Abstract
Objective:
Currently, it is unclear whether the salviae miltiorrhizae (Danshen Salvia) and ligustrazine hydrochloride (Chuanxiong Chuanxiong) (SMLH) injection combined with mecobalamin can improve diabetic peripheral neuropathy (DPN). We conducted a systematic analysis to evaluate the clinical effects of SMLH injection combined with mecobalamin for treating DPN.
Methods:
Seven databases, including PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wan Fang Database (Wang Fang), Chinese Biomedical Literature Database (CBM), and VIP Database for Chinese Technical Periodicals (VIP) were searched for systematic literature retrieval. Each database was searched up to 2020 to identify randomized controlled trials on DPN treated with SMLH injection combined with mecobalamin. We used the RevMan 5.3 and Stata 14.0 software to assess the risk of bias in the included trials.
Results:
A total of 15 publications, including 1349 samples, were reviewed. The total effective rate of SMLH injection combined with mecobalamin was 31% higher than that of mecobalamin alone (95% confidence interval [CI] = 1.23–1.38; P < .00001). The experimental group showed a significant increase in the motor conduction velocity (MCV) of the peroneal nerve (weighted mean difference [WMD] = 4.81, 95% CI 3.53–6.09; P < .00001). In addition, SMLH injection combined with mecobalamin showed a statistical significant effect on the sensory conduction velocity (SCV) of the peroneal nerve (WMD = 5.03, 95% CI = 4.16–5.90; P < .00001), and MCV of the median nerve (WMD = 5.38, 95% CI = 4.05–6.72; P < .00001). The WMD for the change in SCV in the median nerve was 4.89 m/s (95% CI = 3.88–5.89; P < .00001). The P-values of the Egger and Begg tests were 0.967 and 0.961, respectively, indicating no publication bias. Subgroup and sensitivity analyses indicated that the results for MCV and SCV of the peroneal nerve and the median nerve were stable.
Conclusion:
SMLH injection combined with mecobalamin can improve DPN, compared with mecobalamin alone.
Keywords: diabetic peripheral neuropathy, mecobalamin, salviae miltiorrhizae and ligustrazine hydrochloride, systematic analysis
1. Introduction
Diabetes and its complications pose a serious threat to global health, which is expected to increase to 642 million[1–2] by 2040; more than 90% of the affected patients have type 2 diabetes.[3] An aging population and poor lifestyle habits, such as a high-fat/high-sugar diet and a sedentary lifestyle, aggravate the global spread of diabetes.[4] Diabetic peripheral neuropathy (DPN) is a common complication of diabetes mellitus. In recent years, the prevalence of DPN has been increasing. According to current research, approximately 10% to 50% of diabetic patients will develop DPN,[5] which is of serious concern.[5] Pain, numbness, and an abnormal feeling in the limbs and trunk are common clinical manifestations of DPN. Severe cases include glove-like or sock-like sensory disorders and even muscle atrophy. Currently, approximately 50%–70% of non-traumatic amputations are performed due to DPN. Mortality of diabetic patients with neuropathy is expected to reach 25% to 50% in 5 to 10 years.[6–7] The pathogenesis of DPN involves oxidative stress caused by long-term hyperglycemia, vascular injury,[8–9] advanced glycation end products,[10] and the polyol pathway.[11] Early treatment is necessary and can delay the pathological process of DPN.[6] Treatment of DPN includes general treatment, drug treatment, physical treatment, and surgical treatment. The drugs used for DPN include nimodipine; antioxidant α-lipoic acid; epalrestat, an aldose reductase inhibitor; and Chinese medicinal preparations. However, these drugs have limited effects when used alone. Therefore, it is necessary to study the effect of various therapeutic drug combinations.
Traditional Chinese medicine (TCM) is a traditional alternative therapy widely used in China. Based on the TCM theory, DPN can be classified as a “Bi syndrome,” which is mainly caused by blood stasis. Studies have established that Danshen reduces inflammatory reactions and vascular stress,[12] while Chuanxiong promotes vasodilation and eliminates blood stasis.[13–14] Salviae miltiorrhizae (Danshen Salvia) and ligustrazine hydrochloride (Chuanxiong Chuanxiong) (SMLH) injection, a combination of Danshen and Chuanxiong, can activate blood circulation, relieve pain, prevent platelet aggregation, dilate arterial vessels, reduce blood viscosity,and improve circulation.[15–17] SMLH injection has a wide range of clinical applications and has shown beneficial effects on coronary heart disease, cerebral ischemia, cor pulmonale, and diabetic neuropathy,[18–21] demonstrating that SMLH injection can increase blood circulation and alleviate symptoms. Therefore, SMLH injection may help improve DPN by promoting circulation and reducing inflammation. By far, most clinical studies have reported the effects of SMLH injection combined with mecobalamin. However, there is currently no systematic evaluation. In addition, the effects of SMLH injection may vary with the dosage and treatment course. This study aimed to evaluate the effects of SMLH injection combined with mecobalamin in the treatment of DPN, compared with mecobalamin alone.
2. Methods
2.1. Types of research
Published literature meeting all of the following criteria were included in this study:
-
(1)
a randomized controlled trial (RCT);
-
(2)
in terms of intervention measures, the experimental group was treated with SMLH injection combined with mecobalamin;
-
(3)
mecobalamin was used in the control group, and other conditions were the same for both groups.
2.2. Research criteria
DPN was diagnosed based on the diabetes clinical practice guidelines.[22] Exclusion criteria included acute diabetic complications, such as diabetic ketoacidosis and hyperosmolar coma. We excluded pregnant or lactating women, and patients with drug allergies or cancer. Furthermore, studies with incomplete or problematic data and repeated publications were excluded. Since this study used data that was previously published, ethical approval and patient consent were not required.
2.3. Types of intervention
The control group was treated with mecobalamin and other routine primary treatments, such as controlling blood glucose, lowering blood pressure, and regulating blood fat. The treatment group was treated with SMLH injection in addition to treatment administered to the control group.
2.4. Detection index
Detection indices included total effectiveness, motor conduction velocity (MCV), and sensory conduction velocity (SCV) of the peroneal nerve and the median nerve.
2.5. Search strategy
Databases including PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wan Fang Database (Wan Fang), Chinese Biomedical Literature Database (CBM), and VIP Database for Chinese Technical Periodicals (VIP) were searched to retrieve data on RCTs related to interventions performed using SMLH injection combined with mecobalamin on DPN. The retrieval time limit was set to 2020. The search terms and keywords were: “salvia miltiorrhiza and ligustrazine,” “Danshen Chuanxiong,” “diabetic peripheral neuropathy,” and “DPN.” The method of combining free words and subject words was adopted in the retrieval, and the retrieval strategy was adjusted in accordance with the characteristics of different databases for multiple searches, to optimize the recall rate of documents.
2.6. Data processing and analysis
2.6.1. Data screening
Two reviewers independently screened the available publications by topic and abstract. Two reviewers extracted literature data, including basic information on patients, interventions, and experimental results. For points of inconsistency, a third reviewer was consulted. See Figure 1.
Figure 1.
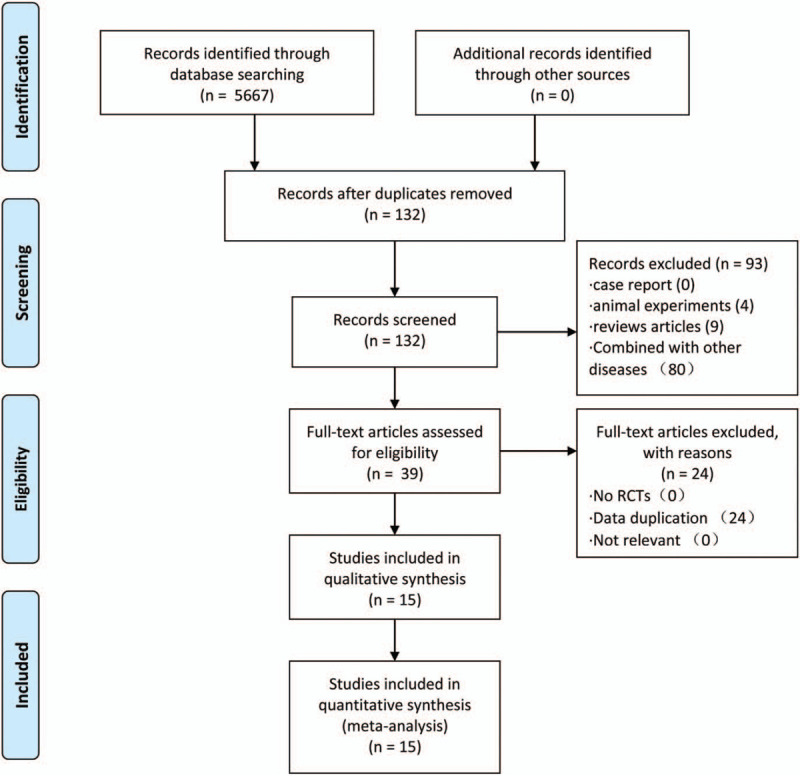
PRISMA flow chart.
2.6.2. Risk of bias analysis
The 2 reviewers independently screened the literature, extracted the basic data, assessed the bias risk, and compared them. In case of disagreement, the third reviewer was consulted. The extraction of basic data mainly included the following content:
-
(1)
basic information included in the study, including the first author and publication time;
-
(2)
baseline characteristics of the study object, including gender and age;
-
(3)
intervention measures, including intervention mode, dosage, and treatment course;
-
(4)
key elements of bias risk assessment; and
-
(5)
outcome indicators and outcome measurement data.
Two evaluators evaluated the bias risk of RCTs according to the manual of the Cochrane system evaluators.
2.6.3. Statistical methods
We used the statistical software RevMan 5.3 and Stata 14.0 from the Cochrane Collaboration. For dichotomous data, we used the risk ratios to analyze the statistics; for continuous data, we used the weighted mean difference (WMD)/standard mean difference (SMD) and the calculated 95% confidence intervals (CIs). The χ2 test was used to test the heterogeneity. If homogeneity was observed (P > .05; I2 < 50%) in the research results, the fixed effect model was used for meta-analysis; if there was a significant statistical heterogeneity (P ≤ .05; I2 ≥ 50%) between the research results, the random effect model was used for meta-analysis.
2.6.4. Heterogeneity and subgroup analysis
If the heterogeneity between studies was large, we analyzed the source of heterogeneity using subgroup analysis. We conducted a subgroup analysis from the perspective of the dose, treatment course, and manufacturer.
2.6.5. Publication bias
We used the Begg test and Egger test to detect publication bias. When P > .05, the deviation was considered not statistically significant.
3. Results
3.1. Research screening results
We searched 5667 articles and excluded 5535 articles unrelated to DPN. Ninety-three studies were excluded based on headlines and abstracts. A total of 84/93 studies were either based on combinations with other drugs and therapies or not compared with mecobalamin, and 9/93 studies were review articles. Thirty-nine articles met the storage requirements. Finally, only 15 studies met the inclusion criteria owing to repeated data in 24 cases.[23–37]
3.2. Research characteristics
The 15 selected studies included 1349 patients with DPN. All the publications were published in Chinese during 2010–2019. No multicenter experiments were reported (Table 1).
Table 1.
Basic characteristics of included studies.
| T | C | |||||||||
| Included studies | Age (yr) | DM course (yr) | n | Intervention | Age (yr) | DM course (yr) | n | Intervention | Treatment Course | Outcomes |
| Zhu MM 2018 | 54.52 ± 10.31 | 8.66 ± 2.13 | 33 | A | 54.96 ± 10.45 | 8.21 ± 2.65 | 33 | F | 2weeks | (1) (2) (3) (4) (5) |
| Chang Y 2013 | – | – | 30 | C | – | – | 30 | F | 2weeks | (1) (2) (3) (4) (5) |
| Li H 2011 | 53.58 ± 6.72 | – | 38 | B | 55.14 ± 8.54 | – | 38 | F | 2weeks | (1) (2) (3) (4) (5) |
| Li JP 2011 | 35–78 | 2–38 | 60 | C | 34–75 | 2–35 | 26 | F | 2weeks | (1) |
| Dai LF 2010 | 54.5 ± 4.2 | 8.2 ± 3.4 | 41 | A | 55.3 ± 3.2 | 9.0 ± 2.5 | 39 | F | 3weeks | (1) (2) (3) (4) (5) |
| Liu L 2019 | 57.44 ± 12.30 | 9.65 ± 2.18 | 50 | D | 58.03 ± 12.78 | 9.88 ± 2.31 | 50 | F | 8weeks | (1) (2) (3) (4) (5) |
| Liang Y 2019 | 53 ± 5 | 3–15 | 35 | C | 54 ± 4 | 2–16 | 35 | F | 2weeks | (1) (2) (3) (4) (5) |
| Li TJ 2011 | 53–82 | 5–13 | 50 | C | 56–78 | 8–15 | 50 | F | 4weeks | (1) (2) (3) (4) (5) |
| Sun H 2013 | 57.6 ± 7.8 | 8.7 ± 2.3 | 57 | C | 59.1 ± 9.2 | 9.1 ± 2.1 | 57 | F | 3weeks | (1) (2) (3) (4) (5) |
| Zhang MX 2016 | 58.2 ± 6.8 | 4.5 ± 0.8 | 42 | B | 56.3 ± 5.5 | 5.2 ± 1.6 | 42 | F | 2weeks | (1) (2) (3) (4) (5) |
| Wang Y-H 2014 | 60.87 ± 8.84 | 16.9 ± 7.04 | 45 | E | 59.74 ± 9.34 | 15.49 ± 6.54 | 43 | F | 4weeks | (1) |
| Peng HX 2011 | 61.3 ± 8.6 | 8.3 ± 4.6 | 56 | A | 62.9 ± 8.9 | 8.5 ± 4.9 | 56 | F | 2weeks | (1) (2) (3) |
| Jiang ZM 2015 | 43–85 | 5–31 | 86 | C | 43–85 | 5–31 | 77 | F | 2weeks | (1) (2) (3) (4) (5) |
| Zhang YJ 2017 | 58.7 ± 7.9 | 8.4 ± 1.7 | 45 | C | 59.2 ± 8.5 | 9.1 ± 1.5 | 45 | F | 3weeks | (1) (2) (3) (4) (5) |
| Wang YH 2014 | 57.23 ± 6.61 | 13.53 ± 2.66 | 30 | D | 58.13 ± 7.05 | 12.83 ± 2.53 | 30 | F | 8weeks | (1) (2) (3) (4) (5) |
Two-thirds of the trials (10 trials) were small sample studies (sample size < 100). The average treatment course in these studies ranged from 2–8 weeks. The treatment course was 2 weeks in 8 trials, 3 weeks in 3 trials, 4 weeks in two trials, and 8 weeks in two trials. All studies evaluated the total effective rate, 13 evaluated the MCV and SCV of the peroneal nerve, and 12 evaluated the MCV and SCV of the median nerve.
3.3. Bias risk
Based on the methodological quality chart (Figs. 2 and 3), only 2 papers mentioned the specific randomization method, 1 article was grouped according to the order of admission with a high risk, and the rest lacked clarity. Adverse reactions were reported in 2 articles. Blinding details were not mentioned in any trial.
Figure 2.
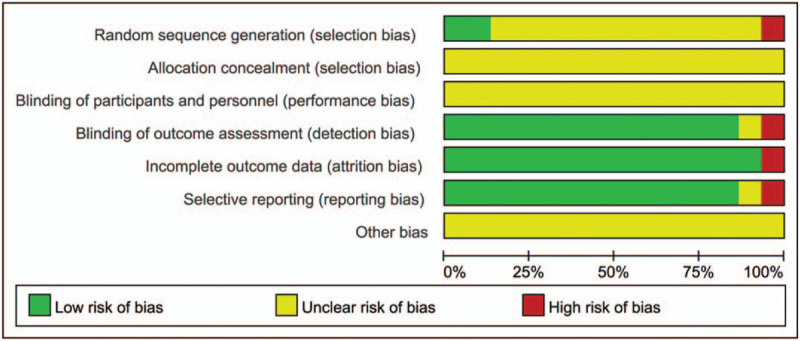
Risk of bias graph.
Figure 3.
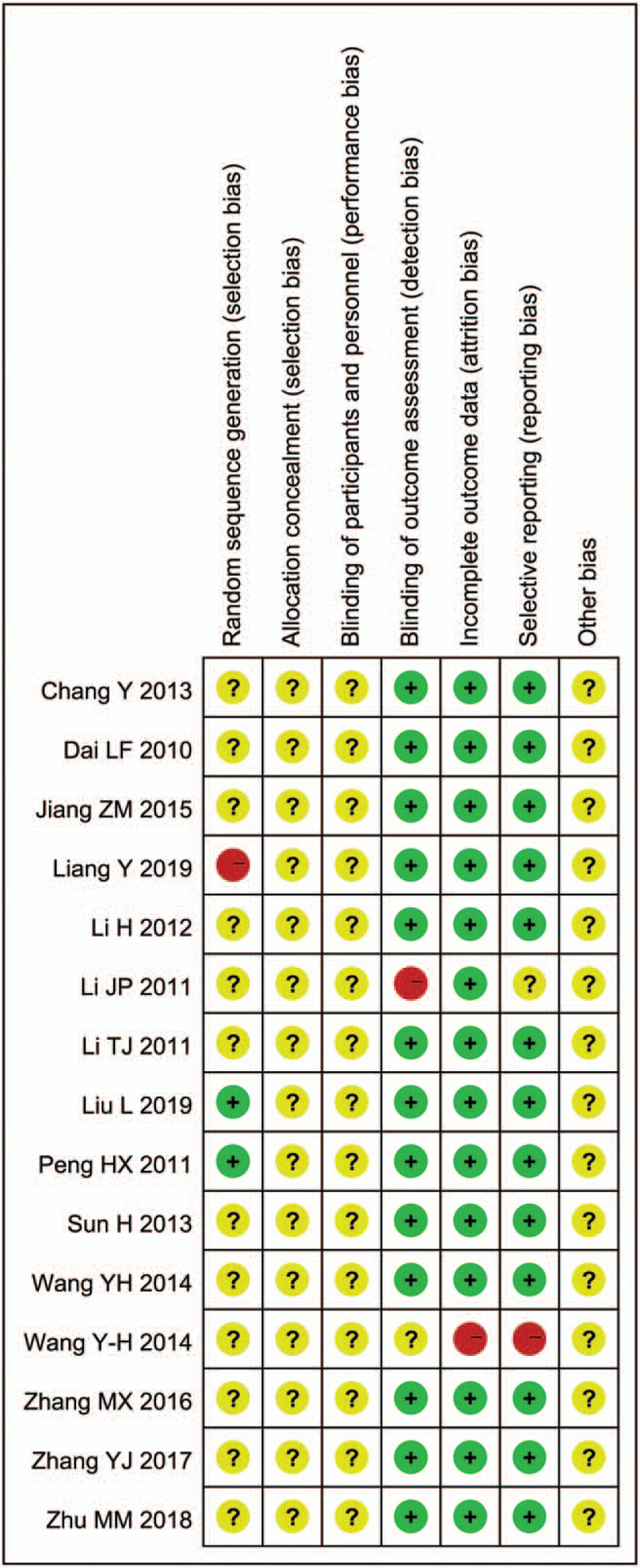
Risk of bias summary.
3.4. Statistical analysis results
3.4.1. Total effective rate
Fifteen trials, including 1349 samples, reported the total effective rate. There was no statistical significance in the heterogeneity test; hence, a fixed effect model was used. The total effective rate was 31% higher than that of the control (95% CI = 1.23–1.38; P < .00001) (Fig. 4).
Figure 4.
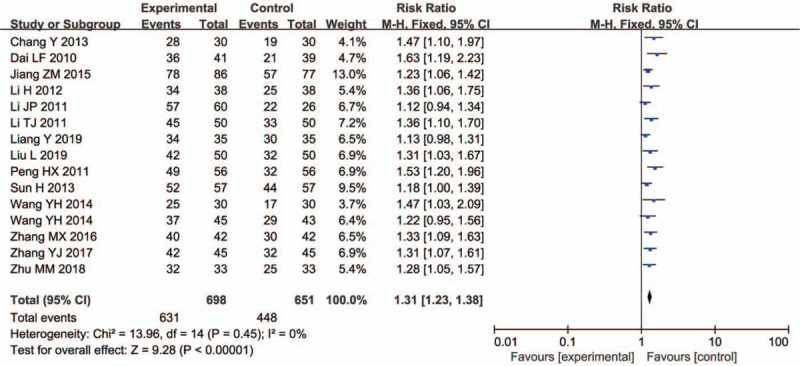
Total effective rate.
3.4.2. Peroneal nerve MCV
Thirteen trials (1175 participants) evaluated the peroneal nerve MCV. Since the heterogeneity was high, we used the random effect model for analysis. The peroneal nerve MCV treated with combined medication showed an increase (WMD = 4.81, 95% CI = 3.53–6.09; P < .00001) (Fig. 5).
Figure 5.
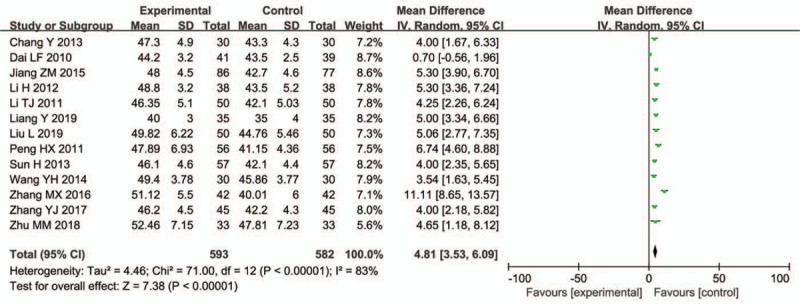
Peroneal nerve MCV.
3.4.3. Peroneal nerve SCV
Thirteen trials (1175 participants) evaluated the peroneal nerve SCV and found a significant difference (WMD = 5.03, 95% CI = 4.16–5.90; P < .00001) (Fig. 6).
Figure 6.
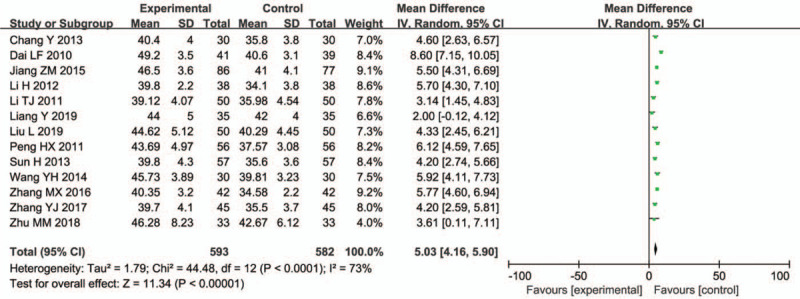
Peroneal nerve SCV.
3.4.4. Median nerve MCV
Twelve trials (1063 participants) reported on the median nerve MCV. The combined medication group showed a significant increase (WMD = 5.38, 95% CI = 4.05–6.72; P < .00001) (Fig. 7).
Figure 7.
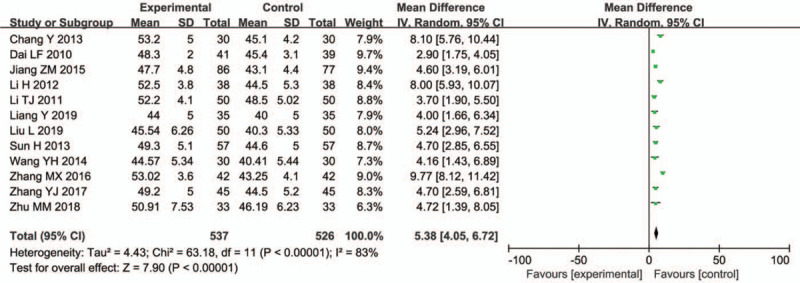
Median nerve MCV.
3.4.5. Median nerve SCV
Twelve studies (1063 participants) reported on the median nerve SCV. In a joint analysis, the WMD in the median nerve SCV change was 4.89 m/s (95% CI = 3.88–5.89; P < .00001; I2 = 73%) (Fig. 8).
Figure 8.
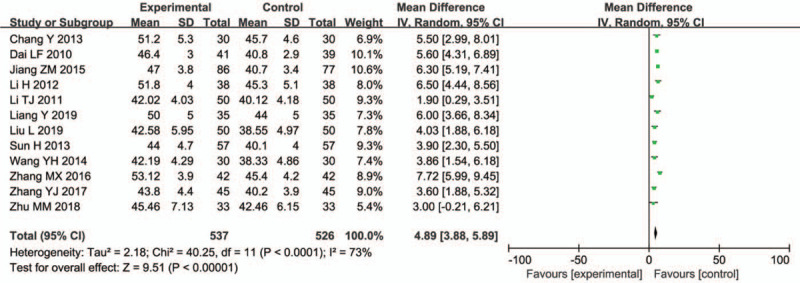
Median nerve SCV.
3.4.6. Subgroup analysis of MCV and SCV of the peroneal nerve and the median nerve
The heterogeneity decreased on subgroup analysis. Subgroup analysis showed that for MCV of the peroneal nerve, the estimated effect of the 15 mL dose was greater than that of other doses. However, this effect did not increase with treatment. In addition, the estimated effect of the Guizhou Baite group was greater than that of the other groups. For SCV of the peroneal nerve, the estimated effect was highest for the 20 mL dose. The group with a 3-week treatment course had the highest expected effect. For MCV and SCV of the median nerve, the group with a 2-week treatment course and the 15 mL dose had the highest expected effect. For most results, the drug produced by Guizhou Baite had a better expected effect than the others (Tables 2 and 3).
Table 2.
Subgroup analyses of the effect of salviae miltiorrhizae and ligustrazine hydrochloride injection for improving peroneal nerve MCV and SCV.
| Peroneal nerve MCV | Peroneal nerve SCV | |||||
| Subgroup | Studies | Patient | MD (95% CI) | Studies | Patient | MD (95CI) |
| Dose | ||||||
| Intravenous infusion 20 mL | 3[23,27,34] | 258 | 3.94 (-0.38–8.25) | 3[23,27,34] | 258 | 6.49 (4.05–8.94) |
| Intravenous infusion 15mL | 2[25,32] | 160 | 8.15 (2.46–13.85) | 2[25,32] | 160 | 5.74 (4.84–6.64) |
| Intravenous infusion 10mL | 6[24,29–31,35–36] | 597 | 4.55 (3.84–5.26) | 6[24,29–31,35–36] | 597 | 4.09 (3.13–5.05) |
| Acupoint injection 1 mL | 2[28,37] | 160 | 4.16 (2.69–5.63) | 2[28,37] | 160 | 5.15 (3.59–6.70) |
| Test for subgroup differences: p = 0.60, I2 = 0% | Test for subgroup differences: P = .06, I2 = 60% | |||||
| Treatment duration | ||||||
| 2 weeks | 7[23–25,29,32,34,35] | 631 | 5.99 (4.46–7.51) | 7[23–25,29,32–35] | 631 | 5.08 (4.18–5.99) |
| 3 weeks | 3[27,31,36] | 284 | 2.83 (0.48–5.19) | 3[27,31,36] | 284 | 5.68 (2.75–8.61) |
| 4 weeks | 1[30] | 100 | 4.25 (2.26–6.24) | 1[30] | 100 | 3.14 (1.45–4.83) |
| 8 weeks | 2[28,37] | 160 | 4.16 (2.69–5.63) | 2[28,37] | 160 | 5.15 (3.59–6.70) |
| Test for subgroup differences: p = 0.12, I2 = 48% | Test for subgroup differences: P = .19, I2 = 37.8% | |||||
| Manufactor | ||||||
| Guizhou Baite | 7[23,25,30–32,34–35] | 715 | 5.84 (4.25–7.44) | 7[23,25,29–32,34–35] | 715 | 5.09 (4.30–5.87) |
| Jilin Sichang | 2[28,36] | 190 | 4.41 (2.98–5.83) | 2[28,36] | 190 | 4.26 (3.03–5.48) |
| Other | 4[24,27,29,37] | 270 | 3.24 (1.05–5.43) | 4[24,27,29,37] | 270 | 5.35 (2.58–8.12) |
| Test for subgroup differences: p = 0.15, I2 = 48.1% | Test for subgroup differences: P = 0.50, I2 = 0% | |||||
Table 3.
Subgroup analyses of the effect of salviae miltiorrhizae and ligustrazine hydrochloride injection for improving peroneal nerve MCV and SCV.
| Subgroup | Median nerve MCV | Median nerve SCV | ||||
| Studies | Patient | MD (95% CI) | Studies | Patient | MD (95CI) | |
| Dose | ||||||
| Intravenous infusion 20 ml | 2[23,27] | 146 | 3.11 (1.97–4.25) | 2[23,27] | 146 | 4.73 (2.33–7.14) |
| Intravenous infusion 15ml | 2[25,32] | 160 | 9.00 (7.28–10.72) | 2[25,32] | 160 | 7.21 (5.89–8.54) |
| Intravenous infusion 10ml | 6[24,29–31,35–36] | 597 | 4.86 (3.77–5.96) | 6[24,29–31,35–36] | 597 | 4.49 (2.97–6.01) |
| Acupoint injection 1ml | 2[28,37] | 160 | 4.80 (3.05–6.54) | 2[28,37] | 160 | 3.95 (2.38 –5.53) |
| Test for subgroup differences: p < 0.001, I2 = 90.4% | Test for subgroup differences: P = 0.007, I2 = 75.2% | |||||
| Treatment duration | ||||||
| 2 weeks | 6[23–25,29,32,34,35] | 519 | 6.61 (4.52–8.70) | 6[23–25,29,32,34,35] | 519 | 6.23 (5.27–7.18) |
| 3 weeks | 3[27,31,36] | 284 | 3.88 (2.56–5.21) | 3[27,31,36] | 284 | 4.46 (3.16–5.76) |
| 4 weeks | 1[30] | 100 | 3.70 (1.90–5.50) | 1[30] | 100 | 1.90 (0.29–3.51) |
| 8 weeks | 2[28,37] | 160 | 4.80 (3.05–6.54) | 2[28,37] | 160 | 3.95 (2.38–5.53) |
| Test for subgroup differences: p = 0.13, I2 = 46.6% | Test for subgroup differences: P < 0.001, I2 = 86.6% | |||||
| Manufactor | ||||||
| Guizhou Baite | 6[23,25,30–32,35] | 603 | 5.95 (3.88–8.03) | 6[23,25,30–32,35] | 603 | 4.97 (3.14–6.80) |
| Jilin Sichang | 2[28,36] | 190 | 4.95 (3.40–6.50) | 2[28,36] | 190 | 3.77 (2.43–5.11) |
| Other | 4[24,27,29,37] | 270 | 4.70 (2.38–7.02) | 4[24,27,29,37] | 270 | 5.36 (4.42–6.31) |
| Test for subgroup differences: p = 0.68, I2 = 0% | Test for subgroup differences: P = 0.16, I2 = 45.3% | |||||
3.5. Publication bias
The P-values of the Egger and Begg tests were 0.967 and 0.961, respectively, indicating no publication bias.
3.6. Sensitivity analysis
Sensitivity analysis by sample size and risk of bias did not show any significant effect on the main outcomes of the nerve conduction velocity (Table 4).
Table 4.
Sensitivity analyses by sample size and risk of bias.
| Study removed from the primary meta-analysis | Number of included studies | RR/WMD (95% CI) | Heterogeneity | |
| Sample size | ||||
| Total effective rate | 10[23–27,29,32–33,36–37] | 5[28,30,31,34,35] | 1.30 (1.19–1.42) | P = 0.43; I2 = 0% |
| Peroneal nerve MCV | 8[23–25,27,29,32,36–37] | 5[26,30,31,34,35] | 5.00 (4.12–5.88) | P = 0.32; I2 = 14% |
| Peroneal nerve SCV | 8[23–25,27,29,32,36–37] | 5[28,30,31,34,35] | 4.73 (3.72–5.74) | P = 0.07; I2 = 54% |
| Median nerve MCV | 8[23–25,27,29,32,36–37] | 4[28,30,31,35] | 4.50 (3.62–5.38) | P = 0.75; I2 = 0% |
| Median nerve SCV | 8[23–25,27,29,32,36–37] | 4[28,30,31,35] | 4.08 (2.02–6.14) | P = 0.0001; I2 = 86% |
| Risk of bias | ||||
| Total effective rate | 3[26,29,33] | 12[23–25,27,28,30–32,34–37] | 1.34 (1.26–1.43) | P = 0.77; I2 = 0% |
| Peroneal nerve MCV | 1[29] | 12[23–25,27,28,30–32,34–37] | 4.80 (3.40–6.20) | P < 0.0001; I2 = 84% |
| Peroneal nerve SCV | 1[29] | 12[23–25,27,28,30–32,34–37] | 5.26 (4.44–6.09) | P = 0.0002; I2 = 69% |
| Median nerve MCV | 1[29] | 11[23–25,27,28,30–32,35–37] | 5.50 (4.07–6.93) | P < 0.0001; I2 = 84% |
| Median nerve SCV | 1[29] | 11[23–25,27,28,30–32,35–37] | 4.79 (3.72–5.87) | P < 0.0001; I2 = 75% |
4. Discussion
The study included 15 RCTs involving 1349 patients with DPN. The analysis revealed that SMLH injection combined with mecobalamin led to an increase in the total effective rate (by 31%), MCV, and SCV of the peroneal nerve and the median nerve. In the subgroup analysis, there was no significant difference in the peroneal nerve MCV in the subgroup with a dose of 20 mL. This may be due to the lack of sample size and the interference of small sample research. However, other doses and treatment courses were stable. The effect value of 15 mL was the highest; however, the sample size was small. The effect value of 10 mL was the second highest, and with a certain sample size, the results were reliable. The results supported that SMLH injection combined with mecobalamin may improve DPN.
An important advantage of this study was a comprehensive literature search, including some recently published experiments. These studies improved the accuracy of the results. In addition, the sensitivity analysis of the peroneal nerve MCV, peroneal nerve SCV, median nerve MCV, and median nerve SCV, which are important evaluation indices of DPN, showed that the preliminary results were stable.
Both salviae miltiorrhiza radix (Danshen) and chuanxiong rhizoma (Chuanxiong) are used in the treatment of ischemic vascular diseases owing to their vascular protective effects.[13–14,38] Danshen is used for its anti-oxidative anti-atherosclerotic, and anti-inflammatory effects.[12] In addition, Danshen exhibits anti-diabetic effects by treating macrovascular and microvascular diseases in preclinical experiments and clinical trials, through an improvement in redox homeostasis and inhibition of apoptosis and inflammation via the regulation of Wnt/β-catenin,[39] TSP-1/TGF-β1/STAT3,[40–42] and JNK/PI3K/Akt[43]. Similarly, Chuanxiong promotes blood vessel dilation and eliminates blood stasis. According to previous research,[44] Danshen has an obvious anti-inflammatory effect on bone marrow macrophages. However, it has minimal effect on human umbilical vein endothelial cells. On the contrary, Chuanxiong has a considerable anti-inflammatory effect on human umbilical vein endothelial cells, but no anti-inflammatory effect on bone marrow macrophages. In toll-like receptor-activated inflammatory responses, SMLH shows a “multi-component multi-target” effect, in the presence of a complementary mechanism. Therefore, SMLH injection combined with mecobalamin can reduce inflammatory reactions through multiple pathways and mechanisms, leading to an improvement in DPN.
There were some limitations to this study. First, most of the included studies had a small sample size, with minimal reports on the implicit and specific random methods of the distribution scheme. A selective bias could be present; the methods were not blinded, which could lead to a measurement bias. Second, all the included publications were in Chinese, which may lead to the risk of bias and increase the limitations of conclusions. This is because Chinese literature tends to publish positive results, while negative results are difficult to publish or do not receive sufficient attention. This could lead to a publication bias, resulting in overestimation or underestimation of the results. Furthermore, most of the studies were completed in 2 weeks, and few studies were conducted for longer than 2 weeks. DPN is a long-term chronic disease that requires long-term treatment. Studies that investigated the treatment course for longer than 2 weeks may require further verification of the effectiveness of SMLH injection combined with mecobalamin.
5. Conclusion
SMLH injection combined with mecobalamin has a better curative effect and better tolerance for patients with DPN, compared with mecobalamin alone. However, the small samples included in some studies may reduce the strength of the evidence. In addition, larger samples, better study design, and RCTs are needed to determine whether SMLH injection combined with mecobalamin, in a prolonged treatment cycle and at other doses, has a perpetual role in improving DPN.
Author contributions
Zhiyuan Deng and Min Liu chaired the design of the study. Manjia Wang and Yaohua Fan were responsible for drafting the paper. Manjia Wang and Yaohua Fan proposed retrieval strategies. Zhiyuan Deng and Manjia Wang were responsible for data collection. All authors approved the final manuscript.
Conceptualization: Zhiyuan Deng.
Data integration: Zhiyuan Deng, Manjia Wang.
Formal analysis: Manjia Wang
Methodology: Yaohua Fan.
Resources: Zhiyuan Deng, Manjia Wang.
Review and editing: Min Liu
Software operation: Zhiyuan Deng.
Original manuscript writing: Zhiyuan Deng, Min Liu.
Footnotes
Abbreviations: DPN = diabetic peripheral neuropathy, MCV = motor conduction velocity, RCTs = randomized controlled trials, SCV = sensory conduction velocity, SMLH = salviae miltiorrhizae (Danshen Salvia) and ligustrazine hydrochloride (Chuanxiong Chuanxiong), WMD = weighted mean difference.
How to cite this article: Deng Z, Wang M, Fan Y, Liu M. Salviae miltiorrhizae and ligustrazine hydrochloride injection combined with mecobalamin for treating diabetic peripheral neuropathy: a protocol for systematic review and meta-analysis. Medicine. 2021;100:3(e24103).
Supported by the project of the National Natural Science Foundation of China (grant No. 81373767).
All research-related data is included in the article or uploaded as supplementary information.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files];
(A = SMLH injection 20 ml combined with mecobalamin; B = SMLH injection 15 ml combined with mecobalamin; C = SMLH injection 10 ml combined with mecobalamin; D = SMLH injection 1 ml combined with mecobalamin; E = SMLH injection 0.5 ml combined with mecobalamin; F = mecobalamin; (1) = total effective rate; (2) = peroneal nerve MCV; (3) = peroneal nerve SCV; (4) = median nerve MCV; (5) = median nerve SCV).
CI = confidence interval, MD= mean difference, MCV = motor conduction velocity, SCV = sensory conduction velocity.
CI = confidence interval, MD= mean difference, MCV = motor conduction velocity, SCV = sensory conduction velocity.
CI = confidence interval, MCV = motor conduction velocity, WMD = weighted mean difference, SCV = sensory conduction velocity.
References
- [1].International Diabetes Federation. IDF Diabetes Atlas-7th Edition. Diabetes Atlas Available at: http://www.diabetesatlas.org/, 2015. [Google Scholar]
- [2].Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes at las: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271. [DOI] [PubMed] [Google Scholar]
- [3].Holman N, Young B, Gadsby R. Current prevalence of type 1 and type 2 diabetes in adults and children in the UK. Diabet Med 2015;32:1119–20. [DOI] [PubMed] [Google Scholar]
- [4].Strasser B, Pesta D. Resistance training for diabetes prevention and therapy: experimental findings and molecular mechanisms. Biomed Res Int 2013. 805217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Oscar A, Tassler PL, A Lee D. Changing the natural history of diabetic neuropathy: incidence of ulcer/amputation in the contralateral limb of patients with a unilateral nerve decompression procedure. Ann Plastic Surg 2004;53:517. [DOI] [PubMed] [Google Scholar]
- [6].Davies M, Brophy S, Williams R, et al. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006;29:1518–22. [DOI] [PubMed] [Google Scholar]
- [7].Cameron NE, Cotter MA. Vascular changes in animal models of diabetic neuropathy. J of Neurochemistry 2010;85:14. [Google Scholar]
- [8].Jamwal S, Sharma S. Vascular endothelium dysfunction: a conservative target in metabolic disorders. Inflamm Res 2018;67:391–405. [DOI] [PubMed] [Google Scholar]
- [9].Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig 2017;8:646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hosseini A, Abdollahi M. Diabetic neuropathy and oxidative stress: therapeutic perspectives. Oxid Med Cell Longev 2013;2013:168039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xin W, Chunyan W, Longjiang Z, et al. Salvianolic acid A shows selective cytotoxicity against multidrug-resistant MCF-7 breast cancer cells. Anti-cancer drugs 2015;26:210–23. [DOI] [PubMed] [Google Scholar]
- [12].XD, ME, Cao YF, et al. Danshen: a phytochemical and pharmacological overview. Chin J Nat Med 2019;17:59–80. [DOI] [PubMed] [Google Scholar]
- [13].Gao HJ, Liu PF, Li PW, et al. Ligustrazine monomer against cerebral ischemia/reperfusion injury. Neural Regen Res 2015;10:832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guo M, Liu Y, Shi DZ. Cardiovascular Actions and Therapeutic Potential of Tetramethylpyrazine (Active Component Isolated from Rhizoma Chuanxiong): Roles and Mechanisms. Biomed Res Int 2016. 2430329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhu Y, Chen X, Huang P, et al. Protective effect of Danshen-ligustrazine on focal cerebral ischemia-reperfusion injury in rats. Mod J Inte Tradit Chin West Med 2011;20:802–4. [Google Scholar]
- [16].Wang Z, Shi A. Effect of Dansbenchuanxiongqin Combined with Fumingpinnon on Treatment of Diabetic Retinopathy. J Med Bes 2009;38:81–3. [Google Scholar]
- [17].Ma Y, Zhou L, Liu Y. Clinical observation of Salviae Miltiorrhizae and Ligustrazine Hydrochloride Injection combined with calcium dobesilate in treatment of diabetic retinopathy. Drugs & Clinic 2020;35:881–4. [Google Scholar]
- [18].Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res 2015;43:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009;4:44–57. [DOI] [PubMed] [Google Scholar]
- [20].Li T, Wang Y, Zhang L, et al. Clinical observation of Salviae Miltiorrhizae and Ligustrazine Hydrochloride Injection combined with atorvastatin in treatment of acute cerebral infarction. Drugs & Clinic 2017;32:1872–5. [Google Scholar]
- [21].Xu Z. Clinical evaluation of Salvia miltiorrhiza and Ligustrazine Injection in the treatment of post circulation ischemic vertigo. Drug Eval Res 2017;40:1334–7. [Google Scholar]
- [22].American Diabetes Association (ADA). Clinical practical guide for diabetes. Chin J of diabetes 2012;02:34–5. [Google Scholar]
- [23].Zhu M, Shan J. Clinical effect of chuanfizin injection combined with mecobalamin in the treatment of diabetic peripheral neuropathy. J Clin Med 2018;5:30–1. [Google Scholar]
- [24].Chang Y. Analysis on clinical effects of Danshen ligustrazine combined with mecobalamine for type 2 diabetic peripheral neuropathy. J Clin Med Pract 2013;17:40–2. [Google Scholar]
- [25].Li H. Clinical analysis of salvia miltiorrhiza ligustrazine joint mecobalamin in the treatment of diabetic peripheral neuropathy. Cin Med and Phar 2012;2:82–3. [Google Scholar]
- [26].Li J, Yao P. Clinical effect of danshen and ligustrazine combined with mecobalamin on diabetic peripheral neuropathy. Clin rational use of drugs 2011;4:55. [Google Scholar]
- [27].Dai L. Effect of danshen ligustrazine and mecobalamin on diabetic peripheral neuropathy in 41 cases. Yunnan J TCM 2010;31:15–8. [Google Scholar]
- [28].Liu L, Jiang C, Zhao Z. Effects of salviae miltiorrhizae and ligustrazine hydrochloride injection on diabetic peripheral neuropathy and oxidative stress response. Chin Tradit and Herbal Drugs 2019;50:2670–4. [Google Scholar]
- [29].Liang Y. Effect of Danshen and ligustrazine injection combined with Mecobalamin on diabetic peripheral neuropathy. J Pract Med Tech 2019;26:1311–2. [Google Scholar]
- [30].Li T. Treatment of diabetic peripheral neuropathy with danshen and ligustrazine injection combined with Mecobalamin. Chin Pharmacist 2011;14:1029–30. [Google Scholar]
- [31].Sun H, Li C. Danshen and ligustrazine injection combined with Mecobalamin in the treatment of diabetic peripheral neuropathy: a clinical observation of 57 cases. Guiding J TCM and Pharmacy 2013;19:29–30. [Google Scholar]
- [32].Zhang M. Clinical observation of danshen and ligustrazine injection combined with mecobalamin in the treatment of diabetic peripheral neuropathy. J Pract TCM 2016;32:985–6. [Google Scholar]
- [33].Wang Y, Mou SM. 45 cases of diabetic peripheral neuropathy treated by injection of Danshen Chuanxiong. Hunan J TCM 2014;30:71–2. [Google Scholar]
- [34].Peng H. Clinical analysis of salvialig a strazine combined with methylcobalamin in treatment of type 2 diabetic peripheral neuropathy. Chin J Postgrad Med 2011;34:18–20. [Google Scholar]
- [35].Jiang Z, Chen Z. Therapeutic effect of the combined treatment of mecobalamin and salvia miltiorrhiza ligustrazine on diabetic peripheral neuropathy. Chin J Prim Med Pharm 2015;22:3467–9. [Google Scholar]
- [36].Zhang Y. Clinical effect of danshen and ligustrazine combined with mecobalamin on diabetic peripheral neuropathy. Frontiers Med 2017;7:226–7. [Google Scholar]
- [37].Wang Y. Acupoint Injection of salvia miltiorrhiza and ligustrazine injection combined cobalt amine clinical research for the treatment of diabetic peripheral neuropathy. Shandong: Shandong University of TCM; 2014. [Google Scholar]
- [38].Wang L, Yu J, Fordjour PA, et al. Danshen injection prevents heart failure by attenuating post-infarct remodeling. J Ethnopharmacol 2017;205:22–32. [DOI] [PubMed] [Google Scholar]
- [39].Xiang X, Cai H, Su S, et al. Salvia miltiorrhiza protects against diabetic nephropathy through metabolome regulation and wnt/(-catenin and TGF-( signaling inhibition. Pharmacol Res 2019;139:26–40. [DOI] [PubMed] [Google Scholar]
- [40].Yu J, Fei J, Azad J, et al. Myocardial protection by Salvia miltiorrhiza Injection in streptozotocin-induced diabetic rats through attenuation of expression of thrombospondin-1 and transforming growth factor-(1. J Int Med Res 2012;40:1016–24. [DOI] [PubMed] [Google Scholar]
- [41].Cui S, Chen S, Wu Q, et al. A network pharmacology approach to investigate the anti-inflammatory mechanism of effective ingredients from Salvia miltiorrhiza. Int Immunopharmacol 2020;81:106040. [DOI] [PubMed] [Google Scholar]
- [42].Jia Q, Zhu R, Tian Y, et al. Salvia miltiorrhiza in diabetes: a review of its pharmacology,phytochemistry, and safety. Phytomedicine 2019;58:152871. [DOI] [PubMed] [Google Scholar]
- [43].Chen Q, Xu T, Li D, et al. JNK/PI3K/Akt signaling pathway is involved in myocardial ischemia/reperfusion injury in diabetic rats: effects of salvianolic acid a intervention. Am J Transl Res 2016;8:2534–48. [PMC free article] [PubMed] [Google Scholar]
- [44].Ye T, Li Y, Xiong D, et al. Combination of Danshen and ligustrazine has dual anti-inflammatory effect on macrophages and endothelial cells. J Ethnopharmacology 2021;266:113425. [DOI] [PubMed] [Google Scholar]


