased signal or flattening / prominence of the sciatic nerve at the sciatic notch with or without increased signal in L5 and/or S1 nerve roots (9).
Initial management of piriformis syndrome includes physical therapy, heat, massage, anti-inflammatory medications, and behavioral modifications. However, if the pain remains uncontrolled, local anesthetic with steroid injection, surgery, or epidural injection have been reported to be effective in treatment (11–13). Onabotulinum toxin A (Botox, Allergan) has been used historically for many disorders of excessive muscle contraction, spasticity, dystonia, muscle pain, myofascial pain, and sacroiliac joint injections (14, 15). The use of Botox for injections in patients with piriformis syndrome has shown positive results. Many small studies and case reports using ultrasound-guided injections have reported pain reduction and improvement in the quality of life after patients received Botox injections to the piriformis muscle (16, 17). Recently, CT-guided injections with Botox for piriformis syndrome has also shown pain reduction compared with baseline (6, 18). However, these studies were not comparative studies evaluating the efficacy of the injection with Botox versus without Botox. In addition, perineural injections of the sciatic nerve and how that may contribute to pain reduction in patients with piriformis syndrome have not been evaluated.
The aim of the study in patients with piriformis syndrome was to determine whether a CT-guided injection of the piriformis muscle with Botox and a perineural injection of the sciatic nerve affect the patients differently than the injections without the administration of Botox. We hypothesized that a CT-guided injection with Botox is more effective in providing pain relief than a CT-guided injection without Botox, resulting in a positive incremental value.
Methods
This is a retrospective cohort study performed following institutional review board approval, protocol number (STU 072013-057). Patient inclusion was not randomized in two different groups and they received the two types of injections as part of their standard care management. For this retrospective evaluation, the informed consent was waived.
Patients
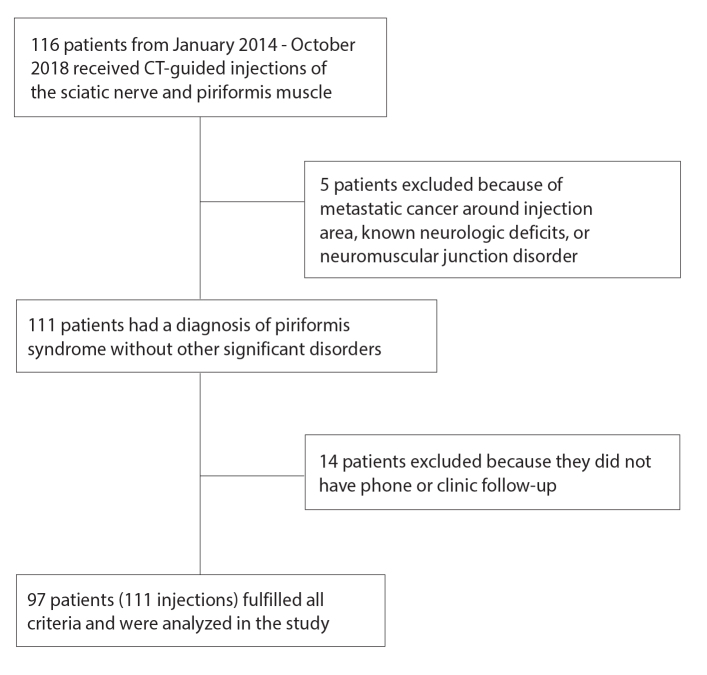
A consecutive series of patients who presented from January 2014 to October 2018 for a CT-guided injection of the piriformis muscle and perineural injection of the sciatic nerve were included. All patients must have had a diagnosis of piriformis syndrome on the basis of clinical findings and/or MRN imaging. Patients must have had both perineural injections of the sciatic nerve and piriformis muscle. Patients were excluded if they did not have clinic follow-up to evaluate symptoms after the injection (Fig. 1). Patient demographic data included sex, age, body mass index (BMI), presenting symptoms, physical examination findings, surgical history, and injection history. The final diagnosis of piriformis syndrome was made by history, physical examination, and MRN findings if they had undergone imaging. Prior clinic injections and other follow-up treatments like surgery and/or radiofrequency ablation (RFA) were also recorded.
Figure 1.
Patient selection flowchart detailing inclusion and exclusion criteria.
MRN lumbosacral plexus protocol
The MRN lumbosacral plexus protocol was performed on a 3 T scanner (Achieva, Ingenia, Philips) using torso XL coil coupled with spine coil elements (Table 1). It included two-dimensional, three-dimesional anatomic, and diffusion imaging sequences, and encompassed evaluation of the lumbosacral spine, lumbosacral plexus and peripheral nerves in the abdomen and pelvis. All included MRNs were read by multiple fellowship-trained musculoskeletal radiologists with 2–8 years’ experience with MRN techniques and reporting. A systematic documentation of the findings had been performed in all the reports as a standard of care, which included findings of bone, spine, muscle, peripheral nerves, masses, and other visceral lesions.
Table 1.
Imaging protocol and parameters for 3 T MRN of the lumbosacral plexus
| Sequence | TR (ms) | TE (ms) | Gap | Turbo factor | Acquisition time | Voxel (mm) | FOV (mm) |
|---|---|---|---|---|---|---|---|
| Axial T1W | 500 | 8 | 10% | 8 | 4 min 39 s | 0.6×0.6×4.0 | 330 |
| Axial T2W SPAIR | 4000 | 60 | 10% | 7 | 6 min 13 s | 1.0×1.0×4.0 | 330 |
| 3D coronal STIR | 2000 | 78 | 0 | 100 | 8 min | 1.5×1.5×1.5 | 380 |
| Sagittal T2W spine | 3500 | 120 | 10% | 19 | 4 min 18 s | 0.9×1.1×4.0 | 280 |
| Axial T2W spine | 3000 | 120 | 10% | 27 | 4 min 19 s | 1.0×1.0×5.0 | 110 |
| Axial DTI | 16100 | 54 | 0 | b-values=0.600 | 5 min | 3.5×3.5×5.0 | 331 |
MRN, magnetic resonance neurography; TR, repetition time; TE, echo time; FOV, field of view; T1W, T1-weighted; T2W, T2-weighted; SPAIR, spectral adiabatic inversion recovery; 3D, three-dimensional; STIR, short T1 inversion recovery; DTI, diffusion tensor imaging.
CT-guided injection technique and follow-up
The injections were performed by musculoskeletal fellows with direct supervision from a musculoskeletal radiologist or by the musculoskeletal radiologists themselves using a standardized template of procedure as described in a previous article by Wadhwa et al. (19). The risks of the procedure were explained in detail to the patient, and informed consent was obtained. The patient was placed on the CT gantry in the prone position. The piriformis muscle and the sciatic nerve were localized using CT guidance. The patient was then prepped and draped in the typical sterile fashion. Under intermittent CT guidance, a 22-guage needle was directed towards the course of the sciatic nerve and a 20-gauge needle was directed into the piriformis muscle. Dilute non-ionic contrast (1–2 cc) was injected to confirm needle tip position. A 4:6:1 mixture of 1% lidocaine, 0.5% bupivacaine, and 100 units of Botox was injected into the piriformis muscle. The patients without Botox only received 4:6 mixture of 1% lidocaine, 0.5% bupivacaine into the piriformis muscle. Both groups of patients received a 2:2:1 mixture of 1% lidocaine, 0.5% bupivacaine, and 4 mg dexamethasone around the sciatic nerve.
After the injection, the patients received a visual analog scale (VAS) pain log to record their pain level until they had a follow-up appointment with either the referring clinician or with the radiologist (Fig. 2). Patients received a telephone call from the staff 48 hours after the injection to follow up on their symptoms and any complications they may have had related to the procedure. At the clinic follow-up, the pain logs were reviewed by the clinician and scanned into the electronic health records. Clinic follow-ups occurred at 1-month, 3-month, and 6-month intervals, or as per the patient’s discretion if the pain returned earlier. At each clinic follow-up, patients self-reported the time when their pain recurred and this was documented in the electronic medical record. Some patients were lost during the clinic follow-up period.
Figure 2.
Patient visual analog scale for CT-guided injection.
Data evaluation
Responses to the CT-guided injections were defined based on published criteria and shown in Table 2 (20, 21). Responses were categorized into three groups: positive block, negative block, and possible positive block. Patient responses at 48 hours were recorded as “positive” and “negative”. Follow-up time to pain-return or the last follow-up without pain were recorded.
Table 2.
Definition of responses to the CT-guided injection of sciatic nerve and piriformis muscle.
| Positive block – must meet all 3 criteria |
|
| Negative block |
|
| Possible positive block |
|
Statistical analysis
Age, BMI, pain level, duration of pain, sex, presence of buttock pain and back pain, pain meds, radiculopathy, FABER (flexion, abduction, external rotation test), FADIR (flexion, adduction, internal rotation test), previous trauma, prior clinic injection, prior imaging for problem muscle change, nerve change, split sciatic, number of CT injection, laterality were all recorded. Continuous variables and categorical variables were represented as median (Q1, Q3) (Q1, 25th percentile; Q3, 75th percentile) and count (percentage) respectively. Wilcoxon-Mann-Whitney tests and chi-square tests were used to test the difference between Botox to identify potential confounders. When testing categorical variables, levels with too few counts were combined when appropriate. p values < 0.2 were considered as confounder and adjusted by inverse probability of treatment weighting (IPTW) via propensity score.
The effect of Botox on 48-hour response and duration of response was tested using weighted chi-square test and weighted Kaplan-Meier analysis. For the pain-free survival analysis, all patients who were not initially pain-free were excluded. P value < 0.05 was considered statistically significant. All analyses were done under SAS 9.4 (SAS institute).
Results
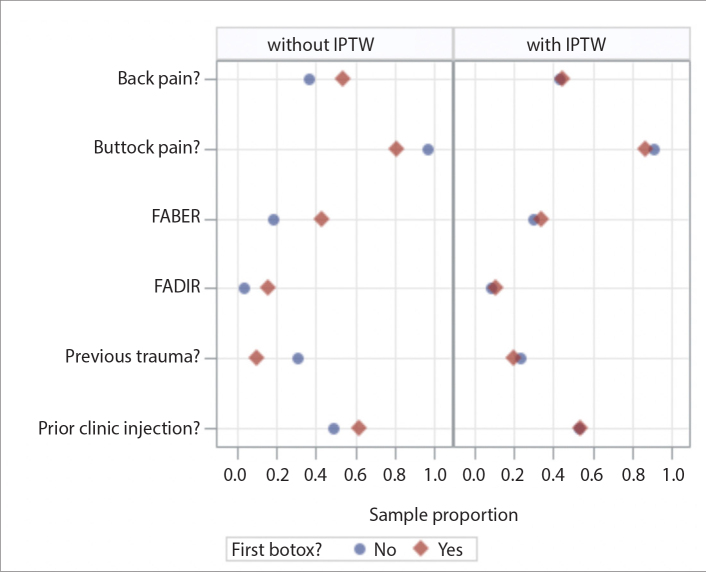
There was a total of 97 patients in the study, and 111 injections, as some patients had bilateral injections. Table 3 summarizes the patient characteristics, history, physical exam findings, and imaging findings. In the study, 84 patients (87%) presented with buttock pain, 56 patients (58%) presented with back pain, and 83 patients (86%) had symptoms of radiculopathy. Also, 55 patients (57%) had prior clinic injections, and 82 patients (85%) received an MRN prior to the CT-guided injections. Of the 82 patients who received an MRN, 18 (22%) showed ipsilateral piriformis hypertrophy (asymmetrical enlargement relative to the unaffected side) and 35 (43%) showed ipsilateral piriformis atrophy (asymmetrically smaller on the affected side with or without fatty infiltration). Finally, 67 patients (82%) had ipsilateral sciatic nerve hyperintensity and 14 patients (17%) showed ipsilateral split sciatic. Proportion of having Botox before and after propensity score weighting are shown in Fig. 3.
Table 3.
Patient characteristics separated based on whether or not they received an injection with Botox
| Without Botox (n=41) | With Botox (n=70) | p | |
|---|---|---|---|
| First injection age (years), median (IQR) | 54 (44–63) | 53 (41–65) | 0.71 |
|
| |||
| BMI (kg/m2), median (IQR) | 25 (22–27) | 25 (22–28) | 0.92 |
|
| |||
| Pain level, median (IQR) | 8 (7–9) | 8 (7–9) | 0.80 |
|
| |||
| Duration of pain (years), median (IQR) | 2.5 (1–4) | 2 (1.5–3) | 0.69 |
|
| |||
| Sex, n (%) | 0.95 | ||
| Female | 26 (63.41) | 44 (62.86) | |
| Male | 15 (36.59) | 26 (37.14) | |
|
| |||
| Buttock pain, n (%) | 0.060 | ||
| No | 2 (4.88) | 12 (17.14) | |
| Yes | 39 (95.12) | 58 (82.86) | |
|
| |||
| Back pain, n (%) | 0.073 | ||
| No | 23 (56.1) | 27 (38.57) | |
| Yes | 18 (43.9) | 43 (61.43) | |
|
| |||
| On pain medications, n (%) | 0.73 | ||
| No | 25 (60.98) | 45 (64.29) | |
| Yes | 16 (39.02) | 25 (35.71) | |
|
| |||
| Radiculopathy, n (%) | 0.69 | ||
| No | 7 (17.07) | 10 (14.29) | |
| Yes | 34 (82.93) | 60 (85.71) | |
|
| |||
| FABER positive, n (%) | 0.13 | ||
| No | 32 (78.05) | 45 (64.29) | |
| Yes | 9 (21.95) | 25 (35.71) | |
|
| |||
| FADIR positive, n (%) | 0.19 | ||
| No | 38 (92.68) | 59 (84.29) | |
| Yes | 3 (7.32) | 11 (15.71) | |
|
| |||
| Previous trauma, n (%) | 0.064 | ||
| No | 30 (73.17) | 61 (87.14) | |
| Fall | 7 (17.07) | 7 (10) | |
| Motor vehicle accident | 3 (7.32) | 2 (2.86) | |
| Cancer | 1 (2.44) | 0 (0) | |
|
| |||
| Prior clinic injection, n (%) | 0.19 | ||
| No | 21 (51.22) | 27 (38.57) | |
| Yes | 20 (48.78) | 43 (61.43) | |
|
| |||
| Muscle changes, n (%) | 0.58 | ||
| No | 21 (51.22) | 32 (45.71) | |
| Hypertrophy | 12 (29.27) | 26 (37.14) | |
| Atrophy | 8 (19.51) | 12 (17.14) | |
|
| |||
| Nerve changes, n (%) | 0.95 | ||
| No | 15 (36.59) | 26 (37.14) | |
| Yes | 26 (63.41) | 44 (62.86) | |
|
| |||
| Split sciatic, n (%) | 0.62 | ||
| No | 35 (85.37) | 62 (88.57) | |
| Yes | 6 (14.63) | 8 (11.43) | |
IQR, interquartile range; BMI, body mass index; FABER, flexion, abduction, external rotation test; FADIR, flexion, adduction, internal rotation test.
Figure 3.
Proportion of patients having Botox injection for their first CT-guided injection before and after propensity score weighting (IPTW, inverse probability of treatment weighting; FABER, flexion, abduction, external rotation test; FADIR, flexion, adduction, internal rotation test).
All variables were comparable between Botox and non-Botox group, back pain, buttock pain, FABER, FADIR, previous trauma and prior clinic injection had p < 0.2 and were considered as possible confounders.
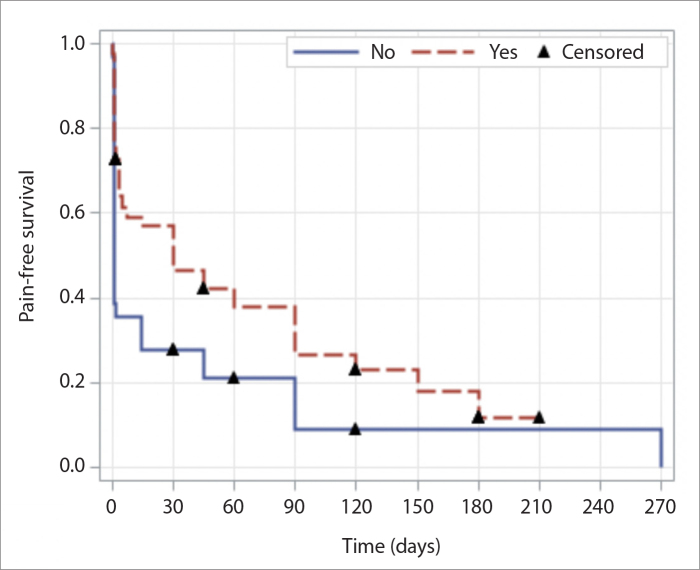
The response to injection after the first CT-guided injection was evaluated. Figs. 4 and 5 show MRN and CT-guided injection images of two patients in the study. Patients in the Botox group had more 48-hour response than patients in the non-Botox group (p < 0.001 with IPTW, p = 0.005 without IPTW) (Table 4). Median pain-free survival for Botox group was 30 days (95% CI: 4–90 days) and was 1 day (95% CI, 1–14 days) for non-Botox group (p = 0.059 with IPTW, p = 0.10 without IPTW). Fig. 6 shows the graph for the pain-free survival analysis.
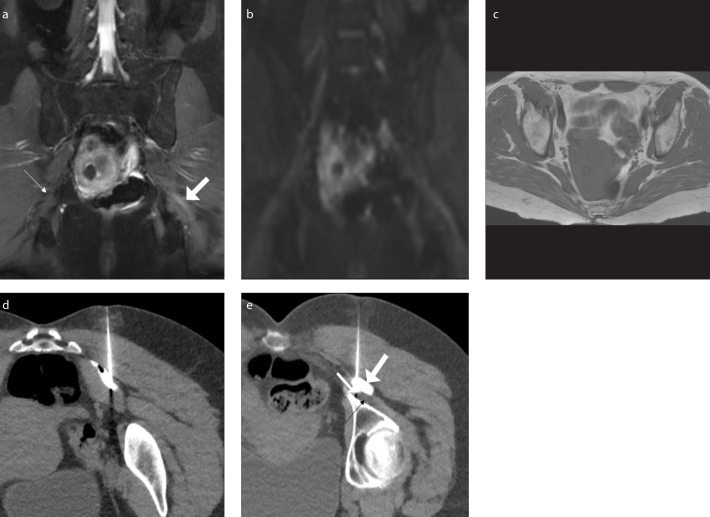
Figure 4. a–e.
A 57-year-old female presenting with buttock pain and back pain and diagnosed with left piriformis syndrome. She received an injection with Botox and had a positive response. Coronal 3D inversion recovery turbo spin-echo (IR TSE) image (a) shows asymmetrically hyperintense left sciatic nerve (thick arrow) at the sciatic notch compared to normal right sciatic nerve (thin arrow). Coronal maximum intensity projection (MIP) DTI image (b=600 s/mm2) (b) shows hyperintense left sciatic nerve. Axial T1-weighted image (c) shows left piriformis hypertrophy. CT-guided injection (d) with Botox and contrast of the left piriformis muscle. CT-guided injection (e) into the sciatic nerve (black arrow) showing the needle tip (thin white arrow), and the contrast (thick white arrow).
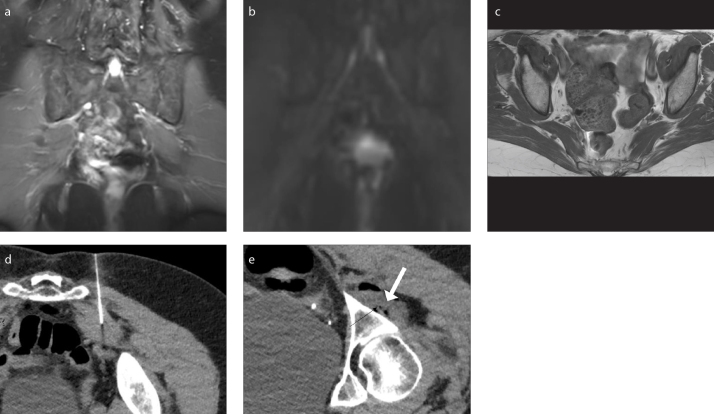
Figure 5. a–e.
A 78-year-old female presenting with buttock pain and back pain and diagnosed with left piriformis syndrome. She received an injection without Botox and had a negative response. Coronal 3D IR TSE image (a) shows normal signal of the sciatic nerves. Coronal MIP DTI image (b=600 s/mm2) (b) shows no enhancement of the sciatic nerves. Axial T1-weighted image (c) shows left piriformis hypertrophy. CT-guided injection (d) without Botox of the left piriformis muscle. Post-injection image (e) of the sciatic nerve (black arrow) and medication mixture (white arrow).
Table 4.
Response of the CT-guided piriformis muscle injection with or without Botox
| Without Botox (n=41), n (%) | With Botox (n=70), n (%) | p | |
|---|---|---|---|
| First injection response | 0.005 | ||
| Negative | 8 (19.51) | 12 (17.14) | |
| Possible positive | 20 (48.78) | 15 (21.43) | |
| Positive | 13 (31.71) | 43 (61.43) | |
Figure 6.
Pain-free survival analysis comparing patients who received Botox to patients who did not receive Botox in the injection.
None of the patients had any complications from the procedure documented at their last follow-up.
Discussion
Piriformis syndrome is a common pain condition involving the buttock and posterior hip that frequently affects a patient’s quality of life. Symptoms are aggravated when in the sitting position with hip flexion, adduction, and internal rotation. Piriformis syndrome is a diagnosis of exclusion, and a combination of history, physical examination, and imaging findings are needed before the diagnosis can be made. In our study, the majority of patients presented with buttock pain (87%), back pain (58%), and radiculopathy (86%). As noted in a review article by Hopayian et al. (22), these frequencies fall within the range documented by many other studies regarding piriformis syndrome. MRN imaging was performed in 85% of patients before they received the CT-guided injection. Of the patients who had an MRN, only 4 did not have any nerve or muscle changes as noted in the report. Previous studies have reported abnormal imaging in the setting of piriformis syndrome (23, 24) and our results validate such findings.
Initial therapy for piriformis syndrome involves conservative options like physical therapy, massages, heat therapy, and anti-inflammatory medications. In our study, all of our patients had undergone conservative treatment options before receiving CT-guided injections. About 43% of the patients had received prior clinic injections through various pain clinics with no success. We had more success as our injections were done under CT guidance and presumably since, we also injected the perineural area around the sciatic nerve in all cases. At our institution, we perform the injections for piriformis syndrome under CT guidance, because it offers soft tissue contrast, which assists in differentiating vessels from nerves (19). In addition, it is less operator dependent than ultrasound. Even though patients are exposed to ionizing radiation during a CT-guided injection as opposed to an MRI-guided injection, the use of the MRI is limited by availability, time, and cost constraints.
This retrospective study confirms our hypothesis that a CT-guided injection using Botox is more effective for pain relief. In our study, 61% of injections with Botox led to a positive response while only 32% of injections without Botox led to a positive response, which meant a reduction in pain score of 50% within the first 24 hours after the injection, sustained response 48 hours later, and no increase in pain over the first 48 hours. A prior study has shown that CT-guided injection of the piriformis muscle with Botox improved pain in 35% of patients at 4 weeks and in 65% of at 8 weeks while there was no response in any of the patients who received only dexamethasone and lidocaine (18). In that study by Yoon et al. (18), the injection was directed solely to the piriformis muscle, and there was no injection to the perineural area of the sciatic nerve. Another study showed no difference in patients who received only local anesthetics versus local anesthetic plus corticosteroids (23). Fanucci et al. (6) showed a 87% rate of pain relief in patients with piriformis syndrome who received a CT-guided injection with Botox; however, they did not compare with patients who received an injection without Botox.
In our study, we showed that median pain-free survival for the non-Botox group was 1 day versus 30 days for the Botox group, a significant difference. Many other studies have shown sustained effects of Botox for many weeks after the piriformis muscle injection (6, 12, 18). Due to the retrospective nature of our study, many patients were lost after the 48 hours follow-up and did not return to clinic. We were unable to serially follow many patients and could only record their last follow-up results. In addition, chronic pain syndromes are multifactorial and since many of them had failed prior injections in clinic, these were complex patients and a psychological component cannot be denied. Comprehensive evaluation of psychological component in a prospective study could shed greater light on these patients with piriformis syndrome.
Some limitations of the study include its retrospective nature. The prescription of Botox was not randomized, which could have introduced potential confounders. While we were not able to identify any confounding variables through our data collection, it is still possible that they exist, so causation is not available. In addition, patients self-reported the response, so it is subject to reporting bias. The patients did have knowledge of whether Botox was administered to them which can further bias the result. Our study can only draw correlations and will facilitate further prospective randomized blinded trials. Finally, piriformis syndrome is a multifactorial diagnosis and difficult to say with certainty that a patient has piriformis syndrome. Our diagnosis was based on clinical history, physical exam, and imaging findings.
In future, prospective randomized blind trials for patients with suspected piriformis syndrome could compare the pain response, range of motion and overall quality of life improvements to injections with and without Botox. There is a significant need to conduct this trial as piriformis syndrome is a debilitating disease greatly affecting patients’ quality of life and ability to perform everyday activities. We hope that our study will facilitate future trials for piriformis syndrome.
Main points
CT-guided injections with botulinum toxin (Botox, Allergan) for patients with piriformis syndrome are more likely to lead to a positive response than CT-guided injections without Botox.
CT-guided injections with Botox for patients with piriformis syndrome are more likely to lead to a longer duration of response than patients who receive a CT-guided injection without Botox.
We showed that median pain-free survival for the non-Botox group was 1 day versus 30 days for the Botox group.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Hopayian K, Song F, Riera R, Sambandan S. The clinical features of the piriformis syndrome: A systematic review. Eur Spine J. 2010;19:2095–2109. doi: 10.1007/s00586-010-1504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller TA, White KP, Ross DC. The diagnosis and management of piriformis syndrome: Myths and facts. Can J Neurol Sci. 2012;39:577–583. doi: 10.1017/S0317167100015298. [DOI] [PubMed] [Google Scholar]
- 3.Cass SP. Piriformis syndrome: A cause of nondiscogenic sciatica. Curr Sports Med Rep. 2015;14:41–44. doi: 10.1249/JSR.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 4.Natsis K, Totlis T, Konstantinidis GA, Paraskevas G, Piagkou M, Koebke J. Anatomical variations between the sciatic nerve and the piriformis muscle: A contribution to surgical anatomy in piriformis syndrome. Surg Radiol Anat. 2014;36:273–280. doi: 10.1007/s00276-013-1180-7. [DOI] [PubMed] [Google Scholar]
- 5.Hernando MF, Cerezal L, Perez-Carro L, Abascal F, Canga A. Deep gluteal syndrome: Anatomy, imaging, and management of sciatic nerve entrapments in the subgluteal space. Skeletal Radiol. 2015;44:919–934. doi: 10.1007/s00256-015-2124-6. [DOI] [PubMed] [Google Scholar]
- 6.Fanucci E, Masala S, Sodani G, et al. CT-guided injection of botulinic toxin for percutaneous therapy of piriformis muscle syndrome with preliminary MRI results about denervative process. Eur Radiol. 2001;11:2543–2548. doi: 10.1007/s003300100872. [DOI] [PubMed] [Google Scholar]
- 7.Filler AG, Haynes J, Jordan SE, et al. Sciatica of nondisc origin and piriformis syndrome: Diagnosis by magnetic resonance neurography and interventional magnetic resonance imaging with outcome study of resulting treatment. J Neurosurg Spine. 2005;2:99–115. doi: 10.3171/spi.2005.2.2.0099. [DOI] [PubMed] [Google Scholar]
- 8.Delaney H, Bencardino J, Rosenberg ZS. Magnetic resonance neurography of the pelvis and lumbosacral plexus. Neuroimaging Clin N Am. 2014;24:127–150. doi: 10.1016/j.nic.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Dessouky R, Xi Y, Scott KM, et al. Magnetic resonance neurography in chronic lumbosacral and pelvic pain: Diagnostic and management impact-institutional audit. World Neurosurg. 2018;114:e77–e113. doi: 10.1016/j.wneu.2018.02.072. [DOI] [PubMed] [Google Scholar]
- 10.Petrasic JR, Chhabra A, Scott KM. Impact of mr neurography in patients with chronic cauda equina syndrome presenting as chronic pelvic pain and dysfunction. AJNR Am J Neuroradiol. 2017;38:418–422. doi: 10.3174/ajnr.A4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirschner JS, Foye PM, Cole JL. Piriformis syndrome, diagnosis and treatment. Muscle Nerve. 2009;40:10–18. doi: 10.1002/mus.21318. [DOI] [PubMed] [Google Scholar]
- 12.Benzon HT, Katz JA, Benzon HA, Iqbal MS. Piriformis syndrome: Anatomic considerations, a new injection technique, and a review of the literature. Anesthesiology. 2003;98:1442–1448. doi: 10.1097/00000542-200306000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Hanania M, Kitain E. Perisciatic injection of steroid for the treatment of sciatica due to piriformis syndrome. Reg Anesth Pain Med. 1998;23:223–228. doi: 10.1136/rapm-00115550-199823020-00020. [DOI] [PubMed] [Google Scholar]
- 14.Soares A, Andriolo RB, Atallah AN, da Silva EM. Botulinum toxin for myofascial pain syndromes in adults. Cochrane Database Syst Rev. 2014:CD007533. doi: 10.1002/14651858.CD007533.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armstrong MW, Mountain RE, Murray JA. Treatment of facial synkinesis and facial asymmetry with botulinum toxin type a following facial nerve palsy. Clin Otolaryngol Allied Sci. 1996;21:15–20. doi: 10.1111/j.1365-2273.1996.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 16.Santamato A, Micello MF, Valeno G, et al. Ultrasound-guided injection of botulinum toxin type a for piriformis muscle syndrome: A case report and review of the literature. Toxins (Basel) 2015;7:3045–3056. doi: 10.3390/toxins7083045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fabregat G, Rosello M, Asensio-Samper JM, et al. Computer-tomographic verification of ultrasound-guided piriformis muscle injection: A feasibility study. Pain Physician. 2014;17:507–513. [PubMed] [Google Scholar]
- 18.Yoon SJ, Ho J, Kang HY, et al. Low-dose botulinum toxin type a for the treatment of refractory piriformis syndrome. Pharmacotherapy. 2007;27:657–665. doi: 10.1592/phco.27.5.657. [DOI] [PubMed] [Google Scholar]
- 19.Wadhwa V, Scott KM, Rozen S, Starr AJ, Chhabra A. CT-guided perineural injections for chronic pelvic pain. Radiographics. 2016;36:1408–1425. doi: 10.1148/rg.2016150263. [DOI] [PubMed] [Google Scholar]
- 20.Giraudeau B, Rozenberg S, Valat JP. Assessment of the clinically relevant change in pain for patients with sciatica. Ann Rheum Dis. 2004;63:1180–1181. doi: 10.1136/ard.2003.015792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dessouky R, Khaleel M, Khalifa DN, Tantawy HI, Chhabra A. Magnetic resonance neurography of the lumbosacral plexus in failed back surgery syndrome. Spine (Phila Pa 1976) 2018;43:839–847. doi: 10.1097/BRS.0000000000002460. [DOI] [PubMed] [Google Scholar]
- 22.Hopayian K, Danielyan A. Four symptoms define the piriformis syndrome: An updated systematic review of its clinical features. Eur J Orthop Surg Traumatol. 2018;28:155–164. doi: 10.1007/s00590-017-2031-8. [DOI] [PubMed] [Google Scholar]
- 23.Lewis AM, Layzer R, Engstrom JW, Barbaro NM, Chin CT. Magnetic resonance neurography in extraspinal sciatica. Arch Neurol. 2006;63:1469–1472. doi: 10.1001/archneur.63.10.1469. [DOI] [PubMed] [Google Scholar]
- 24.Arooj S, Azeemuddin M. Piriformis syndrome--a rare cause of extraspinal sciatica. J Pak Med Assoc. 2014;64:949–951. [PubMed] [Google Scholar]