Abstract
This retrospective cohort study aimed to compare the effectiveness of conventional treatment and ultra-early application of negative pressure wound therapy (NPWT) in patients with snakebites.
Patients who visited the emergency department within 24 hours after a snakebite were assigned to the non- NPWT or NPWT group. Swelling resolution time and rates of necrosis, infection, and operations were compared between the 2 groups. The Stony Brook Scar Evaluation Scale was used to measure short- and long-term wound healing results.
Among the included 61 patients, the swelling resolution time was significantly shorter in the NPWT group than in non- NPWT group (P = .010). The NPWT group showed lower necrosis (4.3% versus 36.8%; P = .003) and infection (13.2% and 4.3%; P = .258) rates than the non- NPWT group. The median Stony Brook Scar Evaluation Scale scores were higher in the NPWT group than in the non- NPWT group (P< .001).
These findings suggest that ultra-early application of NPWT reduces edema, promotes wound healing, and prevents necrosis in patients with snakebites.
Keywords: edema, negative pressure wound therapy, skin necrosis, snakebites, wound infection
1. Introduction
Globally, snakebites affect 5.5 million people per year, around 94,000 of whom die.[1] Snake venoms are typically classified as hemotoxins, neurotoxins, necrotoxins, cardiotoxins, and nephrotoxins according to the different effects exerted.[2] Typically, snake venoms in Far East Asia do not severely affect the human body, and many victims experience mild to severe local symptoms, such as pain, swelling, and necrosis, at the bite area. In Korea, 192 to 621 people are bitten by snakes annually, and on average, 5 people die of snakebites.[3] Venomous snakes in Korea are divided into 2 taxonomic families: Viperidae and Elapidae. The pit viper, which belongs to the Viperidae family, is the most common venomous snake in Korea and has a hemotoxic and necrotoxic venom, although myotoxic and neurotoxic variants of pit vipers do exist.[2,4] Pit viper venom produces local symptoms, such as necrosis, due to hemotoxins and necrotoxins.[5–7] The primary complaints of patients with snakebites are local symptoms, including pain, swelling, and necrosis, in the bite area, especially in appendages such as fingers. These symptoms are caused by necrotoxins that damage the soft tissue and skeletal muscle.[8] Necrotoxin-induced damage can lead to functional and aesthetic complications and may result in disability. Many studies have investigated the treatment of venomous snakebites and systemic complications,[1,2,9] but few studies have examined local complications and active site treatments, especially in the emergency department (ED).[10,11]
Negative pressure wound therapy (NPWT) is a simple and efficient treatment to promote healing in a variety of complicated wounds.[12] NPWT, also called vacuum-assisted wound closure, refers to wound dressing systems that continuously or intermittently apply subatmospheric pressure to the wound, resulting in the application of negative pressure to the surface of a wound, and the placing of a porous dressing in direct contact with the wound, with a drain attached to the vacuum device.[13] NPWT has become a popular treatment modality for the management of many acute and chronic wounds and has been postulated to modulate the inflammatory response caused by soluble factors, such as inflammatory cytokines, collagenases, and elastases, and to accelerate the reduction in local inflammation.[14]
The effects of NPWT on healing are attributable to primary and secondary mechanisms. The primary mechanisms include macro- and micro-deformation of the wound, tissue fluid removal, and changes in the wound environment, and the secondary mechanisms include hemostasis, inflammation control, cellular responses (eg, division, migration, and angiogenesis), formation of granulation tissues, peripheral nerve reactions, and decreased bioburden. NPWT improves regional blood flow and reduces bacterial proliferation, thereby limiting the opportunity for infection. At the cellular level, NPWT facilitates collagen synthesis, angiogenesis, and formation of granulation tissues.[15–17] Moreover, conventional wound dressing changes must be frequently undertaken, whereas NPWT requires a dressing change every 48 to 72 hours, thus reducing clinical staff workload.[13]
A complementary model, wherein many elements of wound healing are more clearly delineated, has recently been presented; in this model, the wound healing process is divided into 2 main stages: the early phase and the cellular phase. The early phase occurs within the first 24 hours after injury and is followed by the cellular phase.[18] We hypothesized that the application of NPWT as early as possible in the ED would promote wound healing and prevent necrosis in snakebite wounds; we defined ultra-early application of NPWT as an application of NPWT within 24 hours after a snakebite. Accordingly, this retrospective cohort study aimed to determine the effects of ultra-early application of NPWT on snakebites by retrospectively comparing the incidence of necrosis and wound healing quality between patients who did and did not receive NPWT.
2. Methods
2.1. Study design and setting
This retrospective study included victims of snakebites who visited the ED of Chungnam National University Hospital, a university-affiliated 1365-bed care referral center in Daejeon, Korea, from March 2015 to February 2020. Hospital admission criteria were defined as follows: snakebite injury and symptoms such as hemorrhagic bullae, swelling, and necrosis. During the study period, the management of patients with snakebites changed from conventional methods to ones that included adjunctive NPWT. In detail, conventional treatment methods were used for patients who visited the ED from March 2015 to June 2018, and NPWT was added as a treatment method for patients who visited from July 2018 to February 2020. The effects of this transition on wound healing outcomes were analyzed. This study was approved by the Institutional Review Board (CNUH 2019–12-034).
2.2. Participants
The inclusion criteria for this study were:
-
(1)
a confirmed diagnosis of envenomation from a snakebite within 24 hours;
-
(2)
progressive worsening of the swelling extremity; local effect scores were used to evaluate swelling and ecchymosis, and patients with a total score ≥3 in both categories were included[19]; and
-
(3)
the presence of hemorrhagic bullae.
The exclusion criteria were:
-
(1)
persons who had a mental illness diagnosed according to the ICD-10 criteria or those who did not cooperate with treatment even if not diagnosed with any mental illness;
-
(2)
currently breastfeeding or pregnant;
-
(3)
refusal of hospitalization; and
-
(4)
patients with mild symptoms (eg, patients with fang wounds, but without swelling, bullae, or necrosis).
2.3. Variables, data sources and quantitative variables
Wound cleaning and dressing were performed for all patients with snakebites who visited the ED during the study period. Conventional treatment was administered to all patients with snakebites who visited the ED from March 2015 through June 2018. These patients, classified as the non-NPWT group, received wound dressing, splint fixation, an antivenom injection, a tetanus antitoxin injection, antibiotics, fluid-replacement therapy, and bullae removal. All patients with snakebites were treated with 6000 IU of antivenom Agkistrodon within 1 hour of ED visit.
From July 2018 until February 2020, all eligible patients with snakebites received NPWT, in addition to conventional treatments (NPWT group), within 24 hours after being bitten. CuraVAC (CGbio, Seongnam-si, Korea) was used for applying NPWT in accordance with standard treatment protocols, with a negative pressure of 125 mm Hg maintained in continuous mode. The sponge replacement cycle was 72 hours, and maintenance of NPWT was undertaken every 72 hours.
The primary outcome was the presence or absence of necrosis; the secondary outcome was wound healing quality, evaluated using the Stony Brook Scar Evaluation Scale (SBSES). The SBSES is designed for short-term evaluation of scarring within 5 to 10 days following stitch removal, and it evaluates the width, height, and color of the wound, along with suturing marks and overall appearance; the scale grades each assessment item with a score of 0 or 1, with a total score ranging from 0 (very poor) to 5 (very good).[20–22] Total SBSES scores of 0–3 are considered poor outcomes, whereas scores of 4 and 5 are considered good outcomes.
All wound follow-ups were conducted in accordance with the institutional wound-evaluation protocol of the study center. Short- and long-term follow-up periods were defined as 7 days and 6 months, respectively, and wound healing status was measured at each follow-up point using the SBSES. Scoring evaluations are shown in Table 1. In addition, both the infection rate and surgical intervention rate were recorded. In patients requiring surgery, a skin flap was used to cover the skin defect. Wound infection was defined as swelling with a focal rise in temperature and fever.[23] Necrosis was defined as appearance of the necrotic tissue on the wound bed after treatment.[24] Hospitalized patients were directly evaluated on the 7th day, and after being discharged, they were subsequently evaluated in the outpatient clinic at the Department of Plastic Surgery. A 6-month follow-up evaluation was undertaken in the outpatient clinic of the Department of Plastic Surgery. Except for the 7-day and 6-month follow-ups, additional follow-ups were performed as needed, depending on wound conditions. All study participants were evaluated by the same physician.
Table 1.
The Stony Brook Scar Evaluation Scale.
| Scar category | Points |
| Width | |
| >2mm | 0 |
| ≤2 mm | 1 |
| Height | |
| Elevated/depressed in relation to surrounding skin | 0 |
| Flat | 1 |
| Color | |
| Darker than surrounding skin | 0 |
| Same color or lighter than surrounding skin | 1 |
| Hatch marks/Suture marks | |
| Present | 0 |
| Absent | 1 |
| Overall appearance | |
| Poor | 0 |
| Good | 1 |
2.4. Statistical analysis
Statistical analysis was conducted using IBM SPSS Statistics, version 19 (IBM Corp., Armonk, NY), and the 2 study groups were compared. The Fisher exact test was used for the analysis of nominal variables, which are expressed as frequencies (percentages). Continuous variables were tested for normal distributions using the Shapiro–Wilk test. Non-normally distributed variables are expressed as median values (interquartile ranges), whereas normally distributed variables are described as means (± standard deviations). The Student t-test was applied for normally distributed data analyses, whereas the non-parametric Mann–Whitney U-test was used for non-normally distributed data analyses. The Wilcoxon singed-rank test was used to compare the SBSES scores of the short- and long-term results. P-values (P) < .05 were considered statistically significant for all analyses.
3. Results
3.1. Participants and descriptive data
Eighty-one patients with snakebite envenomation visited the ED of Chungnam National University Hospital between March 2015 and February 2020. Based on the study eligibility criteria, 20 patients were excluded, and a total of 61 patients were enrolled (Fig. 1). No statistically significant differences in age, sex ratio, location of injury, admission time after injury, and initial wound status were found between the 2 groups (Table 2). In the NPWT group, the median NPWT application time following a snakebite was 7 (4.0–16.0) hours. Blood tests revealed that the C-reactive protein and hemoglobin levels in both groups were normal (Table 2).
Figure 1.
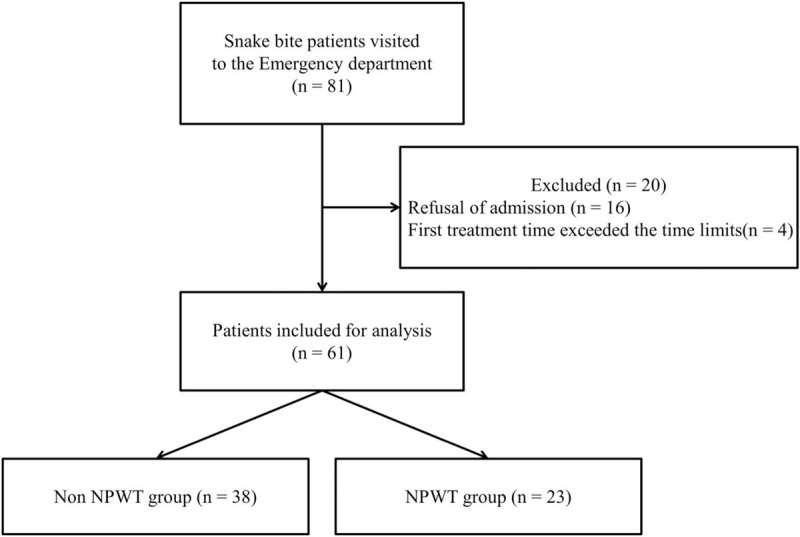
Flow diagram of patient enrollment. A total of 61 patients were enrolled; 38 patients underwent conventional treatment, and 23 received ultra-early NPWT. NPWT, negative pressure wound therapy.
Table 2.
General characteristics, blood test results, and initial wound status of enrolled patients.
| Cohort (n = 61) | Non-NPWT (n = 38) | NPWT (n = 23) | P-values | |
| Male, n (%) | 36 (59.0) | 21 (55.2) | 15 (65.2) | .592 |
| Age, yr median (IQR) | 61 (56–71) | 60 (53.7–69.5) | 66 (58.0–73.0) | .198 |
| Past medical history | ||||
| Hypertension, n (%) | 16 (26) | 9 (23.6) | 7 (30.4) | .765 |
| Diabetes, n (%) | 3 (5) | 0 (0) | 3 (13.0) | .049 |
| Chronic renal disease, n (%) | 1 (2) | 1 (2.6) | 0 (0) | 1.00 |
| Initial laboratory results | ||||
| WBC, x1000/ul, mean (SD) | 8.218 (2.684) | 8.059 (2.821) | 8.480 (2.480) | .413 |
| Hb, g/dl, mean (SD) | 13.80 (1.51) | 13.40 (1.41) | 14.30 (1.54) | .022 |
| NLR, ratio, median (IQR) | 2.75 (1.55–4.37) | 2.31 (1.28–3.84) | 3.94 (1.74–4.91) | .113 |
| CRP, mg/dL, median (IQR) | 0.5 (0.5–0.6) | 0.5 (0.5–0.5) | 0.5 (0.5–0.8) | < .05 |
| BUN, mg/dL, median (IQR) | 16 (13–20) | 17 (13.2–21) | 15 (13–18) | .122 |
| Cr, mg/dL, median (IQR) | 0.76 (0.67–0.93) | 0.76 (0.58–0.93) | 0.76 (0.70–0.94) | .414 |
| INR, ratio, median (IQR) | 0.98 (0.92–1.04) | 1.00 (0.94–1.04) | 0.98 (0.80–1.06) | .290 |
| aPTT, sec, median (IQR) | 28.7 (25.9–31.0) | 29.2 (25.9–31.7) | 28.2 (25.4–30.2) | .188 |
| Antivenin injection time after injury, hr, median (IQR) | 8.0 (4.0–17.0) | 7.5 (4.0–14.6) | 14 (4.0–20.0) | .179 |
| Admission time after injury, hr, median (IQR) | 10 (5–21.5) | 9.0 (5.0–17.3) | 17 (6.0–22.0) | .091 |
| Initial wound status | ||||
| Location | ||||
| Finger, n (%) | 40 (65.6) | 27 (62.5) | 13 (37.5) | .251 |
| Palm, n (%) | 2 (3.3) | 1 (50) | 1 (50) | .718 |
| Foot, n (%) | 14 (23) | 8 (57.1) | 6 (42.9) | .653 |
| Other areas, n (%) | 5 (8.2) | 2 (40) | 3 (60) | .287 |
| Skin color change, n, (%) | 18 (29.5) | 9 (50) | 9 (50) | .160 |
3.2. Primary results
The median duration to the resolution of swelling in the wound area was 4.0 (3.0–5.0) days and 3.0 (3.0–4.0) days in the non-NPWT and NPWT groups, respectively, a statistically significant difference (P = .010; Table 3). Three surgeries were performed in the non-NPWT group, whereas none were performed in the NPWT group; however, this difference was not statistically significant (P = .234; Table 3). Necrosis rates were 36.8% and 4.3% in the non-NPWT and NPWT groups, respectively, and this difference was significant (P= .003; Table 3). There were 12 cases of necrosis and 1 case of finger necrosis in the non-NPWT and NPWT groups, respectively. In addition, patients in the non-NPWT group showed extensive necrosis that required surgery, whereas patients in the NPWT group experienced focal necrosis (Fig. 2). The infection rates were 13.2% and 4.3% in the non-NPWT and NPWT groups, respectively, a not statistically significant difference (P = .258; Table 3).
Table 3.
Treatment results in the 2 study groups.
| Cohort (n = 61) | Non-NPWT (n = 38) | NPWT (n = 23) | Relative risk (95% CI) | P-values | |
| Swelling full resolution time, day, median (IQR) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | 3.0 (3.0–4.0) | .010 | |
| Necrosis, n (%) | 15 (24.6) | 14 (93.3) | 1 (6.7) | 0.660 (0.510–0.885) | .003 |
| Finger, n (%) | 13 (21.3) | 12 (92.3) | 1 (7.7) | 0.602 (0.415–0.873) | .013 |
| Palm, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 | |
| Foot, n (%) | 2 (3.3) | 2 (100) | 0 (0) | 0.750 (0.503–1.119) | .202 |
| Other areas, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 | |
| Infection, n (%) | 6 (9.8) | 5 (83.3) | 1 (16.7) | 0.908 (0.780–1.056) | .258 |
| Initial skin color change | .160 | ||||
| Yes, n (%) | 18 (29.5) | 9 (50) | 9 (50) | ||
| Necrosis, n (%) | 5 (27.8) | 1 (5.6) | 0.500 (0.232–1.076) | .046 | |
| No, n (%) | 43 (70.5) | 29 (67.4) | 14 (32.6) | ||
| Necrosis, n (%) | 9 (20.9) | 0 (0) | 0.690 (0.540–0.880) | .019 | |
| Operation, n (%) | 3 (4.9) | 3 (100) | 0 (0) | 0.921 (0.893–1.011) | .234 |
Figure 2.
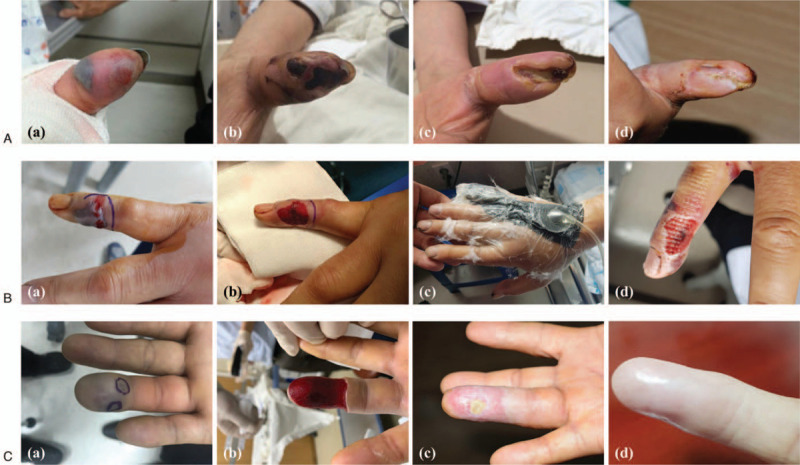
A) A 66-year-old female patient with a snakebite on her left thumb. (A) Traditional treatment. (B) After 15 days, there was progression of necrosis, including hemorrhagic bullae at the bite site. (C) Wound debridement followed by a skin flap graft. (D) After 2 months, the skin defect persisted. B) A 51-year-old man presented with a snakebite on the right little finger. (A) Skin color changes and hemorrhagic bullae were observed during examination in the emergency department. (B) Wound debridement was undertaken. (C) The patient received ultra-early NPWT. (D) On day 3 of hospitalization, the wound healed without necrosis. C) A 61-year-old man with a snakebite on the right fourth finger. (A) A large hemorrhagic bulla and skin color changes were identified during the visit at the emergency department. (B) Removal of hemorrhagic bullae and wound debridement was undertaken. Ultra-early NPWT was applied, and the patient was discharged 3 days later. (C) Outpatient follow-up after 24 days. (D) At the 6-month follow-up, the patient completely recovered without complications. NPWT, negative pressure wound therapy.
3.3. Outcome data
In short- and long-term follow-up evaluations, the median SBSES scores were 4 (3.75–4) and 3 (2.75–3) in the non-NPWT group and 4 (4–5) and 5 (5–5) in the NPWT group (P < .001), respectively (Fig. 3). In particular, the median SBSES scores in the long-term follow-up evaluation were lower in the non-NPWT group than in the NPWT group (P < .001; Fig. 3).
Figure 3.
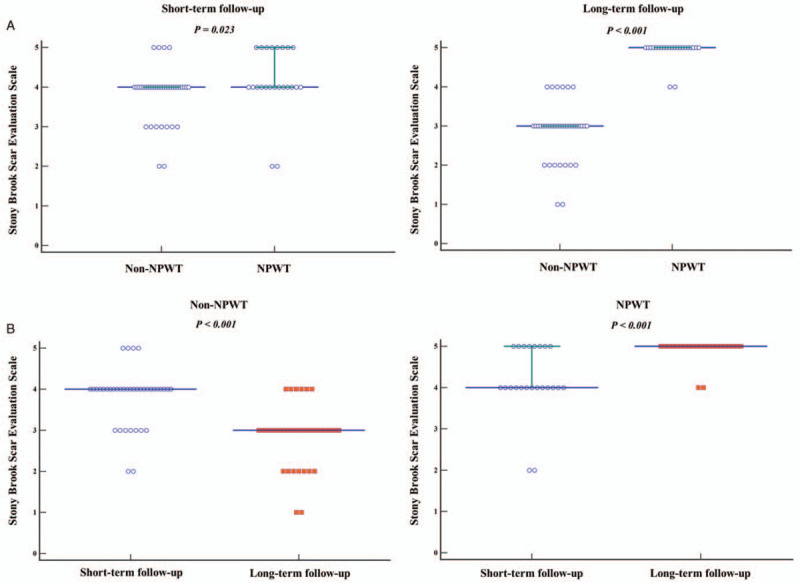
A) Comparison of the Stony Brook Scar Evaluation Scale results between Non-NPWT and NPWT groups using the Mann–Whitney U-test. Solid lines indicate the median values with interquartile ranges. B) Comparison of the Stony Brook Scar Evaluation Scale between short and long-term follow-up evaluations for each group using the Wilcoxon signed-rank test. Solid lines indicate median values with interquartile ranges. Circles indicate the short-term follow-up outcomes, whereas squares indicate long-term follow-up outcomes based on the scores of the scar evaluation scale. NPWT, negative pressure wound therapy.
4. Discussion
In this retrospective study, the NPWT group showed marked decreases in necrosis and time to resolution of swelling, and better cosmetic results than the non-NPWT group. The NPWT group had a higher SBSES score than the non-NPWT group. In particular, the non-NPWT group showed a statistically significant decrease in median SBSES scores, from 4 (3.75–4) to 3 (2.75–4), in the long-term follow-up evaluation than in the short-term follow-up evaluation. Therefore, the non-NPWT group exhibited poorer outcomes than the NPWT group, probably because the ultra-early application of NPWT reduces the wound extent through negative pressure and increases blood flow, which aids wound recovery.[25] In addition, worsening of wounds in the non-NPWT group might be attributable to failed prevention of the progression of necrosis in the ultra-early stages (Fig. 2).
4.1. Interpretations
Snake venoms contain both enzymatic and non-enzymatic proteins, the latter being primarily responsible for the poisoning effects. Following a snakebite, toxins are injected into the body, and local symptoms, such as burning pain, occur within minutes. Pit viper venom produces more local than systemic symptoms due to hemotoxins and necrotoxins.[5] Edema and rashes develop within hours, and swelling becomes severe. Hemorrhagic plaques and bullae develop, and if not properly treated, severe tissue necrosis can occur. Moreover, effects on the lymphatic system are common and may include lymphadenitis and lymphadenopathy, which can lead to serious complications.[26] Therefore, initial treatment in the ED is critically important when treating snakebites. Snakebite treatments involve general wound management, such as irrigation, gauze dressing, and removal of hemorrhagic bullae. However, conventional treatment may lead to long recovery time and poor functional and cosmetic results because of the effects of necrotoxins, which cause skin and soft tissue necrosis. In many cases, the skin or soft tissue defect site needs to be reconstructed through skin transplantation and flap surgery.[26] The bullae formed by the snakebite are filled with toxins and can function as a venous depot inaccessible to the antivenom, and are subsequently able to release it.[27] Accordingly, it is important to remove hemorrhagic bullae and facilitate blood circulation and wound recovery in the bite area.[5,28] However, removal of hemorrhagic bullae alone may not fully lead to wound healing in patients with snakebites, and additional treatment, such as NPWT, is necessary in the ED.
NPWT clinically enhances wound vascular perfusion, formation of granulation tissue, and removal of germs and is associated with increased survival after flap and skin transplantation; in addition, it reduces edema or compartment syndrome due to burns, crush injuries, and necrotizing wounds.[12,13] In patients with snakebites, NPWT may induce toxin entrapment, thereby preventing the necrotoxin from spreading further into the wound; it has been thought that the effect occurs only focally by preventing the necrotoxin from diffusing into the surrounding tissues. Therefore, natural healing can be promoted by sequestering the toxin at the necrotic site. Moreover, NPWT can promote wound healing through the regulation of cytokines or anti-inflammatory profiles and by mechanoreceptor- and chemoreceptor-mediated cell signaling, angiogenesis, extracellular matrix remodeling, and the formation of granulation tissue.[29] In addition, mitochondria are the primary location for cellular respiration, and overexpression of mitochondrial antioxidant manganese superoxide dismutase prevents cell death caused by oxidative stimulation.[30–32] In a recent study, using patient samples and rodent models of acute injury, significant accumulation of mitochondrial antioxidant manganese superoxide dismutase, as well as higher enzymatic activity, was observed in tissues in the NPWT group, and the underlying mechanism is thought to be related to reduced necrosis.[33] Therefore, NPWT can help reduce edema, necrosis, and infection in the distal end of the fingers, which receive less blood flow.
Some studies have demonstrated that NPWT appears safe and effective in managing acute, contaminated wounds in patients meeting sepsis criteria.[34] The clinical efficacy and cost-effectiveness of NPWT have been extensively investigated in several randomized controlled trials and meta-analyses,[35,36] and NPWT has been widely accepted for the treatment of open and infected wounds and for accelerated treatments in many clinics. A previous study found that the application of multiple, small incisions combined with NPWT on the incision sites was effective in controlling the release of inflammatory cytokines and alleviating systemic inflammatory reactions.[37] However, that study only investigated the effects of NPWT on swelling and inflammation but did not specifically examine wound healing and skin necrosis. In the present study, 2 groups of patients with snakebites were analyzed: those who underwent basic, conventional treatment procedures and those who received additional negative pressure therapy within 24 hours after snake bites. We found that necrosis and time to swelling resolution were reduced in the NPWT group. It is thought that by increasing blood flow through negative pressure, nutrients are supplied to areas with circulatory disruption, such as edema. In addition, NPWT may help heal wounds by preventing blood retention or venous and lymphatic fluid retention through the direct influence or extravasation of the venom.[38] Ultra-early application of NPWT resulted in the effective removal of tertiary fluids and debris, thus reducing swelling and necrosis.
4.2. Limitations
This study has some limitations. First, the present study was a single-center study, and the number of study participants was relatively small. Second, this was a retrospective study; thus, it is difficult to fully demonstrate the effectiveness of NPWT in snakebite wounds. Therefore, further multicenter, prospective studies are needed to decisively demonstrate the effects of the ultra-early application of NPWT in snakebite patients.
5. Conclusions
This study found that the ultra-early application of NPWT reduced edema, promoted wound healing, and prevented necrosis in patients with snakebites. The findings demonstrate the high effectiveness of the ultra-early application of NPWT in patients with snake bites. Accordingly, we recommend NPWT application in patients with snakebites in the ED setting as soon as possible to promote wound healing and prevent necrosis.
Author contributions
Conceptualization: Kwan Jae Kim, Jung Soo Park.
Data curation: Kwan Jae Kim, Jung Soo Park, Won Joon Jeong, Se Kwang Oh.
Formal analysis: Jinwoong Lee, Jung Soo Park, Se Kwang Oh.
Investigation: Jin Hong Min, Yeon Ho You, Byung Kook Lee, Dong Hoon Lee.
Methodology: Jin Hong Min, Seung Whan Kim, Yeon Ho You, Dong Hoon Lee.
Project administration: Jin Hong Min, Seung Ryu, Byung Kook Lee, Dong Hun Lee.
Resources: Insool Yoo.
Software: Hong Joon Ahn.
Supervision: Insool Yoo, Yong Chul Cho, Yong Nam In.
Validation: Jung Soo Park, Yong Nam In, Chang Shin Kang, Hyunwoo Kyung.
Visualization: Won Joon Jeong, Yong Nam In, Hyunwoo Kyung.
Writing – original draft: Kwan Jae Kim.
Writing – review & editing: Kwan Jae Kim.
Footnotes
Abbreviations: ED = emergency department, NPWT = negative pressure wound therapy, SBSES = Stony Brook Scar Evaluation Scale.
How to cite this article: Kim KJ, Min JH, Yoo I, Kim SW, Lee J, Ryu S, You YH, Park JS, Jeong WJ, Cho YC, Oh SK, In YN, Ahn HJ, Kang CS, Kyung H, Lee BK, Lee DH, Lee DH. Negative pressure wound therapy for skin necrosis prevention after snakebite in the emergency department: a retrospective cohort study. Medicine. 2021;100:3(e24290).
The authors received no financial support for the research, authorship, and/or publication of this article.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The scale incorporates assessments of individual attributes using a binary response (1 or 0) for each variable, as well as overall appearance, to yield a score ranging from 0 (worst) to 5 (best).
aPTT = activated partial thromboplastin time; BUN, blood urea nitrogen; Cr, creatinine; CRP, C-reactive protein; Hb, hemoglobin; INR, international normalized ratio; IQR, interquartile range; NPWT, negative-pressure wound therapy; SD, standard deviation; WBC, white blood cell.
CI = confidence interval; NPWT = negative pressure wound therapy.
References
- [1].Kasturiratne A, Wickremasinghe AR, de Silva N, et al. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med 2008;5:e218–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Adukauskiene D, Varanauskiene E, Adukauskaite A. Venomous snakebites. Medicina (Kaunas, Lithuania) 2011;47:461–7. [PubMed] [Google Scholar]
- [3].Lim H, Kang HG, Kim KH. Antivenom for snake bite in Korea. J Korean Med Assoc 2013;56:1091–103. [Google Scholar]
- [4].Jun DH, Lee DP, Choi WI. Initial assessment of the snakebites with local effects. Journal of The Korean Society of Emergency Medicine 2004;15:523–30. [Google Scholar]
- [5].Jin SC, Lee JW, Yang SJ, et al. Consideration of factors associated with complications and systemic symptoms of snake bites. Journal of The Korean Society of Emergency Medicine 2008;19:686–96. [Google Scholar]
- [6].Kang H, Lim H, Kim KH. Antivenom for snake bite in Korea. J Korean Med Assoc 2013;56:1091–103. [Google Scholar]
- [7].Rha JH, Kwon SM, Oh JR, et al. Snakebite in Korea: a guideline to primary surgical management. Yonsei Med J 2015;56:1443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Harris JB, Cullen MJ. Muscle necrosis caused by snake venoms and toxins. Electron Microsc Rev 1990;3:183–211. [DOI] [PubMed] [Google Scholar]
- [9].Srirangan A, Pushpakumara J, Wanigasuriya K. A life-threatening complication due to pulmonary haemorrhage following hump-nosed viper bite. BMC pulmonary medicine 2020;20:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pitt KA, Stanley BJ. Negative pressure wound therapy: experience in 45 dogs. Veterinary surgery: VS 2014;43:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rui-Feng C, Li-Song H, Ji-Bo Z, et al. Negative pressure wound therapy for serious dog bites of extremities: a prospective randomized trial. Am J Emerg Med 2016;34:1006–10. [DOI] [PubMed] [Google Scholar]
- [12].Rahmanian-Schwarz A, Willkomm LM, Gonser P, et al. A novel option in negative pressure wound therapy (NPWT) for chronic and acute wound care. Burns 2012;38:573–7. [DOI] [PubMed] [Google Scholar]
- [13].Morykwas MJ, Simpson J, Punger K, et al. Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg 2006;117:121s–6s. [DOI] [PubMed] [Google Scholar]
- [14].Simman R, Forte R, Silverberg B, et al. A comparative histological study of skin graft take with tie-over bolster dressing versus negative pressure wound therapy in a pig model: a preliminary study. Wounds 2004;16:76–80. [Google Scholar]
- [15].Isci E, Canter HI, Dadaci M, et al. The efficacy of negative pressure wound therapy on chemotherapeutic extravasation ulcers: An experimental study. Indian J Plast Surg 2014;47:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kim BS, Choi WJ, Baek MK, et al. Limb salvage in severe diabetic foot infection. Foot & ankle international 2011;32:31–7. [DOI] [PubMed] [Google Scholar]
- [17].Scherer SS, Pietramaggiori G, Mathews JC, et al. The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg 2008;122:786–97. [DOI] [PubMed] [Google Scholar]
- [18].Nguyen DT, Orgill D, Murphy GF. The pathophysiologic basis for wound healing and cutaneous regeneration 2009;25–57. [Google Scholar]
- [19].Scharman EJ, Noffsinger VD. Copperhead snakebites: clinical severity of local effects. Ann Emerg Med 2001;38:55–61. [DOI] [PubMed] [Google Scholar]
- [20].Singer AJ, Arora B, Dagum A, et al. Development and validation of a novel scar evaluation scale. Plast Reconstr Surg 2007;120:1892–7. [DOI] [PubMed] [Google Scholar]
- [21].Min JH, You YH, Cho YC, et al. Comparison of cosmetic appearances after facial lacerations repaired by junior residents and experts. Am J Emerg Med 2019;37:817–22. [DOI] [PubMed] [Google Scholar]
- [22].Min JH, Park KH, Choi HL, et al. Usefulness of direct W-plasty application to wound debridement for minimizing scar formation in the ED. Am J Emerg Med 2017;35:1804–9. [DOI] [PubMed] [Google Scholar]
- [23].Kashha AM, Zailai AH, Alsaadi WS, et al. Causes and management of wound infection. The Egyptian Journal of Hospital Medicine 2017;68:1436–41. [Google Scholar]
- [24].Grey JE, Enoch S, Harding KG. Wound assessment. BMJ 2006;332:285–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Torbrand C, Ugander M, Engblom H, et al. Wound contraction and macro-deformation during negative pressure therapy of sternotomy wounds. J Cardiothorac Surg 2010;5:75–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Park SS, Ryoo HS, Suh IS. Reconstruction of the soft tissue defect of the lower leg by distally based superficial sural artery flap using the endoscope. J Korean Soc Plast Reconstr Surg 2001;28:184–90. [Google Scholar]
- [27].Lin C-C, Wang P-J, Liu C-C. Venom concentrations in blisters and hemorrhagic bullae in a patient bitten by a Taiwan habu (Protobothrops mucrosquamatus). J Revista da Sociedade Brasileira de Medicina Tropical 2019;52. [DOI] [PubMed] [Google Scholar]
- [28].Han SK, Kim IS, Ryu S, et al. The Effectiveness of antivenin in treating snake bites resulting in minimal clinical symptoms. Journal of The Korean Society of Emergency Medicine 2007;18:577–83. [Google Scholar]
- [29].Glass GE, Murphy GF, Esmaeili A, et al. Systematic review of molecular mechanism of action of negative-pressure wound therapy. BJS (British Journal of Surgery) 2014;101:1627–36. [DOI] [PubMed] [Google Scholar]
- [30].Epperly MW, Bernarding M, Gretton J, et al. Overexpression of the transgene for manganese superoxide dismutase (MnSOD) in 32D cl 3 cells prevents apoptosis induction by TNF-alpha, IL-3 withdrawal, and ionizing radiation. Exp Hematol 2003;31:465–74. [DOI] [PubMed] [Google Scholar]
- [31].Epperly MW, Osipov AN, Martin I, et al. Ascorbate as a “redox sensor” and protector against irradiation-induced oxidative stress in 32D CL 3 hematopoietic cells and subclones overexpressing human manganese superoxide dismutase. Int J Rad Oncology, biology, physics 2004;58:851–61. [DOI] [PubMed] [Google Scholar]
- [32].Southgate TD, Sheard V, Milsom MD, et al. Radioprotective gene therapy through retroviral expression of manganese superoxide dismutase. J Gene Med 2006;8:557–65. [DOI] [PubMed] [Google Scholar]
- [33].Bellot GL, Dong X, Lahiri A, et al. MnSOD is implicated in accelerated wound healing upon Negative Pressure Wound Therapy (NPWT): A case in point for MnSOD mimetics as adjuvants for wound management. Redox Biol 2019;20:307–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shweiki E, Gallagher KE. Negative pressure wound therapy in acute, contaminated wounds: documenting its safety and efficacy to support current global practice. Int Wound J 2013;10:13–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Searle R, Milne J. Tools to compare the cost of NPWT with advanced wound care: an aid to clinical decision-making. Wounds UK 2010;6. [Google Scholar]
- [36].Kim JJ, Franczyk M, Gottlieb LJ, et al. Cost-effective alternative for negative-pressure wound therapy. Plast Reconstr Surg Glob Open 2017;5:e1211–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zeng F, Chen C, Chen X, et al. Small incisions combined with negative-pressure wound therapy for treatment of protobothrops mucrosquamatus bite envenomation: a new treatment strategy. Med Sci Monit 2019;25:4495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Huang C, Leavitt T, Bayer LR, et al. Effect of negative pressure wound therapy on wound healing. Current Problems in Surgery 2014;51:301–31. [DOI] [PubMed] [Google Scholar]


