Abstract
Introduction:
Hypertensive intracerebral hemorrhage (HICH) is the most serious complication of hypertension. Clearing intracranial hematoma as soon as possible, reducing brain cell edema, and controlling intracranial pressure could effectively reduce neuron damage, lower patient mortality, and improve patient prognosis. At present, minimally invasive surgery (MIS) has been widely used and plays an important role in the treatment of HICH. However, it is still in controversies about the choice of surgical treatment and medication treatment for HICH. Therefore, we try to conduct a randomized, controlled, prospective trial to observe the efficacy of MIS treatment against HICH compared with medication treatment.
Methods:
Patients will be randomly divided into treatment group and control group in a 1:1 ratio using the random number generator in Microsoft Excel. Stereotactic soft channel minimally invasive intracranial hematoma puncture and drainage treatment and medication treatment will be applied respectively. The outcomes of intracerebral hemorrhage volume, Glasgow coma scale, National Institutes of Health Stroke Scale will be recorded.
Conclusions:
The findings of the study will be helpful for the choice of MIS and conservative treatment when treating HICH patients.
Trial registration:
OSF Registration number: DOI 10.17605/OSF.IO/ME6Y5.
Keywords: hypertensive intracerebral hemorrhage, medication treatment, minimally invasive surgery, protocol, reduction ;
1. Introduction
Hypertensive intracerebral hemorrhage (HICH) is the most serious complication of hypertension, which frequently occurs in middle-aged male, especially in winter and spring. It is caused by the weakening blood vessel due to the damage of small arteries in the brain caused by long-term hypertension.[1,2] HICH is a serious cerebrovascular disease which affects about 4 million people worldwide each year with high mortality and disability rate.[3] Clearing intracranial hematoma as soon as possible, reducing brain cell edema, and controlling intracranial pressure could effectively reduce neuron damage, lower patient mortality, and improve patient prognosis.[4,5]
The bleeding volume in most patients with HICH is 20 to 40 mL, which is a moderate amount. For those patients, either medication treatment or surgical treatment could be applied. Medication treatment for HICH is the most basic therapeutic choice, including controlling blood pressure and glucose, anti-infection, relieving cerebral edema, fluid infusion, nutritional support, and symptomatic treatment.[6] It shows certain curative effects, but it may also prolong the hematoma compression period to damage brain tissue, which could possibly result in serious neurological deficit. Theoretically, surgery has the potential to rapidly remove the hematoma to reduce brain damage. At present, minimally invasive surgery (MIS) has been widely used and plays an important role in the treatment of HICH. The minimally invasive hematoma evacuation is a simple and quick operation, and it could avoid the injury of brain tissues and blood vessels to reducing the risk of postoperative mortality and complications.[4,7,8]
However, it is still in controversies about the choice between surgical treatment and medication treatment for HICH.[9] Although many studies showed that MIS is significantly better than medication treatment,[10] many studies believe that different inclusion criteria could affect the results, and most patients in the surgical treatment group are more severe than those in the medication treatment group, which could also affects the conclusions.[11] In addition, several randomized controlled trials have failed to demonstrate improvement in outcomes of patients in surgical treatment.[12] Therefore, we try to conduct a randomized, controlled, prospective trial to observe the efficacy of MIS treatment against HICH compared with medication treatment.
2. Methods
2.1. Study design
This is a single-center, randomized, controlled clinical trial (CHiCTR), approved by the Ethics Committee of traditional Chinese medicine, and it will be carried out in accordance with the Declaration of Helsinki. A flowchart of this study is shown in Figure 1. The study conforms to the Standard Protocol Recommendations for Interventional Trials 2013 Statement,[13] and the results will be reported according to the CONSORT Statement extension for trials.[14]
Figure 1.
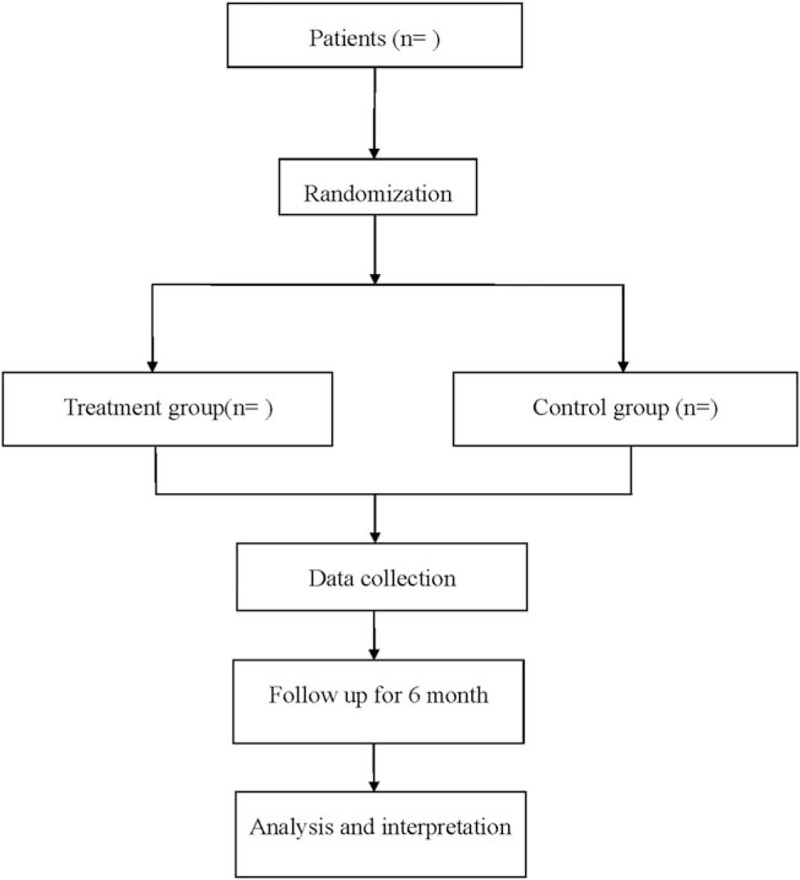
Flow diagram of the study.
The protocol will be in accordance with the Helsinki Declaration and approved by the Clinical Research Ethics Committee of our hospital. This experiment has been registered in the open science framework (OSF) (registration number: DOI 10.17605/OSF.IO/ME6Y5). Before randomization, all patients will sign a written informed consent, and they can freely choose whether to continue the trial at any time.
2.2. Participants
Participants will be mainly recruited from inpatients in Hospital of traditional Chinese medicine. Previous report[15] showed that medication treatment against HICH could reduce intracerebral hemorrhage (ICH) volume in 55% in 7 days. According to our preliminary study, a 7-day-reduction of ICH volume is 82%, and taking α = 0.05 and β = 0.2, a sample size of 38 will be needed. Considering the loss to follow-up, 46 patients will be ultimately recruited.
Inclusion criteria are:
-
(1)
diagnosed with HICH[16];
-
(2)
aged 18 to 75 years old;
-
(3)
with 20 to 40 bleeding volume;
-
(4)
Glasgow coma scale score of 8 to 12;
-
(5)
written informed consent.
Exclusion criteria are:
-
(1)
severe organ dysfunctions or clotting disorders;
-
(2)
complicated with ischemic stroke, or nervous system tumors, or mental illness.
2.3. Randomization and blinding
To minimize selection bias, patients will be randomly divided into treatment group and control group in a 1:1 ratio using the random number generator in Microsoft Excel. The patients and the neurosurgeons will not be blinded, and an independent researcher of no interest with the study will analyze the data.
2.4. Interventions
Stereotactic soft channel minimally invasive intracranial hematoma puncture and drainage treatment will be performed for patients in the treatment group with the following procedure:
-
(1)
Locate the patient's puncture point, puncture angle, and catheter depth based on the level displayed on the preoperative computed tomography.
-
(2)
Mark the puncture point perform local skin anesthesia.
-
(3)
Make a 3 cm coronal linear skin incision lateral to the midline over the frontal area, and penetrate the skull with an electric drill. Insert the No. 12 soft channel drainage tube into the hematoma cavity according to the pre-designed direction and depth, pull out the guide core, and then drainage gently with a 5 mL syringe.
-
(4)
Perform liquefaction and drainage about 4 hours after the puncture with urokinase.
-
(5)
Extubate after the hematoma is basically removed after computed tomography scan.
Conservative treatment will be applied to patients in control group, including controlling blood pressure and glucose, anti-infection, relieving cerebral edema, fluid infusion, nutritional support, and symptomatic treatment.
2.5. Outcome variables
Vital signs and vital organs functions will be closely monitored. The primary outcomes are ICH volume reduction on day 0, 1, 3, and 7, coma condition measured by Glasgow coma scale and neurological function by National Institutes of Health Stroke Scale at week 0, 1, 2, 4, and then every month for 6 month. Secondary outcomes are activity of daily living at week 0, 1, 2, 4, and then every month for 6 month. Length of hospital stay and complications, and all possible adverse events will be recorded.
2.6. Statistical methods
All statistical analyses will be conducted by SPSS version 22 (IBM, Chicago, IL). Measurement data will be described in mean and standard deviation or median and interquartile range, and categorical variables as number and percentage. Different statistic method will be applied according to different data type. P < .05 is considered statistically significant.
3. Discussion
HICH is a spontaneous cerebrovascular disease caused by the rupture of artery, vein or capillary in the brain.[17,18] The hematoma can cause brain cell edema and increased intracranial pressure, and patients would suffer from neurological dysfunction such as decreased limb muscle strength and impaired consciousness. At present, reducing the disability as much as possible and improve the quality of life of patients are critical in the treatment of HICH. In recent years, the application of MIS to remove intracerebral hematomas is more helpful than conventional craniotomy in improving postoperative recovery, reducing postoperative complications, and raising survival rates. MIS offered promise as a means to maximize hematoma evacuation while minimizing damage to normal tissue. Therefore, we try to carry out a prospective trial to compare the difference of ICH volume reduction, neurological function, and activity of daily living between MIS treatment and medication treatment against HICH. We hope that our results will be helpful for the choice between MIS and conservative treatment when treating HICH patients.
Author contributions
Data curation: Jiang Liu, Jing Cheng.
Funding acquisition: Zhengxin Wang.
Investigation: Hongjun Zhou.
Resources: Hongjun Zhou, Chunyan Deng.
Software: Chunyan Deng.
Writing – original draft: Jiang Liu, Jing Cheng.
Writing – review and editing: Jiang Liu, Zhengxin Wang.
Footnotes
Abbreviations: HICH = hypertensive intracerebral hemorrhage, ICH = intracerebral hemorrhage, MIS = minimally invasive surgery.
How to cite this article: Liu J, Cheng J, Zhou H, Deng C, Wang Z. Efficacy of minimally invasive surgery for the treatment of hypertensive intracerebral hemorrhage: a protocol of randomized controlled trial. Medicine. 2021;100:3(e24213).
Informed consent has been obtained from the patient and approval has been obtained from the ethics committee.
This study has been approved by the Ethics committee.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
This work is supported by the Chongqing Science and technology plan project (No. 20160328).
The authors have no conflicts of interest to disclose.
References
- [1].Majidi S, Suarez JI, Qureshi AI. Management of acute hypertensive response in intracerebral hemorrhage patients after ATACH-2 trial. Neurocrit Care 2017;27:249–58. [DOI] [PubMed] [Google Scholar]
- [2].Hu S, Ma Q, Li B, et al. Association of hypothyroidism with hypertensive intracerebral hemorrhage: a case-control study. World Neurosurg 2020;134:e8–11. [DOI] [PubMed] [Google Scholar]
- [3].Feigin V, Lawes CMM, Bennett DA, et al. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol 2003;2:43–53. [DOI] [PubMed] [Google Scholar]
- [4].Tang Y, Yin F, Fu D, et al. Efficacy and safety of minimal invasive surgery treatment in hypertensive intracerebral hemorrhage: a systematic review and meta-analysis. BMC Neurol 2018;18:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nam TM, Kim YZ. A meta-analysis for evaluating efficacy of neuroendoscopic surgery versus craniotomy for supratentorial hypertensive intracerebral hemorrhage. J Cerebrovasc Endovasc Neurosurg 2019;21:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao JL, Du ZY, Sun YR, et al. Intensive blood pressure control reduces the risk of progressive hemorrhage in patients with acute hypertensive intracerebral hemorrhage: a retrospective observational study. Clin Neurol Neurosurg 2019;180:1–6. [DOI] [PubMed] [Google Scholar]
- [7].Luan L, Li M, Sui H, et al. Efficacies of minimally invasive puncture and small bone window craniotomy for hypertensive intracerebral hemorrhage, evaluation of motor-evoked potentials and comparison of postoperative rehemorrhage between the two methods. Exp Therap Med 2019;17:1256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhao XH, Zhang SZ, Feng J, et al. Efficacy of neuroendoscopic surgery versus craniotomy for supratentorial hypertensive intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Brain Behav 2019;9:e01471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Qiu S, Liu T, Cao G, et al. Treatment of intracranial hemorrhage with neuroendoscopy guided by body surface projection. Medicine 2019;98:e15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Teernstra OP, Evers SM, Lodder J, et al. Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA). Stroke 2003;34:968–74. [DOI] [PubMed] [Google Scholar]
- [11].Alqadri SL, Qureshi AI. Management of acute hypertensive response in patients with intracerebral hemorrhage. Curr Atheroscler Rep 2012;14:322–7. [DOI] [PubMed] [Google Scholar]
- [12].Mendelow D, Gregson B, Fernandes H, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005;365:387–97. [DOI] [PubMed] [Google Scholar]
- [13].Chan AW, Tetzlaff JM, Gotzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].W J, YW Efficacy of stereotactic soft channel minimally invasive intracranial hematoma puncture and drainage treatment against hypertensive intracerebral hemorrhage. Anhui Med J 2019;40:892–4. [Google Scholar]
- [16].Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–60. [DOI] [PubMed] [Google Scholar]
- [17].Dowlatshahi D, Chung HS, Reaume M, et al. Prevalence and the predictive performance of the dynamic CT-angiography spot sign in an observational cohort with intracerebral hemorrhage. Medicine 2020;99:e23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jang JH, Cho WS, Hong N, et al. Surgical strategy for patients with supratentorial spontaneous intracerebral hemorrhage: minimally invasive surgery and conventional surgery. J Cerebrovasc Endovasc Neurosurg 2020;22:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


