Supplemental Digital Content is available in the text
Keywords: diseases activity, inflammation, muscle, myopenia, myostatin, rheumatoid arthritis, rheumatoid cachexia
Abstract
Myostatin is a cytokine produced and released by myocytes that might have an outstanding role not only in muscle wasting during cachexia but also in inflammation. Herein we explore the association between myostatin levels and inflammatory parameters in rheumatoid arthritis (RA).
One hundred twenty-seven women without rheumatic diseases and 84 women with a diagnosis of RA were assessed in a cross-sectional study. Outcomes reflecting the activity of the arthritis including Disease Activity Score (DAS28-ESR) and impairment in functioning by the Health Assessment Questionnaire-Disability Index were assessed in RA. We obtained Skeletal muscle mass index (SMI), fat-free mass index (FFMI), and fat mass index using dual-energy x-ray absorptiometry. Serum myostatin was determined by enzyme-linked immunosorbent assay. Myostatin levels were correlated with disease activity and parameters of muscle mass.
The SMI was lower and concentration of myostatin was higher in RA patients than in controls (P = .008 and P < .001, respectively). Myostatin significantly positively correlated with C-reactive protein (rho = 0.48, P < .001), erythrocyte sedimentation rate (rho = 0.28, P = .009), and DAS28-ESR (rho = 0.22, P = .04), and negatively correlated with SMI (rho = −0.29, P = .008), (FFMI) (rho = −0.24, P = .027). In the multivariate logistic regression analysis, levels of myostatin remained associated with disease activity in RA (P = .027).
In our study, myostatin was associated with disease activity in RA patients, suggesting a mechanistic link between myostatin, muscle wasting and inflammation in RA.
1. Introduction
Myostatin which is part of the transforming growth factor-β superfamily, is a cytokine produced and released by myocytes, that negatively regulates skeletal muscle in humans and animal models.[1] Previous work has linked myostatin with muscle wasting in several chronic diseases including rheumatoid arthritis (RA).[2,3] RA is an autoimmune disease characterized by synovial inflammation and joint destruction. Fifty percent of patients with RA can develop muscle loss that will lead to rheumatoid cachexia in around 25% (ranges from 7% to 33%), associated to a poor quality of life.[3,4] Yet, it was suggested that myostatin could be also involved in synovitis and joint damage in animal models of RA.[5] Since RA is a multifactorial disease, it is necessary to explore the potential role of other cytokines, such myostatin, to understand whether it can participate in the joint inflammation. Therefore, the objective of this project was to explore the association of myostatin and parameters of inflammation in RA.
2. Methods
2.1. Study design
This was a cross-sectional study.
Eighty-four female patients fulfilling the 1987 American College of Rheumatology classification criteria for RA were recruited. We excluded patients with pregnancy, thyroid disease, creatinine >1.2 mg/dL, transaminases >2-fold the normal values, cancer, chronic virus infections (such as tuberculosis, human immunodeficiency virus infections, hepatitis B, hepatitis C). A structured chart review was performed for investigating epidemiological characteristics, comorbid diseases, treatments, and other factors potentially related with abnormalities on musculoskeletal mass such as sedentarism. Sedentarism was assessed using a structured questionnaire and it was considered when a person practiced <150 minutes of moderate activity per week or <75 minutes of vigorous-intensity activity per week.[6] Clinical assessment included Disease Activity Score (DAS28) which is a validated composite index that includes measure joint swollen count, tender joint count, a patient's perception of disease severity (assessed with a visual analogue scale that goes from 0 to 100 mm) and erythrocyte sedimentation ratio (ESR).[7] We used DAS28-ESR as disease activity index. This index classifies disease activity into 4 categories: remission <2.6; low disease activity ≥2.6 to ≤3.2; moderate disease activity >3.2 to ≤5.1; and high disease activity >5.1. One of the main objectives of treatment in RA is to achieve low disease activity or remission (DAS28-ESR ≤3.2). Patients with DAS28-ESR >3.2 are considered with moderate or severe disease activity requiring therapeutic modifications.[8] We also included for assessing functional disability the Spanish validated version of the Health Assessment Questionnaire-Disability Index (HAQ-DI).[9]
For the control group, we included 127 females with similar age and body mass index (BMI) and absence of inflammatory or autoimmune disorders, cancer, chronic kidney diseases, endocrinopathies, and active infections.
2.2. Quantification of skeletal muscle mass
Skeletal muscle mass index (SMI) was obtained with a dual-energy x-ray absorptiometry (DXA) (Lunar Prodigy Advance 2000 equipment, General Electric). SMI was computed using the following formula: sum of skeletal muscle mass of extremities (SMME) ([arms + legs]/adjusted by height squared).[10] Rheumatoid cachexia was defined using the Engvall et al criteria according to the following values obtained of the comparison group: fat-free mass index (FFMI) below the 10th percentile and fat mass index (FMI) above the 25th percentile.[11]
2.3. Quantification of serum myostatin levels
Serum concentration of myostatin was measured by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (MyBioSource, Inc., San Diego, CA).
2.4. Ethics
The privacy of the patients was protected, and this study followed the recommendations of the 64th Declaration of Helsinki and, it was conducted under the ethical standards of the Ethics and Research Board of the Centro Medico Nacional de Occidente of Instituto Mexicano del Seguro Social 13-01with approval code: R-2016-1301-93.
2.5. Statistical analysis
We performed all the analyses using SPSS v. 23 statistical software (SPSS, Inc., Chicago, IL). We expressed the quantitative variables as medians and ranges, and qualitative variables such as frequencies and percentages. We used χ2 and Mann–Whitney U test to compare demographic variables, skeletal muscle mass index, and serum concentrations of myostatin between the RA patients and control group for qualitative and quantitative variables, respectively. Also, we used Spearman correlation to determine the strength of association between myostatin and clinical variables in RA patients. To evaluate the levels of myostatin as a good classifier of disease activity in RA, we performed a receiver-operating characteristic (ROC) curve. We performed a multivariate logistic regression analysis including as a dependent variable moderate or severe disease activity adjusted by age, disease duration, SMI, FMI, and the cutoff value of high levels of myostatin based on ROC curve analysis. We considered P < .05 as a result with significance statistic.
3. Results
We included 127 women without rheumatic diseases (controls) and 84 women diagnosticated with RA. We show in Table 1 a comparison between controls and RA patients. The age was similar (60 [24–85] vs 63 [24–89] years’ old, P = .57). RA patients were more sedentary, and a higher percentage were menopausal. BMI and other comorbidities including high blood pressure, diabetes mellitus and smoking were not different between both groups. Concentrations of myostatin in circulation were significantly higher in the RA group than controls (9 [1.2–140] ng/mL vs 3.5 [1–89.9] ng/mL respectively, P < .001). Skeletal muscle mass index was significant diminished in the RA group (5.8 [3.4–10.0] vs 6.2 [3.8–9.0] RA vs controls, respectively, P = .008)).
Table 1.
Comparison of selected characteristics, body composition and myostatin levels between CL group and RA group.
| Variables | CL (n = 127) | RA (n = 84) | P |
| Age, y | 60 (24–85) | 63 (24–89) | .57 |
| Alcoholism, n (%) | 15 (11.8) | 4 (4.9) | .09 |
| Smoking, n (%) | 11 (8. 7) | 8 (9.5) | .83 |
| BMI | 27.8 (16–42) | 27.3 (18–40) | .35 |
| Sedentarism, n (%) | 58 (46) | 52 (61. 9) | .02 |
| Menopause, n (%) | 85 (68) | 76 (90.5) | <.001 |
| Comorbidities | |||
| High blood pressure, n (%) | 41 (33. 3) | 35 (41. 7) | .22 |
| Diabetes mellitus type 2, n (%) | 27 (22.1) | 12 (14.3) | .16 |
| Body composition characteristics | |||
| Weight, kg | 67 (38–97) | 64 (42–98) | .15 |
| Skeletal Muscular Mass Index | 6.2 (3.8–9) | 5.8 (3.4–10) | .008 |
| Laboratory data | |||
| Myostatin, ng/mL | 3.5 (1–89.9) | 9 (1.2–140) | <.001 |
As it is described in Table 2, 12 patients (14.3%) met the criteria for rheumatoid cachexia. To identify whether RA is associated with high levels of myostatin independently of rheumatoid cachexia, we compared RA patients without rheumatoid cachexia (n = 72) versus controls, and we observed that these patients continued to show high levels of myostatin compared to controls (8.05 [1.2–140] vs 3.46 [1.01–89.9] respectively, P < .001).
Table 2.
Clinical variables in patients with rheumatoid arthritis.
| Variables | n = 84 |
| Age, y | 63 (24–89) |
| Years since RA diagnosis | 13 (0–45) |
| HAQ-DI | 0.11 (0–2.7) |
| DAS28-ESR | 2.8 (0.9–7.1) |
| DAS28-ESR moderate-severe (>3.2), n (%) | 31 (36.9) |
| Rheumatoid cachexia, n (%) | 12 (14.3) |
| Treatment | |
| Biological drugs, n (%) | 5 (6) |
| Glucocorticoids, n (%) | 71 (85.5) |
| Dose of glucocorticoids, mg/day | 5 (0–17.5) |
| DMARD, n (%) | 80 (96.4) |
| NSAID, n (%) | 78 (95.1) |
| Laboratory | |
| ∗CRP, mg/dL | 10.2 (0.3–219) |
| ESR, mm/h | 20.5 (1–64) |
| Myostatin, ng/mL | 9 (1.2–140) |
Table 2 shows the characteristics of the RA patient group. The median of HAQ-DI was 0.11 (0–2.7), DAS28-ESR was 2.8 (0.9–7.1), ESR was 20.5 (1–64), CRP was 10.2 (0.3–219). Only 6% were receiving biological therapy, 96% were receiving a synthetic disease-modifying anti-rheumatic drug (DMARD), mostly methotrexate, and 71% were on glucocorticoids (GC), with an average of 5 mg/day (0–17.5). We observed no significant difference in myostatin levels between RA patients with or without cachexia (13.2 [2.5–140.0] vs 8.05 [1.2–140] respectively; P = .35). Fifteen of 24 (62.5%) RA patients with low-muscle mass were sedentary and 37 of 60 (61.7%) RA patients with normal muscle mass were classified as sedentary (P = 1.0). However, 11 of 12 (91.7%) RA patients with cachexia were sedentary, whereas 41 of 72 (56.9%) RA patients without cachexia were classified as sedentary (P = .025).
Table 3 shows the correlation between serum myostatin levels with quantitative variables in RA patients. Higher myostatin levels were correlated with low skeletal muscle mass index (P = 0.008). Interestingly, myostatin concentrations correlated with inflammation parameters of DAS28-ESR (rho = 0.22, P = .04), CRP (rho = 0.40, P < .001), and ESR (rho = 0.28, P = .009). Myostatin concentrations also correlated with years since RA diagnosis (rho = 0.24, P = .02), SMI (rho = −0.29, P = 0.008), FFMI (rho = −0.24, P = .027), and GC daily doses (rho = 0.51, P < .001). However, we did not observe correlation between FMI and myostatin levels (rho = 0.028, P = .80). In data that are not depicted in tables we observed a correlation between SMI with CRP (rho = −0.26, P = .04) and HAQ-DI (rho = −0.21, P = .05). We show the results of a subanalysis aimed at identifying whether previously observed correlations between myostatin levels and parameters of inflammation were independent of the loss of muscle mass observed in rheumatoid cachexia (See Supplemental Digital Content table S1, showing the correlations of serum myostatin concentrations with variables associated with inflammatory activity in the subgroup of patients without rheumatoid cachexia). For this correlation analysis, we excluded the subgroup of patients with rheumatoid cachexia (n = 12). In the results of these analyses, the variables of inflammatory disease activity (DAS28-ESR, CRP, and ESR) remained correlated with myostatin levels.
Table 3.
Correlation of serum myostatin concentrations with epidemiological and body composition variables in Rheumatoid arthritis.
| Myostatin, n = 84 | ||
| Variables | Rho | P |
| Age, y | −0.09 | .37 |
| RA disease duration, y | 0.24 | .02 |
| HAQ-DI (score) | 0.05 | .65 |
| DAS28-ESR (score) | 0.22 | .04 |
| Tender joint counts | 0.11 | .30 |
| Swollen joint counts | 0.19 | .08 |
| Severity of disease activity | 0.03 | .79 |
| Pain perceived by the patient (VAS) | −0.05 | .64 |
| Stiffness perceived by the patient (VAS) | −0.02 | .86 |
| CRP, mg/dL | 0.40 | <.001 |
| ESR, mm/h | 0.28 | .009 |
| Weight, kg | −0.15 | .18 |
| Skeletal muscle mass index | −0.29 | .008 |
| Fat mass index | 0.028 | .80 |
| Free fat mass index | −0.24 | .027 |
| Total glucocorticoid dose, mg/day | 0.51 | <.001 |
We also performed a ROC curve analysis on myostatin levels to evaluate levels of myostatin as a classifier for moderate or severe disease activity (DAS28-ESR >3.2) (Fig. 1). The area under the curve was 0.61. We obtained a sensibility of 61.3% and specificity of 53% to detect moderate or severe disease activity in RA, with a cutoff point of 13 ng/mL of myostatin.
Figure 1.
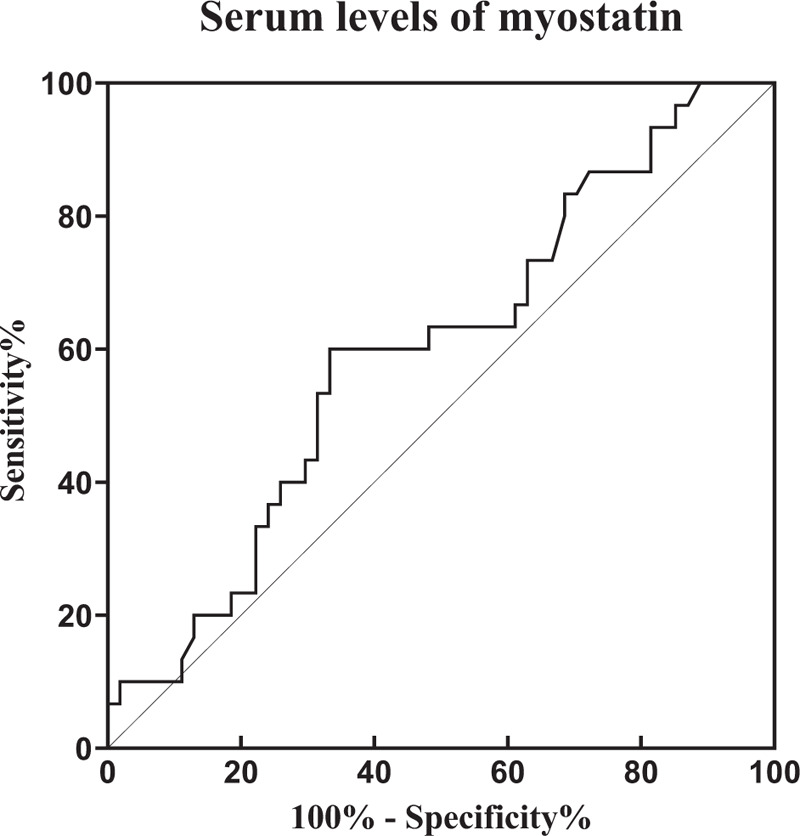
Receiver-operating characteristic curve of serum myostatin levels. The area under the curve was 0.61 with a P = .09. Using cutoff value of ≥13 ng/mL of myostatin, we obtained a sensibility of 61.3% and specificity of 53% to detect moderate or severe disease activity in rheumatoid arthritis patients.
Finally, in the multivariate logistic regression analysis, we identified that myostatin serum levels remain associated with moderate or severe disease activity (DAS28-ESR >3.2) in RA patients after adjusting by age, disease duration, SMI, and FMI (P = .027) (Table 4).
Table 4.
Multivariate logistic regression analysis assessing the factors associated with moderate or severe disease activity.
| Variables | Odds ratio | 95% CI | P |
| Myostatin high levels ≥13 ng/mL | 2.938 | 1.13–7.61 | .027 |
| Duration of disease, y | 1.052 | 1.001–1.107 | .047 |
| Age | — | No significant in the model | — |
| SMI | — | No significant in the model | — |
4. Discussion
Myostatin induces muscle atrophy inhibiting myogenesis and developing myopenia, a clinical condition characterize for wasting of the muscle fibers due to an illness no matter the age.[12] Yet, previous works have also suggested a link between myostatin and inflammation.[5,13,14] RA synovial tissue expresses higher amounts of myostatin compared to normal synovial tissue, and was found to accelerate osteoclast formation and joint destruction in animal models of inflammatory arthritis.[5] In addition, other reports have shown a positive correlation between myostatin and proinflammatory cytokines, such as, IL-1β, and tumor necrosis factor (TNF) expression in synovial fluid of patients with RA. These authors also showed that myostatin is able to induce IL-1β production and to increase TNF expression in synovial fibroblasts, probably through phosphatidylinositol 3-kinase signaling pathway, suggesting a link between this myokine and synovial inflammation.[13,14] In addition, in an experimental model of chronic kidney disease in mice, Zhang et al administered an anti-myostatin peptibody to produce a pharmacological inhibition of myostatin and identify the effects of myostatin suppression on muscle atrophy and chronic inflammation.[15] A significant increase in muscle mass and a suppression of circulating inflammatory cytokines including TNF and IL-6 was observed after the administration of this peptibody, supporting our findings of a connection between myostatin levels, muscle wasting and inflammation.
Our work shows an association between circulating levels of myostatin and myopenia determined by DXA with inflammatory markers in our RA cohort. This result is similar to the association described in a Chinese RA population, which also showed a significant correlation between muscle loss and joint destruction in RA,[16] although our report would be the first one to show correlation between myostatin levels and components of disease activity in RA patients. We found our findings also supported by the study performed by Kerschan-Schindl et al, who observed that RA patients on remission had lower concentration of circulating myostatin compared with active patients.[17]
We also showed a significant correlation between myostatin and CRP and ESR; these correlations remained significant in the subanalysis after excluding patients with rheumatoid cachexia. These results imply that myostatin can be considered as potential biomarker of disease activity independently of severe muscle mass losses. CRP is an acute-phase response molecule which display a high expression in inflammatory condition such as RA and can be induced by some inflammatory cytokines such as IL-6 and IL-1β.[18] Several years ago it was shown that CRP was localized in RA synovial tissue, suggesting a potential pathogenic role of CRP.[19] Interestingly, muscle is a relevant source of IL-6 and this cytokine promotes atrophy and muscle wasting,[20] which could also be important in this association between myostatin, myopenia, and inflammation in our study. Altogether myostatin could be a potential player in RA pathogenesis and a promising target in RA therapy similar to what has been suggested in other diseases.[2]
Although this positive correlation between DAS-28 and myostatin could reflect a potential role of myostatin on inflammation, this could be just a consequence of the sedentarism in patients with high activity and poor response to the treatment. Moreover, GC promotes the expression of myostatin leading to muscle atrophy and waste.[21,22] In addition, muscular waste secondary to GC recovers slowly in older individuals. In our study, 85% of the patients were taken glucocorticoids at low doses and had a median of 63 years’ old, which could also contribute to explain our positive correlation between myostatin and inflammation.
In this study, we observed poor accuracy of myostatin as a biomarker for moderate-severe disease activity considering a cutoff point of 13 ng/mL However, the positive correlation with the parameters of inflammation and negative correlation with the skeletal muscular mass index, suggest a connection between myostatin, muscle wasting, and inflammation in RA. Patients with RA have little physical activity and one possible cause is the pain and inflammation at the time of inclusion. This poor physical activity leads to continuing sedentary lifestyle that contributes to muscle breakdown and increasing circulating proinflammatory cytokines, and then, cachexia, among other comorbidities, which involves patients into a feedback of inflammation-muscle waste-sedentarism. Yet, our multivariate analysis suggests that some of the increase in myostatin in RA patients is due to the inflammation and not just sedentarism and/or cachexia.
This study has some limitations that should be discussed. First, since this is a cross-sectional design, this work was unable to identify a temporal relation between serum myostatin levels and disease activity outcomes in RA patients. Further longitudinal studies following myostatin levels in RA are required to solve this limitation. Although these correlations between myostatin levels and several parameters of inflammation are highly significant we cannot exclude the presence of a possible incidence-prevalence bias, inherent to any cross-sectional study. Another limitation is the presence of some confounders related with myopenia including disease duration, use of GC and comorbid disease that can influence our results. Further follow-up studies assessing myostatin levels in early RA and during different points during the time in the same group of patients with RA are required to establish if the changes on myostatin levels precede the joint inflammation. Finally, this study identified a low frequency of rheumatoid cachexia in our RA patients. However, there are several criteria used for cachexia.[11,23,24] We choose to define rheumatoid cachexia based on the criteria used by Engvall et al.[11] Nevertheless, the low number of patients with presence of rheumatoid cachexia limited the statistical power to observe differences in myostatin levels compared with patients without this complication.
In summary, myostatin was associated with disease activity in RA patients, suggesting a mechanistic link between muscle wasting and inflammation in RA. Yet, future studies are needed to further evaluate whether myostatin positively regulates synovial inflammation and joint damage.
Acknowledgment
The authors thank IMSS Foundation (Fundación IMSS, A.C.) for the support for the research.
Author contributions
Agreement to be accountable for all aspects of the work: Jessica D. Murillo-Saich, Maria Luisa Vazquez-Villegas, Melissa Ramirez-Villafaña, Ana Miriam Saldaña-Cruz, Javier A. Aceves-Aceves, Laura Gonzalez-Lopez, Monica Guma, Jorge I. Gamez-Nava.
Conceptualization: Jorge I. Gamez-Nava, Monica Guma, Jessica D. Murillo-Saich, Laura Gonzalez-Lopez.
Data curation: Maria Luisa Vazquez-Villegas, Melissa Ramirez-Villafaña, Ana Miriam Saldaña-Cruz, Javier A. Aceves-Aceves.
Final approval: Jessica D. Murillo-Saich, Maria Luisa Vazquez-Villegas, Melissa Ramirez-Villafaña, Ana Miriam Saldaña-Cruz, Javier A. Aceves-Aceves, Laura Gonzalez-Lopez, Monica Guma, Jorge I. Gamez-Nava.
Formal analysis: Maria Luisa Vazquez-Villegas.
Founding acquisition and resources: Jorge I. Gamez-Nava, Laura Gonzalez-Lopez
Funding acquisition: Jorge I. Gamez-Nava.
Investigation: Jorge I. Gamez-Nava.
Methodology: Laura Gonzalez-Lopez, Jorge I. Gamez-Nava.
Resources: Laura Gonzalez-Lopez.
Revising the manuscript for important intellectual content: Jorge I. Gamez-Nava, Laura Gonzalez-Lopez, Monica Guma.
Statistical analysis: Maria Luisa Vazquez-Villegas, Melissa Ramirez-Villafaña
Supervision: Laura Gonzalez-Lopez, Monica Guma, Jorge I. Gamez-Nava.
Validation: Jorge I. Gamez-Nava.
Writing – original draft: Jessica D. Murillo-Saich, Jorge I. Gamez-Nava, Laura Gonzalez-Lopez, Monica Guma.
Writing – review & editing: Jessica D. Murillo-Saich, Laura Gonzalez-Lopez, Monica Guma, Jorge I. Gamez-Nava.
Supplementary Material
Footnotes
Abbreviations: 95% CI = 95% confidence interval, BMI = body mass index, CL = controls, CRP = C-reactive protein, DAS28 = disease activity score, DAS28-ESR = Disease activity score using erythrocyte sedimentation rate, DMARD = disease-modifying anti-rheumatic drug, DXA = Dual-energy X ray absorptiometry, ELISA = enzyme-linked immunosorbent assay, ESR = erythrocyte sedimentation rate, FFMI = fat-free mass index, FMI = fat mass index, GC = glucocorticoids, HAQ-DI = Health assessment questionnaire-disability index, IL-1β = interleukin-1 beta, IL-6 = interleukin-6, Kg = kilograms, mg/day = milligrams per day, mg/dL = milligrams/deciliter, mm = millimeters, mm/h = millimeters/hour, n = number, ng/mL = nanograms/milliliter, NSAID = non-steroidal anti-inflammatory drug, P = P value, RA = rheumatoid arthritis, ROC = receiver-operating characteristic, SMI = skeletal muscle mass index, SMME = skeletal muscle mass of extremities, SPSS = Statistical package for the social science, TGF-β = transforming growth factor-Beta, TNF = tumor necrosis factor, VAS = Visual Analogue Scale.
How to cite this article: Murillo-Saich JD, Vazquez-Villegas ML, Ramirez-Villafaña M, Saldaña-Cruz AM, Aceves-Aceves JA, Gonzalez-Lopez L, Guma M, Gamez-Nava JI. Association of myostatin, a cytokine released by muscle, with inflammation in rheumatoid arthritis: A cross-sectional study. Medicine. 2021;100:3(e24186).
The grant was obtained from “Fondo en Investigación en Salud” of Instituto Mexicano del Seguro Social. Grant: FIS/IMSS/PROT/MD16/1565.
JDM-S currently is a recipient of a postdoctoral scholarship supported by the National Institutes of Health (T32AR064194 to JDM-S).
JDM-S and MLV-V participated equally as first authors.
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Qualitative variables are expressed in frequencies (%) and quantitative variables are expressed in median and ranges. BMI = body mass index, CL = control group, RA = rheumatoid arthritis group. Comparison of qualitative variables performed with Chi-square. Comparison of quantitative variables was performed using Mann–Whitney U test. Statistical significance P < .05.
Qualitative variables are expressed in frequencies (%) and quantitative variables are expressed in median and ranges. CRP = C-reactive protein, DAS28-ESR = disease activity score using erythrocyte sedimentation rate, DMARD = synthetic disease-modifying anti-rheumatic drug, ESR = erythrocyte sedimentation rate, HAQ-DI = Health assessment questionnaire-disability index, NSAID = non-steroidal anti-inflammatory drug.
Data of 78 of 84 RA patients of CRP were available at the time of the study.
CRP = C-reactive protein, DAS28-ESR = disease activity score using erythrocyte sedimentation rate, ESR = erythrocyte sedimentation rate, ESR = Erythrocyte sedimentation rate, HAQ-DI = Health assessment questionnaire-disability index, Rho = Spearman correlation, VAS = Visual Analogue Scale. Statistical significance P < .05.
Multivariate analysis logistic regression analysis. Dependent variable moderate or severe disease activity according DAS28-ESR (>3.2). Method forward stepwise. High myostatin levels were considered ≥13 ng/mL (cutoff value obtained of ROC analysis).95% CI = 95% confidence interval, SMI = skeletal muscle mass index. Statistical significance was considered as P < .05.
References
- [1].Rodriguez J, Vernus B, Chelh I, et al. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell Mol Life Sci 2014;71:4361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Smith RC, Lin BK. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr Opin Support Palliat Care 2013;7:352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Torii M, Hashimoto M, Hanai A, et al. Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis. Mod Rheumatol 2019;29:589–95. [DOI] [PubMed] [Google Scholar]
- [4].Santo RCE, Fernandes KZ, Lora PS, et al. Prevalence of rheumatoid cachexia in rheumatoid arthritis: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2018;9:816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dankbar B, Fennen M, Brunert D, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med 2015;21:1085–90. [DOI] [PubMed] [Google Scholar]
- [6].Organization GWH. Global Recommendations on Physical Activity for Health. WHO Press. Available at: https://www.who.int/dietphysicalactivity/physical-activity-recommendations-18-64years.pdf. Published 2010. Accessed 06.19.2020, 2020. [Google Scholar]
- [7].Prevoo ML, van ’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- [8].Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 2012;64:640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cardiel MH, Abello-Banfi M, Ruiz-Mercado R, et al. How to measure health status in rheumatoid arthritis in non-English speaking patients: validation of a Spanish version of the Health Assessment Questionnaire Disability Index (Spanish HAQ-DI). Clin Exp Rheumatol 1993;11:117–21. [PubMed] [Google Scholar]
- [10].Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–63. [DOI] [PubMed] [Google Scholar]
- [11].Engvall IL, Elkan AC, Tengstrand B, et al. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical disability, and low bioavailable insulin-like growth factor. Scand J Rheumatol 2008;37:321–8. [DOI] [PubMed] [Google Scholar]
- [12].Fearon K, Evans WJ, Anker SD. Myopenia-a new universal term for muscle wasting. J Cachexia Sarcopenia Muscle 2011;2:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Su C-M, Hu S-L, Sun Y, et al. Myostatin induces tumor necrosis factor-α expression in rheumatoid arthritis synovial fibroblasts through the PI3K-Akt signaling pathway. J Cell Physiol 2019;234:9793–801. [DOI] [PubMed] [Google Scholar]
- [14].Hu S-L, Chang A-C, Huang C-C, et al. Myostatin Promotes Interleukin-1β Expression in Rheumatoid Arthritis Synovial Fibroblasts through Inhibition of miR-21-5p. Front Immunol 2017;8:1747–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang L, Rajan V, Lin E, et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J 2011;25:1653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lin J-Z, Liang J-J, Ma J-D, et al. Myopenia is associated with joint damage in rheumatoid arthritis: a cross-sectional study. J Cachexia Sarcopenia Muscle 2019;10:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kerschan-Schindl K, Ebenbichler G, Föeger-Samwald U, et al. Rheumatoid arthritis in remission: decreased myostatin and increased serum levels of periostin. Wien Klin Wochenschr 2019;131:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Szalai AJ, van Ginkel FW, Dalrymple SA, et al. Testosterone and IL-6 requirements for human C-reactive protein gene expression in transgenic mice. J Immunol 1998;160:5294–9. [PubMed] [Google Scholar]
- [19].Gitlin JD, Gitlin JI, Gitlin D. Localizing of C-reactive protein in synovium of patients with rheumatoid arthritis. Arthritis Rheum 1977;20:1491–9. [DOI] [PubMed] [Google Scholar]
- [20].Muñoz-Cánoves P, Scheele C, Pedersen BK, et al. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J 2013;280:4131–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schakman O, Kalista S, Barbé C, et al. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 2013;45:2163–72. [DOI] [PubMed] [Google Scholar]
- [22].Wang R, Jiao H, Zhao J, et al. Glucocorticoids enhance muscle proteolysis through a myostatin-dependent pathway at the early stage. PLoS One 2016;11:e0156225–1156225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Masuko K. Rheumatoid cachexia revisited: a metabolic co-morbidity in rheumatoid arthritis. Front Nutr 2014;1:20–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Elkan AC, Engvall IL, Tengstrand B, et al. Malnutrition in women with rheumatoid arthritis is not revealed by clinical anthropometrical measurements or nutritional evaluation tools. Eur J Clin Nutr 2008;62:1239–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


