Supplemental Digital Content is available in the text
Keywords: ATRIA score, atrial fibrillation, CHA2DS2-VASc score, CHADS2 score, secondary prevention, stroke
Abstract
The performance of scoring systems for risk stratification in patients with atrial fibrillation (AF) was not validated well in patients with stroke. The purpose of this study was to evaluate whether the risk scoring systems predict vascular outcomes in stroke patients with AF.
Data were obtained from a nationwide multicenter registry for acute stroke with AF from January 1, 2013, to December 31, 2015. We investigated the predictive power of the CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke scores in stroke patients with AF. The subjects were further stratified into groups according to treatment with or without oral anticoagulants (OACs).
A total of 3112 stroke with AF subjects were included. The rate of recurrent ischemic stroke and any stroke were not associated with the CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke risk scores. The risks of death and major adverse cerebrovascular and cardiovascular events (MACEs) increased sequentially with the increase of each risk score in OAC group. (the range of C-index 0.544–0.558 for recurrent ischemic stroke; 0.523–0.537 for any stroke; 0.580–0.597 for death; 0.564–0.583 for MACEs). However, in the group treated with OACs, all risk scores were significantly associated with the risk of MACEs. The C-statistics of the 4 scoring systems were 0.544 to 0.558, 0.523 to 0.537, 0.580 to 0.597, 0.564 to 0.583, respectively, for recurrent ischemic stroke, any stroke, death, and MACEs.
The performance of the CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke risk scores for the prediction of recurrent stroke was unsatisfactory in stroke patients with AF whereas the performance for the prediction of recurrent stroke was not MACEs or death was good. A new risk stratification scheme that is specific for secondary stroke prevention in the AF population is needed.
1. Introduction
A good prognostication for thromboembolic events in patients with atrial fibrillation (AF) is essential because AF is a significant cause of ischemic stroke with a 5-fold increased risk.[1] Various clinical risk scoring systems for the thromboembolic risk in patients with AF were developed and validated, such as the CHADS2,[2] CHA2DS2-VASc,[3] and ATRIA scores.[4] These scoring systems developed to predict thromboembolic risk and provide a guidance for decision for anticoagulation. Recent clinical practice guidelines are recommending these scoring systems as a guidance for decision of oral anticoagulant (OAC) use in AF patients.[5,6] However, their performance has rarely been validated in stroke patients with AF, especially in real-world setting in the era of newer OAC.
We also analyzed the validity of the Essen stroke score to verify the validity of other scores in this study. The Essen stroke risk score, which was introduced in CAPRIE trial[7] and validated using the data set of the European Stroke Prevention study II[8] is used for prediction of recurrent stroke and combined cerebrovascular events in stroke patient without AF.[9] Therefore, we assumed that the performance of Essen stroke risk score is lower than those of CHADS2, CHA2DS2-VASc, and ATRIA scores for the prediction of vascular events in patients with AF. If the assumption is not correct, then the exclusive value of CHADS2, CHA2DS2-VASc, and ATRIA scores for the prediction of vascular events in AF patients will be unacceptable.
Therefore, we investigated the validity and performance of each scoring system in predicting vascular events along with the incidence rate of vascular events using data obtained from the K-ATTENTION (Korean ATrial fibrillaTion EvaluatioN regisTry in Ischemic strOke patieNts) study.
2. Methods
2.1. Registry data sources
The K-ATTENTION study is a multicenter, cohort study by merging of prospectively collected stroke registries from 11 tertiary hospitals in 5 provinces of South Korea to investigate the diagnosis, treatment, and prognosis of acute stroke patients with AF in a real-world clinical setting. Details of the K-ATTENTION study was described in elsewhere.[10] In brief, subjects over 20 years of age, diagnosed with acute ischemic stroke with AF within 7 days from the onset were included consecutively from each participating center between January 1, 2013, to December 31, 2015. Subjects who were not adequately screened for stroke and arrhythmia and without evidence of cerebral infarction on brain images were excluded. The study protocol was reviewed and approved by the institutional review board of each participating center. Because of the retrospective nature of this study, the ethical board of each center exempted written informed consent.
2.2. Clinical data collection
The K-ATTENTION study collects demographic data (age, sex), physical examinations, vascular risk factors, previous medication history, and vascular outcomes. Vascular outcomes included recurrent ischemic stroke, any stroke, death, and major adverse cerebrovascular and cardiovascular events (MACEs). MACE was defined as a composite of any stroke (ischemic or hemorrhagic), myocardial infarction and death. The 11 tertiary hospitals followed above definition for collect vascular outcomes. We obtained data including date, type, and causes of stroke recurrence, mortality from well-trained research nurses or neurology specialists at each hospital. If the patient did not visit hospital, clinical information was obtained from the patient or their care-givers by telephone interview or by review of medical records. As 1 site did not provide long-term vascular outcome data, all subjects in that site (n = 101) were excluded in this study. And, the censoring date was set at December 31, 2016 or the last date when the investigator had contact with the subject.
2.3. Data management and quality control
All data were collected and uploaded via a web-based electronic data capturing system. All investigators accessed this secure database system and registered mandatory variables related to the answer of primary objects. The collected data were monitored and audited by the quality control team.
2.4. The risk scoring systems
We calculated the CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke risk scores for each subject using the data thus obtained. The CHADS2 score includes congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, prior stroke, or transient ischemic attack (2 points), resulting in a maximum score of 6 points. The CHA2DS2-Vasc score includes congestive heart failure, hypertension, age ≥ 75 years (2 points), diabetes mellitus, previous stroke, or transient ischemic attack (2 points), vascular disease, age 65 to 74 years, and sex (female), resulting in a maximum score of 9 points. The ATRIA score with the previous stroke includes age < 65 years (8 points), age 65 to 84 years (7 points), age ≥ 85 years (9 points), sex (female), congestive heart failure, hypertension, diabetes mellitus, proteinuria, estimated glomerular filtration rate <45 mL/min per 1.73 m2, or end-stage renal disease requiring renal replacement therapy, resulting in a maximum score of 15 points. The Essen stroke risk score includes age 65 to 75 years, age ≥ 75 years (2 points), hypertension, diabetes mellitus, myocardial infarction, prior stroke or transient ischemic attack, smoking, peripheral arterial disease, and other cardiovascular disease (except myocardial infarction and AF), resulting in a maximum score of 9 points.
Based on information in the registries, we calculated the CHADS2 score, CHA2DS2-VASc score, ATRIA score, and Essen stroke risk scores for all subjects at the time of admission for the stroke. Subjects with CHADS2 score ≥ 5 were merged into 1 category, and subjects with CHA2DS2-VASc score 2 and 3 were merged into 1 category, as were those with score ≥7; subjects with ATRIA score 7 and 8 were merged into 1 group, as well as those with score ≥12; subjects with Essen stroke risk score 0 and 1 were combined into 1 category, and so were those with score ≥5.
2.5. Statistical analysis
The data are expressed as mean value and standard deviation, for continuous data, and frequency and percentage for categorical data. For comparisons between the groups, we used Student t test and ANOVA for continuous data and Chi-squared and Fisher exact tests for categorical data. Using the Cox regression model, considering death as a competing risk, the predictive value of the CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke risk scores to the vascular outcome was investigated. Performance for the predictability for vascular outcomes was presented as overall C-index with 95% confidence interval and time-dependent C-index for each scoring system. A separate data management committee managed all data, and an external team performed statistical analyses. All statistical analyses were performed using SAS 9.4 (SAS Institute, NC) and STATA 13 (StataCorp, TX).
3. Results
3.1. Study population
A total of 3112 stroke with AF subjects (mean age 73.5 ± 0.2; 48.6% female) were included, after exclusion of 101 subjects without data on vascular events, among total 3213 subjects included in K-ATTENTION study. Mean duration of follow up was 1399.6 ± 15.8 days (95% confidence interval [CI] 1368.7–1430.5). The baseline characteristics of the subjects are shown in Table 1. A total of 2519 subjects were prescribed OAC after stroke for secondary stroke prevention, while 593 were not. Subjects without OAC treatment, compared to subjects with OAC treatment, were older (76.5 ± 0.4 vs 72.8 ± 0.2 years.), more often women (55.1% vs 47%), had higher initial National Institute of Health Stroke Scale score (13.6 ± 0.3 vs 8.3 ± 0.1), and higher modified Rankin Scale at 90 days (3.7 ± 0.1 vs 2.7 ± 0.0).
Table 1.
Baseline Characteristics.
| OACs (–) (n = 593) | OACs (+) (n = 2519) | P | Total (n = 3112) | |
| Age, yr | 76.5 ± 0.4 | 72.8 ± 0.2 | <.01 | 73.5 ± 0.2 |
| >65 | 73 (12.3) | 455 (18.1) | 528 (17.0) | |
| 65–74 | 152 (25.6) | 824 (32.7) | 976 (31.4) | |
| ≥75 | 368 (62.1) | 1240 (49.2) | 1608 (51.7) | |
| Sex, women | 327 (55.1) | 1335 (53.0) | <.01 | 1511 (48.6) |
| Risk factors | ||||
| Previous Stroke | 207 (34.9) | 839 (33.3) | .46 | 1046 (33.6) |
| Congestive heart failure | 21 (3.5) | 110 (4.4) | .37 | 131 (4.2) |
| Hypertension | 417 (70.3) | 1737 (69.0) | .52 | 2154 (69.2) |
| Diabetes mellitus | 157 (26.5) | 674 (26.8) | .89 | 831 (26.7) |
| Dyslipidemia | 107 (18.0) | 640 (25.4) | <.01 | 747 (24.0) |
| Coronary artery disease | 68 (11.5) | 332 (13.2) | .26 | 400 (12.9) |
| Peripheral artery disease | 6 (1.0) | 32 (1.3) | .61 | 38 (1.2) |
| Initial NIHSS | 13.59 ± 0.3 | 8.28 ± 0.1 | <.01 | 9.29 ± 0.1 |
| mRS at 3 months | 3.73 ± 0.1 | 2.65 ± 0.0 | <.01 | 2.88 ± 0.0 |
| Discharge medication | ||||
| Non-antiplatelet | 346 (58.4) | 1964 (78.0) | 2310 (74.2) | |
| Mono antiplatelet | 178 (30.0) | 474 (18.8) | 652 (21.0) | |
| Dual antiplatelet | 69 (11.6) | 81 (3.2) | 150 (4.8) | |
| Warfarin | 0 (0) | 1818 (72.2) | 1818 (58.4) | |
| NOAC | 0 (0) | 701 (27.8) | 701 (22.5) |
3.2. Distribution of CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke risk scores
The distribution of risk scores according to the various systems is shown in Table 2. The mean CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke risk scores were significantly different between the subjects with OAC treatment (3.4 ± 0.0, 4.8 ± 0.0, 9.1 ± 0.0, and 3.1 ± 0.0, respectively) and those without OAC treatment (3.6 ± 0.0, 5.1 ± 0.1, 9.4 ± 0.1, and 3.3 ± 0.1, respectively).
Table 2.
Distribution of Subjects for Each Score Category in CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke risk scores, According to oral anticoagulation treatment.
| OACs (–), (n = 593) n (%) | OACs (+), (n = 2519) n (%) | Total (n = 3112) n (%) | |
| CHADS2 | |||
| Score 2 | 74 (12.5) | 431 (17.1) | 505 (16.2) |
| Score 3 | 188 (31.7) | 881 (35.0) | 1069 (34.4) |
| Score 4 | 236 (39.8) | 926 (36.8) | 1162 (37.6) |
| Score ≥ 5 | 95 (16.0) | 281 (11.2) | 376 (12.1) |
| CHA2DS2-VASc | |||
| Score 2 or 3 | 74 (12.5) | 459 (18.2) | 533 (17.1) |
| Score 4 | 106 (17.9) | 526 (20.9) | 632 (20.3) |
| Score 5 | 139 (23.4) | 652 (25.9) | 791 (25.4) |
| Score 6 | 193 (32.5) | 608 (24.1) | 801 (25.7) |
| Score ≥ 7 | 81 (13.7) | 274 (10.9) | 355 (11.4) |
| ATRIA | |||
| Score 7 or 8 | 156 (26.5) | 810 (32.8) | 966 (31.6) |
| Score 9 | 172 (29.3) | 829 (33.6) | 1001 (32.8) |
| Score 10 | 133 (22.6) | 477 (19.3) | 610 (20.0) |
| Score 11 | 66 (11.2) | 236 (9.6) | 302 (9.9) |
| Score ≥ 12 | 61 (10.4) | 114 (4.6) | 175 (5.7) |
| Essen stroke | |||
| Score 0 or 1 | 43 (7.3) | 332 (13.2) | 375 (12.1) |
| Score 2 | 114 (19.2) | 479 (19.0) | 593 (19.1) |
| Score 3 | 196 (33.1) | 744 (29.5) | 940 (30.2) |
| Score 4 | 144 (24.3) | 597 (23.7) | 741 (23.8) |
| Score ≥ 5 | 96 (16.2) | 367 (14.6) | 463 (14.9) |
3.3. Association between vascular outcomes and CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke risk scores
Supplemental Table 1, presents the cumulative incidence rates for each outcome. The annualized incidence rates for all outcomes were higher in the non-OAC group than the OAC group.
There was no significant association between the risk of recurrent ischemic stroke or any stroke with the 4 scores (Fig. 1 and Supplemental Table 2). The trend of negative association was consistent in both the OAC group and non-OAC group. However, the risks of death and MACE increased sequentially with the increase of each risk score. The association between risk scores and the risk of death or MACE was different between the OAC and non-OAC group. All 4 scores were predictive death and MACE in the OAC group. However, in the non-OAC group, the CHA2DS2-VASc score (P = .01) and the ATRIA score (P = .03) for death, and the CHA2DS2-VASc score (P = .01) and the ATRIA (P = .02) scores for MACE were predictive. OAC treatment had a significant interaction with the CHADS2, ATRIA, and Essen stroke risk scores in terms of predicting death (P < .01, P < .01, and P < .01, respectively) and with the CHADS2 and Essen stroke risk scores in terms of predicting MACE (P < .01 and P = .03, respectively).
Figure 1.
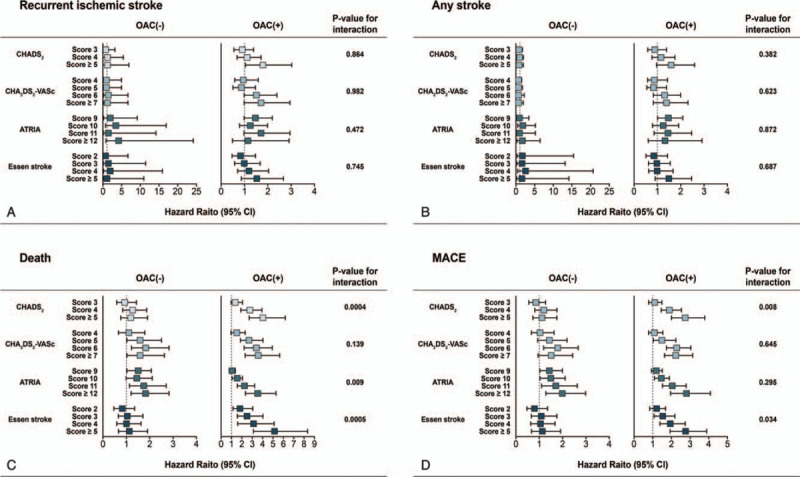
Forest plots for competing risk analysis showing hazard ratios of outcomes stratified by oral anticoagulant treatment history and risk scoring systems.
The overall performance of all scoring systems for the prediction of recurrent ischemic stroke, any stroke, death, and MACE were unsatisfactory (0.54–0.56 for recurrent stroke, 0.52–0.54 for any stroke, 0.58–0.60 for death, and 0.56–0.58 for MACE, respectively; Table 3). Figure 2, which shows the C-index of each scoring system according to the time points, revealed no sizable differences among the scoring systems. Each score in the OAC group showed higher performance (C-index: 0.59–0.63 for death and 0.58–0.60 for MACE; Supplemental Table 3, Supplemental Fig. 1 and 2) than that in the non-OAC group (C-index: 0.51–0.53 for death and 0.51–0.54 for MACE).
Table 3.
Overall C-index for the 4 scoring systems and vascular outcomes.
| C-index (95% confidence interval) | ||||
| CHADS2, | CHA2DS2-VASc | ATRIA | Essen stroke risk | |
| Recurrent Ischemic stroke | 0.54 (0.50–0.59) | 0.56 (0.51–0.60) | 0.55 (0.51–0.60) | 0.55 (0.50–0.60) |
| Any stroke | 0.53 (0.49–0.57) | 0.52 (0.48–0.57) | 0.54 (0.47–0.58) | 0.53 (0.48–0.57) |
| Death | 0.59 (0.60–0.62) | 0.60 (0.58–0.62) | 0.59 (0.56–0.61) | 0.58 (0.56–0.60) |
| MACE | 0.58 (0.57–0.60) | 0.58 (0.56–0.60) | 0.58 (0.57–0.60) | 0.56 (0.54–0.59) |
Figure 2.
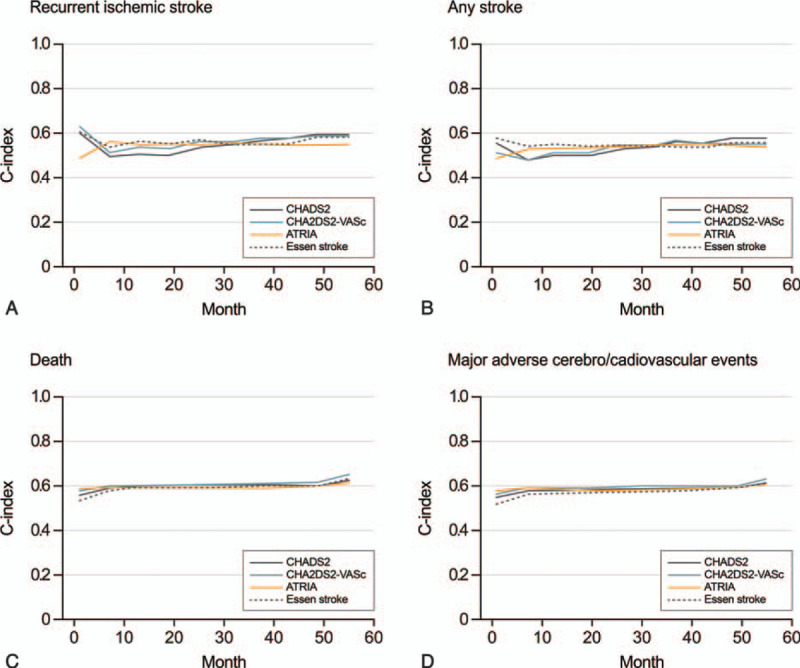
C-Statistics for the 4 scoring systems and vascular outcomes.
4. Discussion
In this study, we investigated the performance of the CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke risk scores in predicting recurrent ischemic stroke, any stroke, death, and MACE in stroke patient with AF, using a nationwide multicenter registry data. The overall performance of all the scoring systems for each outcome was unsatisfactory. In particular, these scoring systems showed poor performance in predicting stroke recurrence. Although these scores showed a better performance in the prediction of death and MACE, the CHADS2, CHA2DS2-VASc, ATRIA scores did not outperform the Essen stroke risk score. Besides, the performance of the CHADS2, ATRIA, and Essen stroke risk scores for death and MACE were different between the OAC-treated and the non-OAC-treated groups.
Previous studies revealed that the CHADS2, CHA2DS2-VASc, ATRIA scores performed reasonably well in the prediction of vascular events and mortality in patients with AF. [11–13] So, in clinical practice, the CHADS2, CHA2DS2-VASc, ATRIA scores have been commonly used for the stratification of future stroke risk in patients with AF. Moreover, current clinical practice guidelines recommend the use of OAC in AF patients with moderate to high risk of a thromboembolic event, based on the CHA2DS2-VASc score.[6] However, the performance of the CHADS2, CHA2DS2-VASc, ATRIA scores in stroke patients with AF was unsatisfactory than our expectation. The initial validation studies for CHADS2,[2] CHA2DS2-VASc,[3] and ATRIA[4] reported that C-statistics for predicting thromboembolic event were 0.82, 0.61, and 0.70, respectively. Recent meta-analyses reported somewhat lower C-statistics for each score than those in initial validation studies (0.58–0.70 for CHA2DS2-VASc and 0.63–0.69 for ATRIA scores).[14,15] However, our results showed even more lower performance than the results of meta-analysis (0.54–0.56 for ischemic stroke, 0.58–0.60 for death, and 0.56–0.58 for MACE, respectively). Considering that the decision whether to use OAC or not depends on the CHA2DS2-VASc score, this predictive value for recurrent stroke seems too low to be acceptable.
The condition of the subjects or geographical region could affect the performance of the CHADS2 and CHA2DS2-VASc scores. The subject of this study were acute stroke patients who are at a higher risk of death and co-morbidity, that can make the performance of the scores weaken. In the Asian population, the performance of the scores was lower than the non-Asian population. A Chinese study reported that the C-statistics of the CHADS2 and CHA2DS2-VASc scores in the China National Stroke Registry were 0.53 to 0.55 for predicting recurrent ischemic stroke, and 0.53 to 0.57 for predicting death in ischemic stroke patients with AF.[16]
In contrast to their poor performance for recurrent ischemic stroke or any stroke, all scores showed moderate performance for death and MACE. Previous studies on stroke patients with AF also reported that a high CHA2DS2-VASc score reflected poor short-term outcome, and higher CHADS2 and CHA2DS2-VASc scores were associated with severe stroke and a worse clinical course.[17,18] Based on another Korean Study, high-risk CHADS2 scores were associated with worse neurological outcomes at discharge, and increased long-term mortality, especially due to vascular causes in stroke patients with AF.[19] Our results are in agreement with these previous studies.
Another interesting finding was that OAC treatment had considerable influence on the performance of each scoring system, especially for the prediction of death or MACE. The performance of every score, except the CHA2DS2-VASc, improved when the subjects were confined to the OAC-treated group. In contrast, the performance of every score was unsatisfactory in the non-OAC-treated group. In clinical practice, the decision to initiate OAC in acute stroke patients can be influenced not only by the risk of stroke or thromboembolism based on stratification by risk scores, but also by other factors such as medical comorbidity, neurological status, or the risk of intracerebral hemorrhage assessed by neuroimaging. Therefore, application of these scores to non-OAC-treated patients seems inappropriate, because the primary target of these scores was to predict stroke or thromboembolic risk in AF patients precisely. However, their performance, in this study, was better for death and MACE.
The 4 scores shared a similar performance in this study because they all share 4 main items, including age, hypertension, diabetes, and previous stroke. Considering that all subjects in this study were acute stroke patients, they had high-risk of stroke or thromboembolism. For example, the CHA2DS2-VASc score outperforms CHADS2 in discriminating very low-risk subjects.[5] However, such discriminative power is not useful in this study, because all subjects were rated at least 2 points by the CHADS2 or CHA2DS2-VASc scores. Furthermore, CHADS2 and CHA2DS2-VASc scores did not outperform the Essen stroke risk score. Vice versa, it was reported that, using the Danish Stroke Registry, the C-statistics of both the Essen and CHA2DS2-VASc scores for predicting recurrent stroke in patients without AF was 0.54 to 0.56.[20] In the Athens Stroke Registry, the CHADS2 and the CHA2DS2-VASc scores were predictive of recurrent stroke and death in non-AF stroke patients.[9] To summarize, all scoring systems, including the Essen stroke risk score, are similarly predictive of the risk of stroke or other vascular events, irrespective of AF, implying that these scoring systems are not specific for AF patients. Therefore, a newer scoring system is required, truly specific for AF patients. Regarding the limitations of these scoring systems, a recent effort to find AF-specific biomarkers that can predict vascular events, such as fibrin clot permeability,[21] N-terminal pro B-type natriuretic peptide,[22] Group/Differentiation Factor-15 (GDF-15),[23] or free fatty acid,[24] could shed light on risk stratification in stroke patients with AF.
4.1. Strengths and limitations
Several limitations pertain to this study. First, all study subjects were ethnically Korean requesting a cautious generalization of the results. Second, the details of oral anticoagulation therapy in individual subjects, such as the type and initiation of OAC used, and the time-in-therapeutic range in the case of treatment with vitamin K antagonist, were not considered. Third, we used baseline risk scores and did not reassess the risk scoring systems every year. A previous study reported that in AF patients, stroke risk as assessed by the CHA2DS2-VASc score is dynamic, and changes over time.[25] However, the primary purpose of this study was to validate the performance of the scoring systems measured at baseline. Finally, the subjects with the previous history of stroke could have a higher risk for recurrent vascular events than those who have single events. In this study, weighting on the previous history of stroke before index event or risk scoring system before index events could improve the prediction for the vascular outcome. However, models using risk scores being set before the index stroke (not counting score for index stroke event) failed to improve c-statistics for the vascular events in comparison with the model using risk scores being set after index stroke (not presented data on the results section).
Despite these limitations, this study is based on one of the largest registries for stroke patients with AF in the Asian population. Using this study, we can raise the issue of the validity of current risk scoring systems in acute stroke patients with AF.
5. Conclusion
We found that, in a real-world dataset, the CHADS2 score, CHA2DS2-VASc score, ATRIA score, and Essen stroke risk score have limited value for the prediction of recurrent stroke in patients with AF. These scoring systems are valid for predicting death and MACE in stroke patients with AF. However, their validity depends on patient characteristics, such as OAC use. Therefore, a new risk stratification scheme that is specific for the AF population in secondary stroke prevention is needed.
Author contributions
Conceptualization: Tae-Jin Song, Bum Joon Kim, Sung Hyuk Heo, Jin-Man Jung, Kyung-Mi Oh, Chi Kyung Kim, Sungwook Yu, Kwang Yeol Park, Jeong-Min Kim, Jong-Ho Park, Jay Chol Choi, Man-Seok Park, Joon-Tae Kim, Yang-Ha Hwang, Jong-Won Chung, Oh Young Bang, Geong-Moon Kim, Yong-Jae Kim, Woo-Keun Seo.
Data curation: Tae-Jin Song.
Formal analysis: Inwu Yu, Seonwoo Kim, Sook young Woo, Hyun Cho.
Investigation: Inwu Yu, Tae-Jin Song, Bum Joon Kim, Sung Hyuk Heo, Jin-Man Jung, Kyung-Mi Oh, Chi Kyung Kim, Sungwook Yu, Kwang Yeol Park, Jeong-Min Kim, Jong-Ho Park, Jay Chol Choi, Man-Seok Park, Joon-Tae Kim, Yang-Ha Hwang, Jong-Won Chung, Oh Young Bang, Geong-Moon Kim, Yong-Jae Kim, Woo-Keun Seo.
Methodology: Sung Hyuk Heo, Jin-Man Jung, Kyung-Mi Oh, Chi Kyung Kim, Sungwook Yu, Kwang Yeol Park, Jeong-Min Kim, Jong-Ho Park, Jay Chol Choi, Man-Seok Park, Joon-Tae Kim, Yang-Ha Hwang, Jong-Won Chung, Oh Young Bang, Geong-Moon Kim, Yong-Jae Kim, Seonwoo Kim, Sook young Woo, Hyun Cho, Woo-Keun Seo.
Project administration: Woo-Keun Seo.
Resources: Sungwook Yu, Kwang Yeol Park, Jeong-Min Kim, Jong-Ho Park, Man-Seok Park, Joon-Tae Kim, Yang-Ha Hwang, Jong-Won Chung, Oh Young Bang, Geong-Moon Kim, Yong-Jae Kim.
Supervision: Woo-Keun Seo.
Visualization: Seonwoo Kim, Sook young Woo, Hyun Cho.
Writing – original draft: Inwu Yu.
Writing – review & editing: Tae-Jin Song, Bum Joon Kim, Sung Hyuk Heo, Jin-Man Jung, Kyung-Mi Oh, Chi Kyung Kim, Sungwook Yu, Kwang Yeol Park, Jeong-Min Kim, Jong-Ho Park, Jay Chol Choi, Man-Seok Park, Joon-Tae Kim, Yang-Ha Hwang, Jong-Won Chung, Oh Young Bang, Geong-Moon Kim, Yong-Jae Kim, Seonwoo Kim, Sook young Woo, Hyun Cho, Woo-Keun Seo.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AF = atrial fibrillation, K-ATTENTION = Korean ATrial fibrillaTion EvaluatioN regisTry in Ischemic strOke patieNts, MACEs = major adverse cerebrovascular and cardiovascular events, OAC = oral anticoagulant.
How to cite this article: Yu I, Song T-J, Kim BJ, Heo SH, Jung J-M, Oh K-M, Kim CK, Yu S, Park KY, Kim J-M, Park J-H, Choi JC, Park M-S, Kim J-T, Hwang Y-H, Chung J-W, Bang OY, Kim G-M, Kim Y-J, Kim S, Woo Sy, Cho H, Seo W-K. CHADS2, CHA2DS2-VASc, ATRIA, and Essen stroke risk scores in stroke with atrial fibrillation: a nationwide multicenter registry study. Medicine. 2021;100:3(e24000).
This study was supported by a grant from Korean Neurological Association (KNA-17-MI-10) and a grant from the National Research Foundation of Korea (NRF-2020M3E5D2A01084891).
WK Seo received honoraria for lectures from Pfizer, Sanofi-Aventis, Otsuka Korea, Dong-A Pharmaceutical Co., Ltd, Beyer, Daewoong pharmaceutical Co., Ltd., Daiichi Sankyo Korea Co., Ltd. Boryung pharmaceutical, study grant from Daiichi Sankyo Korea Co., Ltd., and consulting fee from OBELAB Inc.
The other authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Continuous quantities are shown as mean ± standard deviation. Categorical quantities are shown as frequency (percentage).
MACE = major adverse cerebro/cardiovascular events, mRS = modified Rankin Score, NIHSS = National Institute of Health Stroke Scale, NOAC = non-vitamin K-dependent antagonist oral anticoagulation.
MACE = major adverse cerebro/cardiovascular events.
References
- [1].Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- [2].Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA 2001;285:2864–70. [DOI] [PubMed] [Google Scholar]
- [3].Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro heart survey on atrial fibrillation. Chest 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- [4].Singer DE, Chang Y, Borowsky LH, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc 2013;2:e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360–420. [DOI] [PubMed] [Google Scholar]
- [6].Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- [7].CAPRIE steering committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet (London, England) 1996;348:1329–39. [DOI] [PubMed] [Google Scholar]
- [8].Diener HC, Cunha L, Forbes C, et al. European stroke prevention study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996;143:1–3. [DOI] [PubMed] [Google Scholar]
- [9].Weimar C, Diener HC, Alberts MJ, et al. The Essen stroke risk score predicts recurrent cardiovascular events: a validation within the reduction of atherothrombosis for continued health (reach) registry. Stroke 2009;40:350–4. [DOI] [PubMed] [Google Scholar]
- [10].Song TJ, Baek IY, Woo HG, et al. Characteristics and factors for short-term functional outcome in stroke patients with atrial fibrillation, nationwide retrospective cohort study. Front Neurol 2019;10:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ (Clinical research ed) 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish atrial fibrillation cohort study. Eur Heart J 2012;33:1500–10. [DOI] [PubMed] [Google Scholar]
- [13].van den Ham HA, Klungel OH, Singer DE, et al. Comparative performance of ATRIA, CHADS2, and CHA2DS2-VASc risk scores predicting stroke in patients with atrial fibrillation: results from a national primary care database. J Am Coll Cardiol 2015;66:1851–9. [DOI] [PubMed] [Google Scholar]
- [14].Zhu W, Fu L, Ding Y, et al. Meta-analysis of ATRIA versus CHA2DS2-vasc for predicting stroke and thromboembolism in patients with atrial fibrillation. Int J Cardiol 2017;227:436–42. [DOI] [PubMed] [Google Scholar]
- [15].Xiong Q, Chen S, Senoo K, et al. The CHADS2 and CHA2DS2-vasc scores for predicting ischemic stroke among east Asian patients with atrial fibrillation: a systemic review and meta-analysis. Int J Cardiol 2015;195:237–42. [DOI] [PubMed] [Google Scholar]
- [16].Li SY, Zhao XQ, Wang CX, et al. One-year clinical prediction in Chinese ischemic stroke patients using the CHADS2 and CHA2DS2-VASc scores: the China national stroke registry. CNS Neurosci Ther 2012;18:988–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Giralt-Steinhauer E, Cuadrado-Godia E, Ois A, et al. CHA(2)DS(2)-VASc score and prognosis in ischemic strokes with atrial fibrillation. J Neurol 2012;259:745–51. [DOI] [PubMed] [Google Scholar]
- [18].Hong HJ, Kim YD, Cha MJ, et al. Early neurological outcomes according to CHADS2 score in stroke patients with non-valvular atrial fibrillation. Eur J Neurol 2012;19:284–90. [DOI] [PubMed] [Google Scholar]
- [19].Kim D, Chung JW, Kim CK, et al. Impact of CHADS(2) score on neurological severity and long-term outcome in atrial fibrillation-related ischemic stroke. J Clin Neurol (Seoul, Korea) 2012;8:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Andersen SD, Gorst-Rasmussen A, Lip GY, et al. Recurrent stroke: the value of the CHA2DS2VASc score and the Essen stroke risk score in a nationwide stroke cohort. Stroke 2015;46:2491–7. [DOI] [PubMed] [Google Scholar]
- [21].Drabik L, Wolkow P, Undas A. Fibrin clot permeability as a predictor of stroke and bleeding in anticoagulated patients with atrial fibrillation. Stroke 2017;48:2716–22. [DOI] [PubMed] [Google Scholar]
- [22].Maruyama K, Uchiyama S, Shiga T, et al. Brain natriuretic peptide is a powerful predictor of outcome in stroke patients with atrial fibrillation. Cerebrovasc Dis Extra 2017;7:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Marin F, Roldan V. Biomarkers: GDF-15 and risk stratification in atrial fibrillation. Nature reviews. Cardiology 2015;12:8–9. [DOI] [PubMed] [Google Scholar]
- [24].Choi JY, Jung JM, Kwon DY, et al. Free fatty acid as an outcome predictor of atrial fibrillation-associated stroke. Ann Neurol 2016;79:317–25. [DOI] [PubMed] [Google Scholar]
- [25].Yoon M, Yang PS, Jang E, et al. Dynamic changes of CHA2DS2-VASc score and the risk of ischaemic stroke in Asian patients with atrial fibrillation: a nationwide cohort study. Thromb Haemost 2018;118:1296–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


