Abstract
Objective:
To uncover the function of lncRNA NEAT1 in ovarian cancer (OC) cells and its mechanism.
Methods:
The expression patterns of lncRNA NEAT1 and FGF9 in human OC cells and human ovarian epithelial cells was determined. OC cells were transfected with sh-NEAT1, pcDNA3.1-NEAT1, miR-365 mimic, miR-365 inhibitor or pcDNA3.1-NEAT1 + sh-NEAT1 before cell proliferation rate and cell clone formation rate were measured. After the transfected OC cells were co-cultivated with human umbilical vein endothelial cells (HUVECs), Matrigel angiogenesis assay tested angiogenesis of HUVECs; qRT-PCR and Western blot tested the expressions of vascular endothelial growth factor (VEGF), angiogenin 1 (Ang-1) and matrix metalloproteinase 2 (MMP2). Dual-luciferase reporter assay determined the targeted binding of NEAT1 and FGF9 to miR-365.
Results:
LncRNA NEAT1 and FGF9 are over-expressed in OC cells. Knockdown of NEAT1 or FGF9, or over-expression of miR-365 results in decreased proliferation rate and cell clones as well as inhibited angiogenesis and down-regulated expressions of VEGF, Ang-1 and MMP2. Over-expression of NEAT1 or knockdown of miR-365 can reverse the effect caused by FGF9 knockdown. NEAT1 can down-regulate the expression of miR-365 while up-regulating that of FGF9. Dual-luciferase reporter assay determined that NEAT1 competes with FGF9 for binding to miR-365.
Conclusion:
LncRNA NEAT1 up-regulates FGF9 by sponging miR-365, thus promoting OC cell proliferation and angiogenesis of HUVECs.
Keywords: angiogenesis, cell proliferation, FGF9, NEAT1, ovarian cancer
1. Introduction
Ovarian cancer (OC) is among the most common cancers occurring in female, and it is the second major cause of gynecologic cancer death.[1] OC includes a group of epithelial malignant tumors and approximately 90% OCs are carcinomas with the highest proportion of high-grade serous carcinoma (HGSC).[2] Malignant ovarian lesions are broadly classified into primary and metastatic ovarian tumors; the former can be further divided into epithelial ovarian cancer and nonepithelial neoplasms while metastatic ovarian tumors are most commonly derived from gastric, colon, appendiceal, uterine and breast primary malignancies or rarely exhibit association with acute leukemias or lymphomas.[3] According to the 2013 International Federation of Gynecology and Obstetrics (FIGO) staging for ovarian cancer and its subtypes, the patient survival has a declining tendency with increasing stage.[4] However, early diagnosis of epithelial OC can be difficult and most of the patients are initially diagnosed with advanced epithelial OC.[5,6] Although over the years massive clinical trials have been done for detection and treatment of OC, the overall outcomes in advanced patients are far from satisfactory,[7] which motivates the scholars to determine the potential biomarker and molecular mechanism involved in OC cells so as to provide new ideas for OC treatment.
Some long non-coding RNAs (lncRNAs) are verified to be dysregulated in tumor cells, making themselves potential indicators in cancers.[8] Furthermore, those aberrantly expressed lncRNAs may exert functions in cancer initiation and development.[9] Nuclear enriched abundant transcript 1 (NEAT1) is uncovered for its tumor-promoting role in many cancers, including OC, with the capability of promoting cell aggression and proliferation.[8] NEAT1 is an essential structural foundation to the formation of paraspeckles which are nuclear domains implicated in mRNA regulation.[10] NEAT1 can bind to miR-382-3p to up-regulate ROCK1 which further promoted OC cell metastasis.[11] Up to now, far too little attention has been paid to the detailed molecular mechanism by which NEAT1 accelerates OC cell growth.
Considerable data showed that lncRNAs/microRNAs (miRNAs) crosstalk plays a vital role in tumorigenic processes.[12] The specific interactions are described, with lncRNAs either sponging or directly targeting miRNAs, thus regulating the expression of miRNAs.[9] Any perturbation of these RNA interplays may lead to disease initiation and progression.[13] MiRNAs are reported to have significant biological functions in tumorigenesis.[14] For instance, microRNA-365 (miR-365) acts as a regulatory gene in various malignancies such as non-small cell lung cancer[15] and liver cancer.[16] A previous study claimed that miR-365 was down-regulated in OC cells and inhibited OC tumor growth by targeting Wnt5a.[17] However, whether there is connection between NEAT1 and miR-365 in regulating OC development remains largely unknown.
Furthermore, online software (starBase) predicted that NEAT1 may directly target miR-365 and this prediction was validated by a dual-luciferase reporter assay in our study. MiRNAs play crucial regulatory roles in a variety of physiological and pathological processes, and contribute to mRNA stability and translation as post-transcriptional regulators.[18] Studies have proved that fibroblast growth factor (FGF) plays a pivotal angiogenic role in both normal and tumor cells.[19] Data supported that induction of FGF9 can augment the invasion of OC cells through activating the ERK-signaling pathway.[20] Nevertheless, how FGF9 is activated in OC cells remains to be clarified. Our study further identified FGF9 as a downstream target of miR-365. After measuring the expression pattern of NEAT1, miR-365, and FGF9, we hypothesized that NEAT1 regulating OC cell growth may involve miR-365/FGF9 axis. This study therefore set out to unravel some of the mysteries surrounding the molecular mechanism involved in activities of OC cells.
2. Materials and methods
2.1. Ethical statement
The experiment does not contain animal or human participants, and there is no need for the ethic approval.
2.2. Cell culture
Human OC cells (SKOV3 and OVCAR-3), human ovarian epithelial cells (IOSE80), human umbilical vein endothelial cells (HUVECs) and human embryonic kidney cells (HEK293T) were all obtained from American Type Culture Collection (ATCC, Manassas, Virginia, USA). SKOV3 cells and OVCAR-3 cells were grown in RPMI-1640 culture medium (Hyclone, Novato, CA, USA). IOSE80 cells, HUVECs and HEK293T cells were cultured in DMEM (Gibco, Grand Island, NY, USA). Both RPMI-1640 and DMEM contained 10% fetal calf serum, 1% penicillin and 1% streptomycin. Cells were nurtured in an incubator with 5% CO2 at 37°C. All the cell experiments were conducted in the Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education.
2.3. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
The total RNA was extracted from cells using TRIZOL (Invitrogen, Carlsbad, CA, USA). RNAs (OD260/OD280 values = 1.8 ∼ 2.0) with bright and clear bands at 28S and 18S in gel and the brightness of 28S more than twice that of 18S were selected. These RNA samples (1 μg, 200 ∼ 1000 ng/ml) were reverse-transcribed using a kit (TaKaRa, Tokyo, Japan) based on the instructions. The expression of the genes was detected by Light Cycler 480 (Roche, Indianapolis, IN, USA). Reaction conditions of the qRT-PCR assay were in accordance with the instructions of the fluorescence quantitative PCR kit (SYBR Green Mix, Roche Diagnostics, Indianapolis, IN). The thermal cycle parameters were as follows: 10 seconds at 95°C, followed by 45 cycles of 5 seconds at 95°C, 10 seconds at 60°C and 10 seconds at 72°C, finally extending for 5 minutes at 72°C. Each reaction was tested in triplicate. U6 and 5.8S served as the internal controls for miRNA and β-actin and GAPDH were for mRNA. The experimental data were analyzed using 2-ΔΔCt method. 2-ΔΔCt = experimental group (Ct target gene-Ct internal control)-negative control group (Ct target gene-Ct internal control). The forward and reverse primer sequences used in qRT-PCR are shown in Table 1. The whole qRT-PCR was set 3 independent duplicates.
Table 1.
Primer sequences for reverse transcript polymerase chain reaction.
| Name of primer | Sequences |
| miR-365-F | GCTATTCCTAAAAATCCC |
| miR-365-R | GCAGGGTCCGAGGTATTC |
| U6-F | CTCTCGCTTCGGCAGCACA |
| U6-R | ACGCTTCACGAATTTGCGT |
| NEAT1-F | ACATTGTACACAGCGAGGCA |
| NEAT1-R | CATTTGCCTTTGGGGTCAGC |
| FGF9-F | TCTGTTTGGAAGGCGAGTGT |
| FGF9-R | GCTTGGCAAAGCTTAGGAGG |
| VEGF-F | GTTTCTAGCTGCCTGCCTGG |
| VEGF-R | CCACACCTGGAATCGGCTT |
| Ang-1-F | CGAACCATCTGTCTGCCCTT |
| Ang-1-R | CGAACCATCTGTCTGCCCTT |
| MMP2-F | TAGGACAGCAGCACAGTTGG |
| MMP2-R | GACCAGACATGGGCCAATCA |
| β-actin-F | TGAGCTGCGTTTTACACCCT |
| β-actin-R | GTTTGCTCCAACCAACTGCT |
2.4. Western blot
Proteins were extracted from cells using RIPA lysis buffer (Beyotime, Haimen, China) and the protein concentrations were measured in a BCA kit (Beyotime, Haimen, China). Corresponding volume of protein was mixed with loading buffer (Beyotime, Haimen, China), heated in a boiling water bath for 3 minutes and then separated by 80 V electrophoresis for 30 minutes and 120 V electrophoresis for 1 to 2 hours. Afterwards, the proteins were transferred onto a membrane in an ice-water bath with a current of 300 mA for 60 minutes. After being washed for 1 to 2 minutes, the membrane was confined in blocking liquid for 60 minutes at room temperature or at 4°C overnight. Primary antibodies of β-actin (ab8226, 1:1000), FGF9 (ab206408, 1:1000), VEGF (ab69479, 1:1000), Ang-1 (ab8451, 1:500), or MMP2 (ab97779, 1:500) (Abcam, Cambridge, MA, USA) were incubated with the proteins on a shaking table at room temperature for 1 hour. After being washed for 3 × 10 minutes, the membranes were incubated with the secondary antibodies for 1 hour. Finally, the membranes were subjected to washing (3 × 10 minutes) and allowed for color development with developing solution. The brands were then detected using the chemiluminescence imaging system. The Western blotting was replicated in 3 times.
2.5. Cell transfection
NEAT1 over-expression vectors (pcDNA3.1-NEAT1, 2 μg), NEAT1 interference vectors (sh-NEAT1, 2 μg), FGF9 interference vectors (sh-FGF9, 2 μg) and their negative control, miR-365 mimic (50 nM), miR-365 inhibitor (50 nM) and their negative control were all obtained from GenePharma (Shanghai, China). Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) was used to perform the transfection based on instructions herein. The vectors were inserted with green fluorescent protein (GFP) for measurement of transfection efficiency. Cells with transfection rate above 80% were used for subsequent experiments. The cell transfection was performed in triplicate.
2.6. Cell proliferation assay (CCK-8)
After 24 hours transfection, SKOV3 cells and OVCAR-3 cells were cultivated in a 96-well plate, and each well was added with 100 μl of diluted cell suspension (1 × 105 cells/ml). Each sample was tested in triplicate. After respective incubation for 0 hour, 24 hours, 48 hours, 72 hours and 96 hours, 10 μl of CCK-8 reagent (Tokyo, Dojindo, Japan) was added to each well for another 2 hours of incubation. Finally, the optical density (OD) of the samples was measured at 450 nm. This assay was performed in triplicate.
2.7. Clone formation assay
After 24 hours transfection, the SKOV3 cells and OVCAR-3 cells were separated by pancreatin at 25°C and then centrifuged at 1500 rpm for 5 minutes. After that, cells were resuspended in complete culture medium. A six-well plate with 37°C preheated complete culture medium (2 ml) were inoculated with 1 × 500 cells per well for cell incubation in 5% CO2 at 37°C for 2 to 3 weeks. The cultivation was ended when colonies in the six-well plate became visible. Then the culture solution in the wells was absorbed and the plate was washed twice using PBS. Every well was added with 1.5 ml of methanol and maintained for 15 minutes before the methanol was absorbed to terminate the fixation. Then 1 ml Giemsa staining solution was slowly added along the well wall for reaction in dark place for 20 minutes. Finally, the staining solution was washed with flowing water and the six-well plate was turned over on clean absorbent paper. Cell colonies were counted. This assay was performed in triplicate.
2.8. Matrigel angiogenesis assay
HUVECs (1 × 106) were seeded in the apical chamber (aperture: 0.4 μm, without Matrigel, Millipore, Billerica, MA, USA). Equal amounts of SKOV3 and OVCAR-3 cells in each group were seeded on the 24-well plate in the basolateral chamber. After being cultivated in 5% CO2 at 37°C for 24 hours, HUVECs were taken out for further experiment.
Before the experiment, the 48-well plates (Millipore, Billerica, MA, USA), Matrigel (Corning, Tewksbury, MA, USA) and pipette were pre-cooled at 4°C or dissolved. Matrigel (100 μl) was added to the 48-well plates using the pre-cooled pipette and the plates were placed in an incubator for 30 minutes solidification at 37°C. Each well was added with 2 × 104 HUVECs which were co-cultured with OC cells. After 6 hours of cultivation, the HUVECs were photographed and the length of blood vessels was analyzed by Image J (NIH, Bethesda, MD, USA). This assay was performed in triplicate.
2.9. Dual-luciferase reporter assay
The binding sites of NEAT1 and FGF9 to miR-365 were predicted by starBase (http://starbase.sysu.edu.cn/). The wild and mutated sequences of the binding sites (wt-NEAT1, mut-NEAT1, wt-FGF9 and mut-FGF9) were designed and synthesized according to the prediction. These sequences were inserted into the luciferase reporter vectors (pGL3-Basic) which were then co-transfected into HEK293T cells with either miR-365 mimic or a negative control. After the transfection was finished, the activities of Firefly luciferase and Renilla luciferase were measured. The ratio of Firefly luciferase activity to Renilla luciferase activity is the relative luciferase activity. This assay was performed in triplicate.
2.10. Statistical analysis
Statistical analysis was conducted using GraphPad prism7. All data are presented as mean ± SD (standard deviation). T method was used to analyze discrepancy between 2 groups and One-way analysis of variance was applied for multi-group comparisons. For post-hoc comparisons, we used Dunnett multiple comparisons test. P < .05 was considered statistically significant.
3. Results
3.1. LncRNA NEAT1 and FGF9 are over-expressed in OC cells
Firstly, the expressions of NEAT1 and FGF9 in human OC cells (SKOV3 and OVCAR-3) and human ovarian epithelial cells (IOSE80) were tested. According to the results of qRT-PCR and Western blot, the expressions of NEAT1 and FGF9 in SKOV3 cells and OVCAR-3 cells were significantly higher than in IOSE80 cells (Fig. 1A-C, P < .05).
Figure 1.
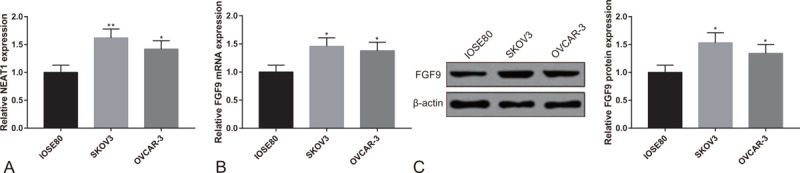
Overexpression of lncRNA NEAT1 and FGF9 in OC cells. Note: (A) qRT-PCR tested the expression of NEAT1 in SKOV3 cells, OVCAR-3 cells and IOSE80 cells. (B) qRT-PCR and (C) Western blot tested the expression of FGF9 in SKOV3 cells, OVCAR-3 cells and IOSE80 cells. ∗P < .05, ∗∗P < .01, compared to IOSE80 group; qRT-PCR, quantitative reverse transcript polymerase chain reaction; OC, ovarian cancer.
3.2. Knockdown of lncRNA NEAT1 suppresses proliferation of OC cells and angiogenesis of HUEVCs
SKOV3 cells and OVCAR-3 cells were transfected with sh-NEAT1 to measure the influences of NEAT1 knockdown on proliferation of SKOV3 cells and OVCAR-3 cells. NEAT1 was significantly under-expressed in SKOV3 cells and OVCAR-3 cells after transfection (Fig. 2A, P < .05), suggesting successful transfection of sh-NEAT1. Additionally, the proliferation activity of SKOV3 cells and OVCAR-3 cells in sh-NEAT1 group was distinctly lower than in sh-NC group and blank group (Fig. 2B, P < .05). Moreover, SKOV3 cells and OVCAR-3 cells in sh-NEAT1 group had weaker colony-forming capacity in comparison to those in sh-NC group and blank group (Fig. 2C, P < .05).
Figure 2.
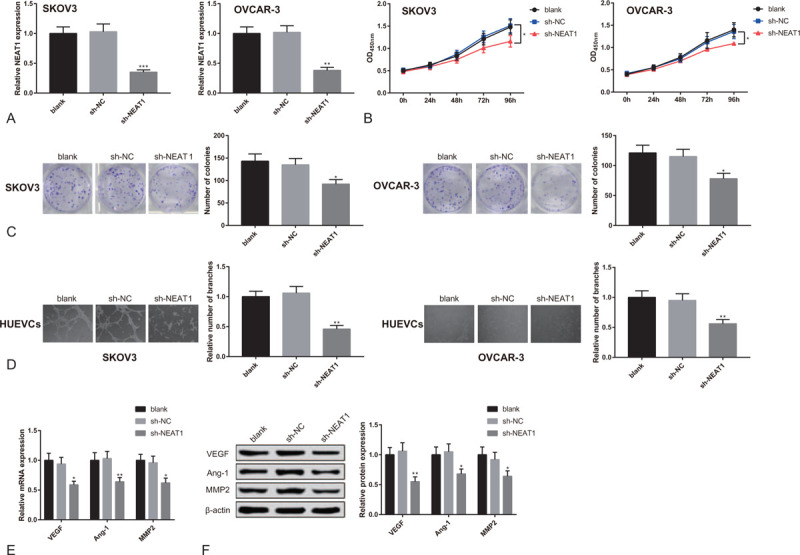
Knockdown of lncRNA NEAT1 suppresses proliferation of OC cells and angiogenesis of HUEVCs. Note: After SKOV3 cells and OVCAR-3 cells were transfected with sh-NEAT1 or sh-NC, (A) qRT-PCR tested the expression of NEAT1; (B) CCK8 detected cell proliferation activity; (C) colonies formation assay counted cell colonies. After HUEVCs were co-cultured with transfected SKOV3 cells and OVCAR-3 cells, (D) Matrigel angiogenesis assay measured the number of vascular branches formed by HUEVCs; (E) qRT-PCR and (F) Western blot analyzed the expressions of VEGF, Ang-1 and MMP2 in HUEVCs. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, compared to sh-NC group or blank group; qRT-PCR, quantitative reverse transcript polymerase chain reaction; OC, ovarian cancer; HUEVCs, human umbilical vein endothelial cells.
After being transfected with sh-NEAT1, SKOV3 cells, and OVCAR-3 cells were co-cultured with HUEVCs to detect the effects of NEAT1 knockdown on angiogenesis of HUEVCs. The results of Matrigel angiogenesis assay showed that the vascular branches formed by HUEVCs in sh-NEAT1 group were remarkably less than in sh-NC group and blank group (Fig. 2D, P < .05). According to the results of qRT-PCR and Western blot, down-regulated expressions of VEGF, Ang-1 and MMP2 in HUEVCs were detected in sh-NEAT1 group (Fig. 2E-F, P < .05). From the above, knockdown of NEAT1 in OC cells can inhibit proliferation of OC cells and angiogenesis of HUEVCs.
3.3. Over-expression of lncRNA NEAT1 promotes proliferation of OC cells and angiogenesis of HUEVCs
We further studied the impact of over-expressed NEAT1 on proliferation of SKOV3 cells and OVCAR-3 cells and on angiogenesis of HUEVCs by transfecting pcDNA3.1-NEAT1 into SKOV3 cells and OVCAR-3 cells. RT-PCR showed that NEAT1 was significantly over-expressed in SKOV3 cells and OVCAR-3 cells (Fig. 3A, P < .05), indicating successful transfection of pcDNA3.1-NEAT1. CCK-8 assay indicated increased cell proliferation rate of SKOV3 cells and OVCAR-3 cells in pcDNA3.1-NEAT1 group (Fig. 3B, P < .05). According to the results of colonies formation assay, the number of cell colonies in pcDNA3.1-NEAT1 group also increased (Fig. 3C, P < .05). Matrigel angiogenesis assay showed that the number of vascular branches formed by HUEVCs grew significantly after pcDNA3.1-NEAT1 transfection (Fig. 3D, P < .05). Additionally, qRT-PCR and Western blot both revealed the elevated expressions of VEGF, Ang-1, and MMP2 in HUEVCs of pcDNA3.1-NEAT1 group (Fig. 3E-F, P < .05). The above results indicate that over-expression of lncRNA NEAT1 in OC cells promotes proliferation of OC cells and angiogenesis of HUEVCs.
Figure 3.
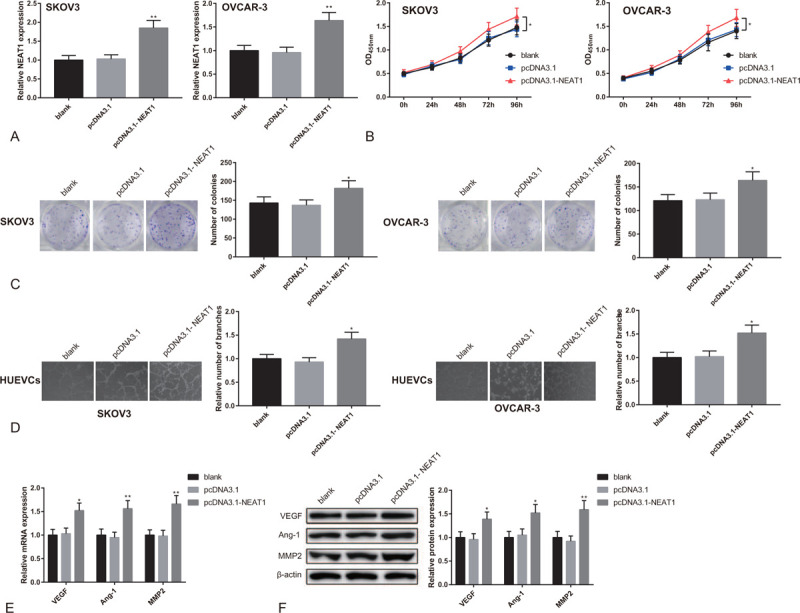
Overexpression of lncRNA NEAT1 promotes proliferation of OC cells and angiogenesis of HUEVCs. Note: After SKOV3 cells and OVCAR-3 cells were transfected with pcDNA3.1-NEAT1 or pcDNA3.1, (A) qRT-PCR detected the expression of NEAT1; (B) CCK8 tested cell proliferation activity; (C) clone formation assay counted cell colonies; (D) Matrigel angiogenesis assay measured the number of vascular branches formed by HUEVCs; (E) qRT-PCR and (F) Western blot analyzed the expressions of VEGF, Ang-1, and MMP2 in HUEVCs. ∗P < .05, ∗∗P < .01, compared to pcDNA3.1 group or blank group; qRT-PCR, quantitative reverse transcript polymerase chain reaction; OC, ovarian cancer; HUEVCs, human umbilical vein endothelial cells.
3.4. LncRNA NEAT1 promotes expression of FGF9 to enhance the proliferation of OC cells and angiogenesis of HUEVCs
We measured the expressions of FGF9 in SKOV3 cells and OVCAR-3 cells after the cells were transfected with sh-NEAT1 or pcDNA3.1-NEAT1. FGF9 was under-expressed in sh-NEAT1 group, while over-expressed in pcDNA3.1-NEAT1 group (Fig. 4A-B, P < .05). This observation indicates that NEAT1 positively regulates the expression of FGF9.
Figure 4.
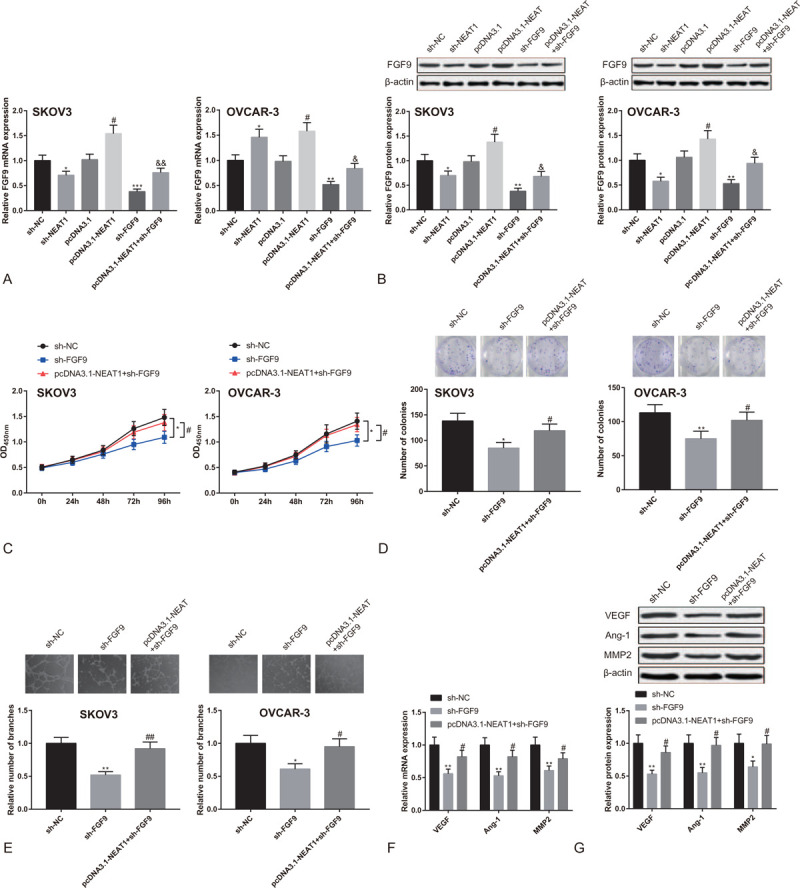
LncRNA NEAT1 promotes proliferation of OC cells and angiogenesis of HUEVCs by regulating FGF9. Note: After SKOV3 cells and OVCAR-3 cells were transfected with sh-NEAT1, pcDNA3.1-NEAT1, sh-FGF9, or pcDNA3.1-NEAT1+sh-FGF9, (A) qRT-PCR and (B) Western blot tested the expression of FGF9. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, compared to sh-NC group; #P < 0.05, compared to pcDNA3.1 group; & P < 0.05, && P < .01, compared to sh-FGF9 group. After SKOV3 cells and OVCAR-3 cells were transfected with sh-FGF9 or pcDNA3.1-NEAT1+sh-FGF9, (C) CCK8 detected cell proliferation activity; (D) clone formation assay measured the number of cell colonies; (E) Matrigel angiogenesis assay tested the number of vascular branches formed by HUEVCs; (F) qRT-PCR and (G) Western blot analyzed the expressions of VEGF, Ang-1 and MMP2 in HUEVCs. ∗P < .05, ∗∗P < .01, compared to sh-NC group; #P < .05, ##P < 0.01, compared to sh-FGF9 group; qRT-PCR, quantitative reverse transcript polymerase chain reaction; HUEVCs, human umbilical vein endothelial cells.
Based on the regulation of NEAT1 over FGF9, we further speculated that NEAT1 might enhance proliferation of OC cells and angiogenesis of HUEVCs through mediating FGF9. Sh-FGF9 or pcDNA3.1-NEAT1+sh-FGF9 were accordingly transfected into SKOV3 cells and OVCAR-3 cells. According to the results of qRT-PCR and Western blot, the expression of FGF9 in sh-FGF9 group was distinctly lower than in sh-NC group, and FGF9 expression in pcDNA3.1-NEAT1+sh-FGF9 group was higher than in sh-FGF9 group (Fig. 4A-B, P < .05). CCK8 assay showed that the proliferation rate of SKOV3 cells and OVCAR-3 cells in sh-FGF9 group was lower than in sh-NC group, and the cell proliferation rate in pcDNA3.1-NEAT1+sh-FGF9 group was much higher than in sh-FGF9 group (Fig. 4C, P < .05). In addition, sh-FGF9 group had attenuated cell clone formation ability than sh-NC group, while the cell formation ability in sh-FGF9 group was suppressed in comparison to pcDNA3.1-NEAT1+sh-FGF9 group (Fig. 4D, P < .05). Matrigel angiogenesis assay showed that the number of vascular branches formed by HUEVCs in sh-FGF9 group was smaller than in sh-NC group, and the vascular branches in pcDNA3.1-NEAT1+sh-FGF9 group were more than in sh-FGF9 group (Fig. 4E, P < .05). Moreover, the expressions of VEGF, Ang-1, and MMP2 in HUEVCs of sh-FGF9 group were lower than in sh-NC group, and these factors were over-expressed in pcDNA3.1-NEAT1+sh-FGF9 group compared to sh-FGF9 group (Fig. 4F-G, P < .05). The above results indicate that knockdown of FGF9 in OC cells can suppress OC cell proliferation and angiogenesis of HUEVCs, which can be reversed by over-expression of NEAT1.
3.5. LncRNA NEAT1 promotes expression of FGF9 via sponging miR-365
We continued to study the mechanism by which lncRNA NEAT1 promotes the expression of FGF9. The starBase (http://starbase.sysu.edu.cn/) found that both NEAT1 and FGF9 could bind with miR-365. Therefore we speculate that NEAT1 regulates the expression of FGF9 by targeting miR-365. To confirm our conjecture, we designed and synthesized wild and mutated sequences of the binding sites of NEAT1 and FGF9 to miR-365 (Fig. 5A). The binding of NEAT1 and FGF9 to miR-365 was validated through the dual-luciferase reporter assay. The results showed that the luciferase activity was decreased in HEK293T cells after wt-NEAT1 or wt-FGF9 were co-transfected with miR-365 mimic (P < .05), whereas the activity remained unaffected in neither mut-NEAT1 nor mut-FGF9 group (Fig. 5B-C). qRT-PCR showed that miR-365 was up-regulated in SKOV3 cells and OVCAR-3 cells after transfection of sh-NEAT1 while down-regulated after transfection of pcDNA3.1-NEAT1 (Fig. 5D, P < .05). The above demonstrates that NEAT1 negatively mediates miR-365 and competes with FGF9 for binding to miR-365.
Figure 5.
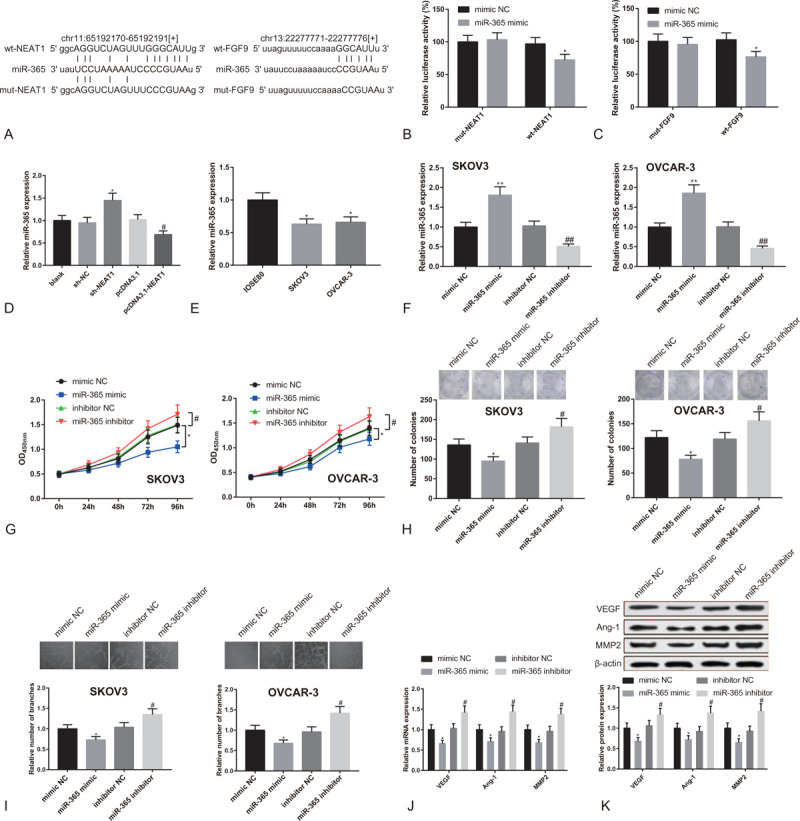
LncRNA NEAT1 competes with FGF9 for binding to miR-365. Note: (A) The wild and mutated sequences of the binding sites of NEAT1 and FGF9 to miR-365; dual-luciferase reporter assay tested (B) the binding of NEAT1 to miR-365, and (C) the binding of FGF9 to miR-365. ∗P < .05, compared to mimic NC group. (D) qRT-PCR detected the expression of miR-365 in OC cells transfected with sh-NEAT1 or pcDNA3.1-NEAT1. (E) qRT-PCR measured the expression of miR-365 in SKOV3 cells, OVCAR-3 cells and IOSE80 cells. ∗P < .05, compared to IOSE80 group. After SKOV3 cells and OVCAR-3 cells were transfected with miR-365 mimic or miR-365 inhibitor, (F) qRT-PCR detected the expression of miR-365; (G) CCK8 tested cell proliferation activity; (H) clone formation assay measured the number of colonies; (I) Matrigel angiogenesis assay tested the number of vascular branches formed by HUEVCs; (J) qRT-PCR and (K) Western blot analyzed the expressions of VEGF, Ang-1 and MMP2 in HUEVCs. ∗P < .05, ∗∗P < .01, compared to mimic NC group or sh-NC group; #P < .05, ##P < .01, compared to inhibitor NC group or pcDNA3.1 group; qRT-PCR, quantitative reverse transcript polymerase chain reaction; HUEVCs, human umbilical vein endothelial cells.
At last, we studied the role of miR-365 in OC cell proliferation and angiogenesis ability of HUEVCs. Firstly, we tested the expression of miR-365 in SKOV3 cells, OVCAR-3 cells and ovarian epithelial cells. MiR-365 was under-expressed in OC cells (Fig. 5E, P < .05). Moreover, in SKOV3 cells and OVCAR-3 cells transfected with miR-365 mimic, the expression of miR-365 was enhanced, while it was lowered down when miR-365 inhibitor was transfected (Fig. 5F, P < .05).
The CCK8 assay showed that the proliferation activity of SKOV3 cells and OVCAR-3 cells was decreased after the transfection of miR-365 mimic, while it was increased when the cells were transfected with miR-365 inhibitor (Fig. 5G, P < .05). Additionally, the clones of SKOV3 cells and OVCAR-3 cells were reduced in miR-365 mimic group, while increased cell clones were found in miR-365 inhibitor group (Fig. 5H, P < .05). According to Matrigel angiogenesis assay, the vascular branches formed by HUEVCs were less in miR-365 mimic group, while increased in miR-365 inhibitor group (Fig. 5I, P < .05). Both qRT-PCR and Western blot showed that the expressions of VEGF, Ang-1, and MMP2 in HUEVCs were inhibited in miR-365 mimic group, while different expression patterns of these genes were displayed in miR-365 inhibitor group (Fig. 5J-K, P < .05). The above results indicate that lncRNA NEAT1 up-regulates the expression of FGF9 by sponging miR-365 to promote proliferation of OC cells and angiogenesis of HUEVCs.
4. Discussion
OC is the second most common gynecologic malignancy which lacks specific symptoms and reliable indicators, thus resulting in late diagnosis and high death rates of this disease.[21] Despite the advancements in platinum chemotherapy, a traditional therapy for epithelial OC, the patient survival has received no distinct improvements over the years.[22] Molecular targeted therapy has surfaced as a high efficient and less toxic treatment strategy for heterogeneous OC, but this promising therapy is now challenged by the absence of valid biomarkers.[23] Therefore, exploration of OC biomarkers and the underlying pathogenic mechanisms can facilitate the development of novel therapeutics of OC. LncRNA-miRNA interaction has been largely investigated in recent studies for revealing the molecular mechanisms involved in regulating cancer progressions.[24] To date, no study has demonstrated the possible interaction between NEAT1 and miR-365 in OC. The present study confirms NEAT1 up-regulates the expression of FGF9 via sponging miR-365, thus promoting OC cell proliferation and angiogenesis ability of co-cultivated HUEVCs.
First of all, up-regulated lncRNA NEAT1 was found in OC cells which promoted OC cell proliferation and angiogenesis of HUEVCs. Consistent with our results, data in a previous study documented that NEAT1 promoted OC cell proliferation by releasing more cells into S phrase of cell cycles and further inhibited apoptosis of OC cells.[25] NEAT1 exerted its biological function by targeting miR-194 to up-regulate ZEB1 which was a tumor suppressor in OC.[26] Another study indicated NEAT1, stabilized by LIN28B, contributed to OC development via binding miR-506.[27] Based on previous studies, the present research also probes into the potential molecular mechanisms by which NEAT1 mediates OC cell proliferation. Although no data are available regarding the angiogenic effects of NEAT1, many lncRNAs have been validated to function as regulators in tumor angiogenesis.[28]
In the subsequent experiments, over-expressed FGF9 was also found in OC cells. After SKOV3 cells and OVCAR-3 cells were transfected with sh-FGF9, the proliferation rate of these cells was decreased, so as the vascular branches formed by HUEVCs. These results demonstrate that FGF9 functions as a promoter of OC cell proliferation and associated angiogenesis. Consistent with our study, Tanner Y et al found that FGF signaling pathway played a pivotal role in malignant development of many cancers.[29] In addition, FGF9 stimulated tumorigenesis through elevating the expression of ERK and AKT in gastric cancer cells.[30] Though the positive role of FGF9 in OC cells is confirmed, whether there is a downstream target of FGF9 in promoting OC cell growth may need further research. A recent study reported that FGF9 contributed to invasion of OC cells through up-regulating the expression of VEGF-A/VEGFR2.[20] Our study demonstrated VEGF in HUEVCs was substantially reduced after OC cells were transfected with sh-FGF9. VEGF has already been authenticated to be a mitogen unique to endothelial cells, with the ability to induce physiological and pathological angiogenesis.[31] Tumor angiogenesis was a process in which new blood vessels were formed to provide oxygen and nutrients for cancer cells, supporting tumor progression and metastasis.[32] It is worth mentioning here that both SKOV3 and OVCAR-3 are cell lines derived from metastatic sites. Enhanced angiogenic capacity of these OC cell lines might contribute to the development of metastatic OC.
Sh-NEAT1 or pcDNA3.1-NEAT1 was transfected into SKOV3 cells and OVCAR-3 cells to investigate the relationship between NEAT1 and FGF9, and increased FGF9 was found in pcDNA3.1-NEAT1 group. The findings indicate that NEAT1 positively regulates the expression of FGF9, but whether NEAT1 directly or indirectly regulates FGF9 was yet unknown. The online prediction suggests that both NEAT1 and FGF9 can combine with miR-365, and this speculation has been validated by the dual-luciferase reporter assay. In addition, we identify miR-365 as a negative regulator of OC cells. The suppressive role of miR-365 was also found in colon cancer, in which miR-365 targeted Cyclin D1 and Bcl-2 to inhibit cell growth and promote apoptosis of colon cancer cells.[33] Moreover, NEAT1 acted as a miR-365 sponge in regulating migration and invasion of oral squamous carcinoma cells.[34] In our study, NEAT1 attenuates the suppressing effect of miR-365 on OC cells by directly targeting miR-365. Furthermore, NEAT1 competes with FGF9 for binding to miR-365, thereby up-regulating the expression of FGF9. Finally, elevated FGF9 contributes to OC cell growth and further angiogenesis.
In summary, we demonstrate that NEAT1 up-regulates the expression of FGF9 to mediate the proliferation and angiogenesis of OC cells by sponging miR-365. This finding may have clinical significance for developing therapeutic targets for molecule-based treatment of OC. However, no in vivo experiment was performed in this study to verify the functions of NEAT1 in vivo. Further experiments could be implemented to facilitate the comprehensive understanding of the role of NEAT1 in OC development. Additionally, Asian ethnicities as a whole had significantly lower OC incidence rates than non-Hispanic whites and significant differences were also observed among various Asian ethnicities.[35] In this regard, racial disparity is also an important issue that is warranted in future research on OC.
Author contributions
Conceptualization: Jialing Yuan, Yang Lingyun.
Data curation: Jialing Yuan, Ke Yi.
Formal analysis: Ke Yi.
Investigation: Jialing Yuan.
Methodology: Yang Lingyun.
Project administration: Yang Lingyun.
Resources: Yang Lingyun.
Software: Yang Lingyun.
Supervision: Yang Lingyun.
Validation: Ke Yi, Yang Lingyun.
Visualization: Jialing Yuan, Ke Yi, Yang Lingyun.
Writing – original draft: Jialing Yuan, Yang Lingyun.
Writing – review & editing: Jialing Yuan, Ke Yi.
Footnotes
Abbreviations: Ang-1 = angiogenin 1, CCK-8 = cell counting kit-8, FGF = fibroblast growth factor, FIGO = International Federation of Gynecology and Obstetrics, GFP = green fluorescent protein, HGSC = high-grade serous carcinoma, HUVECs = human umbilical vein endothelial cells, lncRNAs = long non-coding RNAs, miR-365 = microRNA-365, miRNAs = microRNAs, MMP2 = matrix metalloproteinase 2, NEAT1 = Nuclear enriched abundant transcript 1, OC = ovarian cancer, qRT-PCR = Quantitative reverse transcription-polymerase chain reaction, VEGF = vascular endothelial growth factor.
How to cite this article: Yuan J, Yi K, Yang L. LncRNA NEAT1 promotes proliferation of ovarian cancer cells and angiogenesis of co-incubated human umbilical vein endothelial cells by regulating FGF9 through sponging miR-365: an experimental study. Medicine. 2021;100:3(e23423).
The author declares that they have nothing worth disclosure.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
F = forward, R = reverse.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Gomes Ferreira M, Sancho de Salas M, Gonzalez Sarmiento R, et al. Changes in the management and prognosis of ovarian cancer due to the new FIGO and WHO classifications: a case series observational descriptive study. Seven years of follow-up. Int J Gynecol Cancer 2018;28:1461–70. [DOI] [PubMed] [Google Scholar]
- [3].Konstantinopoulos PA, Awtrey CS. Management of ovarian cancer: a 75-year-old woman who has completed treatment. JAMA 2012;307:1420–9. [DOI] [PubMed] [Google Scholar]
- [4].Rosendahl M, Hogdall CK, Mosgaard BJ. Restaging and survival analysis of 4036 ovarian cancer patients according to the 2013 FIGO classification for ovarian, fallopian tube, and primary peritoneal cancer. Int J Gynecol Cancer 2016;26:680–7. [DOI] [PubMed] [Google Scholar]
- [5].Jayson GC, Kohn EC, Kitchener HC, et al. Ovarian cancer. Lancet 2014;384:1376–88. [DOI] [PubMed] [Google Scholar]
- [6].Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin 2019;69:280–304. [DOI] [PubMed] [Google Scholar]
- [7].Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68:284–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dong P, Xiong Y, Yue J, et al. Long Non-coding RNA NEAT1: a novel target for diagnosis and therapy in human tumors. Front Genet 2018;9:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta 2014;1839:1097–109. [DOI] [PubMed] [Google Scholar]
- [10].Clemson CM, Hutchinson JN, Sara SA, et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell 2009;33:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu Y, Wang Y, Fu X, et al. Long non-coding RNA NEAT1 promoted ovarian cancer cells’ metastasis through regulation of miR-382-3p/ROCK1 axial. Cancer Sci 2018;109:2188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta 2016;1859:169–76. [DOI] [PubMed] [Google Scholar]
- [13].Zhou M, Wang X, Shi H, et al. Characterization of long non-coding RNA-associated ceRNA network to reveal potential prognostic lncRNA biomarkers in human ovarian cancer. Oncotarget 2016;7:12598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Silber J, James CD, Hodgson JG. microRNAs in gliomas: small regulators of a big problem. Neuromolecular Med 2009;11:208–22. [DOI] [PubMed] [Google Scholar]
- [15].Li H, Jiang M, Cui M, et al. MiR-365 enhances the radiosensitivity of non-small cell lung cancer cells through targeting CDC25A. Biochem Biophys Res Commun 2019;512:392–8. [DOI] [PubMed] [Google Scholar]
- [16].Jiang ZB, Ma BQ, Liu SG, et al. miR-365 regulates liver cancer stem cells via RAC1 pathway. Mol Carcinog 2019;58:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang Y, Xu C, Wang Y, et al. MicroRNA-365 inhibits ovarian cancer progression by targeting Wnt5a. Am J Cancer Res 2017;7:1096–106. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [18].Lei S, He Z, Chen T, et al. Long noncoding RNA 00976 promotes pancreatic cancer progression through OTUD7B by sponging miR-137 involving EGFR/MAPK pathway. J Exp Clin Cancer Res 2019;38:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets 2009;9:639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bhattacharya R, Ray Chaudhuri S, Roy SS. FGF9-induced ovarian cancer cell invasion involves VEGF-A/VEGFR2 augmentation by virtue of ETS1 upregulation and metabolic reprogramming. J Cell Biochem 2018;119:8174–89. [DOI] [PubMed] [Google Scholar]
- [21].Lalwani N, Prasad SR, Vikram R, et al. Histologic, molecular, and cytogenetic features of ovarian cancers: implications for diagnosis and treatment. Radiographics 2011;31:625–46. [DOI] [PubMed] [Google Scholar]
- [22].Konstantinopoulos PA, Ceccaldi R, Shapiro GI, et al. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov 2015;5:1137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guan LY, Lu Y. New developments in molecular targeted therapy of ovarian cancer. Discov Med 2018;26:219–29. [PubMed] [Google Scholar]
- [24].Cao MX, Jiang YP, Tang YL, et al. The crosstalk between lncRNA and microRNA in cancer metastasis: orchestrating the epithelial-mesenchymal plasticity. Oncotarget 2017;8:12472–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ding N, Wu H, Tao T, et al. NEAT1 regulates cell proliferation and apoptosis of ovarian cancer by miR-34a-5p/BCL2. Onco Targets Ther 2017;10:4905–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].An J, Lv W, Zhang Y. LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. Onco Targets Ther 2017;10:5377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yong W, Yu D, Jun Z, et al. Long noncoding RNA NEAT1, regulated by LIN28B, promotes cell proliferation and migration through sponging miR-506 in high-grade serous ovarian cancer. Cell Death Dis 2018;9:861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sheng SR, Wu JS, Tang YL, et al. Long noncoding RNAs: emerging regulators of tumor angiogenesis. Future Oncol 2017;13:1551–62. [DOI] [PubMed] [Google Scholar]
- [29].Tanner Y, Grose RP. Dysregulated FGF signalling in neoplastic disorders. Semin Cell Dev Biol 2016;53:126–35. [DOI] [PubMed] [Google Scholar]
- [30].Sun C, Fukui H, Hara K, et al. FGF9 from cancer-associated fibroblasts is a possible mediator of invasion and anti-apoptosis of gastric cancer cells. BMC Cancer 2015;15:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer 2013;13:871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hida K, Maishi N, Torii C, et al. Tumor angiogenesis--characteristics of tumor endothelial cells. Int J Clin Oncol 2016;21:206–12. [DOI] [PubMed] [Google Scholar]
- [33].Nie J, Liu L, Zheng W, et al. microRNA-365, down-regulated in colon cancer, inhibits cell cycle progression and promotes apoptosis of colon cancer cells by probably targeting Cyclin D1 and Bcl-2. Carcinogenesis 2012;33:220–5. [DOI] [PubMed] [Google Scholar]
- [34].Liu X, Shang W, Zheng F. Long non-coding RNA NEAT1 promotes migration and invasion of oral squamous cell carcinoma cells by sponging microRNA-365. Exp Ther Med 2018;16:2243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lee AW, Navajas EE, Liu L. Clear differences in ovarian cancer incidence and trends by ethnicity among Asian Americans. Cancer Epidemiol 2019;61:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


