Abstract
Background:
The perioperative administration of dexmedetomidine may improve the quality of recovery (QoR) after major abdominal and spinal surgeries. We evaluated the effect of an intraoperative bolus of dexmedetomidine on postoperative pain, emergence agitation, and the QoR after laparoscopic cholecystectomy.
Methods:
Patients undergoing elective laparoscopic cholecystectomy were randomized to receive dexmedetomidine 0.5 μg/kg 5 minutes after anesthesia induction (dexmedetomidine group, n = 45) or normal saline (control group, n = 45). The primary outcome was the QoR at the first postoperative day using a 40-item scoring system (QoR-40). Secondary outcomes included intraoperative hemodynamic parameters, postoperative agitation, pain, and nausea and vomiting.
Results:
The heart rate and the mean blood pressure were significantly lower in the dexmedetomidine group than in the control group (P < .001 and .007, respectively). During extubation, emergence agitation was significantly lower in the dexmedetomidine group than in the control group (23% vs 64%, P < .001). The median pain scores in the post-anesthetic care unit were significantly lower in the dexmedetomidine group than in the control group (4 [2–7] vs 5 [4–7], P = .034). The incidence of postoperative agitation, pain, and nausea and vomiting was not different between the groups. On the first postoperative day, recovery profile was similar between the groups. However, the scores on the emotional state and physical comfort dimensions were significantly higher in the dexmedetomidine group than in the control group (P = .038 and .040, respectively).
Conclusions:
A bolus dose of dexmedetomidine after anesthesia induction may improve intraoperative hemodynamics, emergence agitation, and immediate postoperative analgesia. However, it does not affect overall QoR-40 score after laparoscopic cholecystectomy.
Keywords: dexmedetomidine, emergence agitation, laparoscopic cholecystectomy, quality of recovery
1. Introduction
Laparoscopic cholecystectomy is a standard surgical procedure for benign biliary disease. Despite many advantages such as less tissue injury, small surgical incisions, and a shorter hospitalization period,[1] laparoscopic cholecystectomy may cause tissue injury and sympathetic activation due to the surgical stress response.[2] Besides, postoperative pain is the most frequent complaint after laparoscopic cholecystectomy and the most common cause for delayed discharge.[3] Also, emergence agitation may occur during recovery after general anesthesia. It may lead to disorientation, confusion, and altered behavior, leading to self-extubation, which can lead to severe complications, including aspiration pneumonia, bleeding, and hypoxia.[4]
Dexmedetomidine, is a selective α2-receptor agonist with sympatholytic, anxiolytic, sedative, and analgesic effects. It does not cause respiratory disturbance.[5] Several studies have reported that the intraoperative administration of dexmedetomidine resulted in smooth and rapid recovery, reduced postoperative pain, and improved patient satisfaction after surgery.[5–7] When used as an adjuvant intraoperatively, dexmedetomidine attenuates the stress response associated with anesthesia and surgery and maintains hemodynamic stability.[1] Although the perioperative administration of dexmedetomidine may improve the quality of recovery (QoR) score after major abdominal and spinal surgeries,[8,9] the outcomes among patients who undergo laparoscopic cholecystectomy remain poorly investigated.
We evaluated the effect of an intraoperative bolus of dexmedetomidine on emergence agitation, intraoperative hemodynamics, postoperative pain, and postoperative QoR profiles using a 40-item scoring system (QoR-40) in patients undergoing laparoscopic cholecystectomy.
2. Methods
2.1. Study population
All subjects gave written informed consent for inclusion in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Gachon University Gil Hospital (GAIRB2018-017) before patient recruitment. The study was registered at the clinical trials registry, www.ClinicalTrials.gov (NCT03468062). We randomly assigned 90 patients, 20 to 75 years of age, American Society of Anesthesiologists physical status I or II, scheduled to undergo laparoscopic cholecystectomy, to 1 of 2 groups: the control group (n = 45) and the dexmedetomidine group (n = 45) (Fig. 1). We excluded pregnant women, patients with a history of allergy for the study drugs, anxiety or mood disorders.
Figure 1.
Patient's allocation flow diagram. QoR-40 = multidimensional 40-item Quality of Recovery Questionnaire.
2.2. Study protocol
Randomization was performed using a computer-generated randomization sequence (http://www.randomization.com) by an investigator not involved in patient care or perioperative assessment. Allocation results were concealed in sealed opaque envelopes, which were given to an anesthesia nurse not involved with the study. The nurse prepared normal saline or dexmedetomidine in identical syringes according to the patient assignment. Consequently, the surgeons, patients, attending anesthesiologists, investigators, and nursing staff were blinded to the group assignment during the entire study.
Patients received no premedication. Intraoperative monitoring included electrocardiography, pulse oximetry, hemodynamic parameters, and bispectral index (BIS). Anesthesia was induced using lidocaine, 1 mg/kg, propofol 1.5 to 2 mg/kg, fentanyl, 1 μg/kg, and rocuronium, 0.6 mg/kg intravenously. Anesthesia was maintained using 2 to 2.5 vol% of sevoflurane with the end-tidal concentration titrated to maintain the BIS score between 40 and 60. Enrolled patients were randomized to receive either normal saline, 0.125 mL/kg (control group) or dexmedetomidine (Precedex; Hospira, Lake Forest, IL), 0.5 μg/kg at a concentration of 4 μg/mL (dexmedetomidine group) over a period of 5 minutes after induction of anesthesia.
One the first postoperative day (POD), the QoR was assessed using the 40-items multidimensional questionnaire (QoR-40)[10] by one of the investigators. This questionnaire pertains to 5 dimensions of the recovery profile, including physical comfort (12 questions), emotional state (9 questions), psychological support (7 questions), physical independence (5 questions), and pain (7 questions).[10] Each question carries 5 points, and the global score ranges from 40 to 200.
2.3. Outcome Measures
Intraoperative hemodynamic parameters were recorded before anesthesia induction (baseline), at 10 minutes after anesthesia induction (after completion of infusion of the study drug), at 10 minutes and 30 minutes after the creation of pneumoperitoneum, after completion of surgery, and immediately after extubation. Intraoperative hypotension with systolic blood pressure <80 mm Hg or <25% of the baseline value was treated with a bolus of ephedrine 5 mg or phenylephrine 100 μg intravenously. Intraoperative hypertension with systolic blood pressure >150 mm Hg or >25% of the baseline value was treated with a bolus of nicardipine 300 μg intravenously. Any incidence of intraoperative bradycardia with a heart rate (HR) of <50 beats/min was treated with a bolus of atropine 0.5 mg intravenously. An increase in HR of >120 beats/min was treated with a bolus of esmolol 10 mg intravenously. Serum Cr levels were assessed within 2 weeks before surgery (baseline) and on the first POD.
The level of agitation was evaluated using the 7-point Riker sedation-agitation scale (1 = unarousable, 7 = dangerously agitated).[11] Emergence agitation was defined as a sedation-agitation score of ≥5. The highest agitation score of each patient was recorded during extubation and at 10 minutes after arrival in the postanesthetic care unit (PACU).
Postoperative pain was assessed using an 11-point numerical rating scale (NRS) (0 = no pain, 10 = the worst possible pain) at 10-minute intervals after arrival in the PACU and at the time of discharge from the PACU. If the score on the NRS was ≥5, fentanyl, 50 μg, was administered as an intravenous bolus. Postoperative recovery was assessed using the modified Aldrete score.[12] The recovery time was defined as the duration of time between extubation and the point at which criteria for discharge from the PACU were met (Aldrete score ≥9). PONV was assessed using a 4-point scale (0 = no nausea or vomiting, 1 = mild nausea, 2 = moderate to severe nausea requiring antiemetics, 3 = vomiting, retching, or both) at 10-minute intervals during stay in the PACU. Antiemetic medications were administered when if the PONV score was ≥2.
All patients received intravenous patient-controlled analgesia (PCA) with sufentanil 100 to 150 μg (basal infusion rate 2 mL/h and 0.5 mL intermittent bolus with a 15 minutes lock-out interval) for 48 hours after surgery for postoperative pain relief. Patients were informed that when they had moderate to severe pain (NRS ≥5), the analgesic bolus was administered by pressing the button. Ramosetron, 0.3 mg intravenously, was administered after completion of surgery to prevent postoperative nausea and vomiting.
One the first POD, the QoR, NRS score for pain and the PONV score were assessed.
2.4. Statistical analysis
The primary outcome of this study was the global QoR-40 score on the first POD. We calculated the sample size based on the QoR-40 score on the first POD from a previous study that reported a mean score of 174 ± 16.2.[13] Assuming a 10-point improvement in the QoR score in the Dex group, with an α-error of 0.05 and a ß-error of 0.8, the study required 41 patients in each group. Allowing a 10% drop-out rate, 45 patients were recruited in each group.
Data were analyzed using the SPSS 19.0 software (SPSS, Chicago, IL). Variables are presented as mean ± SD, number of patients, or median and interquartile range. Continuous variables that were normally distributed were analyzed using an independent t-test, while non-normally distributed variables were analyzed using the Mann–Whitney U test. Categorical data were analyzed using the Chi-square test or Fisher exact test. Changes in the hemodynamic parameters and bispectral index between groups over time were analyzed using the repeated measures ANOVA with post-hoc Bonferroni correction. A P-value < .05 was considered statistically significant.
3. Results
We enrolled 90 patients in the study. Two patients, including 1 from each group, refused to complete the QoR-40 questionnaire and hence, were not included in the final analysis (Fig. 1). Table 1 depicts patient characteristics and perioperative data.
Table 1.
Patient characteristics and perioperative data.
| Control group (n = 44) | Dex group (n = 44) | P-value | |
| Age (yr) | 46.3 (11.0) | 41.7 (11.0) | .056 |
| Gender (M/F) | 23/21 | 21/23 | .670 |
| Weight (kg) | 69.1 (10.3) | 67.8 (14.8) | .644 |
| Height (cm) | 166 (8) | 167 (9) | .595 |
| BMI (kg/m2) | 25.2 (3.0) | 24.2 (3.5) | .178 |
| Duration of surgery (min) | 66 (23) | 62 (21) | .406 |
| Duration of anesthesia (min) | 88 (23) | 86 (21) | .734 |
Figure 2 depicts intraoperative changes in mean blood pressure (MBP), HR, and BIS. There was a significant difference between groups in the intraoperative changes in the MBP (P = .007) and HR (P < .001). The MBP and HR were significantly lower in the dex group than in the control group (P < .001). There was no significant difference in the change in BIS over time between groups (P = .068).
Figure 2.
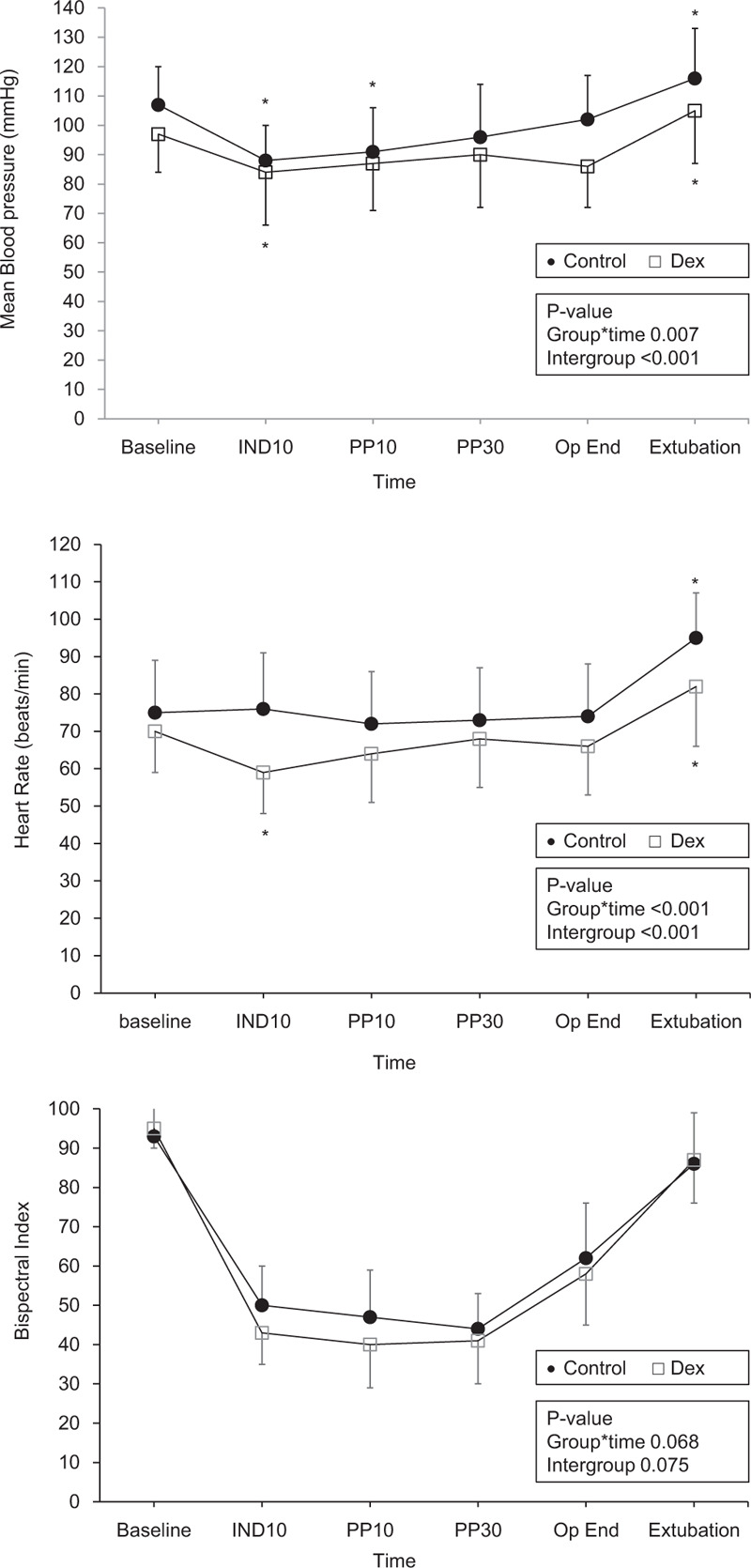
Intraoperative hemodynamic and bispectral index changes. Error bar means standard deviation. The changes in mean blood pressure (MBP, upper frame) and heart rate (HR, middle frame) were significantly different between the control (●, filled circle) and dexmedetomidine (□, empty square) groups. MBP and HR were significantly lower in the dex group than in the control group. Changes in bispectral index were not significantly different between the 2 groups. There are 6 time points of measurement: Baseline, before anesthesia induction; IND10, 10 min after anesthesia induction (at the end of study drug infusion); PP10 and PP30, at 10 min and 30 min after pneumoperitoneum; Op End, at the completion of surgery; extubation, immediately after extubation. ∗P < .05, versus baseline value within the group.
Table 2 presents recovery profiles and pain scores in PACU. The incidence of emergence agitation was significantly lower in the dex group than in the control group during extubation (10 [23%] vs 28 [64%], P < .001) and at admission to the PACU (2 [5%)] vs 14 [32%], P = .01). The recovery time was not different between groups. The pain scores were significantly lower in the Dex group than in the control group upon at admission to the PACU (5 [4–7] vs 4 [2–7]), P = .034) and at discharge from the PACU (3 [3–4.75] vs 3 [2–3.75], P = .019). The requirement for rescue analgesia, PONV scores, and the requirement for rescue antiemetics were not different between groups.
Table 2.
Recovery profile and pain score in the post-anesthetic care unit.
| Control group (n = 44) | Dex group (n = 44) | P-value | |
| Extubation time (min) | 6.8 (3.0) | 8.0 (4.4) | .325 |
| During extubation | |||
| Agitation score | 5 (3–6 [4–5]) | 4 (2–5 [3–4]) | <.001 |
| Emergence agitation | 28 | 10 | <.001 |
| At admission | |||
| Agitation score | 4 (3–5 [4–5]) | 4 (3–5 [4–4]) | <.001 |
| Emergence agitation | 14 | 2 | .001 |
| Recovery time (min) | 24.5 [20–25] | 20 [20–30] | .745 |
| Pain score (NRS) | |||
| At admission | 5 [4–7] | 4 [2–7] | .034 |
| At discharge | 3 [3–4.75] | 3 [2–3.75] | .019 |
| Rescue analgesic (n) | 18 | 13 | .265 |
| PONV (0/1/2/3) | |||
| At admission | 39/4/0/1 | 40/2/2/0 | .976 |
| At discharge | 42/2/0/0 | 39/5/0/0 | .240 |
| Rescue anti-emetic (n) | 1 | 2 | .543 |
Table 3 presents QoR-40 scores and serum creatinine on the first POD. The global QoR-40 score was not significantly different between groups. However, the scores in the emotional state and physical comfort dimensions were significantly higher in the dex group than in the control group (P = .038 and .040, respectively). Serum creatinine levels were similar between the groups at preoperative day and the first POD. Whereas, only in dexmedetomidine group, serum creatinine significantly decreased at the first POD compared to preoperative values (P < .01).
Table 3.
Quality of recovery (QoR) score and serum creatinine on the first postoperative day (POD 1).
| Control group (n = 44) | Dex group (n = 44) | P-value | |
| QoR total score | 150 (26) | 156 (19) | .244 |
| Emotional state | 35 (7) | 38 (4) | .038 |
| Physical discomfort | 46 (8) | 49 (5) | .040 |
| Psychological support | 28 (5) | 27 (5) | .608 |
| Physical independence | 15 (6) | 15 (5) | .985 |
| Pain | 26 (5) | 27 (5) | .453 |
| Serum creatinine (mg/dL) | |||
| Preoperative | 0.68 (0.20) | 0.73 (0.19) | .233 |
| POD 1 | 0.68 (0.20) | 0.68 (0.21)∗ | 1.00 |
4. Discussion
We demonstrated that single-bolus administration of dexmedetomidine 0.5 μg/kg after anesthesia induction may improve intraoperative hemodynamics, emergence agitation, and pain scores in the immediate postoperative period. However, it does not improve the postoperative QoR-40 scores in patients undergoing laparoscopic cholecystectomy.
Dexmedetomidine acts through the α2A-, α2B-, and α2C-receptors in the brain and the spinal cord, which mediate sedative, anxiolytic, analgesic, and sympatholytic effects.[6] Activation of the α2A-receptor in the vasomotor center of the brain stem inhibits the secretion of norepinephrine, leading to hypotension and bradycardia.[6] Activation of α2A- and α2C-receptors in the locus ceruleus leads to sedation, while their activation of in the spinal cord inhibits the secretion of substance P, thus directly inhibiting pain transmission, resulting in the analgesic effect.[6] In this study, the sympatholytic and analgesic effects of dexmedetomidine were confirmed.
Postoperative pain following laparoscopic cholecystectomy is less compared to conventional open surgery; however, pain remains the most frequent complaint, and it may lead to prolonged hospital stay and re-admission.[3] In an earlier study, an intraoperative infusion of dexmedetomidine at 0.5 μg/kg/h alleviated pain and reduced postoperative morphine consumption after laparoscopic cholecystectomy.[14] According to another report, an intraoperative bolus dose of dexmedetomidine 1 μg/kg, followed by an infusion of 0.4 μg/kg/h, reduced the use of rescue analgesics after laparoscopic cholecystectomy.[6] In the present study, a single bolus dose of 0.5 ug/kg of dexmedetomidine resulted in a significant decrease in postoperative pain in the PACU. Previous reports have shown that administration of dexmedetomidine significantly improved the pain component of the QoR-40 score.[7,15] However, in this study, there was no significant difference in the pain component of the QoR-40 score on POD 1. These reduced effect on QoR in this study may be due to the lower dose, earlier administration of dexmedetomidine, and application of PCA. In this study, all patients were given intravenous PCA with continuous infusion and intermittent bolus 48 hours after surgery. Since the patient was able to partly control the pain with intermittent bolus when the pain was severe, the effect of dexmedetomidine on the pain scores after laparoscopic cholecystectomy may have been reduced in this study.
It has been reported that about 20% of adult patients experience emergence agitation after general anesthesia.[4,16] We found that a single bolus of dexmedetomidine 0.5 μg/kg after induction significantly reduced emergence agitation after laparoscopic cholecystectomy without delaying recovery from anesthesia. Our findings are consistent with those of previous studies among patients who underwent laparoscopic cholecystectomy and video-assisted thoracoscopic surgery.[7,17] Pain is an important factor that can lead to emergence agitation.[4,16] In the present study, the decreased incidence of postoperative agitation with dexmedetomidine may have been due to its analgesic effect, as evidenced by the significantly lower NRS scores in the immediate postoperative period. Furthermore, the amelioration of postoperative agitation may also be attributable to the anxiolytic and sympatholytic effects of dexmedetomidine.
We evaluated the QoR at 24 hours after surgery using the QoR-40 scoring system. This questionnaire pertains to 5 dimensions of the recovery profile, including physical comfort, emotional state, psychological support, physical independence, and pain.[17] Each question carries 5 points, and the global score ranges from 40 to 200. The QoR-40 is a reliable measurement tool for evaluating postoperative recovery and has been validated for various types of surgery.[18] Although the global QoR-40 score was not significantly different between the 2 groups on the first POD, the dimensions of emotional state and physical comfort were significantly better with the use of dexmedetomidine. The activation of α2A- and α2C-receptors in the locus ceruleus and the spinal cord leads to anxiolysis, sedation, and analgesia by inhibition of neuronal firing.[19] Besides, dexmedetomidine has anti-cytokine and anti-inflammatory effects,[20] which may indirectly lead to an improvement in the emotional state.[21] An infusion of dexmedetomidine has been shown to reduce anxiety among mechanically ventilated patients in the ICU in the postoperative period.[22] Besides improving the emotional status, the anxiolytic and anti-inflammatory effects may also enhance physical comfort in the postoperative period. The component of physical comfort is assessed based on the presence of restlessness, fatigue, PONV, and dizziness.[18] Shi et al,[23] reported improvement in the sleep pattern and the recovery profile after general anesthesia with the intraoperative use of dexmedetomidine, besides improvement in the global QoR-40 score. The recovery profile is influenced by the presence of postoperative pain, fatigue, and postsurgical endocrine, metabolic, and immune changes.[24] Dexmedetomidine promotes postoperative recovery by inhibiting the surgery-induced stress response.[24] Thus, the reduction of the stress response seems to enhance physical comfort.
The intravenous administration of dexmedetomidine has been reported to reduce the serum catecholamine level by 90% and prevent hemodynamic changes during laryngoscopy and laparoscopy.[25] Other studies have also reported attenuation of the hemodynamic stress responses during laparoscopic cholecystectomy with the intraoperative infusion of dexmedetomidine.[6,25,26] Our results are consistent with the findings of previous reports. We observed that a bolus of dexmedetomidine 0.5 μg/kg alone, without a continuous infusion, resulted in a lower MBP and HR throughout the intraoperative period and during emergence. In the dexmedetomidine group, postoperative serum creatinine levels were significantly reduced compared to preoperative levels. This result might be due in part to the hemodynamic stability of the dexmedetomidine group. However, since it seems to be insufficient to demonstrate the renal protective effect of dexmedetomidine by measuring only serum creatinine, further studies on this might be needed.
There are a few limitations to our study. Some of the patients were on non-opioid analgesics in the preoperative period for abdominal pain, which may have influenced postoperative pain and NRS scores. Severe preoperative pain has been reported as one of the independent predictors of postoperative severe pain.[27] In addition, a high preoperative anxiety has a negative effect on recovery from anesthesia and control of postoperative pain control.[28] Thus, preoperative pain scores and anxiety level may affect the QoR-40 scores during postoperative recovery in this study. Moreover, we did not measure the total amount of analgesics administered from PCA at the first POD (when measuring the QoR-40 scores). To elucidate the effects of dexmedetomidine on QoR or postoperative pain scores, further study might be needed on the association of dexmedetomdine administration and postoperative analgesic or PCA requirements after surgery.
In conclusion, a single bolus dose of dexmedetomidine 0.5 μg/kg after anesthesia induction can attenuate hemodynamic changes in the intraoperative period and during emergence. Besides, it reduces pain and emergence agitation in the PACU in patients who undergo laparoscopic cholecystectomy. However, there was no improvement in the postoperative recovery profile on the 40-item QoR scoring system.
Author contributions
Conceptualization: Jung Ju Choi, Kyungmi Kim, Hee Yeon Park, Hyun Jeong Kwak.
Data curation: Jung Ju Choi, Kyungmi Kim, Hee Yeon Park, Hyun Jeong Kwak.
Formal analysis: Jung Ju Choi, Hyun Jeong Kwak.
Investigation: Jung Ju Choi, Kyungmi Kim, Hee Yeon Park, Young Jin Chang, Kyung Cheon Lee, Kwan Yeong Kim.
Methodology: Hee Yeon Park, Young Jin Chang, Kyung Cheon Lee, Kwan Yeong Kim.
Supervision: Young Jin Chang, Hyun Jeong Kwak.
Writing – original draft: Jung Ju Choi, Hee Yeon Park, Hyun Jeong Kwak.
Writing – review & editing: Hyun Jeong Kwak.
Footnotes
Abbreviations: POD = postoperative day, PONV = postoperative nausea and vomiting, QoR = quality of recovery.
How to cite this article: Choi JJ, Kim K, Park HY, Chang YJ, Lee KC, Kim KY, Kwak HJ. CONSORT the effect of a bolus dose of dexmedetomidine on postoperative pain, agitation, and quality of recovery after laparoscopic cholecystectomy. Medicine. 2021;100:3(e24353).
JJC and KK contributed equally to this study.
The authors report no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Values are means (standard deviation) or numbers of patients. Control group, patients with normal saline infusion after anesthesia induction; Dex group, patients who received dexmedetomidine of 0.5 μg/kg infusion after anesthesia induction. BMI = body mass index.
Values are means (standard deviation) or numbers of patients or medians (minimum-maximum [interquartile ranges]) or numbers of patients. Control group, patients with normal saline infusion after anesthesia induction; Dex group, patients who received dexmedetomidine of 0.5 μg/kg infusion after anesthesia induction. Extubation time, time from the completion of surgery to extubation; Agitation score, Riker scale (1 = unarousable, 2 = very sedated, 3 = sedated, 4 = calm and cooperative, 5 = agitated, 6 = very agitated, 7 = dangerously agitated); NRS, an 11-point numerical rating scale (0 = no pain, 10 = the worst pain); emergence agitation, when the score on the Riker scale was 5 or more. Rescue analgesic, administration of fentanyl 50 μg when NRS was 5 or more; Recovery time, the time from extubation to the time required to meet discharge criteria (Aldrete score of 9 or more).
PONV = postoperative nausea and vomiting (0 = no, 1 = mild, 2 = moderate to severe nausea requiring antiemetics, 3 = retching, vomiting, or both); rescue anti-emetic, when the PONV score was 2 or more.
Values are means (standard deviation). Control group, patients who received normal saline infusion after anesthesia induction; dex group, patients who received dexmedetomidine of 0.5 μg/kg infusion after anesthesia induction. The quality of recovery was assessed using the multidimensional 40-item Quality of Recovery Questionnaire.[18]
P < .05, versus preoperative values within the group.
References
- [1].Vaswani JP, Debata D, Vyas V, et al. Comparative study of the effect of dexmedetomidine vs. fentanyl on haemodynamic response in patients undergoing elective laparoscopic surgery. J Clin Diagn Res 2017;11:UC04–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abdel-Rahman KA, Abd-Elshafy SK, Sayed JA. Effect of two different doses of dexmedetomidine on the incidence of emergence agitation after strabismus surgery: a randomized clinical trial. Rev Bras Anestesiol 2018;68:571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barazanchi AWH, MacFater WS, Rahiri JL, et al. Evidence-based management of pain after laparoscopic cholecystectomy: a PROSPECT review update. Br J Anaesth 2018;121:787–803. [DOI] [PubMed] [Google Scholar]
- [4].Lepousé C, Lautner CA, Liu L, et al. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth 2006;96:747–53. [DOI] [PubMed] [Google Scholar]
- [5].Kang X, Tang X, Yu Y, et al. Intraoperative dexmedetomidine infusion is associated with reduced emergence agitation and improved recovery profiles after lung surgery: a retrospective cohort study. Drug Des Devel Ther 2019;2:871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Khare A, Sharma SP, Deganwa ML, et al. Effect of dexmedetomidine on intraoperative haemodynamics and postoperative analgesia in laparoscopic cholecystectomy. Anesth Essays Res 2017;11:1040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee SH, Lee CY, Lee JG, et al. Intraoperative dexmedetomidine improves the quality of recovery and postoperative pulmonary function in patients undergoing video-assisted thoracoscopic surgery: a CONSORT-prospective, randomized, controlled trial. Medicine 2016;95:e2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ge DJ, Qi B, Tang G, et al. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal colectomy: a CONSORT-prospective, randomized, controlled clinical trial. Medicine 2015;94:e1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bekker A, Haile M, Kline R, et al. The effect of intraoperative infusion of dexmedetomidine on the quality of recovery after major spinal surgery. J Neurosurg Anesthesiol 2013;25:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee JH, Kim DK, Seo DH, et al. Validity and reliability of the Korean version of the Quality of Recovery-40 questionnaire. Korean J Anesthesiol 2018;71:467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Khan BA, Guzman O, Campbell NL, et al. Comparison and agreement between the Richmond Agitation-Sedation Scale and the Riker Sedation-Agitation Scale in evaluating patients’ eligibility for delirium assessment in the ICU. Chest 2012;142:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth 1995;7:89–91. [DOI] [PubMed] [Google Scholar]
- [13].Lee WK, Kim MS, Kang SW, et al. Type of anaesthesia and patient quality of recovery: a randomized trial comparing propofol-remifentanil total i.v. anaesthesia with desflurane anaesthesia. Br J Anaesth 2015;114:663–8. [DOI] [PubMed] [Google Scholar]
- [14].Bielka K, Kuchyn I, Babych V, et al. Dexmedetomidine infusion as an analgesic adjuvant during laparoscopic сholecystectomy: a randomized controlled study. BMC Anesthesiol 2018;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim SY, Kim JM, Lee JH, et al. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. Br J Anaesth 2013;111:222–8. [DOI] [PubMed] [Google Scholar]
- [16].Yu D, Chai W, Sun X, et al. Emergence agitation in adults: risk factors in 2,000 patients. Can J Anaesth 2010;57:843–8. [DOI] [PubMed] [Google Scholar]
- [17].Mostafa MF, Herdan R, Farrag Aly MY, et al. Effect of different doses of dexmedetomidine on stress response and emergence agitation after laparoscopic cholecystectomy: randomized controlled double-blind study. J Anesth Clin Res 2017;8:2. [Google Scholar]
- [18].Myles PS, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth 2000;84:11–5. [DOI] [PubMed] [Google Scholar]
- [19].Maze M, Scarfini C, Cavaliere F. New agents for sedation in the intensive care unit. Crit Care Clin 2001;17:88–97. [DOI] [PubMed] [Google Scholar]
- [20].Memiş D, Hekimoğlu S, Vatan I, et al. Effects of midazolam and dexmedetomidine on inflammatory responses and gastric intramucosal pH to sepsis, in critically ill patients. Br J Anaesth 2007;98:550–2. [DOI] [PubMed] [Google Scholar]
- [21].Gimeno D, Kivimäki M, Brunner EJ, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med 2009;39:413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs 2000;59:263–8. [DOI] [PubMed] [Google Scholar]
- [23].Shi C, Jin J, Pan Q, et al. Intraoperative use of dexmedetomidine promotes postoperative sleep and recovery following radical mastectomy under general anesthesia. Oncotarget 2017;24:79397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bajwa SJ, Gupta S, Kaur J, et al. Reduction in the incidence of shivering with perioperative dexmedetomidine: A randomized prospective study. J Anaesthesiol Clin Pharmacol 2012;28:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Masoori TA, Gupta K, Agarwal S, et al. Clinical efficacy of dexmedetomidine in two different doses to attenuate the hemodynamic changes during laparoscopic cholecystectomy. Int J Res Med Sci 2018;6:959–65. [Google Scholar]
- [26].Bhutia MP, Rai A. Attenuation of haemodynamic parameters in response to pneumoperitoneum during laparoscopic cholecystectomy: a randomized controlled trial comparing infusions of propofol and dexmedetomidine. J Clin Diagn Res 2017;11:UC01–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kalkmana CJ, Visserb K, Moena J, et al. Preoperative prediction of severe postoperative pain. Pain 2003;105:415–23. [DOI] [PubMed] [Google Scholar]
- [28].Ali A, Altun D, Oguz BH, et al. The effect of preoperative anxiety on postoperative analgesia and anesthesia recovery in patients undergoing laparascopic cholecystectomy. J Anesth 2014;28:222–7. [DOI] [PubMed] [Google Scholar]


