Abstract
Background.
Kidney allocation system allows blood type B candidates accept kidneys from A2/A2B donors. There is no mandate by UNOS on which the anti-A2 level is acceptable. We aimed to investigate the safety of kidney transplant in blood group B patients with anti-A2 titers ≤16.
Methods.
We performed 41 A2-incompatible kidney transplants in blood group B recipients between May 2015 and September 2019. Clinical outcomes were compared with a control group of 75 blood group B recipients who received blood group compatible kidney transplantation at the same period.
Results.
Of the 41 recipients, 85% were male, 48% African American, with a median age of 53 (20–73) y. Thirty-eight (93%) were deceased-donor and 3 (7%) were living-donor kidney transplant recipients. Pretransplant anti-A2 IgG titers were 2 in 16, 4 in 9, 8 in 6, and 16 in 5 and too weak to titer in 5 recipients. Eight patients had pretransplant donor-specific antibodies. During a median follow-up of 32.6 mo (6–57.3) patient and graft survival were 100% and 92% in the A2-incompatible kidney transplant group, and 91% and 92% in the blood group compatible group, respectively. Twelve A2-incompatible recipients underwent a 21 clinically indicated kidney biopsies at a median 28 d (6–390) after transplantation. None of the patients developed acute antibody-mediated rejection and 2 patients (5%) had acute T-cell–mediated rejection. Interestingly, peritubular capillary C4d positivity was seen in 7 biopsies which did not have any findings of acute rejection or microvascular inflammation but not in any of the rejection-free biopsies in the control group. C4d positivity was persistent in 5 of those patients who had follow-up biopsies.
Conclusions.
A2-incompatible transplantation is safe in patients with anti-A2 titers ≤16 with excellent short-term kidney allograft outcomes. C4d positivity is frequent in allograft biopsies without acute rejection.
Blood group A is divided into A1 and A2 and approximately 80% of the blood group A is A1. From an antigen perspective, A1 and B blood group donors are considered “major,” and A2 donors “minor” challenges to ABO-incompatible kidney transplantation.1,2 Donor kidneys from individuals of the A2 blood group subtype are inherently less immunogenic as a function of a lower density of the A-antigen immunodominant sugar, N-acetylgalactosamine.3 Blood group A antigen expression is consistently low in the renal cortex and the entire vascular bed endothelium. Proximal and distal tubule and glomerular epithelial staining for A antigens is also very low to nonexistent in kidneys from blood type A2 donors. This explains the weaker antigenicity of A2 and makes donors of A2 group an attractive option to transplant group non-A recipients.
Historically, most blood group B candidates awaiting deceased-donor renal transplantation are African American and Hispanic patients and are less likely to be transplanted than candidates of any other blood group. The likelihood of transplantation after waiting on the list for 2 y was 18.3% for blood group B, 22.4% for blood group O, 38% for blood group A, and 52.6% for blood group AB candidates.4 In fact, in 2013, among blood group B candidates on the kidney transplant waitlist, >70% represented ethnic minorities. Transplanting A2/A2B kidney into B recipients could result in equalization of waiting time between all blood groups and similar patient and allograft survival.5
Given the aforementioned data, UNOS implemented in 2014 a new Kidney Allocation System with new rules to provide greater access to transplantation to blood type B candidates who can safely accept a kidney from an A2/A2B. While A1-incompatible kidney transplantation occurs only in living kidney transplantation and requires desensitization protocols using plasmapheresis, intravenous immunoglobulin (IVIG), rituximab or splenectomy, no desensitization is required in most A2/A2B kidneys to B recipients.6-8 Although blood group A2-incompatible transplantation is regarded as relatively low-risk, significant rejection has been reported in some studies.9,10 The anti-A2 IgG level is important for a successful A2-incompatible transplantation. Currently, there is no mandate by UNOS regarding what anti-A2 titers are acceptable, and as suggested by a comprehensive review, most centers accept anti-A2 titers <8.2 More flexibility in accepting higher anti-A2 titers would further increase the number of A2/A2B to B transplants being performed. In our study, we aimed to investigate the safety of kidney transplant in patients with anti-A2 titers ≤16 and reviewed previous studies of A2-incompatible transplantation reporting anti-A titers.
MATERIAL AND METHODS
Patient Population
Clinical data on kidney transplants in blood group B recipients (A2-incompatible and blood group compatible) that were performed between May 2015 and September 2019 at our institution were collected through retrospective chart review. The study was approved by Albert Einstein Medical School IRB. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism”.
Immunosuppression Protocol
In A2-incompatible kidney transplants, induction therapy was antithymocyte globulin (except 1 patient who received basiliximab) at 1.5 mg/kg for 3 doses. In blood-group-compatible kidney transplant recipients, antithymocyte globulin induction was used if panel reactive antibody titer was >20% and the remaining received basiliximab. Patients with donor-specific anti–HLA antibodies (DSA) received IVIG 0.5 mg/kg for 3 doses, and antithymocyte globulin was given at 1.5 mg/kg for 4 doses. Tacrolimus, mycophenolate mofetil, and prednisone were used for maintenance immunosuppression.
Anti-A2 IgG/IgM Titers
Anti-A2 titers of all patients were studied at the Blood Bank of our institution. Serial dilutions of serum were made with saline. To check for IgG/IgM titers, the samples were suspended with A2 cells, washed with PBS or normal saline, and read immediately after centrifugation for the IgM titer. Dithiothreitol was not used, and for IgM, direct agglutination without enhancement was used. For IgG titers, antihuman IgG was added, samples were incubated for 30 min at 37°C, washed and then centrifuged. The test results are examined macroscopically, graded, and the reactions are recorded as 0–4. The results are reported as the reciprocal of the highest dilution that produces 1+W barely visible macroscopic agglutination.
Methods for DSA, Complement-dependent and FC Cross-match
Anti-HLA antibodies were tested using Luminex HLA Single Antigen Beads (LABScreen products, One Lambda Inc, Canoga Park, CA). The cutoff value for mean fluorescence intensity (MFI) was 1000. The primary method used for cross-match was flow cytometry (FC). Cells were analyzed using an FC500 cytometer (Beckman Coulter, Miami, FL). Patients with preformed low-level DSA or with positive FC cross-match were also analyzed by complement-dependent cytotoxicity (CDC) cross-match. Patients with preformed DSA were accepted for transplantation based on a negative CDC cross-match and an FC cross-match with channel shift values <150 and 250 for T-cell and B-cell cross-match, respectively.
Histopathology
Biopsies were examined by light microscopy using hematoxylin and eosin, periodic acid-Schiff, Masson Trichrome and C4d immunoperoxidase stains. Immunoperoxidase staining for C4d was performed on paraffin embedded sections using a polyclonal rabbit antihuman antibody (Cell Marque) at a dilution of 1:100 with the Dako Envision system. Evaluation of the biopsies was based on the Banff acute and chronic lesion grading system.11
Statistical Analysis
Characteristics of the sample were summarized for each group using descriptive statistics including counts and percentages for categorical variables and medians and range for continuous variables. Comparisons were performed using the Fisher test or the Chi-Square test for categorical variables and the nonparametric Wilcoxon Rank-Sum Test for continuous variables. Statistical analysis was performed with SAS version 9.4 (SAS Institute Inc., Durham, NC)
RESULTS
Demographics
A total of 41 A2-incompatible and 75 blood-group-compatible kidney transplants were performed at our institution during study period, and demographics are summarized at Table 1. Of the 41 A2-incompatible kidney transplant recipients, 35 patients (85%) were male, 20 (49%) were African American, 11 (27%) were Hispanic with a median age of 53 (20–73). Thirty-eight (93%) were deceased-donor and 3 (7%) were living-donor kidney transplant recipients. Four were preemptive transplant recipients, and the rest were on dialysis before transplant with a median time on dialysis of 3 y (0.33–12). Two patients (5%) had a history of prior transplant. Diabetes (41%) and hypertension (34%) were the most common cause of end-stage renal disease. Median body mass index was 29.1 kg/m2 (21.5–41.8). When compared to blood group compatible kidney transplant recipients, the only statistically significant difference in demographic characteristics was more deceased-donor transplant recipients in the A2-incompatible kidney transplant group (93% versus 75%, respectively)
TABLE 1.
Baseline characteristics of A2 incompatible kidney transplant recipients compared to blood group compatible transplants
| A2 incompatible transplant, N =41 | Blood group compatible transplant, N = 75 | P | |
|---|---|---|---|
| Age, median (range), y | 53 (20–73) | 58 (24–79) | 0.70 |
| Sex, male | 35 (85%) | 52(69%) | 0.057 |
| Race | 0.18 | ||
| African American | 20 (49%) | 36 (48%) | |
| Hispanic | 11 (27%) | 11 (15%) | |
| Previous history of transplantation | 2 (5%) | 10 (13%) | 0.21 |
| Preemptive transplant | 4 (10%) | 7 (9%) | 0.94 |
| Median time on dialysis years (range) | 3 (0.33–12) | 3 (0–26) | |
| Type of transplant | 0.024 | ||
| Deceased donor | 38 (93%) | 56 (75%) | |
| Living donor | 3 (7%) | 19 (25%) | |
| Etiology of ESRD | 0.92 | ||
| Diabetes | 17 (41%) | 31 (41%) | |
| Hypertension | 14 (34%) | 28 (37%) | |
| Others | 10 (25%) | 16 (21%) | |
| BMI, median (range), kg/m2 | 29.1 (21.5–41.8) | 27.7 (17.3–45.5) | 0.10 |
| KDPI, median (range), % | 52 (2–86) | 54 (12–96) | 0.70 |
| Cold ischemia time, median (range) h | 30.6 (3–44) | 19.9 (0.2–49.6) | 0.043 |
| Donor age, median (range) y | 42 (16–65) | 36 (2–69) | 0.069 |
| Donor sex, male | 26 (63%) | 47 (63%) | 0.99 |
| Donor race, Caucasian | 26 (63%) | 33 (44%) | 0.032 |
| Donor final creatinine, median (range) mg/dL | 0.8 (0.3–5.4) | 1.41 (0.35–8.08) | 0.012 |
| PHS high-risk donor | 15 (37%) | 19 (25%) | 0.17 |
| Pretransplant PRA 0% | 21 (51%) | 33 (44%) | 0.38 |
| Pretransplant PRA 1%–20% | 6 (15%) | 7 (9%) | |
| Pretransplant PRA 20%–100% | 14 (34%) | 35 (47%) | |
| Pretransplant DSA n, % | 8 (20%) | 16 (21%) | 0.82 |
| Class I | 4 (10%) | 3 (4%) | |
| Class II | 3 (7%) | 11 (15%) | |
| Class I and II | 1 (2%) | 2 (3%) | |
| DSA Class I mean MFI (IQR) | n = 43071 (1076–6232) | n = 55265 (1605–6891) | 0.27 |
| DSA Class II mean MFI (IQR) | n = 42218 (1181–2771) | n = 142054 (1149–14 343) | 0.71 |
| Flow cytometry cross-match positivity, % | 4 (10%) | 7 (9%) | 0.94 |
BMI, body mass index; DSA, donor-specific Anti-HLA antibody; ESRD, end-stage renal disease; KDPI, kidney donor profile index; MFI, mean fluorescence intensity; PHS, public health services; PRA, panel reactive antibody.
The median donor age was 42 y (16–65), 26 (63%) were males, and 26 (63%) were Caucasians in the A2-incompatible kidney transplant group. The median kidney donor profile index was 52 (2–86), and median cold ischemia time was 30.6 (3–44) h. Median donor final creatinine was 0.8 mg/dL (0.3–5.4). Fifteen (37%) patients received Public Health Service high-risk kidneys.
Immunological Profile
The pretransplant anti-A2 IgG titers were 2 in 16, 4 in 9, 8 in 6, and 16 in 5 and too weak to titer in 5 recipients. The pretransplant anti-A2 IgM titers were 2 in 14, 4 in 11, 8 in 6, and 16 in 5 and too weak to titer in 4 recipients (Figure 1). Only 1 patient did not have anti-A2 IgM titers checked pretransplant. In the blood group kidney transplant recipients, 83% had anti-A2 titers less than 1:16, 11% had 1:16, and 6% had more than 1:16.
FIGURE 1.
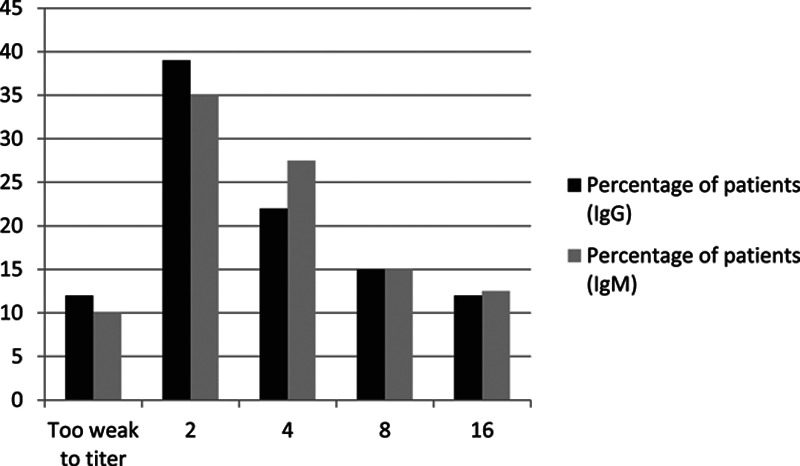
Distribution of anti-A IgG/IgM titers.
Twenty-one patients had no detectable anti-HLA antibodies, 6 had panel reactive antibody 1%–20%, and 14 had panel reactive antibody >20%. Eight patients had pretransplant DSA (4 had class I, 3 class II, and 1 patient both class I and II DSA). Three patients (2 with class I DSA and 1 with both class I and II DSA) had either a positive T-cell and/or B-cell FC cross-match. One patient with no pretransplant DSAs had a positive B-cell FC cross-match, due to a possibly non-HLA antibody. In all cases, channel shift values were <150 and 250 for T-cell and B-cell cross-match, respectively, and CDC cross-match was negative.
There was no statistically significant difference in any immunological profile studied when compared to blood group compatible kidney transplant recipients (Table 1).
Patient and Graft Survival and Clinical Outcomes
Table 2 summarizes clinical outcomes after transplantation. During a median follow-up of 32.6 mo (6–57.3), patient survival was 100%, and graft survival was 92% in A2-incompatible kidney transplant recipients, and it was not statistically significant when compared to blood group compatible kidney transplant recipients (91% and 92%, respectively). Four patients in the A2-incompatible kidney transplant group lost the allograft; 1 due to chronic antibody-mediated rejection (AMR) developed after noncompliance, 1 due to a partially infarcted kidney during surgery, 1 due to early acute T-cell–mediated rejection in setting of antimetabolite withdrawal during an episode of urosepsis and progressed to the end-stage kidney diseasem and the last graft loss was due to acute kidney injury in the setting of COVID-19 infection.
TABLE 2.
Clinical outcomes after A2 incompatible kidney transplantation
| Clinical outcomes | A2 incompatible transplant, N =41 | Blood group compatible transplant, N = 75 | P |
|---|---|---|---|
| Patient survival | 100% | 91% | 0.050 |
| Graft survival | 92% | 92% | 0.99 |
| Acute rejection | |||
| T-cell–mediated rejection | 2 (5%) | 3 (4%) | |
| Antibody-mediated rejection | 0 | 1 (1.3%) | |
| Chronic antibody-mediated rejection | 1 (2%) | 1 (1.3%) | |
| Serum creatinine at the last visit, median (range) mg/dL | 1.3 (0.6–3.2) | 1.35 (0.4–7.0) | 0.74 |
| Spot urine protein/creatinine at the last clinic visit, median (range]) g/g | 0.16 (0–2.4) | 0.19 (0.05–7.9) | 0.22 |
| Spot urine protein/creatinine > 1 g/g | 3 (7%) | 11 (15%) | |
| Cytomegalovirus viremia | 6 (15%) | 11 (15%) | 0.99 |
| BK viremia | 9 (22%) | 15 (20%) | 0.80 |
| Urinary tract infections | 15 (37%) | 37 (49%) | 0.19 |
| Pneumonia | 14 (34%) | 13 (17%) | 0.041 |
| Influenza | 3 (7%) | 14 (19%) | 0.10 |
The median serum creatinine level at the last follow-up was 1.3 mg/dL (0.6–3.2), and 93% of the patients did not have significant proteinuria (<1 g/d). Nine patients developed BK viremia (BKV) (22%), and 6 developed cytomegalovirus viremia (15%). Fifteen patients (37%) developed urinary tract infection and 3 (7%) influenza. The only difference in clinical outcome was more pneumonia was observed in A2-incompatible kidney transplant group (34%) compared with control group (17%) (Table 2).
Biopsy Findings
Twelve patients underwent a total of 21 clinically indicated kidney biopsy for worsening kidney function and/or proteinuria. The median time for biopsy was 28 d (6–390) after transplantation. None of the patients developed acute AMR, and 2 patients (5%) had acute T-cell–mediated rejection type IIA. One patient developed chronic AMR due to noncompliance, and 1 patient had BKV nephropathy. The remaining diagnosis was acute tubular injury (n = 6), normal (n = 2), and 1 patient had infarcted kidney. Out of 8 biopsies with no rejection, C4d positivity was seen in 7 of them and had no microvascular inflammation. Banff C4d score was C4d1 in 2, C4d2 in 2, and C4d3 in 3 biopsies. Table 3 summarizes the pathological diagnosis of kidney biopsies with C4d positivity without evidence of rejection in addition to their respective Banff scores. Out of those 7 patients, only 1 patient with C4d 1+ had a pretransplant DSA (A68 MFI 3071). Five of those 8 patients underwent 1 or more subsequent biopsies, the C4d staining was persistently positive in all 5 patients without histologic findings of acute rejection or microvascular inflammation. The only patient which showed signs of inflammation was the 1 who was diagnosed with BKV nephritis (patient 6).
TABLE 3.
Summary of biopsies with C4d positivity and without rejection with their respective Banff acute allograft injury scores
| Banff scores | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | c4d | t | i | v | g | ptc | ah | mm | ||
| Patient 1 | First biopsy | ATN | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subsequent biopsy | ATN | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Patient 2 | First biopsy | ATN | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Subsequent biopsy | ATN | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Patient 3 | First biopsy | ATN | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subsequent biopsy | ATN | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Patient 4 | First biopsy | ATN | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Subsequent biopsy | ATN | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Patient 5 | One biopsy only | ATN | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Patient 6 | One biopsy only | BKV nephritis | 1 | 1 | 3 | 0 | 0 | 1 | 0 | 0 |
| Patient 7 | First biopsy | Normal | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subsequent biopsy | ATN | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
ATN, acute tubular necrosis; BKV, BK viremia.
Twenty-nine blood group compatible kidney transplant recipients underwent clinically indicated kidney biopsy after transplantation. Acute T-cell–mediated rejection developed in 3 patients (4%) and acute AMR in 1 patient (1.3%) and chronic AMR (1.3%) similar to A2-incompatible kidney transplant recipients (Table 2). C4d positivity was not observed in any kidney biopsies without rejection in blood group compatible kidney transplant recipients.
Donors With Acute Kidney Injury
In the A2-incompatible kidney transplant group, 9 patients received a deceased-donor kidney with donor terminal creatinine >3 mg/dL. Median donor age was 36 y (26–55), cold ischemia time 32.5 h (11.1–43.1), median terminal creatinine 4.2 mg/dL (3.6–5.4), and kidney donor profile index score of 34 (25, 68). Eight patients (89%) developed delayed graft function. During a median follow-up of 16.1 mo (10.6–44.3) of those 9 patients, both patient and graft survival were 100%. None of the patients developed acute rejection and median serum creatinine level at the last follow-up was 1.1 (1.0–1.6) mg/dL.
DISCUSSION
Our results document that A2-incompatible transplantation appears to be safe in patients with anti-A2 titers ≤16 without desensitization with excellent patient survival (100%) and graft survival (90%) at a median follow-up close to 3 y. None of the patients developed acute AMR. Our study will be the first documenting safety of A2-incompatible kidney transplantation in blood group B recipients with anti-A2 IgG/IgM titers of 8 and 16 without plasmapheresis.
Initial experience by Nelson et al10 in 1992 documented the importance of anti-A titer for prediction of acute rejection in A2-incompatible kidney transplantation. There were 24 blood group O and 9 blood group B recipients from 30 A2 and 3 A2B donors. Eight patients (24%) had primary nonfunction or early graft loss and all those patients were blood group O recipients with anti-A titer >8. The authors did not recommend transplantation in patients with anti-A titers >8. We have reviewed 12 publications after this initial manuscript reporting anti-A IgG titers in their A2-incompatible kidney transplantation and summarized at Tables 4 and 5. Anti-A titers were measured by hemagglutination titers in all studies. Anti-A titers were <8 in all the 6 studies of deceased-donor recipients (Table 4). Three studies came from Midwest Organ Bank.5,12,13 An initial study in 1998 reported 18 recipients of A2 donors (10 blood group B and 8 blood group O) between 1986 and 1996.12 Anti-A2 titers were <4 and 4 patients lost the allograft due to rejection; 1 patient at 2 wks and the others more than 21 mo after transplantation. The second study from the same group covered A2-incompatible transplants between 1994 and 2000. There were 41 blood group B patients receiving transplant from 37 A2 and 4 A2B donors.13 Graft survival was 84% at 1 y and 72% at 5 y, but the rejection rate was not reported. The third study extended the study period from 1994 to 2003 and reported the clinical outcomes in 56 patients.5 Death-censored graft survival was 72% at 7 y, and 41% acute rejection and 16% chronic rejection was observed over 10 y. Alkhunaizi et al14 reported 15 A2-incompatible kidney transplants (deceased-donor and 6 living-donor), who underwent plasmapheresis if pretransplant anti-A2 titers were >8. One patient who had anti-A2 titer of 64 and did not undergo plasmapheresis had hyperacute rejection and lost his allograft. There were also 2 other cases of acute rejection. Graft survival at 1 y was 93.3%.14 Williams et al4 reported 101 A2-incompatible kidney transplants to blood group B recipients between September 2002 and July 2008 at 9 donor service areas in the United States. Any potential candidate with an IgG anti-A titer of ≥8 was excluded from the study. Graft survival was 85.4% at 36 mo, comparable to outcomes for blood group B recipients of B kidneys. Acute rejection was 10% at 1 y. Five donor service areas increased the proportion of B transplants during the study period. Shaffer et al15 reported 29 group B recipients with anti-A1 IgG titers <8 and transplanted between 2014 and 2017 at a single center. Patients with anti-A1 IgM titers were >8 and ≤64 received plasmapheresis for 5 d starting postoperative day 1, followed by IVIG 2 g/kg and rituximab 375 mg/m2. Graft survival at 1- and 2-y follow-up was 93% and 88%, respectively.15 There was no information regarding rejection episodes.
TABLE 4.
Review of previous publications regarding A2 incompatible transplantation with reported anti-A IgG titers in deceased-donor transplantation
| Manuscript | No. recipients | Anti-A IgG titers pre-txp | Desensitization | Rejection | Graft survival | Patient survival | Mean serum creatinine |
|---|---|---|---|---|---|---|---|
| Nelson et al12 | 18 (10 group B, 8 group O recipients) | <4 (A2) | No | 4 graft loss due to rejection | 88% at 1 y and 80% at 2 y | 2 patients died at 1 and 21 mo | N/A |
| Alkhunaizi et al14 | 15 (6 group O, 9 group B)—9 deceased donor, 6 living donor | <8 (A1) | Plasmapheresis if A titers > 1/8 | 1 hyperacute rejection in a patient with anti-A2 titer 1/64 and did not receive plasmapheresis, 2 acute rejection | 93.3% at 1 y (combined for both deceased and living donors) | 100% at 1 y (combined for both deceased and living donors) | 1.3 (±0.34) mg/dL at 1 y (combined for both deceased and living donors) |
| Nelson et al13 | 41 (33 A2 to B and 4 A2B to B) | <8 (A2) | No | No data | 84% at 1 y and 72% at 5 y | N/A | N/A |
| Bryan et al5 | 56 (51 A2 to B and 5 A2B to B) | <8 (A1) | No | 41% acute rejection and 16% chronic rejection over 10 y | 72% death-censored graft survival at 7 y | 73.3% at 10 y | N/A |
| Williams et al4 | 101 (all group B recipients) | <8 (A1) | No | 9.7% and 10% acute rejection at 6 mo and 1 y, respectively | 85.4% at 36 mo | 92.5% at 36 mo | 1.4 mg/dL (0.9–2.1) at 1 y |
| Shaffer et al15 | 29 (all group B recipients) | <8 (A1) | Plasmapheresis for 5 d followed by IVIG and rituximab if anti-A2 IgG/M titers > 1/8 and ≤1/64 | No data | 93% at 1 y and 88% at 2 y | 93% at 1 y and 88% at 2 y | 1.7 mg/dL at 1 y and 1.8 mg/dL at 2 y |
IVIG, intravenous immunoglobulin; N/A, not applicable.
TABLE 5.
Review of previous publications regarding A2 incompatible transplantation with reported anti-A IgG titers in living transplantation
| Manuscript | Number of recipients | Anti-A IgG titers pre-txp | Desensitization | Rejection | Graft survival | Patient survival | Mean serum creatinine |
|---|---|---|---|---|---|---|---|
| Alkhunaizi et al14 | 15 (6 group O, 9 group B)—9 deceased donor, 6 living donor | <8 (A1) | Plasmapheresis if A titers > 1/8 | 1 hyperacute rejection in a patient with anti-A2 titer 1/64 and did not receive plasmapheresis, 2 acute rejection | 93.3% at 1 y (combined for both deceased and living donors) | 100% at 1 y (combined for both deceased and living donors) | 1.3 (±0.34) mg/dL at 1 y (combined for both deceased and living donors) |
| Sorensen et al16 | 15 (11 blood group O and 4 blood group B recipients) | 2 had 16, 1 8 and 12 <8 (A1) | No | 3 patients developed rejection | 93.30% at a median 32 mo (range, 7–102) | 93.30% at a median 32 mo (range, 7–102) | Median 1.3 mg/dL (range, 0.9–5.4) |
| Fidler et al17 | 13 (11 blood group O, 2 blood group B) | 4–256 (A2) | If pre-txp A2-titer > 1/64 (plasmapheresis, IVIG, and splenectomy) | 6 early AMR (all in blood group O) | 78% at a median of 25 mo (range, 4–40) | 91% at a median of 25 mo (range, 4–40) | Median 1.3 mg/dL (range, 1.1–1.8) |
| Tydén et al21 | 3 (all blood group O) | 64 initially and 4, 2, and 1 at the time of transplant (A1) | Rituximab, tacrolimus, mycophenolate mofetil, prednisone, and immunoadsorption followed by IVIG | No rejection | 100% at 11, 15, and 34 mo | 100% at 11, 15, and 34 mo | 80, 120, and 168 µmol/L) |
| Sonnenday et al20 | 2 (1 blood group O and 1 blood group B) | 8 and 128 (A2) | Plasmapheresis, CMV hyperimmune globulin and rituximab | No rejection | 100% at 1 y | 100% at 1 y | 1.3 mg/dL at 1 y for both patients |
| Masterson et al8 | 4 (3 blood group O and blood group B) | 3 patients 4 and 1 patient 1 (A2) | No | No rejection | 100% at 36 mo | 100% at 36 mo | N/A |
| Tierney and Shaffer19 | 7 all group O | <8 (A1) | No | 2 acute cellular rejection, 2 acute antibody-mediated rejection and associated with high IgM titers | 85.7% at 1 y | 71.4% at 1 y | 1.4–1.7 mg/dL at 1 y |
AMR, antibody-mediate rejection; CMV, cytomegalovirus; IVIG, intravenous immunoglobulin; N/A, not applicable.
Desensitization protocols were mostly applied in living-donor blood group O or B recipients with high anti-A2 titers. We summarized previous 7 studies involving 59 patients at Table 5. Most of the patients (71%) were blood group O recipients. Sorenson et al reported 15 patients with anti-A IgG titers ≤16 and anti-A IgM titers up to 254.16 Three patients developed acute rejection, and graft survival was 93.3% at a median of 32 mo. The Mayo group reported their experience in A2-incompatible transplantation in a consecutive 3 articles.6,17,18 In their last study involving 13 patients, desensitization was applied if anti-A titers ≥64, which 6 had early AMR (all blood group O).17 Interestingly, in 4 patients who developed AMR, pretransplant anti-A titers were 8. In a report by Tierney and Shaffer,19 7 A2-incompatible living-donor transplantation happened without desensitization if anti-A titers were <8. Two patients developed acute cellular rejection, and 2 others developed acute AMR and associated with high anti-A IgM titers.19 The outcomes of the remaining 3 studies that included 2–4 patients were summarized at Table 4.8,20,21
We and previous studies checked anti-A titers by hemagglutination methods. Although hemagglutination methods carry the risk of interobserver and interinstitutional variability and not standardized, it was suggested that the FC method could have a better reproducibility, but it is not widely used at transplant centers.22 Furthermore, most of the studies reviewed used anti-A1 IgG titers, and only a few studies used A2 donor erythrocytes to determine anti-A titers.12,14,17 We believe that anti-A2 titers should be utilized in decision-making for A2-incompatible transplantation, and anti-A1 should be used in A1-incompatible transplantation. Anti-A1 titers are higher than anti-A2 titers, and using anti-A1 titer in A2-incompatible transplantation might lead to decline a successful transplantation.
Another interesting finding is that 7 of the 8 biopsies stained for C4d without histological evidence of acute AMR or microvascular injury. C4d positivity was persistent at the follow-up biopsies of the 5 patients. None of the blood group compatible transplant recipients had C4d positivity without rejection. This was reported previously in A1-incompatible kidney transplantation and not associated with rejection and decreased allograft survival suggesting accommodation.18,23 In another report, C4d positivity was observed in 94% of biopsies in A1-incompatible kidney transplant recipients with diffuse staining in 66%, and it was not associated with AMR.24 Chow et al25 showed that 52% of protocol biopsies had positive C4d staining without AMR in first 3 mo posttransplant with persistence of C4d positivity in 16% on subsequent biopsies. However, there were only 3 patients who received A2-incomptaible kidneys.25 Our study will be the first and the largest group of biopsies documenting frequent C4d positivity in A2-incompatible kidney transplant recipients without rejection or microvascular inflammation.
An interesting finding is that in recipients receiving a kidney from a donor with acute kidney injury, while most of them developed delayed graft function, they all had a subsequent excellent allograft function. Safety of using deceased-donor kidneys with acute kidney injury has been reported previously.26,27 This finding suggests that expanding the pool of ABO-incompatible kidney to include donors with acute kidney injury is probably safe and will further decrease the waiting time for recipients with blood group B.
Transplanting A2 and A2B kidneys into blood group B recipients has significantly increased the transplantation rate of blood group B5 patients, which has historically the longest waiting time on the deceased-donor kidney transplant waitlist and included more minority patients. In our cohort, 76% of A2-incompatible kidney transplant recipients were African American or Hispanic.
The major limitation of our study is that it is retrospective chart review. The other limitation is we did not monitor anti-A2 titers after transplantation. However, we have not observed any acute AMR to justify posttransplant monitoring of anti-A2 titers. The strength of our study is that it will be the first documenting safety of A2-incompatible kidney transplantation in blood group B recipients with anti-A2 titers of 8 and 16 without plasmapheresis, without development of acute AMR and with excellent allograft survival at a relatively long follow-up in 41 patients. This will also be the first report documenting frequent positive C4d staining without histologic findings of rejection in A2-incompatible kidney transplantation.
Footnotes
Published online 26 January, 2021.
Y.A., J.R., and E.A. participated in research design. Y.A., G.N., P.L.-C., M.A., J.G., L.L.-W., C.P., J.U., A.C., A.C., O.A., M.L., S.G., M.K., J.R., and E.A. participated in the writing of the paper and in the performance of the research. Y.A., G.N., P.L.-C., and E.A. participated in data analysis.
The authors declare no funding or conflicts of interest.
REFERENCES
- 1.Scurt FG, Ewert L, Mertens PR, et al. Clinical outcomes after ABO-incompatible renal transplantation: a systematic review and meta-analysis. Lancet. 2019; 393:2059–2072 [DOI] [PubMed] [Google Scholar]
- 2.Bryan CF, Cherikh WS, Sesok-Pizzini DA. A2 /A2 B to B renal transplantation: past, present, and future directions. Am J Transplant. 2016; 16:11–20 [DOI] [PubMed] [Google Scholar]
- 3.Breimer ME, Mölne J, Nordén G, et al. Blood group A and B antigen expression in human kidneys correlated to A1/A2/B, Lewis, and secretor status. Transplantation. 2006; 82:479–485 [DOI] [PubMed] [Google Scholar]
- 4.Williams WW, Cherikh WS, Young CJ, et al. First report on the OPTN national variance: allocation of A2 /A2 B deceased donor kidneys to blood group B increases minority transplantation. Am J Transplant. 2015; 15:3134–3142 [DOI] [PubMed] [Google Scholar]
- 5.Bryan CF, Winklhofer FT, Murillo D, et al. Improving access to kidney transplantation without decreasing graft survival: long-term outcomes of blood group A2/A2B deceased donor kidneys in B recipients. Transplantation. 2005; 80:75–80 [DOI] [PubMed] [Google Scholar]
- 6.Gloor JM, Lager DJ, Moore SB, et al. ABO-incompatible kidney transplantation using both A2 and non-A2 living donors. Transplantation. 2003; 75:971–977 [DOI] [PubMed] [Google Scholar]
- 7.Tanabe K, Takahashi K, Sonda K, et al. Long-term results of ABO-incompatible living kidney transplantation: a single-center experience. Transplantation. 1998; 65:224–228 [DOI] [PubMed] [Google Scholar]
- 8.Masterson R, Hughes P, Walker RG, et al. ABO incompatible renal transplantation without antibody removal using conventional immunosuppression alone. Am J Transplant. 2014; 14:2807–2813 [DOI] [PubMed] [Google Scholar]
- 9.Stegall MD, Dean PG, Gloor JM. ABO-incompatible kidney transplantation. Transplantation. 2004; 78:635–640 [DOI] [PubMed] [Google Scholar]
- 10.Nelson PW, Helling TS, Shield CF, et al. Current experience with renal transplantation across the ABO barrier. Am J Surg. 1992; 164:541–544. Discussion 544 [DOI] [PubMed] [Google Scholar]
- 11.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999; 55:713–723 [DOI] [PubMed] [Google Scholar]
- 12.Nelson PW, Landreneau MD, Luger AM, et al. Ten-year experience in transplantation of A2 kidneys into B and O recipients. Transplantation. 1998; 65:256–260 [DOI] [PubMed] [Google Scholar]
- 13.Nelson PW, Shield CF, 3rd, Muruve NA, et al. Increased access to transplantation for blood group B cadaveric waiting list candidates by using A2 kidneys: time for a new national system? Am J Transplant. 2002; 2:94–99 [DOI] [PubMed] [Google Scholar]
- 14.Alkhunaizi AM. Renal transplantation across the ABO barrier. Saudi J Kidney Dis Transpl. 2006; 17:311–315 [PubMed] [Google Scholar]
- 15.Shaffer D, Feurer ID, Rega SA, et al. A2 to B kidney transplantation in the post-kidney allocation system era: a 3-year experience with anti-A titers, outcomes, and cost. J Am Coll Surg. 2019; 228:635–641 [DOI] [PubMed] [Google Scholar]
- 16.Sorensen JB, Grant WJ, Belnap LP, et al. Transplantation of ABO group A2 kidneys from living donors into group O and B recipients. Am J Transplant. 2001; 1:296–299 [DOI] [PubMed] [Google Scholar]
- 17.Fidler ME, Gloor JM, Lager DJ, et al. Histologic findings of antibody-mediated rejection in ABO blood-group-incompatible living-donor kidney transplantation. Am J Transplant. 2004; 4:101–107 [DOI] [PubMed] [Google Scholar]
- 18.Park WD, Grande JP, Ninova D, et al. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003; 3:952–960 [DOI] [PubMed] [Google Scholar]
- 19.Tierney J, Shaffer D. Transplantation of ABO A2 kidneys into O recipients: do IgM anti-A1 titers matter? Clin Transplant. 2015; 29:379–382 [DOI] [PubMed] [Google Scholar]
- 20.Sonnenday CJ, Warren DS, Cooper M, et al. Plasmapheresis, CMV hyperimmune globulin, and anti-CD20 allow ABO-incompatible renal transplantation without splenectomy. Am J Transplant. 2004; 4:1315–1322 [DOI] [PubMed] [Google Scholar]
- 21.Tydén G, Kumlien G, Genberg H, et al. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005; 5:145–148 [DOI] [PubMed] [Google Scholar]
- 22.Krishnan NS, Fleetwood P, Higgins RM, et al. Application of flow cytometry to monitor antibody levels in ABO incompatible kidney transplantation. Transplantation. 2008; 86:474–477 [DOI] [PubMed] [Google Scholar]
- 23.Haas M, Segev DL, Racusen LC, et al. C4d deposition without rejection correlates with reduced early scarring in ABO-incompatible renal allografts. J Am Soc Nephrol. 2009; 20:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Setoguchi K, Ishida H, Shimmura H, et al. Analysis of renal transplant protocol biopsies in ABO-incompatible kidney transplantation. Am J Transplant. 2008; 8:86–94 [DOI] [PubMed] [Google Scholar]
- 25.Chow KV, Flint SM, Shen A, et al. Histological and extended clinical outcomes after ABO-incompatible renal transplantation without splenectomy or rituximab. Transplantation. 2017; 101:1433–1440 [DOI] [PubMed] [Google Scholar]
- 26.Torabi J, Graham JA, Choinski K, et al. Young donors with severe acute kidney injury offer an opportunity to expand the donor pool. Am J Surg. 2019; 218:7–13 [DOI] [PubMed] [Google Scholar]
- 27.Hall IE, Akalin E, Bromberg JS, et al. Deceased-donor acute kidney injury is not associated with kidney allograft failure. Kidney Int. 2019; 95:199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]


