Abstract
Background:
As the results of previous systematic reviews and meta-analyses on acupuncture for polycystic ovary syndrome (PCOS) have provided inconsistent evidence. This overview of systematic reviews (SRs) and meta-analyses will aim to critically appraise the methodology and reporting quality of the relevant SRs and meta-analyses with the aim of identifying whether acupuncture could provide an effective treatment for patients with PCOS or not.
Methods:
Electronic databases including MEDLINE via Ovid, EMBASE, PubMed, Cochrane library, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP database), and Wanfang Database will be searched for related SRs and meta-analyses from inceptions to the search date without language restrictions. Two reviewers will independently select SRs and meta-analyses and collect related data, and a third reviewer will be introduced if any disagreement happened during the assessing. The Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) and the latest Assessment of Multiple Systematic Reviews 2 (AMSTAR2) checklists will be employed to evaluate the reporting and methodology quality.
Results:
This overview will be published in a peer-reviewed journal.
Conclusion:
This overview will identify related SRs and meta-analyses of acupuncture for treating PCOS.
Ethics and dissemination:
Ethics approval and patient consent are not required as this study is an overview based on published systematic reviews and meta-analyses.
Keywords: acupuncture, overview, polycystic ovary syndrome, protocol
1. Introduction
Polycystic ovary syndrome (PCOS) is one of the most common cause of an ovulatory infertility conditions in clinic, which is characterized by ovulatory dysfunction, hyperandrogenism, and polycystic ovaries.[1] It is estimated that PCOS prevalence has varied from 5% to 15% among women of reproductive age.[2] PCOS can result in profound, long-term physical, and mental health consequences.[3,4] In addition to an ovulatory infertility, women suffering from PCOS have an increased risk of miscarriage, pregnancy complications, and metabolic abnormalities such as obesity, hypertension, and non-alcoholic fatty liver disease.[5–8] The cause of PCOS is genetic related,[9] and environmental factors such as prenatal exposure to androgens may also play a role in the etiology of PCOS.[10]
Currently, pharmacological therapies for PCOS include clomiphene, which is considered as the first-line treatment to induce ovulation in women with PCOS.[11] However, clomiphene turned out to have a high failure rate of without ovulation, a relatively low cumulative live birth rate, and a high multiple-pregnancy rate.[11] Other pharmacological treatments such as oral contraceptives are used, but it is reported that long-term use of oral contraceptives in women with PCOS may lead to the development of obesity and metabolic abnormalities.[12] Given the fact that clomiphene and oral contraceptives have some side effects during the treatment of PCOS, alternative therapies have become an important supplement for PCOS treatment, among which acupuncture has played an important role. Acupuncture, as an integral part of traditional Chinese medicine (TCM), has gained increased popularity in clinical treatment of PCOS.[13] Although the efficacy of acupuncture for treating PCOS has been proved in clinical practice, some researchers suggest that acupuncture works through placebo effect instead of its real therapeutic effect.[14,15] Several systematic reviews (SRs) were carried out to investigate the effect of acupuncture on PCOS, yet gaining inconsistent conclusions.[16–19] Therefore, it is necessary for us to undertake an overview of SRs and meta-analyses to evaluate the methodology and reporting quality of the SRs and meta-analyses.
2. Methods
2.1. Protocol and registration
This protocol will be undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P),[20] and the study has been registered on OSF platform (https://osf.io/registries) with a registration NO. 10.17605/OSF.IO/Z3BPV.
2.2. Ethics
Ethics application was not required as this study is based on published SRs and meta-analyses.
2.3. Information sources and search strategy
The following 7 electronic databases will be searched for relevant SRs and meta-analyses from their inception to 2019, irrespective of language and publication status: MEDLINE via Ovid, EMBASE, PubMed, Cochrane library, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journal Database (VIP database), and Wanfang Database. The detailed search strategy in PubMed is given in Table 1.
Table 1.
Search strategy draft.
| Number | Entry terms |
| #1 | Ovary syndrome, polycystic [Title/Abstract] |
| #2 | Syndrome, polycystic ovary [Title/Abstract] |
| #3 | Stein-Leventhal syndrome [Title/Abstract] |
| #4 | Stein Leventhal syndrome [Title/Abstract] |
| #5 | Syndrome, Stein-Leventhal [Title/Abstract] |
| #6 | Sclerocystic ovarian degeneration [Title/Abstract] |
| #7 | Ovarian degeneration, sclerocystic [Title/Abstract] |
| #8 | Sclerocystic ovary syndrome [Title/Abstract] |
| #9 | Polycystic ovarian syndrome [Title/Abstract] |
| #10 | Ovarian syndrome, polycystic [Title/Abstract] |
| #11 | Polycystic ovary syndrome [Title/Abstract] |
| #12 | Sclerocystic ovaries [Title/Abstract] |
| #13 | Ovary, sclerocystic [Title/Abstract] |
| #14 | Sclerocystic ovary [Title/Abstract] |
| #15 | or/#1–#14 |
| #16 | acupuncture [MeSH Terms] |
| #17 | acupuncture analgesia [MeSH Terms] |
| #18 | acupuncture analgesia [MeSH Terms] |
| #19 | acupuncture therapy [MeSH Terms] |
| #20 | acupuncture, ear [MeSH Terms] |
| #21 | or/#16–#20 |
| #22 | systematic review [Title/Abstract] |
| #23 | meta-analysis [MeSH Terms] |
| #24 | meta-analysis [Title/Abstract] |
| #25 | or/22–24 |
| #26 | #15 and #21 and #25 |
2.4. Eligibility criteria
The PICOS (participant, intervention, comparison, and study design) principle will be utilized in this study.
2.4.1. Study design
All published SRs and meta-analyses of randomized controlled trials (RCTs) or quasi-RCTs involving acupuncture for PCOS will be included without restriction on language or publication type. Non-RCT SRs and meta-analyses, review comments, overview of SRs, editorials, and guidelines will be excluded.
2.4.2. Participants
Female patients of reproductive age diagnosed with PCOS will be included.
2.4.3. Interventions and comparison
The intervention will include acupuncture as manual acupuncture, electroacupuncture, and warm acupuncture. SRs and meta-analyses with acupuncture combined therapy or acupuncture related treatment like point injection, laser acupuncture, transcutaneous electrical nerve stimulation (TENS), cupping, or blood-letting, will be excluded.
The comparison treatments with sham acupuncture, western medicine, placebo, no treating/waiting list will be included. SRs and meta-analyses with the control group compared different forms of acupuncture treatment will be excluded.
2.4.4. Outcomes
The primary outcomes of this overview will be ovulation induction. Secondary outcomes will be luteinizing hormone/follicle-stimulating hormone, menstrual frequency, birth live, pregnancy, conception, biochemical assessments, anthropometry. Adverse events such as acupuncture fainting, needle twisting and breaking, bleeding, and organ injury will also be taken into account as safety measurement.
2.5. Selection of studies and data extraction process
Two reviewers (HF and HF) will respectively screen the study titles and abstracts to identify potentially eligible SRs and meta-analyses according to the inclusion criteria. The full texts reviews will be obtained and independently screened before final inclusion. If a disagreement on the inclusion of a review cannot be resolved, a third reviewer (XC) will make the final decision after discussion.
Two reviewers (ZL and HF) will independently extract the data of all eligible SRs and meta-analyses by employing the specially designed extraction forms. The following items will be included in the data extraction form: first author, year of publication, country, number of RCTs enrolled, quality assessment tool for RCTs, and characteristics of interventions in control and treatment groups, outcome measures, data synthesis methods, main results, and conclusions. The final extracted data will be cross-checked by the two extractors, and any disagreement regarding the extracted data will be discussed with and adjudicated by a third reviewer (XC). If any data are insufficient or unclear, the first or corresponding author for the study concerned will be contacted via e-mail or telephone to provide additional information. The flow chart is displayed in Fig. 1.
Figure 1.
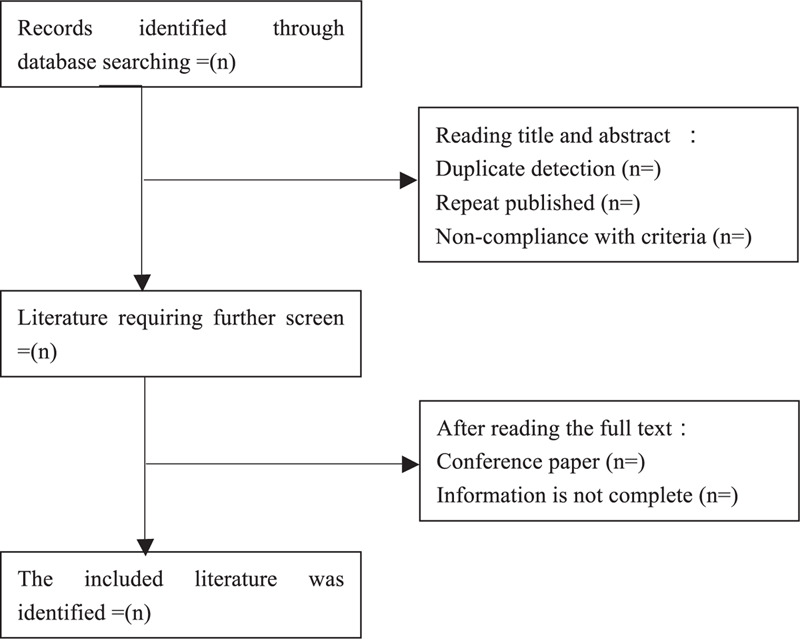
Flow chart of literature selection.
2.6. Quality assessment
The assessment of Multiple Systematic Reviews 2 (AMSTAR2) will be utilized to assess methodological qualities of the included SRs and meta-analyses. AMSTAR is a quality assessment tool for critically assessing the quality of RCTs, while AMSTAR2, which is an update of AMSTAR, can be used to appraise SRs and meta-analyses of intervention trials including both RCTs and non-RCTs.[21] There are 16 items in AMSTAR2. Items 2, 4, 7, 9, 11, 13, and 15 are consider to be more critical than other items, which may affect the production of the systematic review and the validity of the results. The main rule for rating overall quality in the results of SRs and meta-analyses as follows: SRs and meta-analyses with no or 1 noncritical weakness will be rated as high; with >1 noncritical weakness will be rated as moderated; with 1 critical flaw with or without noncritical weakness will be rated as low; with >1 critical flaw with or without noncritical weakness will be rated as critically low.
Two reviewers (ZL and HF) will independently assess the methodological quality of each included SR and meta-analysis by employing the AMSTAR2. Any disagreement during the assessment will be discussed or solved by a third reviewer (XC).
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) will be applied to evaluate the report quality of included SRs and meta-analyses. There are 27 items used to assess whether the reports are standardized or not. Discussion and a third reviewer will be introduced when confronted with disagreement during the assessing process (XC).
2.7. Data synthesis
General characteristics of the included systematic reviews will be summarized and described. PRISMA and AMSTAR2 will be utilized to assess each review to show the risk of bias, and the total percentage and the 95% confidence interval of each item will be calculated.
3. Discussion
With an increasing number of SRs and meta-analyses on acupuncture for treating PCOS in recent years, we would like to figure out whether those SRs and meta-analyses of acupuncture for PCOS have high methodological quality and reporting quality. It is known that systematic reviews and meta-analyses with high quality can help policy-makers or clinical physicians to make better choice and clinical practice, therefore, an overview of SRs and meta-analyses is essential to be done by using AMSTAR2 and PRISMA tools to provide stronger evidence for acupuncture for treating PCOS.
Author contributions
Conceptualization: Zaibo Liao, Huaying Fan.
Data curation: Zaibo Liao, Huayu Fan.
Formal analysis: Huaying Fan.
Investigation: Huaying Fan, Huayu Fan, Xiaohua Chen.
Methodology: Huayu Fan, Xiaohua Chen.
Project administration: Huaying Fan, Xiaohua Chen.
Supervision: Xiaohua Chen.
Validation: Xiaohua Chen.
Writing – original draft: Zaibo Liao, Huaying Fan.
Writing – review & editing: Xiaohua Chen.
Footnotes
Abbreviations: AMSTAR2 = The Assessment of Multiple Systematic Reviews 2, CNKI = China National Knowledge Infrastructure, PCOS = polycystic ovary syndrome, PICOS = participant, intervention, comparison, and study design, PRISMA = The Preferred Reporting Items for Systematic Review and Meta-Analyses, PRISMA-P = Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols, RCTs = randomized controlled trials, SRs = systematic reviews, TCM = traditional Chinese medicine, TENS = transcutaneous electrical nerve stimulation, VIP database = Chinese Scientific Journal Database.
How to cite this article: Liao Z, Fan H, Fan H, Chen X. Acupuncture for polycystic ovary syndrome (PCOS): An overview of a protocol for systematic reviews and meta analyses. Medicine. 2021;100:3(e24218).
ZL and HF have contributed equally to this work.
The authors declare that they have no competing interests.
This work will be financially supported by the Scientific Research Program and Health Planning Commission of Sichuan Province (NO.150156). The funders had no role in study design, data collection and analysis, reporting writing, or decision-making for publication.
The authors have no conflicts of interest to disclose.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- [2].Li R, Zhang Q, Yang D, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod 2013;28:2562–9. [DOI] [PubMed] [Google Scholar]
- [3].Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010;8:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cooney LG, Lee I, Sammel MD, et al. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2017;32:1075–91. [DOI] [PubMed] [Google Scholar]
- [5].Yang R, Yang S, Li R, et al. Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: a meta-analysis. Reprod Biol Endocrinol 2016;14:67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vassilatou E, Vassiliadi DA, Salambasis K, et al. Increased prevalence of polycystic ovary syndrome in premenopausal women with nonalcoholic fatty liver disease. Eur J Endocrinol 2015;173:739–47. [DOI] [PubMed] [Google Scholar]
- [7].Goodman NF, Cobin RH, Futterweit W, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and Pcos Society Disease State Clinical Review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome - Part 2. Endocr Pract 2015;21:1415–26. [DOI] [PubMed] [Google Scholar]
- [8].Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers 2016;2:16057–75. [DOI] [PubMed] [Google Scholar]
- [9].Legro RS, Driscoll D, Strauss JF, 3rd, et al. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci USA 1998;95:14956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Abbott DH, Bacha F. Ontogeny of polycystic ovary syndrome and insulin resistance in utero and early childhood. Fertil Steril 2013;100:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Legro RS, Brzyski RG, Diamond MP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014;371:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Norman RJ, Dewailly D, Legro RS, et al. Polycystic ovary syndrome. Lancet 2007;370:685–97. [DOI] [PubMed] [Google Scholar]
- [13].Smith JF, Eisenberg ML, Millstein SG, et al. The use of complementary and alternative fertility treatment in couples seeking fertility care: data from a prospective cohort in the United States. Fertil Steril 2010;93:2169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu XK, Stener-Victorin E, Kuang HY, et al. Effect of acupuncture and clomiphene in chinese women with polycystic ovary syndrome: a randomized clinical trial. JAMA 2017;317:2502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pastore LM, Williams CD, Jenkins J, et al. True and sham acupuncture produced similar frequency of ovulation and improved LH to FSH ratios in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2011;96:3143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yun L, Liqun W, Shuqi Y, et al. Acupuncture for infertile women without undergoing assisted reproductive techniques (ART): a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lim CED, Ng RWC, Cheng NCL, et al. Acupuncture for polycystic ovarian syndrome. Cochrane Database Syst Rev 2019;7:1–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Qu F, Wu Y, Hu X, et al. The effects of acupuncture on polycystic ovary syndrome: a systematic review and meta-analysis. Eur J Integr Med 2016;8:12–8. [Google Scholar]
- [19].Kim S-H, Hwang D-S, Lee J-M, et al. Recent acupuncture therapy for polycystic ovary syndromes: systematic review. J Oriental Obstetr Gynecol 2014;27:71–82. [Google Scholar]
- [20].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647–7672. [DOI] [PubMed] [Google Scholar]
- [21].Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008–4017. [DOI] [PMC free article] [PubMed] [Google Scholar]


