Abstract
Robotic surgical systems have evolved over time. The da Vinci Xi system was developed in 2014 and was expected to solve the shortcomings of the previous S system. Therefore, we conducted this study to compare these 2 systems and identify if the Xi system truly improves surgical outcomes.
In this retrospective study, a total of 86 patients with unilateral papillary thyroid carcinoma without central lymph node involvement underwent gasless transaxillary hemithyroidectomy using 2 robotic systems, the da Vinci S and Xi. Forty patients were in the da Vinci S group and 46 patients were in the da Vinci Xi group. All surgeries were performed by 1 surgeon (YWC). All surgery video files were analyzed to compare the duration of each surgical step.
The total operation time was significantly shorter in the Xi group than in the S group (153.0 minutes vs 105.7 minutes, P < .01). Time for robot docking was shorter in the Xi group (19.8 minutes vs 10.6 minutes, P < .01), and all procedures performed in the console also required a shorter time in this group. The overall complication rate did not differ significantly (P = .464).
The da Vinci Xi system made robotic thyroidectomy easier and faster without increasing the complication rate. It is a safe and valuable system for robotic thyroidectomy.
Keywords: operative procedures, robotic surgical procedures, surgical injuries, thyroid carcinoma, thyroidectomy
1. Introduction
Since the introduction of robotic surgery, studies have been conducted to compare robots with conventional surgical methods in various areas.[1–3] In the case of thyroidectomy, the first robotic surgery was introduced in 2007 by Chung with the gasless transaxillary approach method.[4–6] Existing research has demonstrated the safety and superiority of robotic surgery, especially in terms of surgical time.[7,8] We have also compared endoscopic and robotic thyroidectomy in detail in a previous study.[9]
Robotic surgical systems have evolved over time, and the da Vinci Xi system was developed in 2014.[10] This newly introduced da Vinci Xi surgical system has improvements with its slimmer body, narrower arms, rotatable boom, and higher definition vision, it was expected to solve the shortcomings of the previous S system. However, little analysis has been reported to determine which procedure of the robotic thyroidectomy is enhanced when using these improvements. Therefore, we conducted this study to compare these 2 systems and identify if the Xi system truly improves surgical outcomes.
The procedure for transaxillary thyroidectomy with the da Vinci S and Xi systems is exactly the same, so we could compare each step of the procedure. The present study reviewed the records of patients with papillary thyroid carcinoma (PTC) who underwent robotic thyroidectomy using these 2 systems by the same surgeon, YWC. We analyzed the recorded working video files and compared these 2 systems in terms of procedure time.
2. Methods
2.1. Patients
Between April 2016 and October 2019, we obtained the records of 86 patients with PTC who underwent robotic hemithyroidectomy at our center in Ansan. Robotic hemithyroidectomy was performed only in patients with unilateral PTC without central lymph node involvement on preoperative radiologic evaluations. Prophylactic ipsilateral central lymph node dissection was performed in all cases.[11]
Since our institute introduced the da Vinci Xi system in December 2018, patients underwent surgery using the da Vinci S system between April 2016 and November 2018. All other patients from December 2018 onward underwent surgery using the da Vinci Xi system. We retrospectively divided patients into 2 groups according to the da Vinci model used. There were 40 patients in the S group and 46 patients in the Xi group. All the surgeries were performed by 1 surgeon, YWC. At the time of surgery, there was no intent to compare the 2 models.
This retrospective study was approved by the Institutional Review Board (registration number: 2020AS0074). The authors declare no potential or actual personal, political, or financial conflicts of interest.
2.2. Surgical procedures
Patients were placed in a supine position with slight neck extension using a shoulder roll. We lifted the patients ipsilateral arm, flexed it, and carefully fixed it to the bar above their head using an elastic band to avoid brachial plexus injury and to shorten the distance between the incision and operation field. An approximate 6-cm incision was made in the inferior axillary fold. Workspace creation was started by dissecting just above the pectoralis major muscle, and this approach proceeded with the process of creating a path between the sternal and clavicular heads of the sternocleidomastoid muscle. When enough space was created, we inserted the wound retractor to maintain the opening during surgery and to protect the skin from unintended burn injuries. Later, an external Chung's retractor was inserted between the omohyoid and strap muscles to maintain the workspace. An additional 8-mm areolar skin incision was made superomedially for the insertion of the trocar for the third robotic arm. When we lifted the strap muscles, the thyroid gland was exposed from the side.
All stages of the hemithyroidectomy procedure following this were the same between the 2 groups except for the robot docking procedure. First, the superior pole of the thyroid was pulled downward and the superior thyroid vessels were dissected individually using an endoscopic energy device. Robot docking was performed at the end of the superior pole dissection. Robotic hemithyroidectomy was then performed. Prograsp forceps, Maryland bipolar forceps, permanent cautery hook, and a 30°-downward stereoscopic scope were used in all surgeries. The inferior pole of the thyroid was dissected along with the central neck lymph nodes. The thyroid was then pulled anteromedially and the ipsilateral parathyroid glands (PTGs) and recurrent laryngeal nerve (RLN) were carefully dissected and preserved. Intraoperative neuromonitoring was not used in any patient. The final procedure for the operation was to divide the thyroid gland at the isthmus, and the excised thyroid lobe was then removed from the trachea. Irrigation and bleeding control were followed, and the surgical wounds were closed with subcuticular sutures using vicryl 3–0 or 4–0 or an absorbable subcuticular stapler after the insertion of a closed suction drain.
2.3. Video file and data analysis
All video files of the surgeries were analyzed to compare the duration of the following surgical steps: flap creation, dissection of the superior pole, robot docking, dissection of the inferior pole, identification of PTGs and RLN, dissection of the thyroid along the trachea, bleeding control, application of the closed suction drain, and wound closure. The duration of each procedure was defined as the time required from the beginning of 1 procedure to the beginning of the next procedure. Each starting point of the procedure is described in Table 1.
Table 1.
Each starting point of the robotic thyroidectomy procedures for video analysis.
| Procedure | The starting point of the procedure |
| Flap creation | Skin incision |
| Dissection of the superior pole | Grabbing the superior pole |
| Robot docking | The end of dissection of the superior pole |
| Dissection of the inferior pole RLN and PTG identification | Retraction of the thyroid |
| Dissection of the thyroid along the trachea | Dissection from the trachea |
| Bleeding control Drain application Wound closure | Undocking robot |
We also analyzed the patients demographic information and pathology, including tumor size, multiplicity, number of sacrificed PTGs, status of cervical lymph nodes, and tumor node metastasis classification according to the 8th American Joint Committee on Cancer.
Complications were also recorded. Skin necrosis within 24 hours of surgery was noted. The incidence of transient RLN palsy was evaluated by the surgeon and determined by the patients symptoms on the first postoperative day. RLN palsy was considered permanent when vocal cord paralysis lasted more than 6 months after surgery. Postoperative bleeding and seroma were defined as evacuation of the hematoma and seroma aspiration, respectively. If occurred, chylous drainage was also noted.
2.4. Statistical analysis
Data were analyzed using SPSS Statistics (version 25.0.0.0; IBM Corp., Armonk, NY, USA). Continuous variables are presented as the means with standard deviations, while categorical variables are presented as percentages. A Student t test was used to compare continuous variables such as age at operation, body mass index, tumor size, harvested lymph nodes, metastatic lymph nodes in Table 2, all variables in Table 3, postoperative hospital stay, and number of sacrificed parathyroid gland in Table 4. A Chi-Squared or Fisher exact test was used to compare categorical variables such as sex, multiplicity, and TNM classification in Table 1, and postoperative complications in Table 2. Differences were considered statistically significant at P values <.05.
Table 2.
Clinical pathological characteristics of patients with PTC who underwent robotic thyroidectomy using the da Vinci S or Xi robotic systems.
| Characteristic | S group (n = 40) | Xi group (n = 46) | P value |
| Age at operation, years (range) | 46.7 (24–62) | 47.3 (26–68) | .769 |
| Sex (%) | |||
| Female | 29 (72.5) | 38 (82.6) | .304 |
| Male | 11 (27.5) | 8 (17.4) | |
| Body mass index | 25.2 (19.1–36.0) | 25.6 (18.4–36.0) | .598 |
| Tumor size, cm (range) | 0.75 (0.3–1.3) | 0.89 (0.1–2.3) | .096 |
| Multiplicity (%) | .158 | ||
| No | 36 (90.0) | 36 (78.3) | |
| Yes | 4 (10.0) | 10 (21.7) | |
| Harvested LNs, n (range) | 3.45 (0–12) | 3.39 (0–13) | .928 |
| Metastatic LNs, n (range) | 0.40 (0–2) | 0.50 (0–6) | .671 |
| T classification (%) | |||
| T1a | 32 (80.0) | 33 (71.7) | .496 |
| T1b | 8 (20.0) | 12 (26.1) | |
| T2 | 0 (0.0) | 1 (2.2) | |
| N classification (%) | |||
| N0 | 30 (75.0) | 38 (82.6) | .434 |
| N1a | 10 (25.0) | 8 (17.4) |
Table 3.
Analysis of the procedure duration of robotic thyroidectomy using the da Vinci S or Xi robotic systems.
| Time frame, min (range) | S group (n = 40) | Xi group (n = 46) | P value |
| Total operative time | 153.0 (106–247) | 105.7 (56–183) | <.001 |
| Flap creation | 36.3 (17–75) | 31.0 (13–65) | .052 |
| Dissection of the superior pole | 11.5 (4–31) | 12.4 (3–45) | .519 |
| Robot docking | 19.8 (8–40) | 10.6 (6–19) | <.001 |
| Dissection of the inferior pole | 37.5 (15–74) | 19.4 (8–40) | <.001 |
| PTG and RLN identification | |||
| Dissection of the thyroid along the trachea | 17.6 (7–33) | 11.6 (4–65) | .001 |
| Bleeding control | 30.4 (24–43) | 20.7 (10–33) | <.001 |
| Drain application | |||
| Wound closure |
Table 4.
Postoperative outcomes in the S and Xi groups.
| S group (n = 40) | Xi group (n = 46) | P value | |
| Postoperative hospital stay, days (range) | 3.75 (2–7) | 3.41 (2–7) | .114 |
| Number of sacrificed PTG, n ± SD | 0.35 ± 0.53 | 0.41 ± 0.50 | .574 |
| Postoperative complications, n (%) | 5 (12.5) | 3 (6.5) | .464 |
| Flap necrosis | 0 (0.0) | 0 (0.0) | |
| Transient RLN palsy | 1 (2.5) | 0 (0.0) | |
| Permanent RLN palsy | 0 (0.0) | 0 (0.0) | |
| Hematoma | 1 (2.5) | 0 (0.0) | |
| Seroma | 2 (5.0) | 3 (6.5) | |
| Chylous drainage | 1 (2.5) | 0 (0.0) |
3. Results
3.1. Clinicopathological characteristics
Patient and tumor characteristics are described in Table 2. The study population included 40 patients in the S group and 46 in the Xi group. The mean patient age in the S group and the Xi group was 46.7 and 47.3 years (P = .769), respectively, with no significant difference between groups. The proportion of female patients, body mass index, and number of harvested and metastatic lymph nodes were also comparable. The mean tumor size was 0.75 cm and 0.89 cm, respectively (P = .096). T- and N-stage classifications also had no significant differences between groups (P = .496 and P = .434, respectively).
3.2. Analysis of procedure duration
The total operation time was significantly shorter for the Xi group than for the S group (153.0 minutes vs 105.7 minutes, P < .01; Table 3). The time for flap creation and superior pole dissection was not different between groups. However, the time for robot docking was shorter in the Xi group than in the S group (19.8 minutes vs 10.6 minutes, respectively, P < .01). All procedures performed in the console required a shorter time in the Xi group. The remaining time from robot undocking was also shorter in the Xi group than in the S group (30.4 minutes vs 20.7 minutes, P < .01). When comparing the combined time of robot docking and console time, the standard deviation (SD) as well as the mean time were shorter in the Xi group than in the S group (74.9 ± 20.8 minutes vs 41.5 ± 15.3 minutes, P < .01; Fig. 1).
Figure 1.
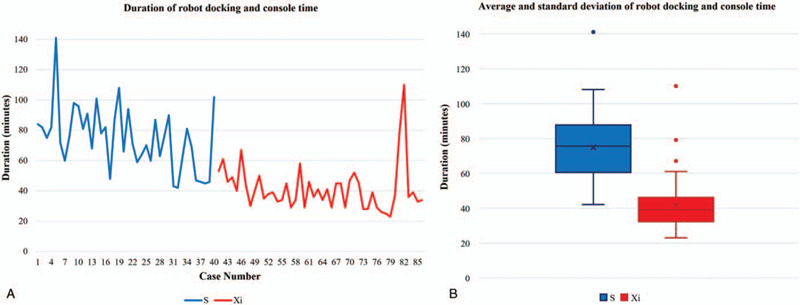
(A) Duration, (B) average and standard deviation of robot docking and console times; standard deviation as well as mean time was shorter in the Xi group than in the S group (74.9 ± 20.8 minutes vs 41.5 ± 15.3 minutes, P < .01).
3.3. Postoperative outcomes
The length of postoperative hospital stay for patients in the S group and the Xi group was comparable (3.75 days vs 3.41 days, P = .114; Table 4). The number of sacrificed PTGs was also comparable between groups (0.35 and 0.41, respectively, P = .574). In the Xi group, there were 3 patients who needed seroma aspiration in the outpatient department, and this was the only complication. In the S group, 1 patient had a transient RLN palsy that recovered within 6 months, 1 patient had a hematoma at the flap site that needed additional hematoma removal, 2 patients had a seroma that needed aspiration, and 1 patient had chylous drainage that resolved within 5 days with a low-fat, high medium-chain triglyceride diet. The overall complication rate did not differ significantly between groups (P = .464).
4. Discussion
As the technology of medical devices has evolved, surgical methods for thyroid surgery have developed tremendously.[4] Since 2007, when robotic systems began their involvement in thyroid surgery, many studies have proven the safety of robotic surgery.[8,12,13] Recently, the advantages of robotic surgery have been at the forefront of discussion in the field of surgery, and the most prominent part has been the shortening of operation times.[14,15] The delicate and steady movements of the robotic arm also make this minimally invasive surgery safe.[16] After the introduction of the da Vinci S followed by the da Vinci Xi, many institutions updated their robotic surgical system from the da Vinci S to the da Vinci Xi, including our institution.
There are several advantages of the da Vinci Xi over the da Vinci S system. The first is the reduction in docking time. In our institution, moving the patient cart of the robotic system during surgery is one of the most difficult surgical steps because the operating room that is assigned for robotic systems is small and rectangular. In addition, since there is only 1 operating room for robotic surgery, it is necessary to move the patient cart several times to perform several operations in 1 day. The da Vinci Xi system overcame these difficulties with its slimmer body and arms and the innovative progression, the rotatable boom.[17] The slimmer body allowed the patient cart of the robotic system to be more easily imported and mobile. The restriction of movement was redeemed with the rotatable boom. During thyroid lobectomy with transaxillary incision, 1 camera port and 3 acting ports are needed to be inserted through a narrow space. With the S system, the arms are thick and commonly collide with 1 another. The da Vinci Xi system overcame these difficulties with its narrower arms with greater reach and slimmer endoscopes that can move between ports. This allowed for increased range of motion of the robotic arms. Therefore, these advantages made robot docking easier and faster.
The second advantage is the reduction in console time. Comparing the overall operative time, the Xi group had a significantly shorter operative time than the S group (153.0 minutes vs 105.7 minutes, P < .01, Table 3). We divided the operation procedure into individual steps and analyzed each step of the procedures (Tables 1 and 3). From the first step of the operation to the end of the dissection of the superior pole of the thyroid, there was no significant difference between groups in terms of operating time. However, after robot docking, all steps of the procedure, including robot docking, required a shorter time in the Xi group. This is probably due to its versatility resulting from the narrower, longer arms and rotatable boom. The improved resolution from the standard definition vision of the S system to the magnified 3D high definition vision of the Xi system also attributed to making the surgery faster and safer.[18,19] This allowed surgeons to see the surgical site with true depth perception and crystal-clear vision. With this improved vision, we could find RLNs and PTGs more easily.
For the duration of robot docking and console time, the actual surgical time using the robotic system was significantly reduced after the introduction of the da Vinci Xi system, while the SD also decreased. This reduction in average and SD indicated that steadier and more stable and rapid operations are possible with the da Vinci Xi system. As the SD of the operating time decreased, we were able to more confidently explain the expected operating time to patients. Schedule management for robotic surgery and the surgery itself have also become more predictable.
There were no significant differences in postoperative outcomes and complications between the 2 groups. After undocking the robot, we performed the same procedures for bleeding control, drain application, and skin closure. The wound closure time was significantly shorter in the Xi group, but it was due to the absorbable subcuticular skin staplers that were only used in this group.
There are a few limitations in this study. First, it is a retrospective, single institution study. In the video file analysis, there may be a few seconds of bias when retrospectively selecting the start and end points of the procedure. Second, the number of patients included is relatively small. However, we were able to see obvious differences between the 2 groups with a small number. Further studies with larger sample sizes may lead to more definitive conclusions. Third, all the surgeries were performed by a single surgeon, so the learning curve could act as a confounder. Figure 1 shows a steep decline rather than gradual decrease after the introduction of the Xi system. Therefore, it can be concluded that this is due to the convenience of the robot, not the experience of the surgeon.
The robotic surgery with the da Vinci Xi system reduced the operative time at every step after robot docking, including the robot docking time. There was no significant difference in postoperative outcomes between the 2 groups. The da Vinci Xi system made robotic thyroidectomy easier and faster without increasing complications. It is a safe and valuable system in robotic thyroidectomy.
Author contributions
Conceptualization: Gil Soo Son, Young Woo Chang.
Data curation: Da Young Yu, Hye Yoon Lee, Woo Young Kim, Hoon Yub Kim, Jae Bok Lee, Gil Soo Son, Young Woo Chang.
Formal analysis: Young Woo Chang.
Funding acquisition: Young Woo Chang.
Investigation: Young Woo Chang.
Methodology: Da Young Yu, Young Woo Chang.
Project administration: Young Woo Chang.
Resources: Young Woo Chang.
Software: Young Woo Chang.
Supervision: Gil Soo Son, Young Woo Chang.
Validation: Hye Yoon Lee, Woo Young Kim, Hoon Yub Kim, Jae Bok Lee, Gil Soo Son, Young Woo Chang.
Visualization: Young Woo Chang.
Writing – original draft: Da Young Yu, Young Woo Chang.
Writing – review & editing: Da Young Yu, Young Woo Chang.
Footnotes
Abbreviations: PTC = papillary thyroid carcinoma, PTGs = parathyroid glands, RLN = recurrent laryngeal nerve, SD = standard deviation.
How to cite this article: Yu DY, Chang YW, Lee HY, Kim WY, Kim HY, Lee JB, Son GS. Detailed comparison of the da Vinci Xi and S surgical systems for transaxillary thyroidectomy. Medicine. 2021;100:3(e24370).
Supported by a Korea University Grant (K1925081).
This retrospective study was approved by the Institutional Review Board of Korea University Medical Center, Ansan. (Registration number: 2020AS0074).
The datasets supporting the conclusions of this article can be shared.
The authors declare that they have no competing interests.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
PTG = parathyroid gland, RLN = recurrent laryngeal nerve.
LN = lymph node, PTC = papillary thyroid carcinoma.
PTG = parathyroid gland, RLN = recurrent laryngeal nerve.
PTG = parathyroid gland, RLN = recurrent laryngeal nerve, SD = standard deviation.
References
- [1].Park EJ, Cho MS, Baek SJ, et al. Long-term oncologic outcomes of robotic low anterior resection for rectal cancer: a comparative study with laparoscopic surgery. Ann Surg 2015;261:129–37. [DOI] [PubMed] [Google Scholar]
- [2].Novara G, Ficarra V, Mocellin S, et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol 2012;62:382–404. [DOI] [PubMed] [Google Scholar]
- [3].Veljovich DS, Paley PJ, Drescher CW, et al. Robotic surgery in gynecologic oncology: program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging. Am J Obstet Gynecol 2008;198:679.e1–9. discussion e9-e10s. [DOI] [PubMed] [Google Scholar]
- [4].Chang EHE, Kim HY, Koh YW, et al. Overview of robotic thyroidectomy. Gland Surg 2017;6:218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kang SW, Jeong JJ, Nam KH, et al. Robot-assisted endoscopic thyroidectomy for thyroid malignancies using a gasless transaxillary approach. J Am Coll Surg 2009;209:e1–7. [DOI] [PubMed] [Google Scholar]
- [6].Tae K, Ji YB, Song CM, et al. Robotic and endoscopic thyroid surgery: evolution and advances. Clin Exp Otorhinolaryngol 2019;12:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lee J, Lee JH, Nah KY, et al. Comparison of endoscopic and robotic thyroidectomy. Ann Surg Oncol 2011;18:1439–46. [DOI] [PubMed] [Google Scholar]
- [8].Tae K, Song CM, Ji YB, et al. Oncologic outcomes of robotic thyroidectomy: 5-year experience with propensity score matching. Surg Endosc 2016;30:4785–92. [DOI] [PubMed] [Google Scholar]
- [9].Chang YW, Lee HY, Ji WB, et al. Detailed comparison of robotic and endoscopic transaxillary thyroidectomy. Asian J Surg 2020;43:234–9. [DOI] [PubMed] [Google Scholar]
- [10].Zirafa CC, Romano G, Key TH, et al. The evolution of robotic thoracic surgery. Ann Cardiothorac Surg 2019;8:210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chang YW, Kim HS, Kim HY, et al. Should central lymph node dissection be considered for all papillary thyroid microcarcinoma? Asian J Surg 2016;39:197–201. [DOI] [PubMed] [Google Scholar]
- [12].Ha TK, Kim DW, Park HK, et al. Comparison of postoperative neck pain and discomfort, swallowing difficulty, and voice change after conventional open, endoscopic, and robotic thyroidectomy: a single-center cohort study. Front Endocrinol (Lausanne) 2018;9:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Koh DH, Jang WS, Park JW, et al. Efficacy and safety of robotic procedures performed using the da Vinci robotic surgical system at a single institute in Korea: experience with 10000 cases. Yonsei Med J 2018;59:975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Patel MN, Hemal AK. Does advancing technology improve outcomes? Comparison of the da Vinci Standard/S/Si to the Xi robotic platforms during robotic nephroureterectomy. J Endourol 2018;32:133–8. [DOI] [PubMed] [Google Scholar]
- [15].Protyniak B, Jorden J, Farmer R. Multiquadrant robotic colorectal surgery: the da Vinci Xi vs Si comparison. J Robot Surg 2018;12:67–74. [DOI] [PubMed] [Google Scholar]
- [16].Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg 2004;239:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ngu JC, Tsang CB, Koh DC. The da Vinci Xi: a review of its capabilities, versatility, and potential role in robotic colorectal surgery. Robot Surg 2017;4:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].van Bergen P, Kunert W, Buess GF. The effect of high-definition imaging on surgical task efficiency in minimally invasive surgery: an experimental comparison between three-dimensional imaging and direct vision through a stereoscopic TEM rectoscope. Surg Endosc 2000;14:71–4. [DOI] [PubMed] [Google Scholar]
- [19].Freschi C, Ferrari V, Melfi F, et al. Technical review of the da Vinci surgical telemanipulator. Int J Med Robot 2013;9:396–406. [DOI] [PubMed] [Google Scholar]


