Abstract
Effects of a large-sized cage with a low metabolizable energy and low crude protein (LME-LCP) diet on growth performance, feed cost, blood biochemistry, and antibody response of growing layers were evaluated. A total of 668 one-day-old female Gushi chicks were randomly allocated into three different cages, namely, large, medium, and small cages, referred to as Group A, Group B, and Group C, respectively, and fed LME-LCP diets. A fourth group of birds raised in small cages and fed a standard diet (STD) was designated Group D i.e. the control. Equal stocking densities were maintained among the four groups throughout the experiment, from 50–20 birds/m2. Large cages with LME-LCP diet (Group A) increased the shank length and girth as chicks grew, improved the activities of serum creatine kinase, and reduced serum triglyceride and cholesterol concentrations. The total feed intake in birds from Group A was higher than those from the other groups at every stage. The total cost (rmb/bird) of feed was 6.70% lower in Group A than that in Group D, which indicated the cost-effectiveness of large cages. In conclusion, large cages with LME-LCP diets have positive effects on body weight, shank growth, and serum biochemical indices of growing Gushi chicks, and can reduce feed costs.
Keywords: blood parameter, growth performance, immune response, large cage, layer
Introduction
Cage design is a component of a hen's environment and plays a critical role in determining its well-being (Widowski et al., 2016). The effect of cage size on chickens has been studied in different ways. Decreased cage space reportedly decreases biological function, egg production, egg weight, body weight, and feed intake, and increases mortality (Sohail et al., 2004; Hartcher and Jones, 2017). Chickens grow more slowly and jostle each other more at higher stocking densities, and a narrow living space can restrain their movements and activities, which leads to osteoporosis (Stamp et al., 2004; Webster, 2004). In contrast, enough free space for movement can allow caged hens to perform most normal patterns of behavior (Mench and Blatchford, 2014). It has been reported that foraging, wing stretching, leg stretching, and tail wagging all increase in frequency when chickens are housed in larger cages (Lay et al., 2011), and that larger spaces for chickens are associated with improved bone mass and bone quality (Eric et al., 2015). Similarly, Meng et al. (2016) found that large furnished cages allowed hens to have stronger or heavier tibias than small furnished cages. Our previous study (Li et al., 2019) indicated that large and medium cages were superior to small cages and were beneficial for the growth and development of birds. Therefore, increasing the space in cages could be advantageous for poultry production.
In addition to the various reported effects of cage size on the performance of chickens, differing diets have also been implicated as having an effect on the growth, carcass traits, and blood serum parameters of hens. Low metabolizable energy (LME) and low crude protein (LCP) diets reportedly enhanced immune functions and feed conversion ratio (Nahashon et al., 2007; Sigolo et al., 2017). Moreover, Kidd et al. (2001) reported that LCP diets improved protein efficiency and increased feed intake. However, few studies have been conducted on the synergistic effect of cage size and diet on chicken performance. Madrid et al. (1981) reported that maintenance energy requirements increased as cage space decreased, whereas Jalal et al. (2006) found that energy intake increased as cage space increased.
Based on the abovementioned findings, cage size plays an important role in enhancing the well-being of chickens and affects their energy needs, and larger cages are expected to improve chicken growth. In this study, we evaluated the effects of a large-sized cage with LME-LCP diets on the growth performance, blood parameters, and immune responses of growing layers.
Material and Methods
The study protocol was approved by the Committee for the Care and Use of Experimental Animals at Anhui Academy of Agricultural Science under permit no. A11-CS06.
Birds and Dietary Treatments
Gushi chickens, originating from central China, are used as a dual-purpose breed. A total of 668 one-day-old female Gushi chicks were obtained from Anhui Wanxi Poultry Development Co., Ltd. (Luan, China) and assigned to two dietary treatments (Table 1): a standard diet (STD) and an LME-LCP diet. The STD contained 12.38 MJ of metabolizable energy (ME)/kg and 20.13% crude protein (CP) from days 1–28, 12.68 MJ of ME/kg and 18.20% CP from days 29–56 and 12.72 MJ of ME/kg and 16.03% CP from days 57–98. The LME-LCP diet contained 2% less CP than the STD at each growth stage and 6.7%, 6.0%, and 5.9% less ME than the STD at the three growth stages, respectively. The diets were provided in mash form, and the feeder space among the treatments was kept identical. Feed and water were provided ad libitum. Feeds were prepared once per week to avoid mildew.
Table 1. Ingredient composition and nutrient levels of experimental diets fed from days 1–98.
| Age (days) | 1–28 | 29–56 | 57–98 | |||
|---|---|---|---|---|---|---|
| Level of diet | STD1 | LME-LCP2 | STD | LME-LCP | STD | LME-LCP |
| Ingredients, g/kg | ||||||
| Corn | 503.2 | 618.1 | 549.0 | 660.1 | 617.2 | 728.2 |
| Soybean meal | 367.3 | 301.2 | 315.3 | 250.1 | 254.2 | 189.2 |
| Soybean oil | 55.0 | 6.2 | 61.2 | 15.3 | 54.1 | 8.1 |
| Limestone | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Salt | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Premix3 | 46.5 | 46.5 | 46.5 | 46.5 | 46.5 | 46.5 |
| Calculated level | ||||||
| ME, MJ/kg | 12.38 | 11.55 | 12.68 | 11.92 | 12.72 | 11.97 |
| CP, % | 20.13 | 18.13 | 18.20 | 16.20 | 16.03 | 14.03 |
| Lysine, % | 1.10 | 1.00 | 1.00 | 0.83 | 0.83 | 0.68 |
| Methionine, % | 0.45 | 0.42 | 0.42 | 0.40 | 0.38 | 0.36 |
| Methionine + cystine, % | 0.82 | 0.75 | 0.74 | 0.71 | 0.68 | 0.62 |
| Calcium, % | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Available phosphorus, % | 0.47 | 0.43 | 0.42 | 0.42 | 0.40 | 0.38 |
STD: Standard diet.
LME-LCP: low metabolizable energy and low crude protein diet.
Premix provided the following per kg of diet: copper, 10.0 mg; iron, 30.0 mg; manganese, 60.0 mg; zinc, 65.0 mg; selenium, 0.3 mg; retinol, 2.7 mg; cholecalciferol, 77.5 mg; tocopheryl acetate, 16.7 mg; menadione, 0.5 mg; thiamine, 5 mg; riboflavin, 2 mg; cyanocobalamin, 0.02 mg; pyridoxine, 3.5 mg; biotin, 0.1 mg; folacin, 1 mg; pantothenic acid, 12 mg; and nicotinic acid, 38 mg.
Cages and Grouping
This study was performed using three different-sized cages for birds: a large cage (1.6×1.6×0.42 m), medium cage (1.2×1.2×0.42 m), and small cage (0.8×0.7×0.37 m). Experimental birds raised in large, medium, and small cages and fed the LME-LCP diet were assigned to Group A, Group B, and Group C, respectively. A fourth group of birds raised in small cages and fed STD was assigned to Group D as a control. The experiment lasted for 14 weeks. The stocking densities of the four groups, which were identical and adjusted with age during the experimental period (Table 2), were 50 birds/m2 (from 1–14 days), 40 birds/m2 (from 15–28 days), 30 birds/m2 (from 29–42 days), 25 birds/m2 (from 43–56 days), 22 birds/m2 (from 57–70 days), and 20 birds/m2 (from 71–98 days).
Table 2. The stocking density of different groups of Gushi chickens during different growth periods.
| Group1 | Age (days) | 1st | 14th | 28th | 42nd | 56th | 70th–98th |
|---|---|---|---|---|---|---|---|
| Group A, large cages, 1.6 m×1.6 m | Number of cages | 2 | 2 | 3 | 3 | 3 | 3 |
| Birds/cage | 128 | 102 | 76 | 64 | 56 | 51 | |
| Total number of birds | 256 | 204 | 228 | 192 | 168 | 153 | |
| Base area of cage (m2) | 2.56 | ||||||
| Group B, medium cages, 1.2 m×1.2 m | Number of cages | 3 | 3 | 5 | 5 | 5 | 5 |
| Birds/cage | 72 | 58 | 43 | 36 | 32 | 29 | |
| Total number | 216 | 174 | 215 | 180 | 160 | 145 | |
| Base area of cage (m2) | 1.44 | ||||||
| Groups C and D, small cages, 0.8 m×0.7 m | Number of cages | 7 | 8 | 11 | 13 | 12 | 13 |
| Birds/cage | 28 | 22 | 17 | 14 | 12 | 11 | |
| Total number | 196 | 176 | 187 | 182 | 144 | 143 | |
| Base area of cage (m2) | 0.56 | ||||||
| Stocking density (birds/m2) | 50 | 40 | 30 | 25 | 22 | 20 | |
Groups A, B, and C were fed a low metabolizable energy and low crude protein (LME-LCP) diet; Group D was fed a standard (STD) diet.
All the birds were reared in the same experimental room. The room temperature was 32°C at the start of the trial and was reduced gradually to 20°C by 21 days of age and kept at that temperature for the remaining experimental period. The light regimen at 1 day old was 24 h light (L):0 h darkness (D), which was stepped down to 9L:15D by 50 days of age, and maintained until the end of the study. Birds were vaccinated in accordance with the standard vaccination schedule.
Measurements and Sampling
On day 1, the chicks were individually weighed, wing-tagged, and separated into different groups. Body weight, shank length, and shank girth were measured every 2 weeks (from 1–14 weeks). Body weight was measured using an electronic scale. Shank length was measured using a digital Vernier caliper while shank girth was measured using a tape rule. Feed intake and mortality per pen were recorded daily.
For each group, 30 birds were randomly selected for blood sampling on days 56, 70, 84, and 98 days. A 4-mL blood sample was collected into two heparinized tubes (2 mL in each tube) from the chickens by wing vein puncture. The time between catching the bird and taking the blood sample did not exceed 45 s. Samples were placed in an ice bath immediately after collection, and then transported to the laboratory for processing. Blood serum was separated by centrifugation at 3000 rpm for 10 min and stored at −20°C until analysis.
The collected serum was assayed for levels of corticosterone (CORT), creatine kinase (CK), triglyceride (TG), total cholesterol (T-CH), blood urea nitrogen (BUN), and glutathione (GSH). The concentrations of these parameters were determined with an automatic biochemical analyzer (Tecan, Männedorf, Switzerland) using commercial laboratory kits (Xinqidi Biotech Co., Wuhan, China). Antibody titers of avian influenza viruses, H5N1 (Re-5 strain) and H9N2 (Re-2 strain), and Newcastle disease virus (NDV) were determined using enzyme-linked immunosorbent assay (ELISA) kits (Mlbio Biotech Co., Shanghai, China) according to the manufacturer's protocol. Antibody titer data were logarithmically transformed (base 2) prior to analysis.
Statistical Analysis
Data were subjected to analysis of variance (ANOVA) using the General Linear Model (GLM) command in SAS version 9.3 statistical software (SAS Institute Inc., Cary, NC, USA). When differences among individual means were found in ANOVA tests (P<0.05), the means were compared using Tukey's multiple test.
Results
Growth Performance
The effects of large cages fed with LME-LCP diet on body weight, shank length, and shank girth of chickens over the 14-week study period are presented in Table 3. The body weight of Group D (fed STD) was significantly higher (P<0.05) than those of Groups A, B, and C, which were fed LME and LCP diets during the early period (from 14–56 days). As the hens aged, the body weights of Groups A, B, and D were similar from days 70–98, while birds from Group C were significantly (P<0.05) lighter in weight than those raised in the other groups. No significant differences were found in shank length between Groups A and D from days 14–70 except on the 42nd day. Large cages (Group A) appeared to increase shank length from 8.08–8.47 cm between 84 and 98 days of age, which were significantly (P<0.05) greater than those observed in Groups C and D. A similar trend was found in the values of shank girth in which chickens from Group A had a greater shank girth than those in the other three groups from days 42–98, while the shank girth of Group C was significantly smaller than those of the other three groups.
Table 3. The body weight,shank length,and shank girth of different groups of Gushi chickens during different growth periods.
| Age (days) | LME-LCP1 > |
STD2 |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group A | N3 | Group B | N | Group C | N | Group D | N | ||
| Mean body weight, g | |||||||||
| 14th | 91.56a | 256 | 89.08a | 216 | 91.94a | 196 | 97.80b | 196 | <0.001 |
| 28th | 189.36a | 204 | 184.31a | 174 | 197.36b | 176 | 210.73c | 176 | <0.001 |
| 42nd | 340.92a | 228 | 331.06ab | 215 | 322.36b | 187 | 356.94c | 187 | <0.001 |
| 56th | 524.17a | 192 | 520.85a | 180 | 507.62a | 182 | 553.04b | 182 | <0.001 |
| 70th | 727.95a | 168 | 706.77a | 160 | 675.62b | 144 | 728.59a | 144 | <0.001 |
| 84th | 870.77a | 153 | 887.15a | 145 | 824.59b | 143 | 866.19a | 143 | <0.001 |
| 98th | 1006.30a | 153 | 1009.99a | 145 | 945.29b | 143 | 1006.95a | 143 | <0.001 |
| Shank length, cm | |||||||||
| 14th | 3.25a | 256 | 3.06b | 216 | 3.10b | 196 | 3.21a | 196 | <0.001 |
| 28th | 4.33ab | 204 | 4.26a | 174 | 4.37b | 176 | 4.26a | 176 | 0.012 |
| 42nd | 5.18a | 228 | 5.12a | 215 | 5.13a | 187 | 5.31b | 187 | 0.002 |
| 56th | 6.47ac | 192 | 6.37ad | 180 | 6.31bd | 182 | 6.57c | 182 | <0.001 |
| 70th | 6.95a | 168 | 6.93a | 160 | 6.80b | 144 | 7.02a | 144 | 0.005 |
| 84th | 8.08a | 153 | 8.05a | 145 | 7.75b | 143 | 7.54c | 143 | <0.001 |
| 98th | 8.47a | 153 | 8.26b | 145 | 8.16b | 143 | 8.26b | 143 | <0.001 |
| Shank girth, cm | |||||||||
| 14th | 1.71a | 256 | 1.73ab | 216 | 1.75b | 196 | 1.75b | 196 | 0.008 |
| 28th | 2.20ac | 204 | 2.17bc | 174 | 2.23ad | 176 | 2.27d | 176 | <0.001 |
| 42nd | 2.70a | 228 | 2.66ab | 215 | 2.61b | 187 | 2.66a | 187 | 0.005 |
| 56th | 2.97 | 192 | 2.94 | 180 | 2.93 | 182 | 2.95 | 182 | 0.400 |
| 70th | 3.24a | 168 | 3.22a | 160 | 3.09b | 144 | 3.20a | 144 | <0.001 |
| 84th | 3.42a | 153 | 3.41a | 145 | 3.34b | 143 | 3.41a | 143 | <0.001 |
| 98th | 3.58a | 153 | 3.55a | 145 | 3.49b | 143 | 3.53ab | 143 | <0.001 |
Means within a day with different superscripts are significantly different (P<0.05).
LME-LCP: low metabolizable energy and low crude protein diet.
STD: standard diet.
N: number of birds.
Feed Cost
The consumption and cost of feed of different groups are shown in Table 4. The total feed intake of Group A was 3775.76 g/bird, which was higher than that observed for the other groups at every stage, while Group C (small cages with LME-LCP diet) had the lowest total feed intake of 3392.04 g/bird. In terms of total feed cost per bird throughout the experiment (from birth to 98 days), the four groups ranked as follows: Group D>Group A>Group B>Group C, with values of 10.60 rmb, 9.89 rmb, 9.47 rmb, and 8.87 rmb, respectively.
Table 4. The consumption and cost of feed per bird among the four groups of Gushi chickens during different growth periods.
| Age (days) | Item | LME-LCP1 |
STD2 |
|||
|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | P-value | ||
| 1–14 | Mean feed intake (g/bird/day) | 10.01 | 9.22 | 9.52 | 9.97 | 0.720 |
| 15–28 | 17.81 | 18.39 | 16.41 | 17.21 | 0.556 | |
| Total feed intake (g/bird) | 389.49 | 386.61 | 362.99 | 380.49 | ||
| Feed prices (rmb/kg) | 2.7 | 3.10 | ||||
| Cost (rmb/bird) | 1.05 | 1.04 | 0.98 | 1.18 | ||
| 29–42 | Mean feed intake (g/bird/day) | 29.35a | 24.51bc | 23.55b | 26.82ac | <0.001 |
| 43–56 | 46.58a | 42.25b | 33.69c | 32.92c | <0.001 | |
| Total feed intake (g/bird) | 1063.12 | 934.52 | 801.38 | 836.41 | ||
| Feed prices (rmb/kg) | 2.7 | 3.07 | ||||
| Cost (rmb/bird) | 2.87 | 2.52 | 2.16 | 2.57 | ||
| 57–70 | Mean feed intake (g/bird/day) | 53.15 | 50.75 | 51.93 | 53.61 | 0.153 |
| 71–84 | 56.30 | 56.31 | 53.55 | 54.78 | 0.200 | |
| 85–98 | 56.48 | 57.19 | 53.63 | 57.42 | 0.264 | |
| Total feed intake (g/bird) | 2323.15 | 2299.48 | 2227.67 | 2321.22 | ||
| Feed prices (rmb/kg) | 2.57 | 2 | .95 | |||
| Cost (rmb/bird) | 5.97 | 5.91 | 5.73 | 6.85 | ||
| 1–98 | Total feed intake (g/bird) | 3775.76 | 3620.61 | 3392.04 | 3538.12 | |
| Total Cost (rmb/bird) | 9.89 | 9.47 | 8.87 | 10.60 | ||
Means in the same time period with different superscripts are significantly different (P<0.05).
LME-LCP: low metabolizable energy and low crude protein diet.
STD: Standard diet.
Blood Parameters
The effects of cage type on blood serum parameters are given in Fig. 1. Birds in large cages that received the LME-LCP diet (Group A) had higher (P<0.05) blood serum concentrations of CK than those in the other groups throughout the experiment but lower concentrations of T-CH and BUN from days 56–84. Similar levels of BUN were found between Groups C and D throughout the experiment. Birds in small cages that received STD (Group D) had lower blood serum concentrations of CK and GSH than those in the other groups throughout the experiment. There were no significant differences (P>0.05) in CORT among the four groups throughout the experiment. The concentrations of TG and TCH were higher in Groups B and C than in Groups A and D (P<0.05) throughout the experiment.
Fig. 1.
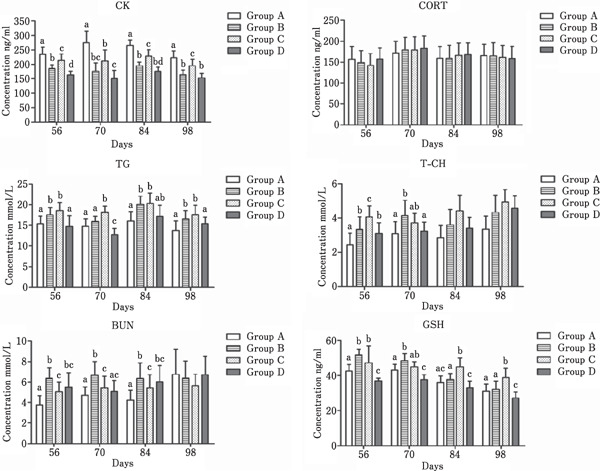
Blood serum concentrations of CK,CORT,TG,T-CH,BUN, and GSH in Gushi chickens from the four groups during different growth periods. Group A, B, and C were large, medium, and small cages, respectively, and fed LME-LCP diet; Group D was small cages and fed STD diet; a–d Means within a day with different superscripts are significantly different (P<0.05).
Antibody Titer
As shown in Table 5, there were no significant differences in immune responses to H5N1 between any groups at 56, 70, and 84 days of age. The antibody titer of H9N2 in Group A was similar to that in the other groups at 56 days of age but was higher at 70, 84, and 98 days. No significant differences in the antibody titer of NDV were observed between Groups A and D throughout the experiment. The antibody titer of NDV in Group B was higher than that in the other groups at 70, 84, and 98 days of age.
Table 5. The antibody titer of the four groups of Gushi chickens during different growth periods.
| Antibody titer1 | Group | Age (days) |
|||
|---|---|---|---|---|---|
| 56th | 70th | 84th | 98th | ||
| H5N1 | A | 9.13 | 10.00 | 9.42 | 6.53 |
| B | 9.24 | 9.60 | 9.54 | 7.28 | |
| C | 9.18 | 9.60 | 8.91 | 6.66 | |
| D | 9.26 | 10.10 | 8.55 | 7.03 | |
| P-value | 0.995 | 0.297 | 0.649 | 0.174 | |
| H9N2 | A | 10.52 | 12.00 | 12.19 | 11.40a |
| B | 10.52 | 11.83 | 12.00 | 11.83a | |
| C | 10.46 | 11.33 | 11.94 | 11.24ab | |
| D | 10.20 | 11.45 | 11.52 | 10.63b | |
| P-value | 0.905 | 0.153 | 0.161 | 0.012 | |
| NDV | A | 6.30 | 9.20 | 9.94ab | 9.90 |
| B | 6.67 | 10.00 | 10.75a | 10.62 | |
| C | 6.86 | 9.57 | 10.62a | 10.00 | |
| D | 6.40 | 9.59 | 9.83b | 10.20 | |
| P-value | 0.445 | 0.068 | 0.047 | 0.115 | |
Means within a day with different superscripts are significantly different (P<0.05).
H5N1: avian influenza viruses H5N1 (Re-5 strain); H9N2: avian influenza viruses H9N2 (Re-2 strain); NDV: Newcastle disease virus; Titers are reported as logarithms (base 2)
Discussion
The differences in various indicators among groups showed the adaptability of birds to different-sized cages. Our previous study (Li et al., 2019) revealed that larger cages would provide more space for birds and promote the growth performance of Jinghong chickens. However, further studies are required to investigate the superiorities of large cage in different breeds. In the present study, different dietary energy and protein contents were used to evaluate the effect of different-sized cages on Gushi chicks.
Theoretically, birds raised in larger cages should have larger space allowances and altered dynamics of space use, which could contribute to growth, than those in small cages at an equal stocking density (birds/m2), which have less activity space (Appleby, 2004; Widowski et al., 2017). Body weight in Group D was greater than that in the other groups from days 14–56, which suggested that energy and protein supply were more important for growth than cage size when birds were young and small. The larger space allowances did not yield apparent benefits, because of the small size of the chicks at this stage; the space available in the small cages was enough for their activities. However, the body weights of chicks were similar between Groups A and D from days 70–98, which indicated that the large cages promoted weight gain during this period despite being fed LME-LCP diet. Moreover, shank length and shank girth were greater in Group A than in the other groups during the late stage of the experiment (from days 84–98), which also reflected the positive effects of large cages on shank growth as the birds aged. These effects were probably due to the larger free space and increased exercise/activity of the birds in the large cages, which are conducive for growth and feed intake (Widowski et al., 2016; Li et al., 2019). Similarly, Widowski et al. (2003) found that increasing space allowance during rearing improved the body weight of pullets. Birds in Group C, which were grown in small cages and fed an LME-LCP diet, had lower body weight, shank length, and shank girth than those in the other groups (from 56–98 days), as expected.
Providing space allowance does have consequences for feed intake in caged laying chickens (Widowski et al., 2017). The feed intake in Groups A and B was higher than that in Group D; this could be due to the relatively larger space in the cage. This is consistent with the finding of Sohail et al. (2004) who reported that increasing cage space per hen increased 14-week average feed intake. Similarly, Jalal et al. (2006) reported that feed intake increased by 6.30 g/hen per day as cage space increased from 342–690 cm2/hen. In addition, birds from Group A had a higher feed intake, which resulted in a lower total cost of feed consumption than that observed for Group D (6.70% reduction per bird). This finding is similar to that in a previous study (Morris, 1968), in which a low-calorie diet could reduce the cost of feeding.
The release of CK is thought to be proportional to the intensity and duration of exercise (Apple, 1981), while exercise can briefly lower serum TG (Oscai et al., 1972) and cholesterol (Johnson et al., 1959) levels. Hens are social animals and sometimes gather under natural conditions (Xiang et al., 2016); larger spaces appear in larger cages, which motivates the activities of birds. In this study, we observed a higher CK level and lower TG and T-CH levels in birds from Group A than in those from Group B and C, which indicated that large-sized cage is conducive for facilitating movements of birds and lowering their blood lipids.
GSH, an important intracellular antioxidant, plays a vital role in antioxidant defense mechanisms (Schulz et al., 2010). Previous studies have stated that small space allowance reduced serum GSH-Px level but did not change the serum GSH level in Ross-308 broiler chickens (Simsek et al., 2009), and plasma GSH-Px levels in Jinghong layer breeder males from small cages were higher than that in those from large and middle cages (Li et al., 2019). In this study, serum GSH levels were lower in the birds from Group D than in those from the other groups throughout the experiment, which did not show the superiority of large-sized cage or low-calorie diets in oxidation resistance. These differences among studies could be attributed to the differences in dietary nutrient levels and chicken breeds.
Low space allowance in cages could induce chronic stress in birds, which affects CORT, an indicator of physiological stress (Villagra et al., 2009). Kang et al. (2013) reported that plasma CORT levels were significantly (P<0.05) lower in hens housed in floor pens with larger space allowance than that in those housed in conventional cages with lower space allowance. Cheng and Muir (2004) reported that laying hens showed a significantly lower plasma CORT level in single bird cages (525 cm2/bird) than that in 10-bird cages (419 cm2 /bird). However, Houshmand et al. (2012) noted a negligible effect of stocking density on CORT in caged broilers, and Thaxton et al. (2006) summarized that stocking density did not cause physiological adaptive changes that were indicative of stress. Similarly, no significant difference in serum CORT levels was found among the four groups in the present study.
Antibody titer of birds was influenced by housing system, stocking density, and cage sizes (Onbaşılar and Aksoy, 2005; Arbona et al., 2011). Birds in large cages showed higher antibody titers against H5N1 and H9N2 avian influenza viruses than those in middle and small cages in our previous study (Li et al., 2019). However, there were no significant differences in antibody titers among the four groups during most of the experimental period in the present study, except the higher level of antibody titers against H9N2 in Group A and B compared to Group D at 98 days of age and the higher antibody titers against NDV in Group B and C as compared with Group D at 84 days of age, which indicated that neither dietary energy or protein nor cage size had great effects on immunity levels. This may be due to the differences in management procedures and environmental conditions in their housing.
In summary, large cages with LME-LCP diets increased shank growth as chicks grew and optimized CK, TG, and T-CH levels in blood serum. The reduction in feed cost by 6.70% per bird compared with the small-sized cages with STD diets indicated the cost-effectiveness of the former conditions. Overall, the current findings provide support for the advantages of large-sized cages with LME-LCP diets during rearing, at least in growing Gushi chickens. Further studies are needed to evaluate the effects of large cage on production performances and welfare traits in larger populations with different chicken breeds.
Acknowledgments
This study was funded by the China Agriculture Research System (CARS-40-K21); the Major Science and Technology Project of Anhui Province (18030701172, 17030701064); and the Nature Science Foundation of Anhui Province (1908085QC115, 1808085MC62). The authors are grateful to Dr. Cheng Zhang at Anhui Agricultural University for his valuable comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Apple FS. Presence of creatine kinase MB isoenzyme during marathon training. New England Journal of Medicine, 305: 764-765. 1981. [DOI] [PubMed] [Google Scholar]
- Appleby MC. What causes crowding? Effects of space, facilities and group size on behaviour, with particular reference to furnished cages for hens. Animal Welfare, 13: 313-320. 2004. [Google Scholar]
- Arbona DV, Anderson KE, Hoffman JB. A comparison of humoral immune function in response to a killed Newcastle's vaccine challenge in caged vs. free-range Hy-Line brown layers. International Journal of Poultry Science, 10: 315-319. 2011. [Google Scholar]
- Cheng HW, Muri WM. Chronic social stress differentially regulates neuroendocrine response in laying hens: II. Genetic basis of adrenal responses under three different social conditions. Psychoneurodenocrinlogy, 29: 961-971. 2004. [DOI] [PubMed] [Google Scholar]
- Eric A, Florence PG, Eric G, Maurice A, Daniel C. Bone mass and bone quality are altered by hypoactivity in the chicken. PLoSOne, 10: e0116763 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartcher KM, Jones B. The welfare of layer hens in cage and cage-free housing systems. World's Poultry Science Journal, 73: 1-15. 2017. [Google Scholar]
- Houshmand M, Azhar K, Zulkifli I, Bejo MH, Kamyab A. Effects of prebiotic, protein level, and stocking density on performance, immunity, and stress indicators of broilers. Poultry Science, 91: 393-401. 2012. [DOI] [PubMed] [Google Scholar]
- Jalal MA, Scheideler SE, Marx D. Effect of bird cage space and dietary metabolizable energy level on production parameters in laying hens. Poultry Science, 85: 306-311. 2006. [DOI] [PubMed] [Google Scholar]
- Johnson D, Mehring AL, Titus HW. Variability of the blood plasma cholesterol of laying chickens. Poultry Science, 38: 1109-1113. 1959. [Google Scholar]
- Kang SY, Ko YH, Moon YS, Sohn SH, Jang IS. Effects of housing systems on physiological and immunological parameters in laying hens. Journal of Animal Science and Technology, 55: 131-139. 2013. [Google Scholar]
- Kidd MT, Gerard PD, Heger J, Kerr BJ, Rowe D, Sistani K, Burnham DJ. Threonine and crude protein responses in broiler chicks. Animal Feed Science and Technology, 94: 57-64. 2001. [Google Scholar]
- Lay DC, Fulton RM, Hester PY, Karcher DM, Kjaer JB, Mench JA, Mullens BA, Newberry RC, Nicol CJ, O'Sullivan NP, Porter RE. Hen welfare in different housing systems. Poultry Science, 90: 278-294. 2011. [DOI] [PubMed] [Google Scholar]
- Li JY, Liu W, Ma RY, Li Y, Liu Y, Qi RR, Zhan K. Effects of cage size on growth performance, blood biochemistry and antibody response in layer breeder males during rearing stage. Poultry Science, 98: 3571-3577. 2019. [DOI] [PubMed] [Google Scholar]
- Madrid A, Maiorino PM, Reid BN. Cage density and energy utilization. Nutrition Reports International, 23: 89-93. 1981. [Google Scholar]
- Mench JA, Blatchford RA. Determination of space use by laying hens using kinematic analysis. Poultry Science, 93: 794-798. 2014. [DOI] [PubMed] [Google Scholar]
- Meng FY, Chen DH, Li X, Li JH, Bao J. The effect of large or small furnished cages on behaviors and tibia bone of laying hens. Journal of Veterinary Behavior, 17: 69-73. 2016. [Google Scholar]
- Morris TR. The effect of dietary energy level on the voluntary calorie intake of laying birds. British Poultry Science, 9: 285-295. 1968. [Google Scholar]
- Nahashon SN, Adefope N, Amenyenu A, Wright D. Effect of varying metabolizable energy and crude protein concentrations in diets of pearl gray Guinea fowl pullets. 2. Egg production performance. Poultry Science, 86: 973-982. 2007. [DOI] [PubMed] [Google Scholar]
- Onbaşılar EE, Aksoy FT. Stress parameters and immune response of layers under different cage floor and density conditions. Livestock Production Science, 95: 255-263. 2005 [Google Scholar]
- Oscai LB, Patterson JA, Bogard DL, Beck RJ, Rothermel BL. Normalization of serum triglycerides and lipoprotein electrophoretic patterns by exercise. American Journal of Cardiology, 30: 775-780. 1972. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. European Journal of Biochemistry, 267: 4904-4911. 2010. [DOI] [PubMed] [Google Scholar]
- Sigolo S, Zohrabi Z, Gallo A, Seidavi A, Prandini A. Effect of a low crude protein diet supplemented with different levels of threonine on growth performance, carcass traits, blood parameters, and immune responses of growing broilers. Poultry Science, 96: 2751-2760. 2017. [DOI] [PubMed] [Google Scholar]
- Simsek UG, Dalkilic B, Ciftci M, Yuce A. The influences of different stocking densities on some welfare indicators, lipid peroxidation (MDA) and antioxidant enzyme activities (GSH, GSH-Px, CAT) in broiler chickens. Journal of Animal and Veterinary Advances, 8: 1568-1572. 2009. [Google Scholar]
- Sohail SS, Bryant MM, Roland DA. Effect of reducing cage density on performance and economics of second-cycle (Force Rested) commercial leghorns. Journal of Applied Poultry Research, 13: 401-405. 2004. [Google Scholar]
- Stamp DM, Donnelly CA, Jones TA. Chicken welfare is influenced more by housing conditions than by stocking density. Nature, 427: 342-344. 2004. [DOI] [PubMed] [Google Scholar]
- Thaxton JP, Dozier WA, Branton SL, Morgan GW, Miles DW, Roush WB, Lott BD, Vizzier-Thaxton Y. Stocking density and physiological adaptive responses of broilers. Poultry Science, 85: 819-824. 2006. [DOI] [PubMed] [Google Scholar]
- Villagra A, Ruiz de la Torre JL, Chacon G, Lainez M, Torres AG, Manteca X. Stocking density and stress induction affect production and stress parameters in broiler chickens. Animal Welfare, 18: 189-197. 2009. [Google Scholar]
- Webster AB. Welfare implications of avian osteoporosis. Poultry Science, 83: 184-192. 2004. [DOI] [PubMed] [Google Scholar]
- Widowski T, Classen H, Newberry R, Petrik M, Schwean-Lardner K, Cottee S, Cox B. Recommended code of practice for the care and handling of pullets, layers and spent fowl: Poultry (layers). The code of practice for the care and handling of pullets and laying Hens. Vol. 3 pp. 17-29. Canadian Agri-Food Research Council; 2003. [Google Scholar]
- Widowski TM, Hemsworth PH, Barnett JL, Rault JL. Laying hen welfare I. Social environment and space. World's Poultry Science Journal, 72: 333-342. 2016. [Google Scholar]
- Widowski TM, Caston LJ, Caseytrott TM, Hunniford ME. The effect of space allowance and cage size on laying hens housed in furnished cages, Part II: Behavior at the feeder. Poultry Science, 96: 3816-3823. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Chen D, Li J, Bao J. Effects of furnished cage type on behavior and welfare of laying hens. Asian-Australasian Journal of Animal Sciences, 29: 887-894. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


