Supplemental Digital Content is available in the text
Keywords: B coagulans, Bristol stool form scale (BSFC), constipation, diarrhea, Digestive Symptom Frequency Questionnaire, irritable bowel syndrome
Abstract
Goals:
To evaluate safety and efficacy of Bacillus coagulans LBSC [DSM17654] in irritable bowel syndrome (IBS) through a prospective, interventional, randomized, double-blind, and placebo-controlled, CONSORT compliant clinical trial.
Background:
Bacteriotherapy shows promising impact on alleviating clinical conditions of IBS and associated functional gastrointestinal disorders. B coagulans LBSC is a genetically and phenotypically safe probiotic strain used in this study to study its impact on ameliorating IBS symptoms and improving quality of life.
Methods:
In this interventional, randomized, double-blind, placebo-controlled clinical study, total 40 subjects (18–65 years) were screened through Rome IV criteria and randomized into 2 groups, that is, interventional and placebo arm (n = 20/arm). Similar dosages were received by both the arm, that is, placebo (vehicle) and interventional arm (B coagulans LBSC, 6 billion/d) for a period of 80 days. Study completed with per protocol subjects (n = 38) and results were considered to evaluate the primary and secondary endpoints.
Results:
Assessment through Digestive Symptom Frequency Questionnaire 5 point Likert scale showed significant improvement in interventional arm compared to placebo on symptoms such as bloating/cramping, abdominal pain, diarrhea, constipation, stomach rumbling, nausea, vomiting, headache, and anxiety. Maximum of “no symptoms” cases and mild to moderate gastrointestinal symptoms along with improved stool consistency were from interventional arm tested following IBS severity scoring system and Bristol stool form scale. Upper gastrointestinal endoscopy revealed no clinical difference of gastrointestinal mucosa between both the arms. B coagulans LBSC was well tolerated with no serious adverse events.
Conclusions:
B coagulans LBSC was safe for human consumption and efficacious in alleviating overall pathophysiological symptoms of IBS and thereby improving inclusive quality of life evaluated.
1. Introduction
Irritable bowel syndrome (IBS) is a chronic gastrointestinal disorder characterized by severe abdominal pain and/or discomfort associated with changes in bowel frequency and/or stool consistency. It is the most commonly diagnosed functional gastrointestinal disorder (FGID) accompanied with recurrent abdominal pain for at least 1 day per week in previous 3 months and associated with pain/discomfort during defecation (Rome IV criteria).[1–3] IBS classes such as diarrhea-predominant (IBS-D), constipation-predominant (IBS-C), mixed bowel habits (IBS-M), and un-subtyped (IBS-U) have been defined based on the stool pattern.[4]
Global prevalence of IBS is estimated to 15% to 45% of general population of all age group.[5] Meta-analyses revealed that IBS is more common among women than men (2:1) and mostly starts in early adulthood.[6] Many scientific groups recognized that compromised gut microbiome, small-bowel bacterial overgrowth (SIBO), altered gut motility, mucosal inflammation, visceral hypersensitivity, and impaired neuro-endocrinal communications could be the underlying etiology for IBS pathogenesis.[4,7]
Current treatments of IBS majorly include dietary [low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs)] and antibiotic interventions.[8–10] A low FODMAP diet is however controversial due to inherent limitations like dietary restrictions, required close monitoring by an expert dietitian, potential nutritional deficiencies, reduction in gut microbiota diversity, lack of predictors of response, dearth of alternative dietary, pharmacological and psychological interventions for IBS.[11] Compared to FODMAPs intervention, antibiotic treatment is more common practice for treating IBS; however, few clinical reports highlighted the significant association of antibiotic use with increased risks of IBS.[12,13] Use of antibiotics promotes horizontal gene transfer (hgt) and confers antibiotic resistance to otherwise sensitive microorganisms.[14] Broad-spectrum antibiotics such as beta-lactam, cephalosporin, and macrolide are known to either eliminate or alter gastrointestinal microbiota; narrowing in microbial diversity which has a magnitude of adverse impacts on IBS.[15,16]
Numerous studies have described the mechanism of IBS onset as a shift from “normal and healthy” gut to “dysbiosed and unhealthy” gut where gut microbial community plays a pivotal role.[17–19] Healthy microbiome-modulated intestinal homeostasis is therefore a fundamental therapeutic paradigm where probiotics could offer promising healthcare solution for IBS.[20] Probiotics are live bio-therapeutics[21] which offer promising route for treating various gastrointestinal ailments like diarrhea, indigestion, nutrient malabsorption, SIBO, inflammatory bowel disease (IBD), ulcerative colitis, Crohn's disease, etc, without a risk of spreading antibiotic resistance in microorganisms.
The systematic interventions of lactic acid-producing Bacillus probiotics are well documented in different community-based meta-analysis and ensued significant reduction of IBS symptoms, especially by a monoculture or mixed culture probiotic formulations. The lactic acid-producing Bacillus probiotics, like, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus paracasei, Bifidobacterium longum, Bifidobacterium lactis, Streptococcus thermophillus, Bacillus coagulans are mainly included in the formulations.[22] Among all, Bacillus coagulans is determined as a commonly used heterolactate live biotherapeutic for various gastrointestinal disorders including IBS.[23]
Strains of B coagulans are spore-former and widely studied probiotic for production of antimicrobials like coagulin and lactosporin, immune-modulation and modulation of gut microbiome.[24] The naturally encapsulated coating around the cell protects it from various drought conditions like high temperature, desiccation, osmolarity, etc. This highly resilient allochthonous probiotic can survive and proliferate in gastric acid, pepsin, pancreatin, ions, digestive enzymes, bile, and mucin of gastrointestine.[25] The efficacy of probiotic B coagulans varies among strains; differs as per dosages used in finished formulations and severity of clinical conditions to exhibit the intended health benefits.[26]
The therapeutic activity of B coagulans is well established for various ailments like diarrhea, antibiotic-associated diarrhea, hypercholesterolemia, immune modulation, IBS, IBD, Clostridium difficile-induced colitis, dysbiosis, Helicobacter pylori infection, gingivitis, necrotizing enterocolitis, and other FGID's.[23,24,26,27] Some strains of B coagulans have shown clinical efficacy by significantly improving the bowel pattern, diarrheal duration and frequency, abdominal pain, and bloating associated with IBS through randomized control trials (RCTs) studies.[28–34] However, few of these clinical studies have also reported about variable effect of B coagulans strains for IBS symptoms instead of complete relive of symptoms subset. Very few reports are available on quality of life (QoL) findings associated with IBS, which directed current study to evaluate the efficacy and safety of B coagulans LBSC [DSM17654] in relieving IBS and associated symptoms. This probiotic strain has been previously reported for its genomic safety[35] and clinical efficacy.[24,26] The frequency and severity of gastrointestinal symptoms, stool consistency, and QoL in the intervened (test) and control (placebo) group were assessed as outcome variables.
2. Patients and methods
2.1. Formulation and method of analysis
The investigational product (IP) was the active ingredient, B coagulans LBSC mixed with the excipient. The strength of the active ingredient was 2 billion spores per gram per sachet, which was supplied by Advanced Enzyme Technologies Ltd., Thane, India. The placebo contained only excipient, maltodextrin (1.00 g). Both, the investigational and placebo products complied with the specifications (Supplement: Method of Analysis).[26] The packaging and labeling for both the products were same except the coded batch numbers used for differentiation.
2.2. Ethics and informed consent
Trial was registered in the Clinical Trial Registry India (CTRI/2018/02/011654) and conducted with the written ethical approval from Sri Venkateshwara Hospital, Bangalore and People Tree Hospital Clinical Research Center, Bangalore. Approved study protocol was designed in accordance with Declaration of Helsinki (WMA 2000),[36] ICH-harmonized tripartite guideline for good clinical practice (ICH 1996)[37] and Indian Council of Medical Research Guidelines for Biomedical Research on Human subjects (ICMR 2006)[38]; same was followed with no further amendments during trial. Written and oral information was provided to all participating subjects in understandable language. Every subject has given written informed consent to investigator after understating the objective of this trial, including possible risks and benefits.
2.3. Study design and selection of study subjects
The prospective interventional trial was randomized, double-blinded, parallel, placebo controlled and had a total of 5 visits to the clinical site by the study subjects. Subject selection was based on the defined inclusion and exclusion criteria as follows.
Inclusion Criteria: Subjects were included based on following criteria –
-
(1)
Male and females (18–65 years) with diagnosis of IBS as per Rome IV criteria associated with following symptoms for more than last 3 months; like abdominal discomfort such as mild pain, cramping, bloating, altered bowel habit indicated by frequent diarrhea or constipation and functional dyspepsia;
-
(2)
written informed consent by study participants.
Exclusion Criteria: Subjects those were on antibiotics or laxatives within the preceding 6 weeks, Symptoms of IBD, acute GI tract infection, fever, abdominal mass, signs of bowel obstruction, history of colon cancer or diverticulitis, infection from human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), celiac disease, scleroderma and gastroparesis and hypothyroidism were excluded from the study (Supplement: Details of Exclusion and Withdrawal Criteria). In addition, subjects having adverse effects or serious adverse effects, pregnancies, disease emergencies, concomitant therapy, and study protocol violation are considered for withdrawal of subjects from the trial.
2.4. Samples, randomization, treatments, and procedures
A total of 40 subjects consisting 20 randomized subjects in each of the tests and the placebo group were enrolled for the current study. The test group – G (n = 20) was on B coagulans LBSC with 2 billion colony-forming unit activity (Supplement: Method of Analysis) powder (carrier maltodextrin) thrice a day, whereas, control (placebo) group – H (n = 20) was only on maltodextrin with similar dosing schedule. Treatment duration was up to 80 days and total study duration did not exceed 90 days. The subject randomization (followed by SAS random number generation method), treatment allocation, and procedures are represented in Figure 1. All supportive treatments were, if required, administered to the subjects as deemed necessary by the investigator, but no antibiotics were recommended along with the study drug. No changes or amendments were made to the approved protocol after the trial commenced and no interim data analysis was done during the study period.
Figure 1.
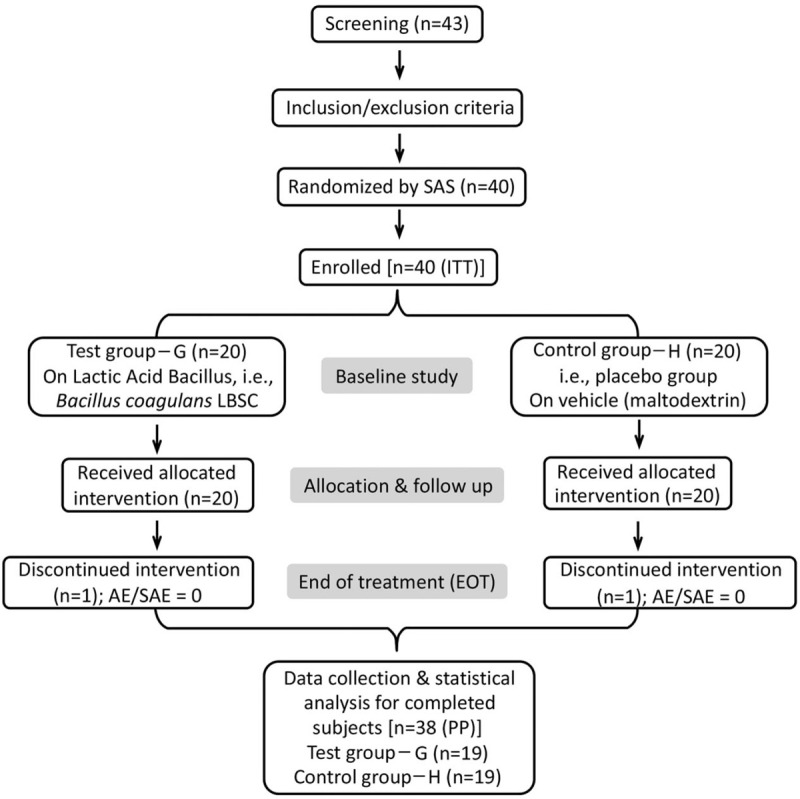
The schematic diagram of current clinical study. The study enrolled “intension to treat” 40 subjects through the inclusion and exclusion criteria screening followed by the SAS random number generation method. Upon discontinuation of one subject from each group, total 38 subjects were considered per protocol for evaluation. No subject reported for adverse events or serious adverse events. The study compliance was checked at every follow-up visit alongside study evaluation and assessment by a team of physician-investigator and officers.
2.5. Endpoints: efficacy and safety variables
Primary endpoints were set to study the efficacy outcomes like assessment of change in gastrointestinal symptom's frequency, assessment using Digestive Symptom Frequency Questionnaire (DSFQ) on 5-point Likert scale (0 = never, 1 ≤ 1 episode/wk; 2 ≤ 3 episodes/wk; 3 ≥ 3 episodes/wk; 4 = daily episodes), change in gastrointestinal symptom's severity using IBS severity scoring system (IBS-SSS) and change in stool consistency using Bristol stool form scale. Secondary endpoints were for safety evaluations of B coagulans LBSC; measured by evaluating physical examination and vitals, systematic biomarkers, adverse events (AEs) or serious adverse events (SAEs), and Quality of life (QoL) questionnaire. The QoL was assessed based on yes or no responses for pain scale on abdominal pain, diarrhea, constipation, bloating and flatulence, vomiting and nausea, perception of mental well-being, and influence on daily life. Results were processed to a binary numerical response and analyzed through principal component analysis (PCoA).[26] Hematological and hepatic biomarkers were analyzed following the standard medical test protocols. The AE is defined as any medically untoward event detected in clinical study subject after use of the study agents, whether or not caused by the use of the agents. Whereas, SAE is defined as any untoward medical incidence which is life-threatening and results into death or hospitalization, disability or incapacity, and congenital anomaly.
2.6. Sample power and statistical analysis
Data was analyzed with 5% significance level (confidence interval 95%) and maintaining a minimum power of 80% for study using SAS software, version 9.1. The differences within the groups were assessed using t-test, whereas, ANOVA and χ2 test were used for differences between groups. Efficacy analysis was performed for the per-protocol (PP) population. Separate analyses were performed for primary and secondary endpoints. The entire statistical analysis was performed as per the statistical analysis plan.
3. Results
Enrollment of first patient and treatment of the last patient were completed in November 2018 and July 2019, respectively. Trial was initiated with the participation of total 40 “intention to treat” which was screened from a total of 43 (n = 43) patients. With total 2 patients lost to follow up (dropped out); trial was completed with total 38 PP population. Of 2 treatment arms, that is, Test-G and Placebo-H, each arm was allocated with 20 subjects. Total 12 females (F) and 28 males (M) took part in the trial (Test G, 7F:13 M and Placebo H, 5F:15 M). No significant difference was observed in the demographic parameters of both treatment groups (Table 1). Principal investigator and clinical trial team assessed study regulations at each visit along with all the safety and efficacy assays as per the schedule of events (Table 2). Efficacy analyses were performed on PP population, that is, full analysis set of 38 patients. The clinical trial was concluded after the follow-up visit (at Visit 05) of the last enrolled patient and completion of target sample size according to the study procedures.
Table 1.
The demographic details of subjects of two treatment arms participated in the current clinical trial and their descriptive statistics. Values expressed as mean ± SD.
| Treatment groups | |||
| Parameters | Test-G | Placebo-H | P value [95% CI] (P < .05) |
| Subjects number (n) | 20 | 20 | |
| Gender [n (%)] | |||
| Male | 13 (65.00%) | 15 (75.00%) | |
| Female | 7 (35.00%) | 5 (25.00%) | |
| Age (years) [min/max] | 36.20 ± 9.81 [21/56] | 34.80 ± 11.06 [18/55] | 0.674 [−8.092–5.292] |
| Height (cm) [min/max] | 162.38 ± 19.82 [88.97/185.40] | 164.84 ± 9.99 [141.00/183.00] | 0.623 [−7.587–12.507] |
| Weight (kg) [min/max] | 65.93 ± 10.87 [47.00/90.08] | 64.65 ± 9.17 [48.90/81.00] | 0.689 [−7.717–5.157] |
| Body Mass Index (kg m−2) [min/max] | 23.37 ± 3.23 [16.05/30.04] | 24.03 ± 2.99 [19.30/29.70] | 0.506 [−1.332–2.652] |
| Diet | |||
| Mixed | 20 (100.00%) | 20 (100.00%) | |
| Vegetarian | 0 (−) | 0 (−) | |
| Smoker/alcohol drinkers | 0 (−) | 0 (−) | |
Table 2.
Schematic schedule of the clinical trial conducted for irritable bowel syndrome (IBS) patients.
| Visits | Visit 01 (Day 0)∗ | Visit 02 (Day 1)† | Visit 03 (Day 40)‡ | Visit 04 (Day 80)§ | Visit 05 (Day 90)|| | Visit 06¶ |
| Informed consent | √ | |||||
| Demography | √ | |||||
| Inclusion/exclusion criteria | √ | |||||
| Vital signs | √ | √ | √ | √ | √ | √ |
| Physical examination | √ | √ | √ | √ | √ | |
| Medical/surgical history | √ | |||||
| Pregnancy test | √ | |||||
| Rome IV criteria | √ | |||||
| Laboratory tests | √ | √ | ||||
| Upper GI endoscopy | √ | √ | ||||
| IP Dispensing | √ | √ | ||||
| Issue Dairy card | √ | √ | ||||
| Concomitant medicationschecking | √ | √ | √ | √ | √ | |
| IBS-SSS Questionnaire and (VAS) | √ | √ | √ | √ | ||
| Bristol stool form scale (BSFC) | √ | √ | ||||
| DSFQ | √ | √ | √ | √ | √ | |
| Compliance check | √ | √ | √ | |||
| AE/SAE assessment | √ | √ | √ | √ | √ |
4. Primary endpoint: efficacy evaluation
4.1. Change in gastrointestinal symptom's frequency assessment
DSFQ on 5-point Likert scale has been used to assess the gastrointestinal symptoms frequency of subjects and to compare the difference between Test-G and Placebo-H arm.[39] Frequency of various gastrointestinal symptoms like bloating/cramping, abdominal pain, diarrhea or constipation, stomach rumbling, nausea, vomiting, headache, and anxiety were assessed for both Test-G and Placebo-H arm at baseline (visit 2) and end of treatment (EOT) (visit 4) (Table 2). Boating and cramping symptoms were improved in Test-G [mean difference (MD) −1.21, 95% CI (−1.74, −0.68)] compared to Placebo-H [MD −0.26, 95% CI (−0.79, 0.27)] after B coagulans LBSC treatment (IP) and changes [MD −0.95, 95% CI (−1.69, −0.19)] were found statistically significant (P = .0148). Relive from abdominal pain was significant (P < .0001) in Test-G (B coagulans LBSC) [MD −1.74, 95% CI (−2.44, −1.03)] compared to Placebo-H; where no symptoms of improvement were recorded (Table 3). Statistically significant improvement in diarrhea and constipation was noted after B coagulans LBSC treatment (Test-G) [MD −1.47, 95% CI (−1.89, −1.05)] compared to Placebo-H [MD −0.53, 95% CI (−0.94, −0.10)] (P = .0027).
Table 3.
Comparative DSFQ scores evaluated in IBS subjects based on 5-point Likert scale at baseline and EOT from both B coagulans LBSC (Test-G) and vehicle (Placebo-H) arm (±SD).
| Parameters | Arms | Baseline | EOT | Mean change | P value | 95% CI |
| Bloating and cramping | Placebo - H | 1.42 ± 0.69 | 1.16 ± 0.60 | −0.26 | .0148 | −0.79, 0.27 |
| Test - G | 2.21 ± 1.36 | 1.00 ± 0.82 | −1.21 | −1.74, −0.68 | ||
| Between groups (G-H) | −0.95 | −1.69, −0.19 | ||||
| Abdominal pain | Placebo - H | 1.84 ± 0.69 | 1.42 ± 0.61 | −0.42 | <.0001 | −0.92, 0.07 |
| Test - G | 2.84 ± 1.07 | 0.68 ± 0.95 | −2.16 | −2.65, −1.66 | ||
| Between groups (G-H) | −1.74 | −2.44, −1.03 | ||||
| Diarrhea and constipation | Placebo - H | 1.79 ± 0.86 | 1.26 ± 0.73 | −0.53 | .0027 | −0.94, −0.10 |
| Test - G | 2.68 ± 1.06 | 1.21 ± 0.79 | −1.47 | −1.89, −1.05 | ||
| Between groups (G-H) | −0.95 | −1.54, −0.35 | ||||
| Stomach rumbling | Placebo - H | 1.58 ± 0.96 | 1.00 ± 0.67 | −0.58 | .021 | −1.20, 0.04 |
| Test - G | 1.90 ± 1.33 | 0.26 ± 0.73 | −1.63 | −2.26, −1.01 | ||
| Between groups (G-H) | −1.05 | −1.93, −0.16 | ||||
| Nausea | Placebo - H | 0.74 ± 0.73 | 0.79 ± 0.79 | 0.05 | .0031 | −0.39, 0.50 |
| Test - G | 1.16 ± 1.34 | 0.21 ± 0.71 | −0.95 | −1.39, −0.49 | ||
| Between groups (G-H) | −1.00 | −1.63, −0.36 | ||||
| Vomiting | Placebo - H | 0.26 ± 0.56 | 0.32 ± 0.48 | 0.05 | .0386 | −0.37, 0.47 |
| Test - G | 0.79 ± 0.63 | 0.21 ± 0.71 | −0.58 | −1.01, −0.16 | ||
| Between groups (G-H) | −0.63 | −1.23, −0.03 | ||||
| Headache | Placebo - H | 1.37 ± 0.68 | 1.00 ± 0.67 | 0.74 | .0003 | 0.28, 1.19 |
| Test - G | 1.90 ± 1.33 | 0.26 ± 0.93 | −0.53 | −0.98, −0.07 | ||
| Between groups (G-H) | −1.26 | −1.90, −0.61 | ||||
| Anxiety | Placebo - H | 1.26 ± 0.93 | 1.00 ± 0.58 | −0.26 | .0177 | −0.84, 0.31 |
| Test - G | 1.58 ± 1.58 | 0.32 ± 0.95 | −1.26 | −1.84, −0.69 | ||
| Between groups (G-H) | −1.00 | −1.82, −0.18 |
Results showed that subjects from Test-G arm (B coagulans LBSC) were relived from stomach rumbling [MD −1.63, 95% CI (−2.26, −1.01)] than Placebo-H [MD −0.58, 95% CI (−1.20, 0.04)]. Improvement in stomach rumbling [MD −1.05, 95% CI (−1.93, −0.16)] was found statistically significant (P = .021) between both groups analyzed through baseline to EOT. Simultaneously, nausea and vomiting conditions were improved after treatment with B coagulans LBSC in Test-G, whereas, these symptoms were worsened in Placebo-H. Nausea was reduced in Test-G [MD −0.95, 95% CI (−1.39, −0.49)] than Placebo-H [MD +0.05, 95% CI (−0.39, 0.50)] and the change between group [MD −1.00, 95% CI (−1.63, −0.36)] was significant (P = .0031) (Table 3). Vomiting was reduced in Test-G from baseline (0.79) to EOT (0.21) [MD −0.58, 95% CI (−1.01, −0.16)]; while, increased in Placebo-H [MD +0.05, 95% CI (−0.37, 0.47)] with overall significant intragroup difference [MD −0.63, 95% CI (−1.23, −0.03)] (P = .0386). Headache was reduced in Test-G, that is, on B coagulans LBSC treatment [MD 0.53, 95% CI (-0.98, -0.07)], whereas, increased in Placebo-H [MD +0.74, 95% CI (0.28, 1.19)] with statistically significant MD between both the groups [MD −1.26, 95% CI (−1.90, −0.61)] (P = .0003). Additionally, anxiety was reduced after B coagulans LBSC treatment (Test-G) compared to Placebo-H. Differences between 2 group means were statistically significant for anxiety score [MD −1.00, 95% CI (−1.82, −0.18)] (P = .0177) (Table 3).
4.2. Change in gastrointestinal symptom's severity using IBS-SSS
Severity of gastrointestinal symptoms of IBS subjects was measured following the Rome Foundations IBS-SSS universal questionnaire.[40] Three severity categories were considered, viz, mild, moderate, and severe. At baseline, all subjects from Test-G arm reported mild (10) and moderate (9) level of severity, which was reduced after B coagulans LBSC treatment and maximum subjects reported relief from symptoms; no symptoms (12), mild (6) and moderate (1) at EOT (Fig. 2). Reduction in severity in Test-G was found statistically significant between baseline and EOT (P < .0001). Whereas, subjects from Placebo-H reported mild (14) and moderate (5) severity at baseline; and maximum subjects (78.94%) reported with mild severity of IBS at EOT (Fig. 2). Maximum cases “no symptoms” [63.16%, 95% CI (0.41–0.81)] were reported from Test-G, that is, B coagulans LBSC treated arm than Placebo-H [21.05%, 95% CI (0.08–0.43)] arm at EOT [ARR, −0.42 (95% CI, −0.64 to −0.11); RR, 3.00 (95%CI, 1.18–7.65); OR, 6.43 (95%CI, 1.52–27.24)].
Figure 2.
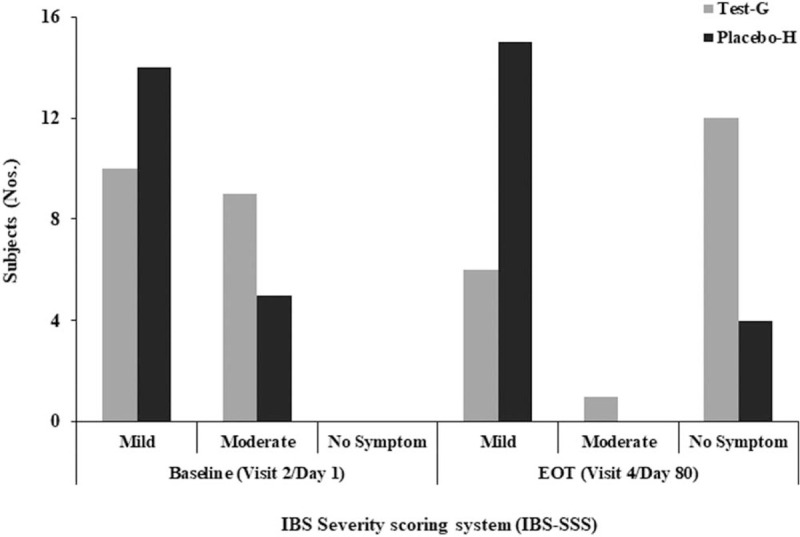
Change in IBS-SSS from baseline (visit 2/d 1) to end of treatment (visit 4/d 80) in Test-G and Placebo-H. The Rome Foundation IBS-SSS universal questionnaire has been considered with 3 severity categories, viz, mild, moderate, and severe as examined by the investigator. IBS-SSS = IBS-Severity Scoring System.
4.3. Change in stool consistency using Bristol stool form scale
Stool consistency of subjects from both Test-G and Placebo-H had following characteristics at base line as per Bristol form stool scale; inflammation, lacking fiber, normal to slight constipation, and highly constipated. However, stool consistency was significantly improved in interventional arm (Test-G) after B coagulans LBSC treatment compared to Placebo-H arm. In Test-G arm, at baseline and EOT, the number of subjects for inflammation was 2 and zero, for lacking fiber 7 and 1, normal stool consistency 2 and 12, slight constipation 2 and 6, for severe constipation 6 and zero, respectively (Fig. 3). None reported for severe constipation in Test-G arm at EOT. A quite similar stool consistency profile in Placebo-H arm reported as follows, 1 IBS patient reported with inflammation, 4 reported for lacking fiber, 6 reported for normal, 5 with slight constipation, and 3 have reported for severe constipation at EOT (Fig. 3). Maximum cases [63.15%, 95% CI (0.41–0.81)] of normal stool consistency were from Test-G compared to Placebo-H [31.57%, 95% CI (0.15–0.54)] at EOT [ARR, −0.32 (95% CI, −0.56 to −0.001); RR, 2.00 (95%CI, 0.95–4.22); OR, 3.71 (95%CI, 0.97–14.23)]. Improvement in stool consistency in Test-G arm was found statistically significant from baseline to EOT (P = .0002), whereas, it was insignificant in Placebo-H arm (P = .1989).
Figure 3.
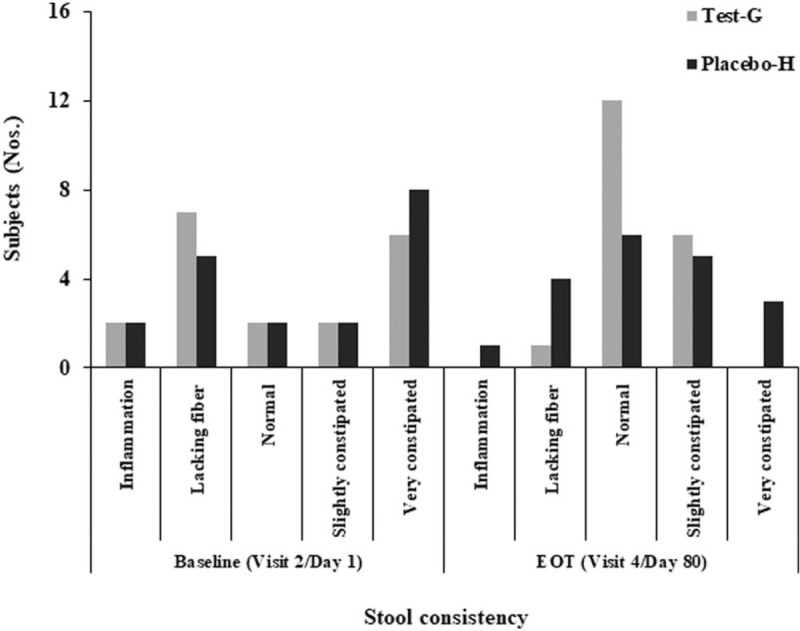
Change in stool consistency from baseline (visit 2/d 1) to end of treatment (visit 4/d 80) in Test-G and Placebo-H. The Bristol stool form scale is used to assess the stool consistency as reported by total number of subjects.
5. Secondary endpoints: safety evaluation
5.1. Assessment of adverse effect and serious adverse effects and systematic biomarkers
Vital examinations like pulse, respiratory rate, systolic blood pressure, diastolic blood pressure, and body temperature were carried out for all subjects at each visit by the principal investigator (Supplement: Table 1). No significant differences (P < .05) were observed in vital parameters in both Test-G and Placebo-H arm [Pmin = .059 (Fmax = 4.833) and Pmax = .962 (Fmin = 0.002)] among all visits and results remained within the normal range. No AE or SAE's were reported related to IP. Biochemical and hematological parameters were studied at baseline and EOT in both Test-G and Placebo-Harm. Biochemical parameters included serum glutamic-oxaloacetic transaminase, serum glutamic pyruvic transaminase, and creatinine whereas, hematological parameters comprised parameters like total red blood cells and total leucocyte count, eosinophils, basophils, neutrophils, lymphocytes, monocytes, hematocrit, erythrocyte sedimentation rate, and platelet counts (Supplement: Table 2). No statistically significant differences [Pmin = .0454 (95% CI, −2.69 to −0.03) and Pmax = .9857 (95% CI, 5.66–5.56)] were obtained between Test-G and Placebo-H arm both at baseline and EOT for hematological and biochemical parameters. Additionally, hematological and biochemical estimation results were within standard range of reference values. No significant visual changes were observed in the upper GI endoscopy of patients in both Test-G and Placebo-H arm at baseline and EOT.
5.2. Assessment of quality of life by Visual Analogue Scale assessments
Modulation in QoL parameters is visualized through two-dimensional PCoA in Test-G and Placebo-H arm at baseline and EOT. Processed responses clearly indicated improvement in parameters like abdominal pain, diarrhea, constipation, bloating and flatulence, vomiting and nausea, perception of mental well-being and influence on daily life in Test-G arm, that is, treated with B coagulans LBSC. However, Placebo-H showed either no or mild improvement or worsened unbearable pain at all pain scale. Vector scaling and singular value decomposition with imputation is used to calculate PCoA. In Test-G, X (PC1) and Y (PC2) – axis explains respectively 90.1% and 9.9% of the total variance; whereas, 47.4% and 19.4% in Placebo-H (Fig. 4).
Figure 4.
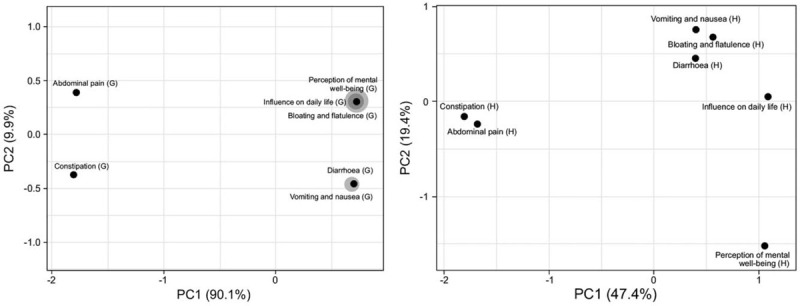
Clustering of multivariate data using principal component analysis analysis of responses for different variables of quality of life from Test-G (G) and Placebo-H (H) arms at end of treatment (visit 04). Variables considered are— abdominal pain, diarrhea, constipation, bloating and flatulence, vomiting and nausea, perception of mental well-being, and influence on daily life.
6. Discussion
Probiotics offer promising therapeutic solution for various health conditions including gastrointestinal illnesses like diarrhea, SIBO, IBS, IBD, ulcerative colitis, Crohn's disease, etc. Microbial dysbiosis, that is, imbalance in gastrointestinal microbiome is a known cause for prognosis of such gastrointestinal conditions.[24] Bacteriotherapy with probiotics helps in reprogramming the microbial balance of gut and restore healthy and complex host-microbiota interactions.[41] Clinical efficacies of probiotics are strain dependent and therapeutic effectiveness depends on specific clinical circumstances like digestive and nondigestive disorders.[29,42] Variability in study designs sometimes demonstrated variable probiotic efficacies in gastrointestinal ailments like IBS, IBD, and diarrhea.[21,29,43–46]
This CONSORT compliant, interventional, randomized, placebo-controlled trial (RCT) has evaluated the therapeutic impact of B coagulans LBSC on IBS in human subjects. B coagulans LBSC [DSM17654; GenBank: CP022701.1 & GenBank: ATW84696.1 (gyrB)] is previously reported as a genomically safe probiotic microorganism,[35] efficacious for treating acute diarrhea and abdominal discomforts[26] and can modulate gut microbiome in human subjects.[24]
The intervention of B coagulans LBSC (Test-G), compared to placebo-H, showed significant improvement in all gastrointestinal symptoms, notably in abdominal pain (mean change −1.74; P < .0001), headache (mean change −1.26; P = .0003), and stomach rumbling (mean change −1.05; P = .0210). In a previous trial on IBS using a symbiotic composition with B coagulans, Rogha et al[31] reported on reduction in abdominal pain and diarrhea but no change in constipation frequency after 12 weeks compared to placebo. An earlier study with B coagulans (GBI-30, 6086) also demonstrated relief from abdominal pain and bloating in IBS patients (n = 44) after 8 weeks of intervention.[29] Another study (n = 61) on the same strain (GBI-30, 6086) is reported to reduce average number of bowel movements per day in patients with diarrhea-predominant irritable bowel syndrome (IBS-D) compared to placebo. However, the study was not able to assess any severity scores and QoL due to large variability in baseline scores.[30] In a similar RCT (n = 52), Urgesi et al[47] demonstrated efficacy and the safety of Colinox (a medical device contained combination of simethicone and B coagulans) in the treatment of bloating predominant intrusive IBS. Both intragroup and intergroup results showed significant reduction of bloating, discomfort, and pain in Colinox group compared to placebo (P < .0001).
Current study demonstrated that B coagulans LBSC significantly improved other IBS-associated symptoms like bloating and cramping, diarrhea and constipation, nausea, vomiting, and anxiety from baseline compared to Placebo-H. Similarly, Sudha et al[48] reported significant improvement in stool consistency in children with IBS (n = 141) as well as reduction in abdominal discomfort, bloating, staining, urgency, incomplete evacuation, and passage of gas after 8 weeks of treatment with B coagulans Unique IS-2 (P ≤ .0001). An analogous study with Unique IS-2 strain on IBS adults (n = 136) also helped in reducing abdominal discomfort/pain intensity and increasing complete spontaneous bowel movements.[34] However, supplementation of B coagulans MTCC 5856 (2 billion per g) was reported to improve the clinical conditions of diarrhea predominant irritable bowel syndrome (IBS-D) (n = 36) treated for 90 days. Majeed et al[33] reported improvement in IBS-D clinical conditions included symptoms like bloating, vomiting, diarrhea, abdominal pain, and stool frequency (P < .01) compared to placebo group. The data presented in the present study and other published information, in general, establishes that B coagulans is efficacious in treating the IBS symptoms.
Comprehensiveness in improvement of DSFQ symptoms are often coupled with IBS-SSS category, which showed maximum cases of “no symptoms” (63.16%) after B coagulans LBSC treatment compared to mild and moderate severity observed in Placebo (21.05%) (P < .0001). This score 63.16% observed in this study is higher than other B coagulans strains as reported in other literature.[34,49] A multispecies probiotic containing 5 strains of lactic acid bacteria and bifidobacteria [Lactobacillus casei LMG101 and Lactobacillus plantarum CECT4528 (5 billion CFU), Bifidobacterium animalis subsp. lactis Bi1, B. breve Bl10, Bifidobacterium breve Bbr8 (10 billion CFU) was reported to significantly decrease the IBS-SSS as compared with placebo (P < .001) along with gastrointestinal symptom rating scale.[50] Similarly, a multistrain probiotic Bio-Kult (14 different bacterial strains) also showed an improvement of 69% IBS-SSS compared to placebo (47%) after 16 weeks.[51] Though a randomized triple-blind trial (n = 340) conducted by Lyra et al[49] which reported on relief of IBS severity in all three groups, viz, placebo, active low-dose (1 billion per g), and active high-dose (10 billion per g) of Lactobacillus acidophilus NCFM for 12 weeks. However, no significant difference was observed between placebo and active groups; and respectively, 28.4%, 25.0%, and 26.5% of volunteers considered adequately relieve from IBS symptoms. Noteworthy, alleviations from IBS severity by B coagulans LBSC (63.16%) was equally efficacious with B coagulans Unique IS2 which showed 60% decrease in severity score after 8 weeks.[34]
In consequence of the improvement in IBS-SSS, maximum patients treated with B coagulans LBSC attained normal stool consistency at EOT from baseline as well as in comparison to placebo. This observation is in line with a previous clinical study on B coagulans LBSC, where stool consistency was improved in patients with acute diarrhea by right balance in stool water content and regular complete spontaneous bowel movements.[26] Notably, stool consistency ensues due to improvement in stool texture, colors, odor, and bowel frequency which can be correlated with other clinically efficacious strains of B coagulans in related gastrointestinal ailments.[33,34,47,52] Further, amelioration of gastrointestinal symptoms along with improved stool frequency and intestinal transit are reported for other than spore forming Bacillus probiotics; which predominantly belong to genus Bifidobacterium and Lactobacillus.[53,54] Mechanisms of such effects were largely unexplored until the compositions of gut microbiota and their role are elucidated in various gastrointestinal dysbiosis including IBS. In a double blind RCT (n = 88), Yoon et al[54] have shown L plantarum LRCC5193 treatment improved stool consistency in IBS-C; may be due to increased abundance of L plantarum in gut which inversely hastened gut transit time and bolus movement. Increasing evidences support the notion of probiotic's favorable actions which is due to reprogramming of gut microbiota, elevation of anti-inflammatory microbial groups, competitive exclusion of pathogens, competitions for nutrients, production of short chain fatty acids, modulation of local epithelial immunity, and augmentation of tight junction proteins.[55,56] In a recent study, B coagulans LBSC is reported to modulate gut microbiome of IBS patients comprehended by whole genome metagenome analysis. B coagulans LBSC treatment showed positive modulation in gut microbiota, especially upregulation of phyla such as Actinobacteria and Firmicutes, whereas downregulation of Bacteroids, Proteobacteria, Streptophyta, and Verrucomicrobia. Subsequently, it altered various microbiota associated metabolic pathways to create the normalcy of gut microenvironment which can be put together for ameliorative effects of the strain as alternative therapeutic supplementation.[24]
Both Test-G and Placebo-H groups reveal no significant intergroup and intragroup difference in vital signs, hematological, and serum biochemical results. Simultaneously, no study drug-related AEs or SAEs were noted during the trial, which concludes that investigational product is safe to use. Further, a dose of 2 billion CFU of B coagulans LBSC for thrice a day was found to be safe and well-tolerated by the study participants for an 80 days study duration and it was with in recommended dose for healthy human (0.1 × 109–36.4 × 109 CFU/person/d) described in various studies.[57] The endoscopy of upper gastrointestinal tract of both the arms appeared normal with no sign of inflammation, which further supports safety of B coagulans LBSC.
PCoA plot showed highly modulated Quality of life (QoL) parameters at baseline and at EOT after B coagulans LBSC treatment. Contrarily, Placebo-H showed either mild or no improvement at EOT. In an earlier communication, B coagulans LBSC has been described as effective in improving QoL parameters in acute diarrhea patients.[26] Strains of B coagulans are largely known for modulating QoL in several disease conditions including subtypes of IBS, diarrhea, FGIDs, rheumatoid arthritis, etc in children and adults.[29–33,48,58,59] Mechanism behind the therapeutic health benefits by probiotic B coagulans LBSC is mainly the modulation of gut microbiome and associated metabolic normalcy, better digestion and immune homeostasis, and improvement in intestinal health.[24]
In conclusion, probiotic B coagulans LBSC [DSM17654] with a dose of 2 × 109 CFU for thrice a day was well tolerated, found safe, and showed significant alleviation in IBS-associated clinical symptoms like bloating/cramping, abdominal pain, diarrhea, constipation, stomach rumbling, nausea, vomiting, headache, and anxiety, compared to placebo group. B coagulans LBSC treatment improved stool consistency, decreased the severity, and conferred better QoL to IBS patients. No report of adverse and serious adverse effects, no usage of rescue medicine further confirmed the safety and efficacy of B coagulans LBSC; which could be used as a therapeutic supplement in the management of IBS pathophysiology and improving QoL in adults.
Acknowledgments
The authors are grateful and would like to acknowledge Mr. V.L. Rathi and Mr. M. Kabra at Advanced Enzyme Technologies Ltd. for supporting this study.
Author contributions
Conceptualization: Anil K. Gupta, Chiranjit Maity.
Formal analysis: Chiranjit Maity.
Investigation: Chiranjit Maity.
Validation: Anil K. Gupta.
Writing – original draft: Chiranjit Maity.
Writing – review & editing: Anil K. Gupta, Chiranjit Maity.
Supplementary Material
Footnotes
Abbreviations: AE = adverse events, BSFC = Bristol stool form scale, DSM = Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, EOT = end of treatment, FGID = functional gastrointestinal disorder, FODMAPs = fermentable oligosaccharides, disaccharides, monosaccharides and polyols, IBD = inflammatory bowel disease, IBS = irritable bowel syndrome, IBS-SSS = IBS-Severity Scoring System, IP = investigational product, MD = mean difference, PCoA = principal component analysis, PP = per-protocol, QoL = quality of life, RCT = randomized control trials, SAE = serious adverse events.
How to cite this article: Gupta AK, Maity C. Efficacy and safety of Bacillus coagulans LBSC in irritable bowel syndrome: a prospective, interventional, randomized, double-blind, placebo-controlled clinical study [CONSORT Compliant]. Medicine. 2021;100:3(e23641).
This study was solely funded by Advanced Enzyme Technologies Limited, Thane (India).
Trial registration: This trial was conducted in compliance with the applicable ethical standards Harmonized Tripartite Guidelines for Good Clinical Practice (GCP), the relevant sections of Good Laboratory Practice (GLP), local laws and regulations and the provisions of the Declaration of Helsinki. The EC approvals were obtained from sites. The trial was registered with Clinical Trials Registry - India (CTRI) [Reference No.: CTRI/2018/02/011654 and Dated: 01/02/2018] before patients enrolment begun.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article [and its supplementary information files].
VAS = Visual Analogue Scale.
Visit 01 was the screening day.
Visit 02 was the baseline day, that is, first day of treatment.
Visit 03 was the fortieth day of treatment.
Visit 04 was the eightieth day of treatment, that is, end of treatment (EOT).
Visit 05 was the follow-up visit.
Visit 06 was the unscheduled visit.
Symptoms considered were bloating and cramping, abdominal pain, diarrhea and constipation, stomach rumbling, nausea, vomiting, headache, and anxiety. Intergroup mean difference [(Test-G) – (Placebo-H)] for all the symptoms was analyzed through ANOVA and 95% confidence interval (CI) estimation.
References
- [1].Talley NJ. A unifying hypothesis for the functional gastrointestinal disorders: really multiple diseases or one irritable gut? Rev Gastroenterol Disord 2006;6:72–8. [PubMed] [Google Scholar]
- [2].Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med 2017;6:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Babu S. A young woman with diarrhea predominant IBS showing improvement with probiotics. Med Update 2019;27:16–8. [Google Scholar]
- [4].Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hadjivasilis A, Tsioutis C, Michalinos A, et al. New insights into irritable bowel syndrome: from pathophysiology to treatment. Ann Gastroenterol 2019;32:554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mayer EA. Clinical practice. Irritable bowel syndrome. N Engl J Med 2008;358:1692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chong PP, Chin VK, Looi CY, et al. The microbiome and irritable bowel syndrome - a review on the pathophysiology, current research and future therapy. Front Microbiol 2019;10:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Staudacher HM, Irving PM, Lomer MCE, et al. Mechanisms and efficacy of dietary FODMAP restriction in IBS. Nat Rev Gastroenterol Hepatol 2014;11:256–66. [DOI] [PubMed] [Google Scholar]
- [9].Frissora CL, Cash BD. Review article: the role of antibiotics vs. conventional pharmacotherapy in treating symptoms of irritable bowel syndrome. Aliment Pharmacol Ther 2007;25:1271–81. [DOI] [PubMed] [Google Scholar]
- [10].Basseri RJ, Weitsman S, Barlow GM, et al. Antibiotics for the treatment of irritable bowel syndrome. Gastroenterol Hepatol 2011;7:455–93. [PMC free article] [PubMed] [Google Scholar]
- [11].Molina-Infante J, Serra J, Fernandez-Banares F, et al. The low-FODMAP diet for irritable bowel syndrome: Lights and shadows. Gastroenterol Hepatol 2016;39:55–65. [DOI] [PubMed] [Google Scholar]
- [12].Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 2001;1:101–14. [DOI] [PubMed] [Google Scholar]
- [13].Quigley EMM. Bacterial flora in irritable bowel syndrome: role in pathophysiology, implications for management. J Dig Dis 2007;8:2–7. [DOI] [PubMed] [Google Scholar]
- [14].Lacy BE. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med 2016;9:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jernberg C, Löfmark S, Edlund C. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiol 2010;156:3216–23. [DOI] [PubMed] [Google Scholar]
- [16].Villarreal AA, Aberger FJ, Benrud R, et al. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. Wis Med J 2012;111:17–20. [PubMed] [Google Scholar]
- [17].Menees S, Chey W. The gut microbiome and irritable bowel syndrome. F1000Res 2018;7: F1000 Faculty Rev-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].James SC, Fraser K, Young W, et al. Gut microbial metabolites and biochemical pathways involved in irritable bowel syndrome: effects of diet and nutrition on the microbiome. J Nutr 2019; 10.1093/jn/nxz302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterol 2019;157:97–108. [DOI] [PubMed] [Google Scholar]
- [20].Black CJ, Ford AC. Probiotics for treating irritable bowel syndrome: are bugs the best drugs? Gastroenterol 2018;155:2019–21. [DOI] [PubMed] [Google Scholar]
- [21].Food and Agriculture Organization and World Health Organization Expert Consultation. Evaluation of health and nutritional properties of powder milk and live lactic acid bacteria. Córdoba, Argentina. 2001. [Google Scholar]
- [22].Dale HF, Rasmussen SH, Asiller OO, et al. Probiotics in irritable bowel syndrome: an up-to-date systematic review. Nutrients 2019;11:2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jurenka JS. Bacillus coagulans: monograph. Altern Med Rev 2012;17:76–81. [PubMed] [Google Scholar]
- [24].Maity C, Gupta AK, Saroj DB, et al. Impact of a gastrointestinal stable probiotic supplement Bacillus coagulans LBSC on human gut microbiome modulation. J Diet Suppl 2020; 10.1080/19390211.2020.1814931 [DOI] [PubMed] [Google Scholar]
- [25].Mu Y, Cong Y. Bacillus coagulans and its applications in medicine. Benef Microbes 2019;10:679–88. [DOI] [PubMed] [Google Scholar]
- [26].Maity C, Gupta AK. A prospective, interventional, randomized, double-blind, placebo-controlled clinical study to evaluate the efficacy and safety of Bacillus coagulans LBSC in the treatment of acute diarrhea with abdominal discomfort. Eur J Clin Pharmacol 2019;75:21–31. [DOI] [PubMed] [Google Scholar]
- [27].Hungin APS, Mulligan C, Pot B, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice – an evidence based international guide. Aliment Pharmacol Ther 2013;38:864–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].La Rosa M, Bottaro G, Gulino N, et al. Prevention of antibiotic-associated diarrhea with Lactobacillus sporogens and fructo-oligosaccharides in children. A multicentric double-blind vs placebo study. Minerva Pediatr 2003;55:447–52. [PubMed] [Google Scholar]
- [29].Hun L. Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad Med 2009;121:119–24. [DOI] [PubMed] [Google Scholar]
- [30].Dolin BJ. Effects of a proprietary Bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods Find Exp Clin Pharmacol 2009;31:655–9. [DOI] [PubMed] [Google Scholar]
- [31].Rogha M, Zahiri-Esfahani M, Zargarzadeh AH. The efficacy of a synbiotic containing Bacillus coagulans in treatment of irritable bowel syndrome: a randomized placebo-controlled trial. Gastroenterol Hepatol Bed Bench 2014;7:156–63. [PMC free article] [PubMed] [Google Scholar]
- [32].Saneian H, Pourmoghaddas Z, Roohafza H, et al. Synbiotic containing Bacillus coagulans and fructo-oligosaccharides for functional abdominal pain in children. Gastroenterol Hepatol Bed Bench 2015;8:56–65. [PMC free article] [PubMed] [Google Scholar]
- [33].Majeed M, Nagabhushanam K, Natarajan S, et al. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant irritable bowel syndrome: a double blind randomized placebo controlled pilot clinical study. Nutr J 2016;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Madempudi RS, Ahire JJ, Neelamraju J, et al. Randomized clinical trial: the effect of probiotic Bacillus coagulans Unique IS2 vs. placebo on the symptoms management of irritable bowel syndrome in adults. Sci Rep 2019;9:12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Saroj DB, Gupta AK. Genome based safety assessment for Bacillus coagulans strain LBSC (DSM 17654) for probiotic application. Int J Food Microbiol 2020;318:108523. [DOI] [PubMed] [Google Scholar]
- [36].World Medical Association (WMA) Declaration of Helsinki. Ethical principles for medical research involving human subjects. 52nd WMA general assembly. Scotland, Edinburgh. 2000. [Google Scholar]
- [37].International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for good clinical practice [E6(R1)], June 1996. [Google Scholar]
- [38].Indian Council of Medical Research (ICMR). Ethical guidelines for biomedical research on human participants. New Delhi. 2006. Available at: http://www.cns.iisc.ac.in/wordpress/wpcontent/uploads/2017/01/ethical_guidelines.pdf [access date March 2, 2018]. [Google Scholar]
- [39].Azpiroz F, Guyonnet D, Donazzolo Y, et al. Digestive symptoms in healthy people and subjects with irritable bowel syndrome: validation of symptom frequency questionnaire. J Clin Gastroenterol 2015;49:e64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Drossman DA, Chang L, Bellamy N, et al. Severity in irritable bowel syndrome: a Rome foundation working team report. Am J Gastroenterol 2011;106:1749–59. [DOI] [PubMed] [Google Scholar]
- [41].Azad MAK, Sarker M, Li T, et al. Probiotic species in the modulation of gut microbiota: an overview. Biomed Res Int 2018;2018:9478630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bubnov RV, Babenko LP, Lazarenko LM, et al. Specific properties of probiotic strains: relevance and benefits for the host. EPMA J 2018;9:205–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Aragon G, Graham DB, Borum M, et al. Probiotic therapy for irritable bowel syndrome. Gastroenterol Hepatol 2010;6:39–44. [PMC free article] [PubMed] [Google Scholar]
- [44].Dai C, Zheng CQ, Jiang M, et al. Probiotics and irritable bowel syndrome. World J Gastroenterol 2013;19:5973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sniffen JC, McFarland LV, Evans CT, et al. Choosing an appropriate probiotic product for your patient: an evidence-based practical guide. PLoS One 2018;13:e0209205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Brüssow H. Probiotics and prebiotics in clinical tests: an update. F1000Res 2019;8: F1000 Faculty Rev-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Urgesi R, Casale C, Pistelli R, et al. A randomized double-blind placebo-controlled clinical trial on efficacy and safety of association of simethicone and Bacillus coagulans (Colinox®) in patients with irritable bowel syndrome. Eur Rev Med Pharmacol Sci 2014;18:1344–53. [PubMed] [Google Scholar]
- [48].Sudha MR, Jayanthi N, Aasin M, et al. Efficacy of Bacillus coagulans Unique IS2 in treatment of irritable bowel syndrome in children: a double blind, randomised placebo controlled study. Benef Microbes 2018;9:563–72. [DOI] [PubMed] [Google Scholar]
- [49].Lyra A, Hillilä M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol 2016;22:10631–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Francavilla R, Piccolo M, Francavilla A, et al. Clinical and microbiological effect of a multispecies probiotic supplementation in celiac patients with persistent IBS-type symptoms: a randomized, double-blind, placebo-controlled, multicenter trial. J Clin Gastroenterol 2019;53:e117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ishaque SM, Khosruzzaman SM, Ahmed DS, et al. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea predominant irritable bowel syndrome. BMC Gastroenterol 2018;18:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Minamida K, Nishimura M, Miwa K, et al. Effects of dietary fiber with Bacillus coagulans lilac-01 on bowel movement and fecal properties of healthy volunteers with a tendency for constipation. Biosci Biotechnol Biochem 2015;79:300–6. [DOI] [PubMed] [Google Scholar]
- [53].Miller LE, Ouweh AC, Ibarra A. Effects of probiotic-containing products on stool frequency and intestinal transit in constipated adults: systematic review and meta-analysis of randomized controlled trials. Ann Gastroenterol 2017;30:629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yoon JY, Cha JM, Oh JK, et al. Probiotics ameliorate stool consistency in patients with chronic constipation: a randomized, double-blind, placebo-controlled study. Dig Dis Sci 2018;63:2754–64. [DOI] [PubMed] [Google Scholar]
- [55].O’Toole PW, Cooney JC. Probiotic bacteria influence the composition and function of the intestinal microbiota. Interdiscip Perspect Infect Dis 2008;175285:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kho ZY, Lal SK. The human gut microbiome – a potential controller of wellness and disease. Front Microbiol 2018;9:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].GRAS Notice (GRN) No. 597.Bacillus coagulans SNZ1969 spore preparation. Available at: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=597 [access date February 2018). 2015. in press. [Google Scholar]
- [58].Kalman DS, Schwartz HI, Alvarez P, et al. A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol 2009;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Mandel DR, Eichas K, Holmes J. Bacillus coagulans: a viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement Altern Med 2010;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


