Abstract
Background.
Sensitization remains a barrier to heart transplantation (HT). Perioperative desensitization strategies have been described; however, a paucity of evidence exists to demonstrate efficacy and safety in HT.
Methods.
This single-center, retrospective study consisted of adults who received an HT. Perioperative desensitization was initiated if virtual crossmatch or flow-cytometry crossmatch was positive. Therapy consisted of plasmapheresis, intravenous immunoglobulin, and rabbit antithymocyte globulin. Historical controls received standard immunosuppression or induction. The primary endpoint was survival at 12 mo. Secondary endpoints included freedom from acute rejection, cardiac allograft vasculopathy (CAV), and infectious complications.
Results.
Of the 104 patients included, 48 received no induction, 46 received induction, and 10 underwent perioperative desensitization. No differences were observed in the primary endpoint at 12 mo (90.0% versus 97.9%, P = 0.25 for desensitization versus no-induction; 90.0% versus 100%, P = 0.72 for desensitization versus induction). Rates of acute rejection were lower with induction and desensitization compared with no-induction. There were no significant differences in CAV between the groups. Infectious complications were also similar among the groups (10.0% versus 16.7%, P = 0.62 for desensitization versus no-induction; 10.0% versus 30.4%, P = 0.34 for desensitization versus induction).
Conclusions.
This study suggests that a perioperative desensitization strategy triggered by positive virtual crossmatch or flow-cytometry crossmatch allows for successful transplantation of sensitized HT recipients and results in acceptable rates of survival, rejection, CAV, and infection at 12 mo.
BACKGROUND
Advances in modulating the recipient immune response following heart transplantation (HT) have led to substantial improvements in rates of acute rejection and survival.1 However, even with these advancements, pretransplant sensitization, as measured by calculated panel-reactive antibody (cPRA), remains largely unchanged.2
Sensitization results from the production of alloantibodies directed against HLA and may be triggered by blood transfusions, multiparity, prior transplantation, or implantation of a durable mechanical assist device.3,4 Although sensitization does not guarantee donor-recipient incompatibility, it has been associated with reduced access to HLA-compatible organs and increased waitlist time, which may result in clinical deterioration before transplantation.5 Conversely, transplantation in the presence of donor-specific antibody (DSA) or elevated cPRA is associated with increased rates of acute rejection, cardiac allograft vasculopathy (CAV), and graft failure.6-8
Desensitization protocols before transplant have been proposed as a strategy for mitigating risk, and various combinations of IVIg, plasma exchange (PLEX), rituximab, or bortezomib, have been used.4,9 Although these strategies have demonstrated reductions in panel-reactive antibody (PRA) and successful transplantation into sensitized individuals, patients on the waitlist are exposed to potentially unnecessary treatment if they are not transplanted. Notably, these therapies carry both high cost and poor tolerability.
Recent approaches using perioperative PLEX and IVIg have demonstrated acceptable rates of acute rejection and survival.10–12 The following report details our experience with the transplantation of 10 HT recipients despite a positive crossmatch using a defined perioperative desensitization protocol. We assessed patient survival and the incidence of acute rejection and compared desensitized patients with 2 historical cohorts: patients receiving induction with lymphocyte-depleting agents and those receiving standard immunosuppression without induction.
MATERIALS AND METHODS
Study Population
This single-center, retrospective cohort study consisted of adult patients between the ages of 18 and 80 y who received an HT between January 1, 2012 and March 1, 2019. Patients were excluded if they received multiorgan transplantation or had a history of prior transplantation. HT recipients were categorized into 3 groups: no-induction, induction with a lymphocyte-depleting agent, and perioperative desensitization.
Alloantibody Characterization and Crossmatching
A virtual crossmatch (VXM) was performed on both recent (ie, <60 d old) and historic serum specimens for most HT recipients. The VXM was reported as positive when DSA to either HLA class I or II antigen was detected in the current or historic specimen with a mean fluorescence index (MFI) value ≥1000. All physical crossmatches were performed using the flow cytometry method. In most cases, the flow-cytometry crossmatch (FXM) was performed post–organ acceptance, unless a local donor was available. FXM was performed using pretransplant sera. The shift median channel value cutoffs were determined through evaluation of donor cells with negative control serum. Shifts ≤2 standard deviations from the mean were considered negative, shifts ≥3 standard deviations were positive, and shifts between 2 and 3 standard deviations were considered equivocal. Dilutional studies were performed to assess for prozone effect. cPRA was also calculated before transplantation. The MFI cutoff for a positive reaction was at least 2 times the average fluorescence of a negative control. The average negative control with our assay was an MFI of ≤500, and thus our assay needed an MFI of ≥1000 to indicate positivity. This was adapted from an ELISA-fluorescence formula established with a local control.
Immunosuppression and Monitoring
Patients receiving no-induction received methylprednisolone 500 mg intraoperatively and 125 mg every 8 h on postoperative day (POD) 0, followed by a taper over 6 mo. Mycophenolate mofetil (MMF) 2000 mg/d or equivalent was started on POD 0 and an initial tacrolimus concentration of 10–15 ng/mL was targeted.
In the induction group, alemtuzumab, or rabbit antithymocyte globulin (rATG) was typically used to delay calcineurin inhibitor initiation, a decision based on physician’s discretion and often due to renal insufficiency. Patients treated with alemtuzumab received 30 mg on POD 0 and 4, along with methylprednisolone 500 mg. MMF 2000 mg/d or equivalent was started on POD 0 and an initial tacrolimus concentration of 8–10 ng/mL was targeted. Following POD 4, patients received prednisone 10 mg/d, which was tapered over time. Patients treated with rATG received 1.5 mg/kg/d to target a total dose of 6 mg/kg. Corticosteroids were given with each rATG dose and gradually tapered over 6 mo. Maintenance immunosuppression was similar to those receiving alemtuzumab. Immunosuppression protocols are detailed further in Appendix (SDC, http://links.lww.com/TXD/A304).
A protocol for perioperative desensitization was implemented at our institution in 2015. The decision to initiate perioperative desensitization was based on positive VXM or FXM. Patients received methylprednisolone 1000 mg and 3 intraoperative exchanges of plasma volume initiated at the induction of anesthesia and completed before reperfusion. If the FXM was positive for T- and B-cells or if there was an isolated B-cell crossmatch, patients received rATG for a goal total dose of 5 mg/kg. Four additional PLEX sessions were completed postoperatively. Following PLEX, 1 g/kg of IVIg was administered. Corticosteroids were given with each dose of rATG and were tapered over 12 mo. MMF 2000 mg/d or equivalent was initiated on POD 0, and tacrolimus (goal 10–15 ng/mL) was started on POD 1. DSA was repeated on POD 21; this could be completed earlier if there was concern for rejection (Figure 1). In patients with isolated T-cell FXM or isolated B-cell FXM with DSA <1000 MFI, rATG induction was used with no further PLEX or IVIg.
FIGURE 1.
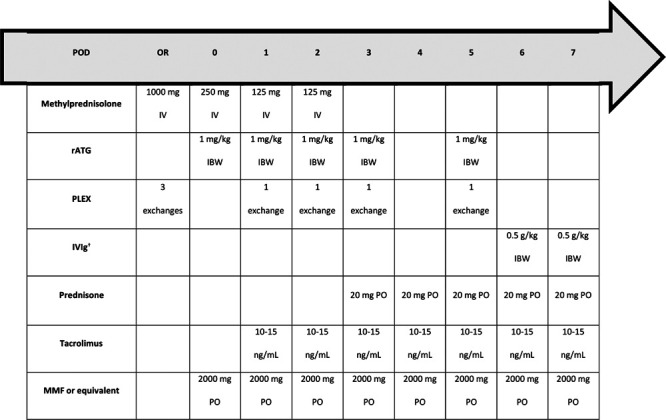
Desensitization protocol. This treatment algorithm was followed if T- and B-cell FXM were positive with DSA MFI ≥1000, or if there was a positive isolated B-cell FXM with DSA MFI ≥1000. †Maximum single IVIg dose of 40 g/d. DSA, donor-specific antibody; FXM, flow cytometry crossmatch; IBW, ideal body weight; IV, intravenous; MFI, mean fluorescence intensity; MMF, mycophenolate mofetil; OR, operating room; PLEX, plasma exchange; PO, by mouth; POD, postoperative d; rATG, rabbit antithymocyte globulin; VXM, virtual crossmatch.
All patients, regardless of immunosuppression pathway, received prophylaxis against opportunistic infections consisting of valganciclovir, sulfamethoxazole/trimethoprim, and nystatin. Surveillance endomyocardial biopsy (EMB) was performed weekly for the first month, biweekly during months 2 and 3, monthly between months 4 and 6, and every 3 mo between months 7 and 12.
Outcomes
The cohort was divided into 3 groups: no-induction, induction, and desensitization. The primary endpoint was survival at 12 mo. Secondary endpoints included freedom from antibody-mediated rejection (AMR) and acute cellular rejection (ACR), Grade ≥2 ACR and AMR, International Society of Heart and Lung Transplant (ISHLT) CAV1, and infectious complications. Assessment of ACR and AMR was conducted according to the ISHLT grading scale.13,14 CAV was diagnosed by intravascular ultrasound and was graded according to the ISHLT standard nomenclature.15 Infectious complications included the first incidence of cytomegalovirus (CMV) viremia or disseminated disease, Clostridium difficile infection, fungal infection, donor-derived infection, or infection with an atypical bacterium.
Statistical Analysis
Categorical variables are presented as frequency (percentage), and continuous variables are presented as mean ± SD or median (25th–75th percentile). Nonparametric continuous data were analyzed using Kruskal-Wallis 1-way ANOVA with a Dunn’s test to assess stochastic dominance. Categorical variables were analyzed using chi-squared or Fischer’s exact test. Cox proportional hazards models were used for time-to-event outcomes, and event curves were generated using the Kaplan-Meier method. No power calculations were conducted given the small size of this exploratory analysis. Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, Inc; San Diego, CA) and SPSS version 23 (IBM Corp; Armonk, NY). The local institutional review board approved the study.
RESULTS
A total of 155 consecutive patients were assessed, of whom 51 were excluded (19 received multiorgan transplants, 2 were retransplanted, 3 were <18 y of age, and 27 were transplanted within 12 mo and lacked complete data at the time of analysis). Of the 104 remaining patients, 48 received no induction therapy, 46 received induction (33 received alemtuzumab and 13 received rATG), and 10 underwent perioperative desensitization. Baseline characteristics were similar between groups with the exception of renal function and low-density lipoprotein levels. Median glomerular filtration rate was significantly lower in the induction group compared with the no-induction group [58.3 mL/min (43.6–92.3) versus 85.7 mL/min (61.9–107); P < 0.05], which corresponded to a higher prevalence of chronic kidney disease (45.7% versus 16.7%; P < 0.05). Baseline low-density lipoprotein was significantly lower in the desensitization group compared with the induction group [48 mg/dL (46–59) versus 77.5 mg/dL (57–96.8); P < 0.05]. Most patients were White males; nonischemic cardiomyopathy and left ventricular assist device explantation were common (Table 1).
TABLE 1.
Baseline characteristics
| Desensitization (n = 10) | No induction (n = 48) | Induction (n = 46) | P | |
|---|---|---|---|---|
| Age | 54 (48.5–66.3) | 61.5 (51.3–67.8) | 55.5 (47.8–64) | 0.29 |
| Race | 0.35 | |||
| White | 3 (30.0) | 27 (56.3) | 23 (50.0) | |
| Black | 7 (70.0) | 19 (39.6) | 19 (41.3) | |
| Other | 0 (0.0) | 2 (4.2) | 4 (8.7) | |
| Male | 6 (60.0) | 37 (77.1) | 38 (82.6) | 0.29 |
| Heart failure pathogenesis | 0.28 | |||
| Ischemic | 5 (50.0) | 15 (31.3) | 21 (45.7) | |
| Nonischemic | 5 (50.0) | 33 (68.8) | 25 (54.3) | |
| Left-ventricular assist device | 7 (70.0) | 27 (56.3) | 24 (52.2) | 0.59 |
| Cold ischemic time (min) | 158 (147–246.3) | 154.5 (117.3–193) | 149 (121.5–179.3) | 0.37 |
| Donor age (y) | 36 (24–50) | 30 (21–42.5) | 34 (27.8–47) | 0.18 |
| Listing time (d) | 189 (87–380.5) | 137 (46–270.5) | 82 (24.3–224.3) | 0.44 |
| Index length of stay (d) | 17.4 (12.5–30.8) | 18 (13–25) | 19 (12.8–29) | 0.81 |
| Immunologic risk | ||||
| cPRAa,b (%) | 99 (93–99.8) | 30 (0.5–56) | 18 (0–59) | 0.0003 |
| Pretransplant class I DSA ≥ 1000 MFIa,b | 6 (60.0) | 3 (6.3) | 1 (2.2) | <0.0001 |
| Pretransplant class II DSA ≥ 1000 MFIa,b | 4 (40.0) | 4 (8.3) | 4 (8.7) | 0.01 |
| Comorbidities | ||||
| Hypertension | 7 (70.0) | 35 (72.9) | 29 (63.0) | 0.59 |
| Hyperlipidemia | 3 (30.0) | 23 (47.9) | 26 (56.5) | 0.29 |
| Diabetes | 5 (50.0) | 14 (29.2) | 24 (52.3) | 0.06 |
| Chronic kidney diseasec | 3 (30.0) | 8 (16.7) | 21 (45.7) | 0.01 |
| Proteinuria | 3 (30.0) | 8 (16.7) | 8 (17.4) | 0.60 |
| CMV status | ||||
| Donor CMV+ | 4 (40.0) | 27 (56.3) | 27 (58.7) | 0.56 |
| Recipient CMV+ | 6 (60.0) | 28 (58.3) | 25 (54.3) | 0.90 |
| High-risk mismatch | 1 (10.0) | 12 (25.0) | 12 (26.1) | 0.55 |
| Laboratory values | ||||
| Serum creatinine (mg/dL)c | 1.48 (1.04–1.81) | 1.00 (0.85–1.23) | 1.35 (0.96–1.81) | 0.002 |
| Glomerular filtration rate (mL/min)c | 57.4 (39.3–88.4) | 85.7 (61.9–107.0) | 58.3 (43.6–92.3) | 0.007 |
| White blood cell count (K/mm3) | 6.7 (4.9–12.4) | 8.3 (5.8–13.4) | 7.4 (6.2–10.8) | 0.63 |
| Platelet count (K/mm3) | 213 (149–257) | 153 (133.3–205.5) | 180 (127.5–232.3) | 0.16 |
| Hemoglobin A1c (%) | 5.8 (5.6–6.4) | 5.6 (5.2–6.3) | 5.9 (5.4–6.7) | 0.32 |
| Total cholesterol (mg/dL) | 115 (111–124) | 130.5 (112.3–161.8) | 146.5 (110.3–165.8) | 0.20 |
| Triglycerides (mg/dL) | 127 (50.5–171.5) | 109.5 (69–138.8) | 95 (62–141.5) | 0.61 |
| High-density lipoprotein (mg/dL) | 46 (29–57.5) | 40 (30.5–49.5) | 37.5 (29–61.8) | 0.83 |
| Low-density lipoprotein (mg/dL)a | 48 (46–59) | 66 (48–85.5) | 77.5 (57–96.8) | 0.04 |
Data are presented as median (25th–75th percentile) or n (%). Bold indicates statistically significant difference.
aP < 0.05 for comparison of desensitization cohort to induction cohort.
bP < 0.05 for comparison of no-induction cohort to desensitization cohort.
cP < 0.05 for comparison of no-induction cohort to induction cohort.
CMV, cytomegalovirus; cPRA, calculated panel reactive antibody; DSA, donor-specific antibody; MFI, mean fluorescence index.
Desensitization Therapy
Patients in the desensitization group were significantly more sensitized before HT compared with both induction and no-induction groups (Table 1). Median cPRA was 99% (93–99.8) in the desensitization group. Of these patients, 60% had pretransplant class I DSA with MFI ≥ 1000 and 40% had pretransplant class II DSA with MFI ≥ 1000. Eight patients had positive VXM. A VXM was not performed in 2 patients as prospective FXM was available. Crossmatch results and DSA of the desensitization group are depicted in Table 2. Desensitized patients received a mean of 5.1 ± 1.9 sessions of PLEX, 0.7 ± 0.5 g/kg of IVIg, and 4.6 ± 1.6 mg/kg of rATG. There were no significant differences in surgical times among the groups. In the group receiving perioperative desensitization, median surgical time was 499.5 min (460.3–688.8), median bypass time was 181 min (145.8–231), and median cross clamp time was 98 min (83.3–106.3). There were few differences in overall transfusion requirements within the first 7 d posttransplant, although patients receiving desensitization required significantly more transfusions of fresh frozen plasma (FFP) [19 units (8.3–24.8) for desensitization versus 7 units (4–8) for no-induction versus 5.5 units (3–8) for induction; P = 0.02]. Surgical and transfusion data are detailed further in Appendix (SDC, http://links.lww.com/TXD/A304).
TABLE 2.
Donor-specific antibody and crossmatch results of desensitized patientsa,b
| Patient | VXM | T-cell FXM | B-cell FXM | DSA | Pretransplant MFI | Day 21 MFI | Month 12 MFI | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | PositiveA3, B41, B45, C16, C17 | Positive | Positive | B45, Bw6 | 8396 | 2784 | 486 | No rejection to dateAlive 3 y and 6 mo |
| B41, Bw6 | 7698 | 1052 | 288 | |||||
| Cw16 | 1108 | 858 | 304 | |||||
| Cw17 | 1134 | 884 | 532 | |||||
| 2 | Never assessed | Positive | Positive | B13, Bw4 | 1700 | 98 | 2 | Grade 2R ACR at 83 dGrade 1R ACR at 323 dAlive 2 y and 4 mopost-HT |
| B35, Bw6 | 9939 | 4829 | 14 | |||||
| Cw4 | 1856 | 533 | 86 | |||||
| 3 | PositiveDR4, DRw51 | Negative | Positive | DR4 | 2589 | 1310 | 338 | No rejection to dateAlive 2 y and 6 mopost-HT |
| DRw51 | 2816 | 1190 | 2723 | |||||
| DQ6 | 1076 | 494 | 52 | |||||
| 4 | Never assessed | Positive | Equivocal | A1 | 2986 | Not drawn | 147 | No rejection to dateAlive 2 y and 6 mopost-HT |
| DR4 | 2512 | Not drawn | 0 | |||||
| 5 | PositiveB35, B72, DP1 | Positive | Positive | B51, Bw4 | 1349 | 2218 | 274 | Grade 1 pAMR at 10 dRequired VA-ECMO for PEA arrest and biventricular dysfunctionAlive 2 y and 5 mopost-HT |
| B53, Bw4 | 2188 | 3451 | 0 | |||||
| Cw4 | 1024 | 125 | 0 | |||||
| Cw16 | 5802 | 596 | 0 | |||||
| DP1 | 0 | 1541 | 43 | |||||
| 6 | PositiveB37, DQ5 | Positive | Negative | B37, Bw4 | 5566 | 552 | 88 | No rejection to dateAlive 1 y and 11 mopost-HT |
| DQ5 | 1025 | 509 | 382 | |||||
| 7 | PositiveDR13, DQ6 | Negative | Negative | DQ6 | None | Not drawn | 3273 | No rejection to dateAlive at 1 y and 10 mo |
| 8 | PositiveA3, DR103, DR11, DQ5 | Equivocal | Equivocal | A3 | 8354 | 454 | Deceased | Withdrawal of care on POD 49 after complicated VA-ECMOcourse for PGDBiopsy with ischemic necrosis |
| DR103 | 2780 | 245 | ||||||
| DQ5 | 11 377 | 2356 | ||||||
| 9 | PositiveA1 | Positive | Positive | A1 | 8185 | 8185 | 6898 | No rejection to dateAlive at 1 y and 5 mo post-HT |
| 10 | PositiveDR4 | Negative | Positive | DR4 | 1222 | 1139 | 1345 | No rejection to dateAlive at 1 y and 5 mo post-HT |
aThe VXM was reported as positive when DSA to either HLA class I or II antigen was detected in the current or historic specimen with an MFI value ≥1000.
bFor the FXM, the shift median channel cutoffs were determined through evaluation of donor cells with negative control serum. Shifts ≤2 standard deviations from the mean were considered negative, shifts ≥3 standard deviations were positive, and shifts between 2 and 3 standard deviations were considered equivocal. A 5% false positive and 5% false negative rate is expected with the FXM.
ACR, acute cellular rejection; DSA, donor-specific antibody; FXM, flow cytometry crossmatch; HT, heart transplant; MFI, mean fluorescence index; pAMR, pathologic antibody-mediated rejection; PEA, pulseless electrical activity; PGD, primary graft dysfunction; POD, postoperative d; VA-ECMO, veno-arterial extracorporeal membrane oxygenation; VXM, virtual crossmatch.
Patient and Graft Outcomes
There was no significant difference in the primary endpoint of patient survival at 12 mo (Figure 2). Rates of acute rejection were numerically lower in the induction and desensitization groups compared with the no-induction group; this difference was not statistically significant. Freedom from ACR and AMR was also similar (Figure 3). There was no significant difference in freedom from ISHLT CAV1. Patient and graft outcomes are summarized in Table 3.
FIGURE 2.
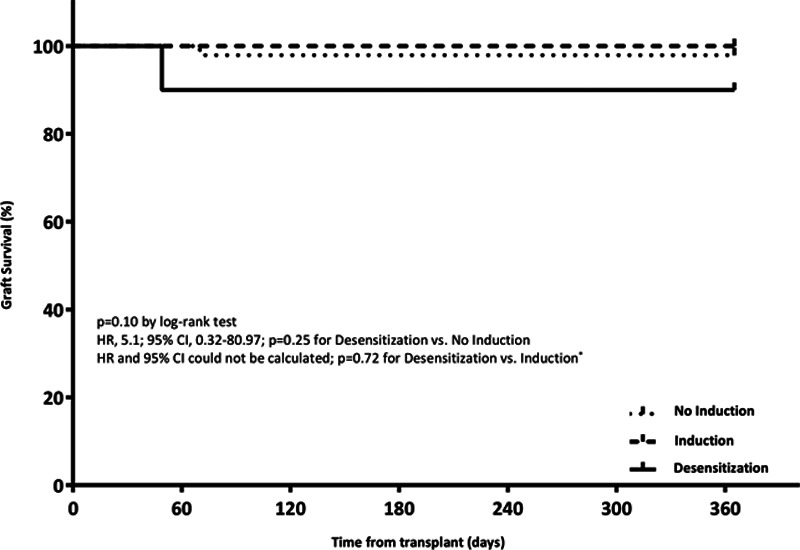
Survival at 12 mo. *HR and 95% CI could not be determined as there were zero events in at least 1 of the groups.
FIGURE 3.
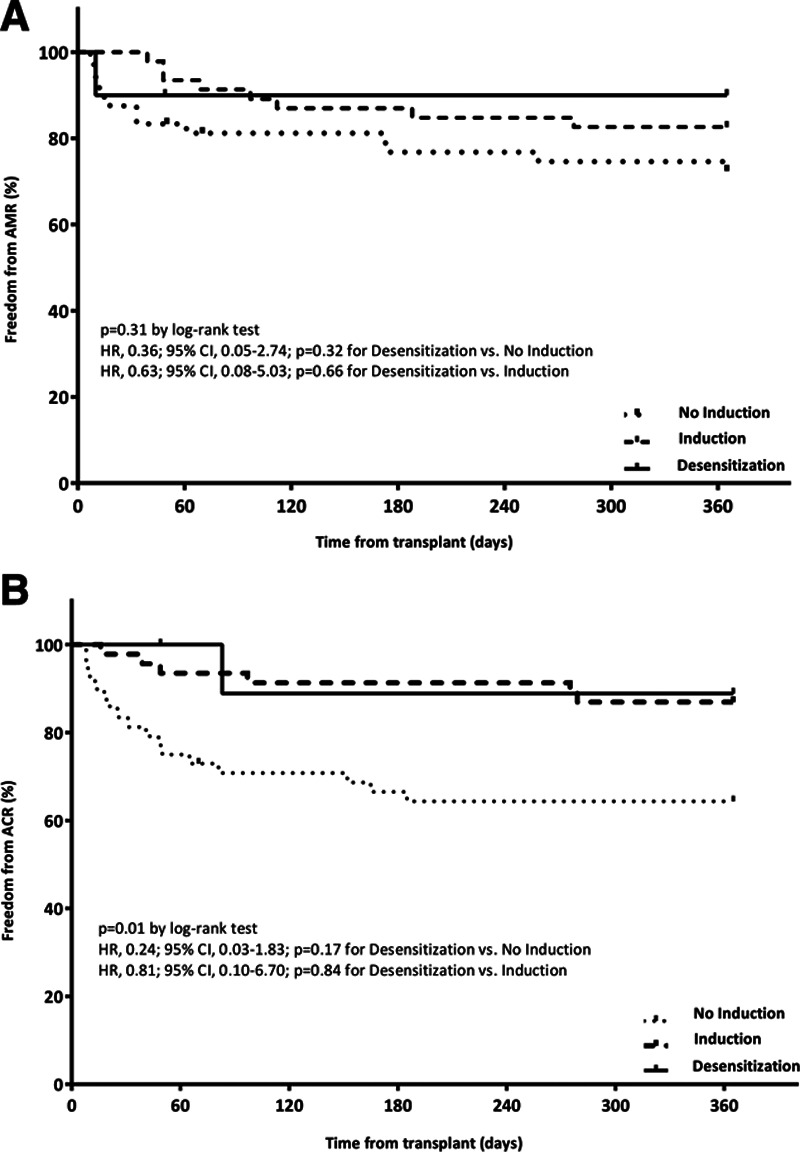
Freedom from antibody-mediated rejection and acute cellular rejection at 12 mo. A, Freedom from any AMR at 12 mo. B, Freedom from any ACR at 12 mo. ACR, acute cellular rejection; AMR, antibody-mediated rejection.
TABLE 3.
Graft outcomes at 12 mo
| Desensitization (n = 10) | No induction (n = 48) | HR (95% CI) | P a | |
|---|---|---|---|---|
| Graft failure | 1 (10.0) | 1 (2.1) | 5.1 (0.32-80.97) | 0.25 |
| Rejection | ||||
| Any severity | 2 (20.0) | 23 (47.9) | 0.36 (0.08-1.51) | 0.16 |
| Grade ≥2 rejection | 1 (10.0) | 8 (16.7) | 0.59 (0.07-4.69) | 0.62 |
| Acute cellular rejection | ||||
| Any severity | 1 (10.0) | 17 (35.4) | 0.24 (0.03-1.83) | 0.17 |
| Grade ≥2R | 1 (10.0) | 5 (10.4) | 1.01 (0.12-8.62) | 0.99 |
| Antibody-mediated rejection | ||||
| Any severity | 1 (10.0) | 13 (27.1) | 0.36 (0.05-2.74) | 0.32 |
| Grade ≥2 pAMRb | 0 (0.0) | 3 (6.3) | – | 0.62 |
| ISHLT CAV1 | 2 (20.0) | 12 (25.0) | 0.79 (0.20-3.17) | 0.76 |
| Desensitization (n = 10) | Induction (n = 46) | HR (95% CI) | P | |
| Graft failureb | 1 (10.0) | 0 (0.0) | – | 0.72 |
| Rejection | ||||
| Any severity | 2 (20.0) | 11 (23.9) | 0.93 (0.21-4.18) | 0.92 |
| Grade ≥2 rejection | 1 (10.0) | 1 (2.2) | 5.42 (0.34-86.78) | 0.23 |
| Acute cellular rejection | ||||
| Any severity | 1 (10.0) | 6 (13.0) | 0.81 (0.10-6.70) | 0.84 |
| Grade ≥2Rb | 1 (10.0) | 0 (0.0) | – | 0.75 |
| Antibody-mediated rejection | ||||
| Any severity | 1 (10.0) | 8 (17.4) | 0.63 (0.08-5.03) | 0.66 |
| Grade ≥2 pAMRb | 0 (0.0) | 1 (2.2) | – | 0.78 |
| ISHLT CAV1 | 2 (20.0) | 7 (15.2) | 1.43 (0.25-8.26) | 0.65 |
aCox proportional hazards models were used for the analysis of these time-to-event outcomes.
bHR and 95% CI could not be determined as there were 0 events in at least 1 of the groups.
CAV, cardiac allograft vasculopathy; ISHLT, International Society of Heart and Lung Transplantation; pAMR, pathologic antibody-mediated rejection.
Concomitant Maintenance Immunosuppression
There were significant differences in maintenance immunosuppression across the 3 groups (Table 4). Compared with the no-induction group, the induction group was on lower MMF doses, whereas the desensitization cohort received similar MMF doses. Because of delay in tacrolimus initiation in the desensitization and induction cohorts, tacrolimus trough concentrations were lower compared with the no-induction cohort at POD 7 (desensitization 4.8 ng/mL versus no-induction 8 ng/mL, P < 0.05; induction 4 ng/mL versus no-induction 8 ng/mL, P < 0.05). Tacrolimus trough concentrations in the desensitization cohort remained at or below goal during the 12 mo of follow-up. Desensitization patients were on similar steroid doses compared with the no-induction group through 12 mo; however, all desensitization patients were off steroids at 12 mo.
TABLE 4.
Concomitant maintenance immunosuppression
| Desensitization (n = 10) | No induction (n = 48) | Induction (n = 46) | P | |
|---|---|---|---|---|
| Mycophenolate dose (mg/d) | ||||
| D 7a | 2000 (2000–2000) | 2000 (2000–2000) | 2000 (2000–2000) | 0.01 |
| Mo 1a,b | 2000 (2000–2500) | 2000 (2000–2500) | 2000 (1000–2000) | <0.0001 |
| Mo 3a,b | 2000 (2000–2500) | 2000 (2000–2000) | 1750 (500–2000) | 0.0001 |
| Mo 6a | 2000 (1750–2500) | 2000 (1500–2000) | 1000 (500–2000) | 0.002 |
| Mo 12a | 1000 (1000–2000) | 2000 (1000–2000) | 1000 (1000–2000) | 0.04 |
| Tacrolimus trough concentration (ng/mL) | ||||
| D 7a,c | 4.8 (2.4–7.2) | 8 (6.4–10.4) | 4 (1.8–7) | <0.0001 |
| Mo 1a | 9.3 (6.6–11.4) | 10.7 (8.3–14.1) | 7.9 (5.3–9.9) | 0.0002 |
| Mo 3a | 8.7 (5.9–11.3) | 12 (10–14.3) | 8.4 (6.9–10.8) | 0.0002 |
| Mo 6b | 12.5 (8.8–14.7) | 10 (7.5–13.1) | 8.5 (6.6–10.6) | 0.02 |
| Mo 12a | 7.3 (5.9–11.2) | 9.8 (7.8–13.2) | 8 (5.3–10.3) | 0.01 |
| Corticosteroid dose (mg/d) | ||||
| Cumulative at d 7 | 2103 (1936–2431) | 2049 (1915–2094) | 1994 (1300–2249) | 0.46 |
| D 7a,b | 20 (20–25) | 40 (20–50) | 10 (7.5–20) | <0.0001 |
| Mo 1a,b | 17.5 (13.8–20) | 17.5 (15–20) | 10 (5–10) | <0.0001 |
| Mo 3a | 7.5 (7.5–15) | 10 (7.5–12.5) | 5 (5–10) | 0.0001 |
| Mo 6 | 5 (2.5–5) | 5 (2.5–7.5) | 5 (2.5–5) | 0.11 |
| Mo 12c | 0 (0–0) | 2.5 (0–5) | 0 (0–5) | 0.02 |
Myconolate and corticosteroid doses are standardized to mycophenolate mofetil and prednisone equivalents, respectively. Bold indicates statistically significant difference.
aP < 0.05 for comparison of no-induction cohort to induction cohort.
bP < 0.05 for comparison of desensitization cohort to induction cohort.
cP < 0.05 for comparison of desensitization cohort to no-induction cohort.
Additional Outcomes
There was no significant difference in infection among the 3 groups (Figure 4). CMV viremia made up the majority of infections, with a numerically higher incidence in the induction group. Use of CMV and Pneumocystis jirovechi pneumonia prophylaxis was similar among the desensitization cohort when compared with induction and no-induction cohorts. Few differences in laboratory parameters emerged over the study period. Patients receiving perioperative desensitization had significantly lower leukocyte counts at 7 d compared with those receiving no induction [9.7 K/mm3 (6.9–12.5) versus 13.2 K/mm3 (10.9–16.1); P < 0.05]. Desensitized patients also had significantly lower platelet counts at 7 d compared with both the no-induction [72 K/mm3 (61.5–113.5) versus 150.5 K/mm3 (118.5–204); P < 0.05] and induction [72 K/mm3 (61.5–113.5) versus 129 K/mm3 (102–177.8); P < 0.05] cohorts. These outcomes are detailed in Appendix (SDC, http://links.lww.com/TXD/A304).
FIGURE 4.
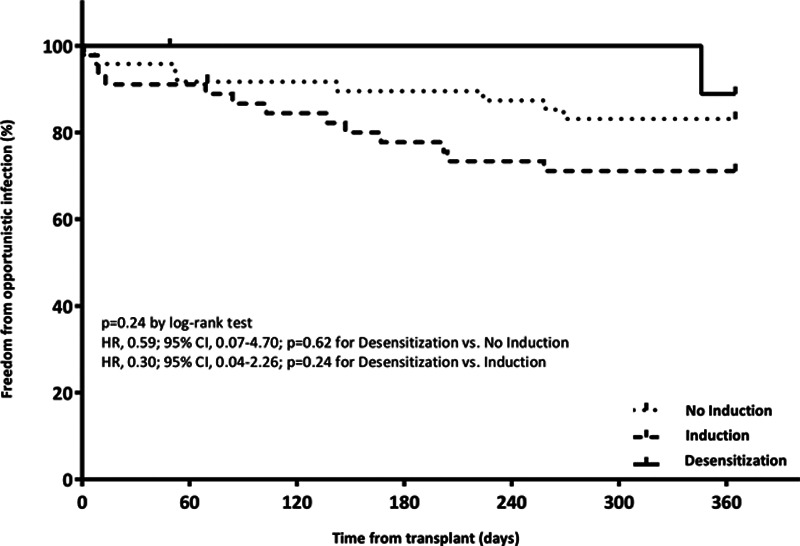
Freedom from infectious complication at 12 mo.
DISCUSSION
In the present study, we describe the outcomes of HT recipients who have received a perioperative desensitization strategy initiated after positive VXM or FXM. HT recipients who received perioperative desensitization demonstrated acceptable rates of survival, rejection, CAV, and infectious complications at 12 mo when compared with those who received either standard immunosuppression or induction therapy. Notably, these patients would not have been transplanted at our institution before implementation of this desensitization protocol. To our knowledge, this is the first study in HT to compare outcomes of perioperative desensitization triggered by a positive VXM or prospective FXM to an unsensitized cohort.
In the first study evaluating a perioperative PLEX, IVIg, and rATG strategy, desensitization of 35 HT recipients (PRA >10%), led to significant decreases in PRA. However, 7 patients demonstrated little change or even increase in PRA following therapy.11 Additionally, the effect on positive crossmatch by complement-dependent cytotoxicity (CDC) or flow-cytometry was heterogenous. Of the 12 patients with positive T- and B-cell crossmatch before transplant, 8 had persistent positivity postdesensitization. Nonetheless, overall survival was significantly higher than 277 historical controls (P = 0.0414) at a mean follow-up of 21.2 mo.
More recently, desensitization appeared efficacious in lung transplant recipients with positive DSA (MFI >1200) or high PRA (≥30%).10 All patients (53 with positive DSA) received 8 exchange sessions, rATG 3–5 mg/kg, and IVIg 1 g/kg. Compared with PRA-positive with no DSA and unsensitized patients, there was no difference in 1- or 5-y allograft survival. Additionally, ISHLT grade ≥2 rejection was less common in patients who were DSA- or PRA-positive compared with unsensitized patients. Despite similar outcomes, 53% of DSA-positive recipients had at least 1 persistent DSA posttransplant and 56% developed de novo DSA.
A follow-up study was conducted in 4 highly sensitized HT recipients with positive VXM.12 Mean class I and II PRA before transplant was 96% and 66%, respectively, and all patients had positive T- and B-cell FXM with high-level DSA for at least 3 mo. At a median follow-up of 556 d, all patients survived. One patient developed primary graft dysfunction requiring veno-arterial extracorporeal membrane oxygenation (VA-ECMO) for 10 d. Three of 4 patients experienced ISHLT grade >2R ACR during the first 12 mo, with 1 patient also developing ISHLT pathologic AMR (pAMR) 2 at 2 wk.
Another recent study in HT used a similar perioperative desensitization protocol in sensitized patients (88 patients with pretransplant HLA antibodies with an MFI 500–1000 and 194 patients with an MFI > 1000).16 In patients with an MFI > 1000, the protocol consisted of PLEX (1 pretransplant session, followed by 4 daily postoperative sessions) and IVIg 2 g/kg. At a median follow-up of 4.06 y, the group with an MFI > 1000 demonstrated lower rejection-free survival and a 4-fold increase in the risk of AMR. Additionally, overall survival rates were found to only be 81.1% and 73.6% at 1 and 3 y, respectively.
Compared with previous studies, the present study has several important differences. First, our study compared rates of survival and acute rejection with those of 2 unsensitized control groups receiving either no induction or induction. The majority (n = 33) of patients in the induction cohort received alemtuzumab. Several previous studies have demonstrated reductions in ACR with alemtuzumab, especially ISHLT grade ≥2, compared with patients receiving no induction.17,18 Despite high cPRA and positive VXM and FXM in our desensitized patients, there was no difference in biopsy-proven rejection at 12 mo compared with our induction cohort. This suggests that transplantation into a sensitized cohort can be accomplished safely with acceptable rates of rejection within the first year, contrary to previous studies that have shown dramatically increased rejection rates.16
Second, selection of patients for desensitization at our institution was typically driven by positive VXM. Although use of VXM has strong negative and positive predictive value for determining incompatible CDC crossmatch (92% and 79%, respectively), there are minimal data for use of FXM in HT.19 In kidney transplantation, VXM results have been correlated with FXM and CDC crossmatch results.20,21 Additionally, previous studies have demonstrated a high correlation between positive FXM and graft loss in kidney transplant recipients with a specificity of 86%.20 Of the 8 patients with positive VXM in our cohort, 3 had positive T- and B-cell FXM, 2 had isolated positive B-cell FXM, 1 had isolated positive T-cell FXM, 1 had equivocal T- and B-cell FXM, and 1 had negative T- and B-cell FXM. Although these data suggest that VXM is an imperfect predictor of positive FXM, utility remains in its application. For example, patient 7 had a positive VXM to DQ6 and DR13 and a negative T- and B-cell FXM; antibody to the DQ6 antigen reappeared at month 12.
Finally, we reported details that suggest acceptable tolerability of perioperative desensitization. At 12 mo, no significant differences in rates of infectious complications were observed. The infection rate was largely driven by CMV viremia in all cohorts. Numerically fewer patients in the desensitization group had baseline CMV high-risk status (donor-positive/recipient-negative), which may account for the numerically lower rates of CMV viremia in this cohort. Additionally, there were few differences in laboratory parameters. Patients receiving desensitization had significantly lower leukocyte and platelet counts at 7 d compared with those receiving no induction, which was expected given the known adverse effects of rATG. Platelet count was also significantly lower at 7 d in desensitized patients compared with those receiving induction, which may be a consequence of platelet removal by PLEX. Desensitized patients received significantly more FFP transfusions by POD 7 compared with both induction and no-induction groups. This was expected given the coagulopathy that ensues following PLEX, and our desensitization protocol, which entails scheduled FFP transfusion during the final intraoperative exchange and on POD 1 and 2. Importantly, there were no differences in the transfusion of other blood products, nor were there any episodes of clinically significant bleeding events related to PLEX.
Although survival and rejection were similar among the 3 groups, there are several findings warranting further discussion. Patient 8 experienced severe biventricular dysfunction immediately postoperatively requiring VA-ECMO. The EMB demonstrated ischemic necrosis. Interestingly, both T- and B-cell FXM were equivocal despite high-level DSA to A3, DR103, and DQ5. Despite reduced-dose IVIg and fewer sessions of PLEX, DSA MFI decreased dramatically. Ultimately, care was withdrawn on POD 49 because of inability to wean from VA-ECMO. This patient’s outcome might have been confounded by donor-related factors (ie, old donor age of 59 y, prolonged ischemic time of 312 min) and not necessarily because of the detrimental effect of desensitization or positive crossmatch. Overall, our results suggest that our cohort overcame the potential risks of perioperative desensitization, including intraoperative volume shifts, coagulopathy, and substantial immunosuppression.
Patient 5 was the only desensitized patient to experience pAMR, which occurred on POD 10. The initial postoperative course was complicated by cardiac arrest requiring VA-ECMO. Initial biopsy demonstrated pAMR 1, which persisted on subsequent EMBs for 2 mo. The initial VXM was positive for antibody against B35, B72, and DP1. Interestingly, the FXM demonstrated Class I DSA against B51, B53, Cw4, and Cw16 with no DSA against Class II antigens ≥1000 MFI. Following desensitization, there was a reduction in Cw16 and Cw4 but a rebound in B51 and B53. Additionally, DSA to DP1 reemerged. This case demonstrates that the use of PLEX, IVIg, and rATG perioperatively is imperfect given the rebound in antibody production. Additionally, the significance of individual DSA is unknown and further studies are needed to elucidate the clinical relevance of DSA in relation to graft outcomes.
Three patients in the desensitization cohort demonstrated persistent DSA ≥1000 MFI at 12 mo. Patient 2 and 10 had DSA to class II antigens, whereas patient 9 had evidence of class I DSA at 12 mo. Although none of these patients had evidence of AMR throughout the study, these findings indicate that perioperative desensitization does not completely eliminate DSA. Despite research suggesting that de novo DSA is a poor predictor of concurrent pAMR, antibodies to class II antigens in this cohort were still of particular concern given their association with an increased risk of future AMR.22 Long-term follow-up will be necessary to adequately assess the impact of persistent DSA on survival, acute rejection, and CAV. Despite such risks, these patients would not have been transplanted at our center before implementation of the crossmatch-triggered desensitization strategy. Importantly, desensitized patients in our cohort were listed for heart transplant for a median of 189 d, which was numerically longer than the no-induction and induction groups (137 and 82 d, respectively). One previous study demonstrated reduction in mean waitlist time from 129 ± 246 d to 59 ± 78 d after implementation of a prospective VXM strategy for donor allocation.23
There are several limitations of the present study. First, this study is retrospective by design and was not powered to detect differences in the rates of survival and acute rejection. Studies with adequate power are not likely feasible as the use of perioperative desensitization is infrequent and nuanced. Second, there are limitations to using a VXM-guided approach as this tool is not a perfect predictor of physical crossmatch. However, we did see evidence of antibody from a VXM reappear at 12 mo suggesting that it may still be useful in risk-stratification. Third, only a few subtypes of infection were analyzed. Although there were instances in which patients were empirically treated with antibiotics, we elected to not include these in the analysis as no clear source or organism was identified. Fourth, perioperative desensitization at our institution was implemented late in the study period; thus, differences in surgical technique and postoperative management may confound these results. Fifth, patients receiving induction mostly received alemtuzumab, which is infrequently used by HT centers. Nonetheless, alemtuzumab has demonstrated improved graft outcomes in several studies.17,18 Given similar rates of rejection and survival when comparing our desensitized patients with those receiving induction therapies, we feel comfortable that our perioperative strategy results in acceptable 1-y outcomes. Finally, CDC crossmatch was not performed. It is possible that lower levels of DSA would result in positive VXM or FXM, but negative CDC crossmatch. However, the high cPRA, and positive VXM or FXM would have precluded transplantation of these individuals before implementation of this desensitization protocol. Our threshold MFI of ≥1000 for cPRA may be lower than what some centers may use; however, it is important to note that MFI is not truly quantitative and many factors, both biological and technical, can influence MFI. Our results suggest that this strategy is feasible, avoids undue toxicity, and improves access for sensitized individuals. It is important to note that desensitization is nuanced and careful evaluation of crossmatch results, MFI strength, class of HLA antibodies, and overall transplant risk is imperative before proceeding with this protocol. Further evaluation of this protocol in a population with a positive CDC crossmatch is needed to fully understand the effectiveness in the most sensitized patients.
CONCLUSION
This study suggests that a strategy of perioperative desensitization triggered by a positive VXM or prospective FXM allows for successful transplantation of sensitized HT recipients and results in acceptable rates of survival, acute rejection, CAV, and infection at 12 mo when compared with unsensitized control groups receiving no induction or lymphocyte-depleting induction therapy. Studies with longer follow-up periods are needed to further assess the impact of this strategy on the rates of CAV, acute rejection, and long-term graft survival.
ACKNOWLEDGMENTS
The authors of this article would like to acknowledge Dr Debra L. KuKuruga for her expert guidance on interpretation of crossmatch and DSA results. Additionally, the authors would like to acknowledge Dr Bartley P. Griffith, Dr Zachary N. Kon, and Dr Erika D. Feller for their work with implementation of the desensitization protocol at our institution.
Footnotes
Published online 26 January, 2021.
The authors declare no funding or conflicts of interest.
M.E.P. participated in research design, performance of the research, data analysis, and writing of the article. S.E.G., S.H., R.J.M., and B.R. participated in research design, performance of the research, and writing of the article. B.N.R. participated in research design, data analysis, and writing of the article. V.-K.T. and D.J.K. participated in research design and writing of the article.
REFERENCES
- 1.Khush KK, Cherikh WS, Chambers DC, et al. ; International Society for Heart and Lung Transplantation. The international thoracic organ transplant registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult heart transplantation report-2018; focus theme: multiorgan transplantation. J Heart Lung Transplant. 2018; 37:1155–1168 [DOI] [PubMed] [Google Scholar]
- 2.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2017 annual data report: heart. Am J Transplant. 2019; 19Suppl 2323–403 [DOI] [PubMed] [Google Scholar]
- 3.Betkowski AS, Graff R, Chen JJ, et al. Panel-reactive antibody screening practices prior to heart transplantation. J Heart Lung Transplant. 2002; 21:644–650 [DOI] [PubMed] [Google Scholar]
- 4.Patel J, Everly M, Chang D, et al. Reduction of alloantibodies via proteasome inhibition in cardiac transplantation. J Heart Lung Transplant. 2011; 30:1320–1326 [DOI] [PubMed] [Google Scholar]
- 5.Chih S, Patel J. Desensitization strategies in adult heart transplantation-will persistence pay off? J Heart Lung Transplant. 2016; 35:962–972 [DOI] [PubMed] [Google Scholar]
- 6.Kaczmarek I, Deutsch MA, Kauke T, et al. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp Clin Transplant. 2008; 6:229–235 [PubMed] [Google Scholar]
- 7.Nwakanma LU, Williams JA, Weiss ES, et al. Influence of pretransplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg. 2007; 84:1556–1562discussion 1562 [DOI] [PubMed] [Google Scholar]
- 8.Kobashigawa JA, Sabad A, Drinkwater D, et al. Pretransplant panel reactive-antibody screens. are they truly a marker for poor outcome after cardiac transplantation? Circulation. 1996; 949 SupplII294–II297 [PubMed] [Google Scholar]
- 9.Kobashigawa JA, Patel JK, Kittleson MM, et al. The long-term outcome of treated sensitized patients who undergo heart transplantation. Clin Transplant. 2011; 25:E61–E67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tinckam KJ, Keshavjee S, Chaparro C, et al. Survival in sensitized lung transplant recipients with perioperative desensitization. Am J Transplant. 2015; 15:417–426 [DOI] [PubMed] [Google Scholar]
- 11.Leech SH, Lopez-Cepero M, LeFor WM, et al. Management of the sensitized cardiac recipient: the use of plasmapheresis and intravenous immunoglobulin. Clin Transplant. 2006; 20:476–484 [DOI] [PubMed] [Google Scholar]
- 12.Alhussein M, Moayedi Y, Posada JD, et al. Peri-operative desensitization for highly sensitized heart transplant patients. J Heart Lung Transplant. 2018; 37:667–670 [DOI] [PubMed] [Google Scholar]
- 13.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation working formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant. 2013; 32:1147–1162 [DOI] [PubMed] [Google Scholar]
- 14.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005; 24:1710–1720 [DOI] [PubMed] [Google Scholar]
- 15.Mehra MR, Crespo-Leiro MG, Dipchand A, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010; 29:717–727 [DOI] [PubMed] [Google Scholar]
- 16.Coutance G, d’Orio V, Belin L, et al. Favorable outcome of an exclusively posttransplant prophylactic strategy after heart transplantation in recipients with high immunological risk. Transplantation. 2019; 103:1439–1449 [DOI] [PubMed] [Google Scholar]
- 17.Teuteberg JJ, Shullo MA, Zomak R, et al. Alemtuzumab induction prior to cardiac transplantation with lower intensity maintenance immunosuppression: one-year outcomes. Am J Transplant. 2010; 10:382–388 [DOI] [PubMed] [Google Scholar]
- 18.Gale SE, Ravichandran B, Ton VK, et al. Alemtuzumab induction versus conventional immunosuppression in heart transplant recipients. J Cardiovasc Pharmacol Ther. 2019; 24:435–441 [DOI] [PubMed] [Google Scholar]
- 19.Stehlik J, Islam N, Hurst D, et al. Utility of virtual crossmatch in sensitized patients awaiting heart transplantation. J Heart Lung Transplant. 2009; 28:1129–1134 [DOI] [PubMed] [Google Scholar]
- 20.Ho EK, Vasilescu ER, Colovai AI, et al. Sensitivity, specificity and clinical relevance of different cross-matching assays in deceased-donor renal transplantation. Transpl Immunol. 2008; 20:61–67 [DOI] [PubMed] [Google Scholar]
- 21.Piazza A, Ozzella G, Poggi E, et al. Virtual crossmatch in kidney transplantation. Transplant Proc. 2014; 46:2195–2198 [DOI] [PubMed] [Google Scholar]
- 22.Clerkin KJ, Farr MA, Restaino SW, et al. Donor-specific anti-HLA antibodies with antibody-mediated rejection and long-term outcomes following heart transplantation. J Heart Lung Transplant. 2017; 36:540–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanagida R, Czer LS, Reinsmoen NL, et al. Impact of virtual cross match on waiting times for heart transplantation. Ann Thorac Surg. 2011; 92:2104–2110; discussion 2111 [DOI] [PubMed] [Google Scholar]


