Abstract
Background:
Previous publications studied the correction about folate intake and ovarian cancer risk, with inconsistent results. This meta-analysis aimed to explore the association between folate intake and ovarian cancer risk using the existing published articles.
Method:
We searched for relevant studies in electronic databases of PubMed, Web of Science, Embase, Cochrane, and Wanfang databases from inception to May 31, 2020. The overall relative risk (RR) and its 95% confidence intervals (95% CI) were pooled using a random-effect model.
Results:
A total of 12 articles with 6304 ovarian cancer cases were suitable for the inclusion criteria. The evaluated of the ovarian cancer risk with total folate intake and dietary folate intake were reported in 6 articles and 10 articles, respectively. Overall, highest category of dietary folate intake compared with lowest category had nonsignificant association on the risk of ovarian cancer (RR = 0.90, 95% CI = 0.77–1.06). The association was not significant between total folate intake and ovarian cancer risk (RR = 1.06, 95% CI = 0.89–1.27). The results in subgroup analyses by study design and geographic location were not changed either in dietary folate intake analysis or in total folate intake analysis.
Conclusion:
Our meta-analysis demonstrates that folate intake had no significant association on the risk of ovarian cancer. Study design and geographic location were not associated with ovarian cancer while some other related factors were not investigated due to the limited information provided in each included study. Therefore, further studies are needed to verify our results.
Keywords: folate, meta-analysis, ovarian cancer, risk
1. Introduction
Based on American cancer statistics in 2019, ovarian cancer is the 11th most common cancer, with approximately 22,530 newly diagnosed ovarian cancer cases, and the 5th leading cause of cancer-related death, with estimated 13,980 ovarian cancer deaths.[1] Ovarian cancer is a diverse and genomic complex disease, which has attracted worldwide attention.[2] Previous meta-analyses had confirmed that ovarian cancer was related to genetic factors,[3,4] as well as dietary factors.[5–7] Back in 1999, Kushi et al [8] performed a study about total folate intake on ovarian cancer risk. They concluded that highest category versus lowest category of total folate intake had an increase but nonsignificant relationship on ovarian cancer risk. Since then, many relevant publications assessed the association between folate intake and ovarian cancer risk. Zhang et al[9] found an inverse association between dietary folate intake and ovarian cancer risk, while some researchers failed to obtain a significant relationship between them.[10,11] The results of already published studies between folate intake and ovarian cancer risk were inconsistent. Therefore, this meta-analysis aimed to investigate the effect of folate intake on ovarian cancer risk by combining all the studies that met our inclusion criteria.
2. Methods
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Statement.[12]
2.1. Search strategy
Two independent authors searched PubMed, Web of Science, Embase, Cochrane, and Wanfang databases from inception to May 31, 2020 for all related papers. The search terms were as follows: “folate” OR “folic acid” combined with “ovarian cancer” OR “ovarian tumor.” Wherever possible, we searched for references to relevant articles to identify potential information that had not already been retrieved. This study did not require approval by an ethics review committee because it is a meta-analysis. The discrepancies in the search process by the 2 independent authors were discussed by a third author.
2.2. Inclusion criteria
The relevant papers about the effect of folate on the risk of ovarian cancer were included if they meet the following criteria: Patients: all the patients should be diagnosed as ovarian cancer with order than 18 years; Study design: case-control, cross-sectional, or cohort studies; Interested and outcomes: the studies should relationship of folate intake on the risk of ovarian cancer; Data: the study should provide the available data of relative risk (RR) and 95% confidence intervals (CI).
2.3. Exclusion criteria
The following exclusion criteria were used: Animal studies; Literature reviews, or Case reports; Duplicate publication; No available data about RR and 95% CI.
2.4. Data extraction
Two investigators independently reviewed the whole content of each eligible literature, including supplements, and extracted the data using a data extraction sheet. The following contents was included in the extraction sheet: first author; year of publication; study design; age of patients; country; total folate intake or dietary folate intake; number of patients and participants enrolled; category of highest compared with lowest; RR and their 95% CI; and other necessary information. The discrepancies in the data abstracted by the 2 independent authors were discussed by a third author.
2.5. Quality assessment
The Newcastle-Ottawa-Scale (NOS) was used for evaluating the quality of each study.[13] Two authors independently rated for each included study. Any discrepancies in ratings were reconciled by the third rater.
2.6. Statistical analysis
The data of the analysis were extracted from the selected literature, and all meta-analysis were performed using Review Manager 5.0. Statistical heterogeneity was analyzed using Cochran Q test and inconsistency (I2) statistics; P < .10 or I2 > 50% indicate significant heterogeneity.[14,15] The overall RR and 95% CI were pooled using the random-effect model.[16] In addition, to assess publication bias, visual observations using the funnel plot[17] and the Egger test.[18] Sensitivity analysis was used to explore whether 1 single study had the essential effect on the overall RR. For all analyses, P < .05 was referring to indicate statistical significance. The power of each component study was estimated using the effect size of the largest study in a meta-analysis and the power calculation was based on an algorithm using a noncentral t distribution.
3. Results
3.1. Literature search and study characteristics
A total of 3421 records were identified from all searched databases and 1 additional record was identified in the references of a review. There were 1652 articles that were retained after excluding duplicates in different databases. After assessing the titles and abstracts, 51 articles were reviewed in full-text. Furthermore, 39 articles were excluded for the following reasons: reviews; no available odds ratio or RR; animal studies; letter to the editors. Finally, 12 articles were included in the final analysis.[8–11,19–26] The flowchart of the trial selection process is shown in Figure 1. Six articles come from United States, 1 from Sweden, 1 from Canada, 1 from Italy, 1 from Mexico, 1 from Australia, and 1 from China. Six articles were with cohort design and 6 with case-control design. All of the 8 studies had relatively high quality (over 6 stars), with an average NOS score of 7.42. The characteristics of all included studies were summarized in Table 1.
Figure 1.
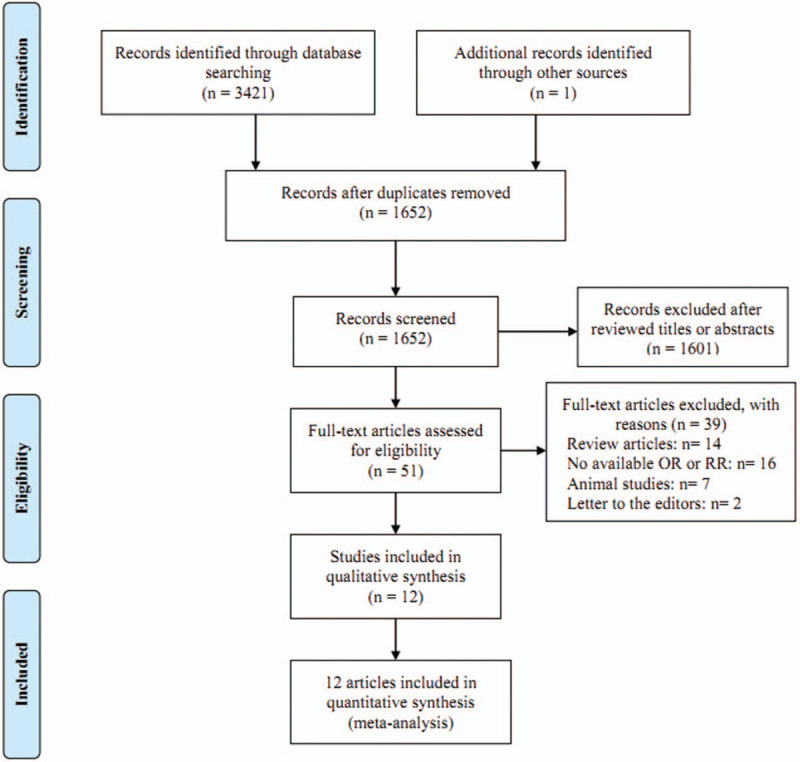
Flow chart of meta-analysis for exclusion/inclusion of studies.
Table 1.
The basic characteristics of all included studies.
| First author | Year | Country | Study design | Age | Quality score | Participants | Cases (%) | Noncases (%) | Folate | Categories (μg/d) | RR (95% CI) |
| Chang et al | 2007 | United States | Cohort | <84 | 7 | 97,275 | 280 (0.29%) | 96995 (99.71%) | Total folate intake | >711 vs ≤272 | 0.81(0.49–1.32) |
| Harris et al | 2012 | United States | PBCC | 54 ± 12 | 8 | 3899 | 1910 (48.99%) | 1989 (51.01%) | Dietary folate intakeTotal folate intake | Q4 vs Q1Q4 vs. Q1 | 0.88(0.74–1.06)0.90(0.75–1.08) |
| Kelemen et al | 2004 | United States | Cohort | 55–69 | 7 | 27,205 | 147 (0.54%) | 27058 (99.46%) | Dietary folate intakeTotal folate intake | ≥347 vs <238≥541 vs<257 | 1.45(0.83–2.53)1.73(0.90–3.33) |
| Kushi et al | 1999 | United States | Cohort | 55–69 | 6 | 29,083 | 139 (0.48%) | 28944 (99.52%) | Total folate intake | >488.5 vs. <240.9 | 1.63(0.97–2.76) |
| Larsson et al. | 2004 | Sweden | Cohort | 38-76 | 7 | 61,084 | 266 (0.44%) | 60818 (99.56%) | Dietary folate intake | ≥204 vs. <155 | 0.67(0.43–1.04) |
| McCann et al | 2003 | United States | PBCC | 40–85 | 7 | 820 | 124 (15.12%) | 696 (84.88%) | Dietary folate intake | >425 vs <236 | 0.82(0.38–1.77) |
| Navarro Silvera et al | 2006 | Canada | Cohort | 40–59 | 8 | 49,613 | 264 (0.53%) | 49349 (99.47%) | Dietary folate intake | >357 vs <248 | 0.78(0.44–1.40) |
| Pelucchi et al | 2005 | Italy | HBCC | NA | 8 | 3442 | 1031 (29.95%) | 2411 (70.05%) | Dietary folate intake | Q5 vs Q1 | 0.98(0.67–1.44) |
| Salazar-Martinez et al | 2002 | Mexico | HBCC | 20–79 | 7 | 713 | 84 (11.78%) | 629 (88.22%) | Dietary folate intake | ≥322 vs ≤197 | 1.70(0.95–3.05) |
| Tworoger et al | 2006 | United States | Cohort | 30–55 | 8 | 80,254 | 481 (0.6%) | 79773 (99.4%) | Dietary folate intakeTotal folate intake | Q5 vs Q1Q5 vs Q1 | 0.76(0.52–1.12)1.13(0.83–1.53) |
| Webb et al | 2011 | Australia | PBCC | 18–79 | 8 | 2777 | 1363 (49.08%) | 1414 (50.92%) | Dietary folate intakeTotal folate intake | >366 vs <252>546 vs <334 | 1.0(0.8–1.24)1.05(0.84–1.30) |
| Zhang et al | 2012 | China | HBCC | 47.2 ± 7.5 | 8 | 433 | 215 (49.65%) | 218 (50.35%) | Dietary folate intake | >310 vs <200 | 0.54(0.32–0.94) |
3.2. Dietary folate intake and ovarian cancer risk
Ten studies[9–11,20–26] comprising 5885 cases were carried out to assess the association between dietary folate intake and ovarian cancer risk. After pooling the data, it showed that highest category of dietary folate intake compared with lowest category had nonsignificant association on the risk of ovarian cancer (RR = 0.90, 95% CI = 0.77–1.06, I2 = 38.8%, Pfor heterogeneity = .099) (Fig. 2). The power value of the study was 0.83.
Figure 2.
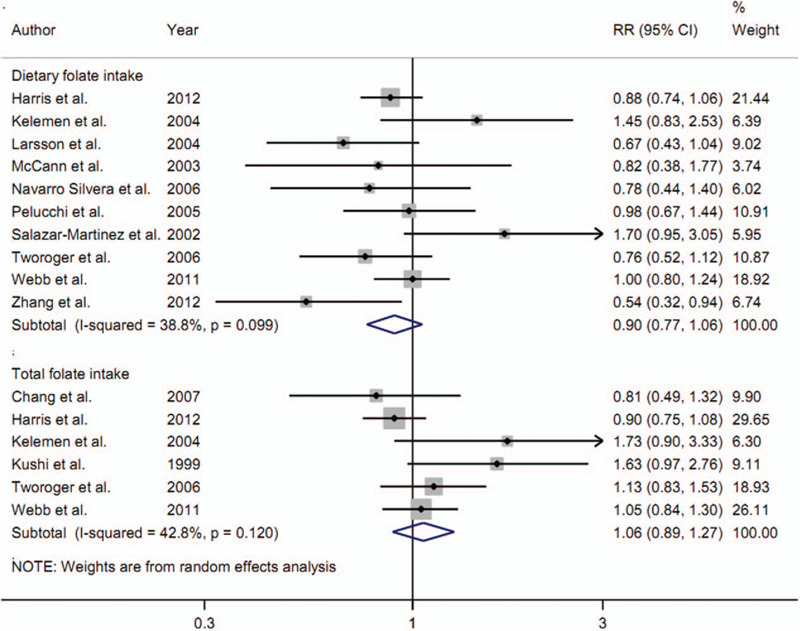
The forest plot about dietary folate intake and total folate intake on ovarian cancer risk.
The subgroup analysis by study design was performed. The result was not significant either in cohort studies (4 studies; RR = 0.84, 95% CI = 0.62–1.15, I2 = 40.0%, Pfor heterogeneity = .172) or in case-control studies (6 studies; RR = 0.93, 95% CI = 0.77–1.13, I2 = 44.3%, Pfor heterogeneity = .110). Six studies were from North America, and the association between dietary folate intake and ovarian cancer risk was not significant (RR = 0.97, 95% CI = 0.76–1.22, I2 = 40.5%, Pfor heterogeneity = .135). Two studies were from Europe, and the result was not changed (RR = 0.82, 95% CI = 0.57–1.19, I2 = 38.6%, Pfor heterogeneity = .202).
The results by funnel plot (Fig. 3) and Egger test (t = 0.05, P = .963) showed no statistical evidence of publication bias was found.
Figure 3.

Funnel plot for the analysis of publication bias between dietary folate intake and ovarian cancer risk.
Sensitivity analysis indicated that no singly study had essential effect on the overall results.
3.3. Total folate intake and ovarian cancer risk
Six studies[8,10,11,19,25,26] involving 4320 cases assessed the association about total folate intake on the risk of ovarian cancer. Overall, the association was not significant between total folate intake and ovarian cancer risk (RR = 1.06, 95% CI = 0.89–1.27, I2 = 42.8%, Pfor heterogeneity = .120) (Fig. 2).
Hierarchical analysis by study design was performed, and the result was not significant either in cohort studies (4 studies; RR = 1.21, 95% CI = 0.89–1.65, I2 = 40.8%, Pfor heterogeneity = .167) or in case-control studies (2 studies; RR = 0.96, 95% CI = 0.83–1.12, I2 = 11.4%, Pfor heterogeneity = .288). Five of the included studies reported in North America, and the summary RR on the risk of ovarian cancer was 1.10 (95% CI = 0.86–1.41, I2 = 53.7%, Pfor heterogeneity = .071).
Publication bias was not found while evaluated by Egger test (t = 1.75, P = .155). Sensitivity analysis showed no singly study had essential effect on the overall results.
4. Discussion
Findings from the current meta-analysis using 12 articles suggested that highest category of dietary folate intake compared with lowest category had nonsignificant association on the risk of ovarian cancer. The association was not significant between total folate intake and ovarian cancer risk. Results in subgroup analyses by study design and geographic location were not changed in either dietary folate intake or total folate intake. No publication bias was found in all the analyses.
Folic acid is a water-soluble vitamin that existed naturally in green leafy vegetables, cereals, beans, and fruits.[27,28] It plays an important role in DNA synthesis, integrity, and stability. In addition, folic acid plays a central role in DNA methylation.[27,28] There were 2 potential mechanisms that folic acid deficiency could cause ovarian cancer. First, it can induce the incorporation of uracil into DNA, thereby disrupting DNA integrity and DNA repair. Second, it can alter key tumor suppressor genes and proto-oncogenes expression through altering DNA methylation.[29,30]
As shown in Figure 2, dietary folate intake had a marginal inverse association on ovarian cancer risk, but, total folate intake had an increased but nonsignificant relationship on ovarian cancer risk. In our meta-analysis, total folate intake was defined as dietary folate intake plus supplementary folate intake. Therefore, the amount of total folate intake was more than that in dietary folate intake. The categories of folate intake showed in Table 1 indicated that almost all the highest amount of total folate was more than 500 μg/d while the highest amount of dietary folate intake was between 300 to 400 μg/d. Thus, further studies with supplementary folate intake on ovarian cancer risk were required to assess whether supplementary folate intake was associated with ovarian cancer risk.
A number of manuscripts already include multiple micronutrients in addition to folate intake. There are 3 studies (Harris et al in 2012, Webb et al in 2011, Tworoger et al in 2006) carried out to assess the association between vitamin B6 and ovarian cancer risk. Meanwhile, 3 studies (Harris et al in 2012, Salazar-Martinez et al in 2002, Webb et al in 2011) were carried out to assess the association between vitamin B12 and ovarian cancer risk. However, the association was not significant either in vitamin B6 intake (RR = 0.95, 95% CI = 0.72–1.24) or vitamin B12 intake (RR = 0.93, 95% CI = 0.73–1.19).
Some limitations existed in our analysis. First, we only performed the subgroup analyses by geographic location and study design due to the limited data in the every included study. Second, we could not do the dose–response analysis due to the limited data in each study, as dose–response relationship needing detailed cases, participants, and amount in each category of folate intake. The further related studies about folate intake on ovarian cancer risk are required to explore the dose–response relationship. Third, although we performed the subgroup analysis by geographic location, almost all the studies (8 out of 12) were from North America, only 2 studies from Europe, 1 study from Oceania, and 1 study from Asia. Thus, more studies conducted in some other populations, other than North Americans, are needed to further assess the association about folate intake on ovarian cancer risk. Fourth, the quantification of dietary and total folate micronutrient intake may have been too crude to reflect actual folic acid status in our meta-analysis. However, we did not perform the analysis about folate levels on the risk of ovarian cancer due to the limited studies published. Therefore, more studies about folate levels on the risk of ovarian cancer were warranted to further explore these associations. Fifth, examination of only 1 micronutrient may not reflect the entire picture of folic acid levels and status while folic acid levels are influenced by many factors. In addition to dietary and supplemental folate intake, medications, comorbidities (e.g., inflammatory conditions), and genetic factors, the carbon metabolism pathway includes B vitamins, homocysteine, and methyltransferases. However, the limited information provided in each individual study was restricted for the further analysis on this section. We only calculated the overall RR using each included study because this was a meta-analysis. Therefore, further original studies were required to explore the related factors on the risk of ovarian cancer.
5. Conclusion
Our meta-analysis demonstrates that folate intake had no significant association on the risk of ovarian cancer. Study design and geographic location were not associated with ovarian cancer while some other related factors were not investigated due to the limited information provided in each included study. Therefore, further studies are needed to verify our results.
Footnotes
Abbreviations: CI = confidence intervals, NOS = Newcastle-Ottawa-Scale, RR = relative risk.
How to cite this article: Wang K, Zhang Q, Yang J. The effect of folate intake on ovarian cancer risk: a meta-analysis of observational studies. Medicine. 2021;100:3(e22605).
The authors have no conflicts of interest to disclose.
This study was supported by the National Key Research and Development Program of China (2018YFC1004800), and The Key Research and Development Program of Zhejiang Province (2017C03022).
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
CI = confidence intervals, HBCC = hospital-based case-control studies, NA = not available, PBCC = population-based case-control studies, Q1 = quartile 1, Q4 = quartile 4, Q5 = quartile 5, RR = relative risk.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [2].Coleman RL, Monk BJ, Sood AK, et al. Latest research and treatment of advanced-stage epithelial ovarian cancer. Nat Rev Clin Oncol 2013;10:211–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jin Y. Association between EPHX1 polymorphism rs1051740 and the risk of ovarian cancer: a meta-analysis. Artif Cells Nanomed Biotechnol 2019;47:2338–42. [DOI] [PubMed] [Google Scholar]
- [4].Feng Y, Peng Z, Liu W, et al. Evaluation of the epidemiological and prognosis significance of ESR2 rs3020450 polymorphism in ovarian cancer. Gene 2019;710:316–23. [DOI] [PubMed] [Google Scholar]
- [5].Pang Y, Wang W. Dietary protein intake and risk of ovarian cancer: evidence from a meta-analysis of observational studies. Biosci Rep 2018;38:BSR20181857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Long Y, Fei H, Xu S, et al. Association about dietary vitamin C intake on the risk of ovarian cancer: a meta-analysis. Biosci Rep 2019;40:BSR20192385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leng Y, Zhou H, Meng F, et al. Association of vitamin E on the risk of ovarian cancer: a meta-analysis. Biosci Rep 2019;39:BSR20193311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kushi LH, Mink PJ, Folsom AR, et al. Prospective study of diet and ovarian cancer. Am J Epidemiol 1999;149:21–31. [DOI] [PubMed] [Google Scholar]
- [9].Zhang L, Liu W, Hao Q, et al. Folate intake and methylenetetrahydrofolate reductase gene polymorphisms as predictive and prognostic biomarkers for ovarian cancer risk. Int J Mol Sci 2012;13:4009–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Harris HR, Cramer DW, Vitonis AF, et al. Folate, vitamin B (6), vitamin B(12), methionine and alcohol intake in relation to ovarian cancer risk. Int J Cancer 2012;131:E518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kelemen LE, Sellers TA, Vierkant RA, et al. Association of folate and alcohol with risk of ovarian cancer in a prospective study of postmenopausal women. Cancer Causes Control 2004;15:1085–93. [DOI] [PubMed] [Google Scholar]
- [12].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [14].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med 2004;23:1663–82. [DOI] [PubMed] [Google Scholar]
- [16].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [17].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [18].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chang ET, Lee VS, Canchola AJ, et al. Diet and risk of ovarian cancer in the California Teachers Study cohort. Am J Epidemiol 2007;165:802–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Larsson SC, Giovannucci E, Wolk A. Dietary folate intake and incidence of ovarian cancer: the Swedish Mammography Cohort. J Natl Cancer Inst 2004;96:396–402. [DOI] [PubMed] [Google Scholar]
- [21].McCann SE, Freudenheim JL, Marshall JR, et al. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J Nutr 2003;133:1937–42. [DOI] [PubMed] [Google Scholar]
- [22].Navarro Silvera SA, Jain M, Howe GR, et al. Dietary folate consumption and risk of ovarian cancer: a prospective cohort study. Eur J Cancer Prev 2006;15:511–5. [DOI] [PubMed] [Google Scholar]
- [23].Pelucchi C, Mereghetti M, Talamini R, et al. Dietary folate, alcohol consumption, and risk of ovarian cancer in an Italian case-control study. Cancer Epidemiol Biomarkers Prev 2005;14:2056–8. [DOI] [PubMed] [Google Scholar]
- [24].Salazar-Martinez E, Lazcano-Ponce EC, Gonzalez Lira-Lira G, et al. Nutritional determinants of epithelial ovarian cancer risk: a case-control study in Mexico. Oncology 2002;63:151–7. [DOI] [PubMed] [Google Scholar]
- [25].Tworoger SS, Hecht JL, Giovannucci E, et al. Intake of folate and related nutrients in relation to risk of epithelial ovarian cancer. Am J Epidemiol 2006;163:1101–11. [DOI] [PubMed] [Google Scholar]
- [26].Webb PM, Ibiebele TI, Hughes MC, et al. Folate and related micronutrients, folate-metabolising genes and risk of ovarian cancer. Eur J Clin Nutr 2011;65:1133–40. [DOI] [PubMed] [Google Scholar]
- [27].Aune D, Deneo-Pellegrini H, Ronco AL, et al. Dietary folate intake and the risk of 11 types of cancer: a case-control study in Uruguay. Ann Oncol 2011;22:444–51. [DOI] [PubMed] [Google Scholar]
- [28].Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology 2006;131:1271–83. [DOI] [PubMed] [Google Scholar]
- [29].Gallus S, La Vecchia C. Is there a link between diet and esophageal cancer? Nat Clin Pract Gastroenterol Hepatol 2007;4:2–3. [DOI] [PubMed] [Google Scholar]
- [30].Blount BC, Mack MM, Wehr CM, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A 1997;94:3290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


