Supplemental Digital Content is available in the text.
Abstract
Background.
Surveillance biopsies permit early detection of subclinical inflammation before clinical dysfunction, but the impact of detecting early subclinical phenotypes remains unclear.
Methods.
We conducted a single-center retrospective cohort study of 441 consecutive kidney transplant recipients between 2015 and 2018 with surveillance biopsies at 6 months post-transplant. We tested the hypothesis that early subclinical inflammation (subclinical borderline changes, T cell-mediated rejection, or microvascular injury) is associated with increased incidence of a composite endpoint including acute rejection and allograft failure.
Results.
Using contemporaneous Banff criteria, we detected subclinical inflammation in 31%, with the majority (75%) having a subclinical borderline phenotype (at least minimal inflammation with mild tubulitis [>i0t1]). Overall, subclinical inflammation was independently associated with the composite endpoint (adjusted hazard ratio, 2.88; 1.11-7.51; P = 0.03). The subgroup with subclinical borderline inflammation, predominantly those meeting the Banff 2019 i1t1 threshold, was independently associated with 5-fold increased hazard for the composite endpoint (P = 0.02). Those with concurrent subclinical inflammation and subclinical chronic allograft injury had worse outcomes. The effect of treating subclinical inflammation was difficult to ascertain in small heterogeneous subgroups.
Conclusions.
Subclinical acute and chronic inflammation are common at 6 months post-transplant in kidney recipients with stable allograft function. The subclinical borderline phenotype with both tubulitis and interstitial inflammation was independently associated with poor long-term outcomes. Further studies are needed to elucidate the role of surveillance biopsies for management of allograft inflammation in kidney transplantation.
Kidney transplantation is the optimal treatment for most patients with end-stage renal disease. While there have been significant improvements in short-term acute rejection and allograft survival rates, these have not led to improved long-term allograft survival.1,2 The majority of late allograft failures are attributable to late acute rejection, especially antibody-mediated rejection (AMR).3-5 Traditionally, late acute rejection is diagnosed with an indication or for-cause biopsy performed to investigate clinical dysfunction. However, the decision to biopsy is usually based on noninvasive estimates of allograft function that lack sensitivity,6 and often detect established phenotypes of late acute rejection that are difficult to treat and hasten allograft failure.7,8
Some transplant centers rely on surveillance biopsies that are performed at prespecified time points to detect early subclinical rejection before clinical dysfunction.9-11 Surveillance biopsies detect multiple phenotypes of subclinical inflammation (SCI), including borderline T cell-mediated rejection (SC-B-TCMR), acute T cell-mediated rejection (SC-TCMR), and AMR (SC-AMR). Recent studies indicate that early detection of subclinical rejection identifies patients at increased risk for allograft failure who might benefit from increased immunosuppression exposure.9,12,13 However, the long-term consequences of detecting and treating SCI remain controversial.14 This was reflected in recent surveys of US transplant centers, of which <25% performed surveillance biopsies in selected patients, citing the low yield of actionable information as the primary reason for nonperformance.10,15
Previous studies of subclinical rejection have been largely conducted in ethnically homogeneous cohorts, often at lower risk for rejection, and with varying histologic definitions detected at different time points post-transplant.13,16-20 The incidence of subclinical rejection in these studies varied widely from 2.6% to 61% and was diagnosed between 1 and 12 months post-transplant. Long-term outcomes in patients with early subclinical rejection are variable, ranging from no differences compared to those with normal surveillance to worse renal allograft function, chronic allograft injury, fibrosis, and graft survival.9,16,18,20-23
Since 2015, our center has performed universal surveillance kidney allograft biopsies at 6 months post-transplant to detect relevant subclinical pathology. The decision of how best to treat subclinical findings has been left to the discretion of the treating physician. This practice has generated a large and heterogeneous “real-world” kidney transplant cohort treated with modern immunosuppression protocols. The purpose of this study was to test 2 hypotheses: (1) early SCI is associated with increased rates of subsequent acute rejection, allograft failure, and death and (2) the impact of SCI on kidney transplant outcomes differs according to recipient characteristics and individual phenotypes of subclinical rejection.
MATERIALS AND METHODS
Study Design
We performed a retrospective cohort study of all consecutive 6-month surveillance biopsies performed at our center from May 1, 2015 (the start of our universal surveillance biopsy program) to December 31, 2018. We identified surveillance biopsies by reviewing a clinical pathology database of all kidney transplant biopsies performed during the study period. Biopsies were identified as surveillance if they were documented as such in the medical record or occurred at 6 ± 1 months post-transplant when estimated glomerular filtration rate (eGFR) was within 25% of recent baseline values, consistent with recent studies of surveillance biopsies.9,16,24,25 Participants were included if they were >18 y of age at the time of ABO-compatible kidney transplantation. Participants were excluded if there was no surveillance biopsy performed at 6 months post-transplant, if they underwent desensitization for an ABO- or HLA-incompatible kidney transplant, or if they tested positive for HIV before transplant. The Institutional Review Board at the University of Alabama approved this study (Institutional Review Board protocols 150825006, 160105002, and 170428001). All study procedures adhered to the guidelines set forth in the Declaration of Helsinki.
Immunosuppression and Surveillance
Participants received induction immunosuppression with rabbit anti-thymocyte globulin (4.5 mg/kg total dose), alemtuzumab, or basiliximab. Maintenance immunosuppression included tacrolimus (target trough levels 8–11 ng/mL until month 3, then 6–8 ng/mL until month 6, then 4–6 ng/mL thereafter), mycophenolate mofetil (target dose 1000 mg BID throughout), and/or prednisone (tapering to 10 mg daily by week 4–6) in nearly all participants. Allograft function was assessed using the chronic kidney disease epidemiology collaboration equation to calculate eGFR.26 Donor-specific antibody (DSA) surveillance was performed at the time of 6-month surveillance biopsy using Luminex single antigen bead assays for class I (A, B, and C loci) and II (DR and DQ loci) HLA antigens (One Lambda, Canoga Park, CA). Our HLA laboratory uses a mean fluorescence intensity (MFI) cutoff of >1500 MFI for a positive DSA but reports “weak” or “probable” DSA between 500 and 1500 MFI. We considered a positive DSA as >1500 MFI. These cutoffs were developed internally by our HLA laboratory director (V.H.-D.) and validated longitudinally against flow cytometry crossmatch testing according to consensus guidelines.27,28
Surveillance biopsies were initially scored for clinical use according to Banff 2013, 2015, or 2017 criteria as appropriate for the timing of each biopsy and processed in standard fashion.29-31 C4d staining of peritubular capillaries was assessed by immunohistochemistry techniques as part of routine clinical care. We defined SC-B-TCMR a priori using a contemporaneous Banff scoring threshold of i0t1, SC-TCMR using a threshold of i2t2 (or v > 0 if present), and SC-AMR as g + ptc > 1 with detectable DSA (regardless of C4d scores). Subsequently, we found only 4 participants with SC-AMR (C4d-positive or C4d-negative), so we redefined all cases with g + ptc > 1 as subclinical microvascular injury (SC-MVI) regardless of C4d or DSA status. All surveillance biopsies that did not meet the thresholds for SC-B-TCMR, SC-TCMR, or SC-MVI were defined as no major surveillance abnormalities (NMA).
Recent studies support a higher minimum threshold of i1t1 for defining B-TCMR,17,32 which was incorporated into the 2019 Banff consensus guidelines.33 The updated guidelines were published after all of our surveillance biopsies had been clinically classified and managed using the i0t1 threshold for SC-B-TCMR. In addition to our a priori definition of SC-B-TCMR (i0t1 threshold), we reclassified SC-B-TCMR biopsies using the more stringent threshold of i1t1 to study the impact of the change in diagnostic criteria between the Banff 2017 and 2019 definitions.
Exposures and Outcomes
The primary exposure was SCI at 6 months post-transplant, defined as any of the following lesions in a surveillance biopsy: SC-B-TCMR (i0t1), SC-TCMR, or SC-MVI. SCI was modeled as a categorical exposure variable. Secondary exposures modeled as continuous variables included recipient age at transplant, donor age, Kidney Donor Profile Index, calculated panel reactive antibodies at transplant, eGFR, urine protein-to-creatinine ratio, tacrolimus trough level at 6 months post-transplant, and the number of HLA-A, -B, -DR, and -DQ mismatches between donor and recipient. Secondary exposures modeled as categorical variables included recipient sex, donor type (deceased or living donor), recipient race (Black or non-Black), cause of end-stage renal disease (glomerular, diabetes, all others), pretransplant diabetes mellitus, pretransplant hepatitis C, immunosuppression regimen, repeat transplantation, de novo class I and II DSA (present or absent), C4d staining in peritubular capillaries (present or absent), BK viruria or viremia during year-1 post-transplant (present or absent), and subclinical BK virus-associated nephropathy (SC-BKVAN) on the 6-month surveillance biopsy (present or absent).
We considered individual Banff severity scores (tubulitis [t], interstitial inflammation [i, total inflammation [ti], inflammation in areas of interstitial fibrosis and tubular atrophy [i-IFTA] intimal arteritis [v], glomerulitis [g], peritubular capillaritis [ptc] tubular atrophy [ct], interstitial fibrosis [ci], chronic glomerulopathy [cg], chronic vasculopathy [cv]; each ranging 0–3)31,34 and a composite subclinical chronic injury score (ci + ct + cg + cv) as secondary exposures modeled as ordinal variables. We also studied treatment of surveillance biopsy findings (increasing immunosuppression versus observing without a change in immunosuppression) as an exploratory binary categorical exposure variable. Management of subclinical pathology was at the discretion of the treating physician and included an increase in maintenance immunosuppression, pulse intravenous corticosteroids, or rabbit anti-thymocyte globulin.
The primary endpoint was prespecified a priori as a triple composite outcome of clinical acute rejection (TCMR, AMR, or mixed rejection), death-censored allograft loss (preemptive retransplantation or return to dialysis), or death with a functioning allograft. Clinical acute rejection was defined using Banff consensus criteria in indication biopsies performed for allograft dysfunction after the 6-month surveillance biopsy. Secondary outcomes were prespecified a priori as each component of the primary composite endpoint, eGFR at 12 and 24 months post-transplant, and an eGFR decline of >30% between 6 and 24 months post-transplant.35
Statistical Analysis
Continuous variables were compared between groups using Student t test, Mann-Whitney U test, or 1-way ANOVA as appropriate for the normality of the data distribution and the number of comparator groups. Categorical variables were compared using a chi-square test or Fisher exact test as appropriate for the number of participants in each subgroup. Survival distributions for the primary composite endpoint and each component were compared between groups using Kaplan-Meier methods and the log-rank test. Cases that did not experience the endpoint by the end of the study period or were lost to clinical follow-up were censored for time-to-event analyses. The primary composite endpoint was also modeled using Cox proportional hazards regression to adjust the univariable relationship between surveillance findings and the primary endpoint for clinical covariates. SCI phenotypes (including both i0t1 and i1t1 SC-B-TCMR definitions), Banff chronic injury scores (ci, ct, cg, and cv), and clinical covariates that were associated with the primary endpoint by univariable analysis at P < 0.10 were entered into a multivariable Cox model. We did not include Banff acute injury scores (t, v, i, g, ptc) in the Cox model because of concerns for multicollinearity with SCI and its phenotypes that are defined by these scores. We included the total inflammation score as a covariate in the models to capture inflammation in areas with scarring.34 We also performed a sensitivity analysis where cases of SC-BKVAN were excluded from the SCI group. Visual inspection of log-log plots was used to confirm the proportionality of hazards assumption. All statistical tests were 2-tailed with statistical significance defined as P < 0.05. All analyses were performed using SPSS Statistics version 25 (IBM, Armonk, NY).
RESULTS
Cohort Characteristics
We reviewed 1,254 consecutive biopsies from 940 kidney transplant recipients during the study period, of which 441 had 6-month surveillance biopsies and met inclusion criteria. Median (minimum, maximum) follow-up was 29 months (6–55 mo) post-transplant. The cohort was highly diverse with 62% male sex, 60% deceased donor, and 53% Black race. The majority of participants received induction with rabbit anti-thymocyte globulin and maintenance immunosuppression with tacrolimus and mycophenolate ± prednisone. Significant bleeding after a surveillance biopsy was a rare event, with <5% of biopsies associated with any form of postprocedural bleeding. Of the bleeding episodes, 90% were gross hematuria that resolved spontaneously, with the remaining 10% requiring hospitalization for further observation. Patient and allograft survival were excellent at 98% each; rejection-free survival after surveillance was 96%. Complete clinical and demographic data and the outcomes for the entire cohort are presented in Tables 1–3, respectively.
TABLE 1.
Pretransplant demographic and clinical data
| Parameter | All (n = 441) | SCI (n = 137) | NMA (n = 304) | P | ||
|---|---|---|---|---|---|---|
| SC-B-TCMR (n = 102) | SC-TCMR (n = 15) | SC-MVI (n = 20) | ||||
| Recipient age at transplant (y) | 49 ± 0.6 | 49 ± 1 | 53 ± 3 | 50 ± 3 | 49 ± 1 | 0.35 |
| Donor age at transplant (y) | 38 ± 0.6 | 38 ± 1 | 41 ± 3 | 41 ± 3 | 38 ± 1 | 0.55 |
| Donor KDPI | 40 ± 1.5 | 38 ± 3 | 46 ± 9 | 41 ± 7 | 40 ± 2 | 0.68 |
| Sex (male/female) | 273/168 | 68/34 | 10/5 | 14/6 | 181/123 | 0.13 |
| Donor type (deceased/living) | 265/176 | 64/38 | 10/5 | 15/5 | 176/128 | 0.15 |
| Recipient race (Black/non-Black) | 234/207 | 54/48 | 10/5 | 10/10 | 160/144 | 0.79 |
| Cause of ESRD, n (%) | ||||||
| Glomerular | 147 (33) | 35 (34) | 6 (40) | 6 (30) | 100 (33) | 0.83 |
| Diabetes | 108 (25) | 21 (21) | 4 (27) | 6 (30) | 77 (25) | |
| Other | 186 (42) | 46 (45) | 5 (33) | 8 (40) | 127 (42) | |
| Pretransplant diabetes, n (%) | 148 (34) | 31 (30) | 5 (33) | 6 (30) | 106 (35) | 0.39 |
| Pretransplant hepatitis C, n (%) | 10 (2) | 3 (3) | 0 (0) | 0 (0) | 7 (2) | 0.80 |
| Repeat transplant, n (%) | 42 (10) | 10 (10) | 2 (13) | 4 (20) | 26 (9) | 0.30 |
| cPRA at transplanta | 17 ± 1.5 | 18 ± 3 | 22 ± 9 | 40 ± 10 | 16 ± 2 | 0.05 |
| PRA < 20%, n (%) | 335 (77) | 78 (77) | 11 (73) | 9 (47) | 237 (79) | 0.31 |
| PRA 20%–80%, n (%) | 54 (12) | 13 (13) | 2 (13) | 5 (26) | 34 (11) | |
| PRA > 80%, n (%) | 48 (11) | 11 (11) | 2 (13) | 5 (26) | 30 (10) | |
| HLA-A and -B mismatch | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.4 | 2.5 ± 0.4 | 2.8 ± 0.8 | 0.93 |
| HLA-DR and -DQ mismatch | 2.3 ± 0.1 | 2.5 ± 0.1 | 1.9 ± 0.5 | 2.2 ± 0.2 | 2.3 ± 0.1 | 0.50 |
acPRA data were available from 437 participants.
Comparison of pretransplant demographic and clinical data between groups. The first column shows data for the entire cohort. The P represent comparisons between the SCI group (n = 137) and the NMA group (n = 304) by Mann-Whitney U test (continuous variables) or chi-square/Fisher exact test (categorical variables). Non-normally distributed continuous variables are presented as mean ± SE.
cPRA, calculated panel reactive antibodies; ESRD, end-stage renal disease; KDPI, Kidney Donor Profile Index; NMA, no major surveillance abnormalities; PRA, panel reactive antibodies; SC-B-TCMR, subclinical borderline T cell-mediated rejection; SCI, subclinical inflammation; SC-MVI, subclinical microvascular injury; SC-TCMR, subclinical T cell-mediated rejection.
TABLE 3.
Outcomes according to surveillance phenotypes
| Outcome | All (n = 441) | SCI (n = 137) | NMA (n = 304) | P | ||
|---|---|---|---|---|---|---|
| SC-B-TCMR (n = 102) | SC-TCMR (n = 15) | SC-MVI (n = 20) | ||||
| Triple composite endpoint, n (%) | 30 (7) | 14 (14) | 0 (0) | 5 (25) | 11 (4) | 0.0001 |
| Acute rejection after surveillance, n (%) | 16 (4) | 8 (8) | 0 (0) | 3 (15) | 5 (2) | 0.001 |
| TCMR | 4 (1) | 3 (3) | 0 (0) | 0 (0) | 1 (0.3) | 0.01 |
| AMR/mixed | 12 (3) | 5 (5) | 0 (0) | 3 (15) | 4 (1) | |
| Death-censored graft failure, n (%) | 10 (2) | 7 (7) | 0 (0) | 2 (10) | 1 (0.3) | 0.0002 |
| Death, n (%) | 9 (2) | 2 (2) | 0 (0) | 1 (5) | 6 (2) | 1.00 |
| Estimated GFR (mL/min/1.73 m2), 12 mo | 49 ± 0.6 (n = 388) | 54 ± 2 | 47 ± 4 | 55 ± 5 | 58 ± 1 | 0.06 |
| Estimated GFR (mL/min/1.73 m2), 24 mo | 49 ± 0.6 (n = 238) | 57 ± 3 | 49 ± 4 | 55 ± 7 | 58 ± 2 | 0.26 |
| Estimated GFR decline >30%, 6–24 mo, n (%) | 16/238 (7) | 3 (6) | 1 (14) | 0 (0) | 12 (7) | 0.78 |
Comparison of outcomes after the 6-mo surveillance biopsy. The first column shows outcomes for the entire cohort. The P represent comparisons between the SCI group and the NMA group by Mann-Whitney U test (continuous variables) or chi-square/Fisher exact test (categorical variables). Non-normally distributed continuous variables are presented as mean ± SE.
AMR, antibody-mediated rejection; GFR, glomerular filtration rate; NMA, no major surveillance abnormalities; SC-B-TCMR, subclinical borderline T cell-mediated rejection; SCI, subclinical inflammation; SC-MVI, subclinical microvascular injury; SC-TCMR, subclinical T cell-mediated rejection; TCMR, T cell-mediated rejection.
Characteristics of SCI Phenotypes
SCI was detected in 137 of 441 (31%) of participants at 6 months post-transplant, including 102 (23%) with SC-B-TCMR by the i0t1 threshold (29 [7%] by i1t1 threshold) and 15 (3%) with SC-TCMR. There were only 4 (1%) participants with SC-AMR (g + ptc > 1 with detectable DSA; C4d-positive or C4d-negative). Due to the infrequency of SC-AMR, we grouped all cases with Banff g + ptc scores > 1 (n = 20; 5%) as SC-MVI regardless of C4d or DSA status. Of the 304 biopsies with NMA, 280 (92%) had Banff i, t, v, g, ptc, and cg scores all = 0. The remaining 24 NMA biopsies all had i, t, v scores = 0 and either g, ptc, or cg = 1. Excluding these 24 biopsies from the NMA group did not change the results of our main analysis (data not shown). Conversely, adding these 24 cases to the SC-MVI group attenuated the association between SC-MVI and the primary endpoint, although it remained statistically significant in univariable modeling (data not shown). Compared to those with NMA, participants with SCI were more likely to have higher calculated panel reactive antibodies at transplant and higher urine protein-to-creatinine ratio at biopsy, DSA+ positivity at biopsy, and BK virus reactivation during year-1 post-transplant (Tables 1 and 2).
TABLE 2.
Post-transplant demographic and clinical data
| Parameter | All (n = 441) | SCI (n = 137) | NMA (n = 304) | P | ||
|---|---|---|---|---|---|---|
| SC-B-TCMR (n = 102) | SC-TCMR (n = 15) | SC-MVI (n = 20) | ||||
| Induction immunosuppression, n (%) | ||||||
| Rabbit anti-thymocyte globulin | 302 (69) | 70 (69) | 10 (67) | 13 (65) | 209 (69) | 0.19 |
| Alemtuzumab | 90 (20) | 17 (17) | 3 (20) | 4 (20) | 66 (22) | |
| Basiliximab | 49 (11) | 15 (14) | 2 (13) | 3 (15) | 29 (9) | |
| Maintenance immunosuppression, n (%) | ||||||
| Tacrolimus/MMF | 429 (97) | 99 (97) | 13 (87) | 18 (90) | 299 (98) | 0.11 |
| Tacrolimus/azathioprine | 5 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (0.5) | |
| Cyclosporine/MMF | 1 (0.5) | 1 (1) | 1 (7) | 1 (5) | 2 (1) | |
| Other | 6 (1.5) | 2 (2) | 1 (7) | 1 (5) | 2 (1) | |
| Estimated GFR (mL/min/1.73 m2), 6 mo | 56 ± 0.8 | 57 ± 2 | 49 ± 3 | 56 ± 4 | 57 ± 1 | 0.59 |
| Urine protein-to-creatinine ratio, 6 mo | 0.18 ± 0.01 | 0.20 ± 0.03 | 0.23 ± 0.05 | 0.40 ± 0.09 | 0.15 ± 0.01 | 0.004 |
| Tacrolimus trough (ng/mL), 6 mo | 7.3 ± 1.1 | 7.3 ± 0.3 | 7.0 ± 0.6 | 7.1 ± 0.5 | 7.3 ± 0.1 | 0.43 |
| DSA, no. positive/no. assessed (%) | 28/406 (7) | 10/96 (10) | 1/14 (7) | 4/20 (20) | 13/276 (5) | 0.01 |
| Class I DSA | 15/406 (4) | 5/96 (5) | 0/14 (0) | 2/20 (10) | 8/276 (3) | 0.03 |
| Class II DSA | 13/406 (3) | 5/96 (5) | 1/14 (7) | 2/20 (10) | 5/276 (2) | |
| BK viremia during year-1 post-transplant, n (%) | 118 (27) | 45 (44) | 10 (67) | 6 (30) | 57 (19) | <0.0001 |
| BK viruria during year-1 post-transplant, n (%) | 152 (35) | 50 (51) | 10 (67) | 9 (45) | 83 (28) | <0.0001 |
Comparison of post-transplant demographic and clinical data between groups. The first column shows data for the entire cohort. The P represent comparisons between the SCI group (n = 137) and the NMA group (n = 304) by Mann-Whitney U test (continuous variables) or chi-square/Fisher exact test (categorical variables). Non-normally distributed continuous variables are presented as mean ± SE.
DSA, donor-specific antibody; GFR, glomerular filtration rate; MMF, mycophenolate mofetil; NMA, no major surveillance abnormalities; SC-B-TCMR, subclinical borderline T cell-mediated rejection; SCI, subclinical inflammation; SC-MVI, subclinical microvascular injury; SC-TCMR, subclinical T cell-mediated rejection.
Subclinical chronic allograft injury lesions were often present by 6 months. In particular, 129 (29%) had ci + ct ≥ 2, 15 (3%) had cg > 0, and 172 (39%) had cv > 0. Of the chronic injury scores, cg and cv were similarly distributed between SCI, race, and donor groups. However, ci + ct ≥ 2 was detected significantly more often in SCI, Black race, and deceased donor groups versus their respective comparator groups (40% versus 24%, 35% versus 23%, and 37% versus 17%, respectively; P ≤ 0.001 for all). Overall, a composite Banff chronic injury score (ci + ct + cv + cg) was ≥2 in 48% of participants (Table S1, SDC, http://links.lww.com/TXD/A311). Subclinical i-IFTA was present in 234 (53%) participants (226 with i-IFTA = 1) but was not a significant determinant of the composite endpoint (8% in i-IFTA > 0 versus 4 % in i-IFTA= 0; P = 0.09). Additionally, the presence of i-IFTA did not affect the relationship between SCI and the primary endpoint (data not shown).
Finally, in spite of prospective frequent monitoring protocols for BK virus polymerase chain reaction in blood and urine, SC-BKVAN was detected in 21 of 441 (5%) of 6-month surveillance biopsies, with 20 of 21 cases having concordance for SCI. SC-BKVAN overlapped with all 3 SCI phenotypes, but the majority had SC-B-TCMR (split equally between i0t1 and i1t1 definitions).
SCI and Outcomes
The primary composite endpoint occurred in 14% with SCI compared to 4% in NMA (P = 0.0002; Figure 1). The incidence of the primary outcome was highest in the SC-MVI subgroup, followed by the SC-B-TCMR (i1t1) and SC-B-TCMR (i0t1) subgroups; no participants in the SC-TCMR subgroup met the primary outcome (Table 3; Figures 2 and 3). Acute rejection after surveillance and death-censored allograft failure were equally common in the SCI group; death with a functioning allograft was rare (Table 3). SCI was independently associated with a nearly 3-fold greater hazard for the composite endpoint (Figure 1; Table 4). The strength, but not magnitude, of this association was mildly attenuated when cases with SC-B-TCMR (i0t1) were classified as NMA (data not shown). Within the SCI group, only SC-B-TCMR (i1t1) had an independent association with the primary endpoint in multivariable analysis, with a 5-fold increased hazard after adjustment for clinical covariates (Table 4; Figure 3). SCI was not associated with lower eGFR by 24 months post-transplant (Table 3). Finally, in spite of its frequent detection in surveillance biopsies, we found no association between SC-BKVAN and the primary composite endpoint (Figure S1, SDC, http://links.lww.com/TXD/A311). The association between SCI and the primary composite endpoint was maintained after removing all SC-BKVAN cases (Figure S2, SDC, http://links.lww.com/TXD/A311).
FIGURE 1.
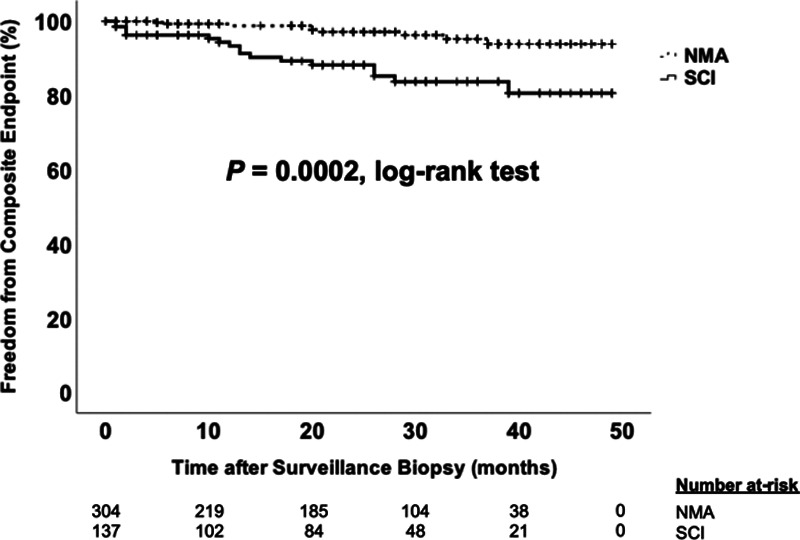
Time to composite endpoint according to presence of subclinical inflammation (SCI). Kaplan-Meier plot comparing time to the composite endpoint between the SCI group and the no major surveillance abnormalities (NMA) group using the log-rank test. Hatch marks represent censored cases in each group.
FIGURE 2.
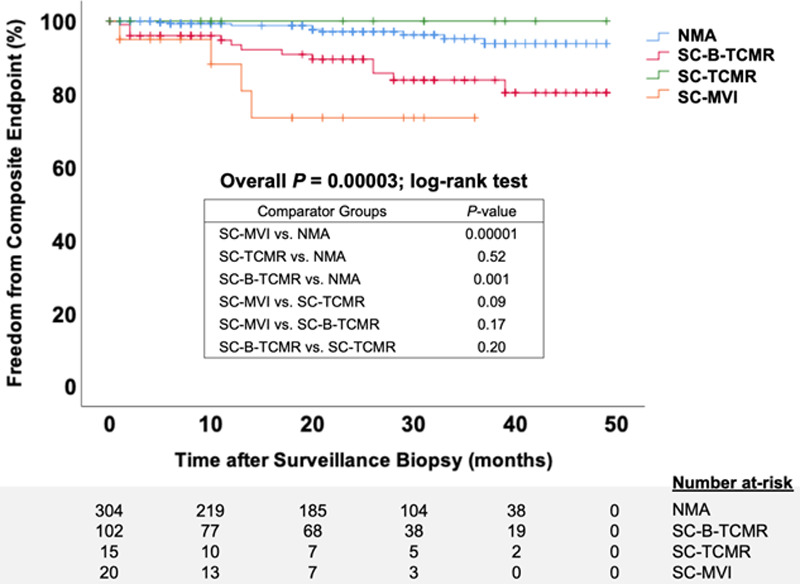
Time to composite endpoint by subclinical inflammation phenotypes. Kaplan-Meier plot comparing time to the composite endpoint between each subclinical inflammation phenotype by the log-rank test. The box shows the P values for comparisons between subgroups. Hatch marks represent censored cases in each group. NMA, no major surveillance abnormalities; SC-B-TCMR, subclinical borderline T cell-mediated rejection (using the i0t1 threshold); SC-MVI, subclinical microvascular injury; SC-TCMR, subclinical T cell-mediated rejection.
FIGURE 3.
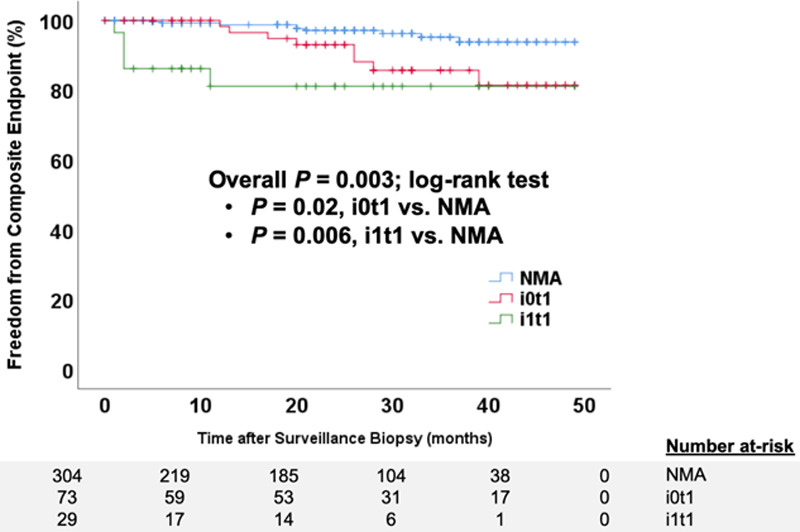
Time to composite endpoint according to different thresholds for subclinical borderline T cell-mediated rejection (TCMR). Kaplan-Meier plot comparing time to the composite endpoint between subclinical borderline TCMR cases diagnosed with an i1t1 threshold, subclinical borderline cases with an i0t1 threshold, and cases with no major surveillance abnormalities (NMA) by the log-rank test. Hatch marks represent censored cases in each group.
TABLE 4.
Multivariable Cox model of the composite endpoint
| Parameter | Univariable HR (95% CI) | P | Multivariable HR (95% CI) | P |
|---|---|---|---|---|
| Subclinical inflammation (vs no major abnormalities) | 4.15 (1.85-9.32) | 0.001 | 2.88 (1.11-7.51) | 0.03 |
| SC-B-TCMR (i0t1 threshold) | 3.15 (1.21-8.16) | 0.02 | 2.39 (0.82-6.95) | 0.11 |
| SC-B-TCMR (i1t1 threshold) | 7.01 (2.34-20.94) | 0.0005 | 5.32 (1.37-20.7) | 0.02 |
| SC-TCMR | — | — | — | — |
| SC-MVI | 9.41 (2.87-30.85) | 0.0002 | 3.18 (0.73-13.83) | 0.12 |
| ti score (0–3) | 1.96 (1.17-3.29) | 0.01 | 1.18 (0.52-2.71) | 0.69 |
| ci score (0–3) | 1.54 (0.82-2.92) | 0.18 | ||
| ct score (0–3) | 1.94 (0.87-4.29) | 0.11 | ||
| cg score (0–3) | 4.08 (1.23-13.62) | 0.02 | 3.93 (0.98-15.72) | 0.05 |
| cv score (0–3) | 1.62 (1.06-2.47) | 0.03 | 1.28 (0.77-2.12) | 0.35 |
| Age at transplant (per year) | 0.99 (0.95-1.02) | 0.37 | ||
| Black race (vs non-Black race) | 1.69 (0.72-4.00) | 0.23 | ||
| Deceased donor (vs living donor) | 2.51 (0.93-6.75) | 0.07 | 2.24 (0.83-6.05) | 0.11 |
| Male sex (vs female sex) | 0.68 (0.30-1.54) | 0.36 | ||
| Pretransplant diabetes mellitus (vs no diabetes) | 0.89 (0.37-2.17) | 0.80 | ||
| Repeat transplant (vs first transplant) | 0.74 (0.17-3.17) | 0.69 | ||
| DSA at surveillance (vs no DSA) | 2.10 (0.62-7.07) | 0.23 | ||
| Estimated GFR (per 1 mL/min/1.73 m2) | 0.99 (0.97-1.01) | 0.52 | ||
| Urine protein-to-creatinine ratio at surveillance (per unit) | 7.74 (3.05-19.61) | <0.0001 | 4.85 (1.53-15.35) | 0.007 |
Multivariable Cox proportional hazards model of a composite endpoint of acute rejection after surveillance, death-censored allograft failure, and death with a functioning allograft. We tested the association between subclinical inflammation phenotypes (with SC-B-TCMR defined using the Banff 2017 i0t1 threshold as well as the Banff 2019 i1t1 threshold), individual Banff chronic injury scores, and clinical covariates with the composite endpoint in univariable models. We excluded Banff acute injury scores (except for ti) because of collinearity with subclinical inflammation phenotypes. All covariates that were significantly associated with the composite endpoint at P < 0.10 by univariable modeling were force entered into a multivariable Cox model. There were no outcome events in the SC-TCMR phenotype (reflected as “—“ in the table). The final Cox model was significant at P < 0.0001, df 9, and chi-square 50.98.
cg, chronic glomerulopathy; CI, confidence interval; ci, interstitial fibrosis; ct, tubular atrophy; cv, chronic vasculopathy; DSA, donor-specific antibody; GFR, glomerular filtration rate; HR, hazard ratio; SC-B-TCMR, subclinical borderline T cell-mediated rejection; SC-MVI, subclinical microvascular injury; SC-TCMR, subclinical T cell-mediated rejection; ti, total inflammation.
Exploring the Effect of Treatment on Outcomes After SCI
In the SC-B-TCMR (i0t1) subgroup, only 36% were managed with increased immunosuppression, with most receiving an increase in maintenance steroids or targeting a higher tacrolimus trough target range. We saw similar proportions in the subset of SC-B-TCMR cases meeting the i1t1 threshold. By comparison, in the SC-TCMR and SC-MVI subgroups, the vast majority of cases were treated with pulse dose intravenous steroids. In exploratory analyses, we could not detect a difference in the incidence of the primary outcome in treated versus observed SCI. Likewise, we found no difference in the primary outcome with treatment of SC-B-TCMR, SC-TCMR, or SC-MVI subgroups. Importantly, the small sample sizes should caution the interpretation of these data, as we were likely underpowered to detect differences according to treatment status (Figure 4; Table S2, SDC, http://links.lww.com/TXD/A311).
FIGURE 4.
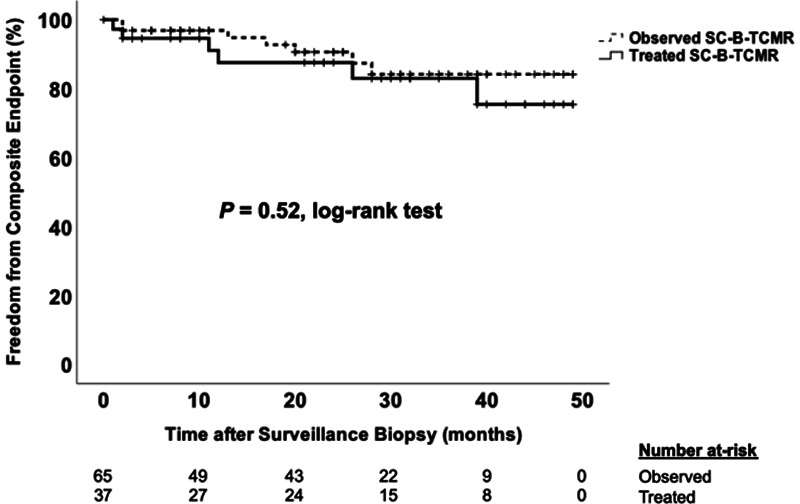
Time to composite endpoint by treatment status of subclinical borderline T cell-mediated rejection (SC-B-TCMR). Kaplan-Meier plot comparing time to the composite endpoint in the SC-B-TCMR group (using i0t1 threshold) that was treated with increased immunosuppression compared to the group that was observed expectantly without changes to immunosuppression by the log-rank test. Hatch marks represent censored cases in each group. Repeating the analysis using the SC-B-TCMR subgroup defined by the i1t1 threshold did not change the results (data not shown).
Subclinical Chronic Allograft Injury and Outcomes
The primary composite endpoint occurred in 9% with ci + ct ≥ 2 versus 4% with ci + ct < 2 (P = 0.05), 20% with cg > 0 versus 5% with cg = 0 (P = 0.05), and 8% with cv > 0 versus 5% with cv = 0 (P = 0.11). Given these trends, we modeled the relationship between a composite subclinical Banff chronic injury score (ci + ct + cg + cv) and the primary endpoint, which had good prognostic performance (Figure 5A). We applied the Youden index to identify a cutoff of ci + ct + cg + cv ≥ 2 for optimal prognostic performance for the primary endpoint. Those with a subclinical composite chronic injury score ≥ 2 had an 9% incidence of the primary endpoint compared to 3% with a score < 2 (P = 0.002) and had a 3-fold increased hazard for the primary endpoint after adjusting for SCI and multiple clinical covariates (Figure 5B). Cases with concomitant SCI (using our a priori definition) and a subclinical composite chronic injury score ≥ 2 had the greatest adjusted hazard for the composite endpoint compared to all other subclinical phenotypes (Figure 5C).
FIGURE 5.
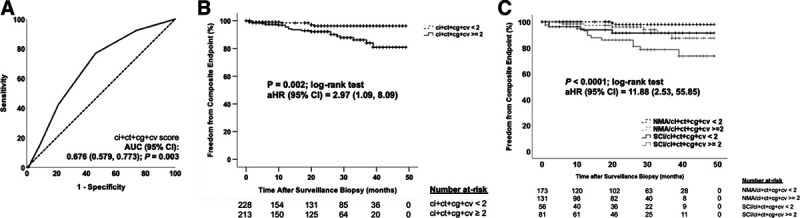
Subclinical chronic allograft injury scores at 6 mo are prognostic for the composite endpoint. A, Receiver operating characteristic (ROC) curve analysis depicting good prognostic performance of the composite Banff chronic allograft injury score (ci + ct + cg + cv) for the primary composite endpoint. The dotted line represents the line of identity. The area under the ROC curve (AUC) was significant at P = 0.003. B, Kaplan-Meier plot comparing time to the composite endpoint between the group with subclinical ci + ct + cg + cv score ≥ 2 vs < 2 by the log-rank test. Categorizations were based on the optimal cutoff value determined by the Youden index in (A). The adjusted hazard ratio (aHR) with 95% confidence interval (CI) for subclinical ci + ct + cg + cv score ≥ 2 and the primary endpoint was derived from a multivariable Cox proportional hazards regression. The association between the subclinical ci + ct + cg + cv score ≥ 2 and primary endpoint was independent of subclinical inflammation (SCI), age at transplant, race, donor type, sex, pretransplant diabetes status, repeat transplantation, and the presence of donor-specific antibody (DSA) at the surveillance biopsy. The final model used forced entry of the same clinical covariates used in Table 4 and was significant at P = 0.01 with chi-square = 21.28 and 9 df. Hatch marks represent censored cases in each group. C, Further characterization of the association between subclinical pathology and the composite endpoint, illustrated in a Kaplan-Meier plot comparing time to the composite endpoint between 4 subgroups: (1) cases with no major surveillance abnormalities (NMA) and subclinical ci + ct + cg + cv score < 2 (black dotted line), (2) cases with NMA and subclinical ci + ct + cg + cv score ≥ 2 (gray dotted line), (3) cases with SCI and subclinical ci + ct + cg + cv score < 2 (solid black line), and (4) cases with SCI and subclinical ci + ct + cg + cv score ≥ 2 (solid gray line) by the log-rank test. The same modeling approach was used as in (B) to derive the aHR for the composite endpoint. The aHR represents the association between the SCI and subclinical ci + ct + cg + cv score ≥ 2 group compared to the NMA and subclinical ci + ct + cg + cv score < 2 reference group. The final model was significant at P = 0.01 with chi-square = 21.77 and 9 df. cg, chronic glomerulopathy; ci, interstitial fibrosis; ct, tubular atrophy; cv, chronic vasculopathy.
DISCUSSION
We present the findings from 1 of the largest cohort studies of consecutive kidney transplant recipients implementing a universal surveillance biopsy program. The key findings of our study include: (1) SCI inclusive of SC-B-TCMR, SC-TCMR, and SC-MVI was relatively common by 6 months and was associated with a nearly 3-fold increased hazard for our composite endpoint, (2) SC-B-TCMR, when reclassified according to the Banff 2019 i1t1 threshold, had an independent association with long-term outcomes compared to our a priori i0t1 threshold, and (3) a higher burden of subclinical chronic allograft injury was independently associated with poor long-term outcomes.
Previous studies of subclinical pathology have used varying definitions of SCI and deployed surveillance biopsies at different times post-transplant. Anil Kumar et al22 reported similar enrichment for Black race and deceased donors as in our study. Overall, they reported SCI in only 7%–9% of recipients at 6 months post-transplant, but their definition of SCI excluded SC-B-TCMR that was the most common phenotype in our cohort and others.9,13,16,22 The incidence of SCI and subsequent outcomes were similar between Black and non-Black recipients as in our study. Gloor et al19 found a comparatively low rate of SCI (2.6%) at 3 months post-transplant in a low immunologic risk cohort managed with a modern tacrolimus-based immunosuppression regimen. Notably, their definition of SCI also excluded cases with SC-B-TCMR and predated contemporary Banff classifications of MVI/AMR. Kee et al13 reported a higher incidence of SCI compared to our cohort (31% versus 47%); their definition of SCI included SC-B-TCMR but not SC-MVI/AMR. However, the majority of SCI was detected at 1 month post-transplant and could have reflected residual ischemia-reperfusion injury.36 Finally, Mehta et al16 defined SCI as Banff i + t > 0 but excluded SC-TCMR and SC-MVI/AMR, a strategy that detected SCI in 65% of recipients at 3 months post-transplant. Their definition of SCI skewed toward milder degrees of inflammation (40% had i > 0 but t = 0) but was still associated with increased de novo DSA, chronic allograft injury scores, and subsequent acute rejection. To our knowledge, ours is the first study to use a comprehensive definition of SCI including modern Banff definitions of SC-B-TCMR, SC-TCMR, and SC-MVI in a large consecutive cohort enriched for clinical and racial diversity.
The independent association between early SCI and poor long-term outcomes agrees with some,9,13,16,18,20 but not all21,36 prior surveillance studies. The variability in surveillance biopsy practices, definitions of SCI, and outcome ascertainment between centers10 creates difficulties in comparing results between studies. Nonetheless, our findings are in parallel to those from our pediatric surveillance biopsy cohort9 and the recent Clinical Trials in Organ Transplantation-08 study24 that each confirmed an association between a comprehensive definition of early SCI and poor subsequent outcomes. We propose that future studies of subclinical pathology use a similarly comprehensive definition of SCI that includes B-TCMR, TCMR, and AMR classifications from the most recent Banff report,33 which will allow for reporting of outcomes by individual SCI phenotypes along with easier comparisons of results between studies. Importantly, our SC-B-TCMR data agree with the recent reports by Nankivell et al17,37 where B-TCMR with minimal interstitial inflammation (i0t1) had better long-term outcomes compared to the more stringent i1t1 threshold, which supports the application of the Banff 2019 i1t1 threshold for B-TCMR to surveillance biopsies as well as indication biopsies.33
Although multiple studies have identified early SCI as an important clinical biomarker for long-term outcomes, many transplant programs have not adopted surveillance biopsies into routine practice, often citing a low diagnostic yield.10 Indeed, recent studies using a comprehensive definition of SCI still find no subclinical rejection in 60%–80% of surveillance biopsies.9,24 Therefore, noninvasive screening tools would be advantageous to identify patients for whom surveillance biopsies would produce the highest diagnostic and prognostic yield. Emerging noninvasive biomarkers may prove better at targeting surveillance biopsies to patients that will experience the optimal risk-benefit ratio.24,38,39
While identifying SCI is important for risk stratification, the impact of treating SCI has remained unclear. We explored this issue with available data but were likely underpowered to detect differences in outcomes according to treatment. Most clinicians opted to treat SC-TCMR and SC-MVI, albeit not with therapies targeting humoral immunity in the latter. In univariable analysis, SC-TCMR had excellent outcomes after treatment, but SC-MVI had poor long-term outcomes as in a previous study.18 As reported by Orandi et al,12 perhaps the SC-MVI group would have fared better after treatment with therapeutic plasma exchange, as compared to intravenous methylprednisolone that we used in the majority of SC-MVI. Notably, the majority of SC-B-TCMR cases (regardless of the definition used) were managed expectantly without changes to immunosuppression. We detected no differences in treated versus observed SC-B-TCMR cases, contrary to our previous findings in children.9 However, the majority of treated pediatric SC-B-TCMR cases received intravenous methylprednisolone rather than increased maintenance immunosuppression as in the current study.9 Ultimately, our exploratory analyses were unable to clarify the long-term impact of treating subclinical pathology.
We found that a composite of subclinical chronic allograft injury scores was independently prognostic for long-term outcomes, with worse outcomes in cases with both SCI and subclinical chronic allograft injury. Moreso et al40 also reported that concomitant subclinical acute and chronic injury was associated with worse outcomes than either phenotype in isolation. Although mild subclinical i-IFTA was a common finding at 6 months, we did not detect an association with long-term outcomes as in indication biopsies from the long-term deterioration of kidney allograft functions study.41
Our study had several strengths, including a relatively large cohort of consecutive 6-month surveillance biopsies that detected the full spectrum of subclinical phenotypes and allowed for robust model building with adjustment for multiple clinical covariates. Our cohort was clinically heterogeneous and enriched for populations at increased risk for poor outcomes, including large proportions of Black recipients and deceased donor transplants. Despite these strengths, we concede some limitations including a relatively short period of clinical follow-up during which the primary composite endpoint occurred in <10% of participants. Also, while we analyzed surrogate endpoints such as eGFR, we did not obtain serial DSA assessments or perform follow-up biopsies in each participant that could have produced further insights into the effects of SCI over time. While the retrospective cohort design allowed us to collect a thorough set of clinical covariates, we did not collect all variables that could have affected our outcomes of interest. Treatment of subclinical phenotypes was not randomized and occurred in small, likely underpowered subgroups; treatment decisions may have been influenced by several factors that cannot be easily ascertained in a retrospective study.
In summary, we showed that SCI is a relatively common finding at 6 months post-transplant that identifies a high-risk group of kidney recipients for poor subsequent outcomes. Further studies are needed to determine the extent to which SCI and related phenotypes, especially SC-B-TCMR, are merely a biomarker of future rejection and allograft failure, or also identify an opportunity for early intervention to improve long-term outcomes.
ACKNOWLEDGMENTS
The authors are grateful to Paul MacLennan for assistance with data collection and analysis.
Supplementary Material
Footnotes
Published online 26 January, 2021.
M.E.S. conceived and designed the study, performed the research, participated in data analysis, wrote the original draft of the article, and revised subsequent drafts of the article. G.A. performed the research, participated in data analysis, and revised subsequent drafts of the article. M.B., E.K., S.S.R., H.F., R.S.G., V.H.-D.., B.A.J., C.E.K., V.K., S.M., S.O., F.F., and G.T. performed the research and revised subsequent drafts of the article. R.B.M. conceived and designed the study, performed the research, participated in data analysis, and revised subsequent drafts of the article. All authors approved the final version of the article for publication.
The authors declare no conflicts of interest.
This study was supported by K23 DK101690, R01 DK126807 (both to M.E.S.) and Dean’s Impact Funds from UAB School of Medicine (M.E.S. and R.B.M.). M.B., E.K., and S.S.R. were supported by the Kidney Undergraduate Research Experience (KURE) Program (R25 DK115353).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
Portions of these data were originally presented in abstract form at the 27th International Congress of The Transplantation Society; June 30–July 5, 2008; Madrid, Spain as well as the 2018 and 2019 American Transplant Congress; June 2–6, 2018; Seattle, WA and June 1–5, 2019; Boston, MA, respectively.
REFERENCES
- 1.Lodhi SA, Lamb KE, Meier-Kriesche HU. Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. Am J Transplant. 2011; 11:1226–1235 [DOI] [PubMed] [Google Scholar]
- 2.Meier-Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004; 4:378–383 [DOI] [PubMed] [Google Scholar]
- 3.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012; 12:388–399 [DOI] [PubMed] [Google Scholar]
- 4.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009; 9:527–535 [DOI] [PubMed] [Google Scholar]
- 5.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010; 90:68–74 [DOI] [PubMed] [Google Scholar]
- 6.Bosma RJ, Doorenbos CR, Stegeman CA, et al. Predictive performance of renal function equations in renal transplant recipients: an analysis of patient factors in bias. Am J Transplant. 2005; 5:2193–2203 [DOI] [PubMed] [Google Scholar]
- 7.Matas AJ, Fieberg A, Mannon RB, et al. Long-term follow-up of the DeKAF cross-sectional cohort study. Am J Transplant. 2019; 19:1432–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gourishankar S, Leduc R, Connett J, et al. Pathological and clinical characterization of the ‘troubled transplant’: data from the DeKAF study. Am J Transplant. 2010; 10:324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seifert ME, Yanik MV, Feig DI, et al. Subclinical inflammation phenotypes and long-term outcomes after pediatric kidney transplantation. Am J Transplant. 2018; 18:2189–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta R, Cherikh W, Sood P, et al. Kidney allograft surveillance biopsy practices across US transplant centers: a UNOS survey. Clin Transplant. 2017; 31:e12945. [DOI] [PubMed] [Google Scholar]
- 11.Cosio FG, Grande JP, Wadei H, et al. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant. 2005; 5:2464–2472 [DOI] [PubMed] [Google Scholar]
- 12.Orandi BJ, Chow EH, Hsu A, et al. Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant. 2015; 15:489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kee TY, Chapman JR, O’Connell PJ, et al. Treatment of subclinical rejection diagnosed by protocol biopsy of kidney transplants. Transplantation. 2006; 82:36–42 [DOI] [PubMed] [Google Scholar]
- 14.Nankivell BJ, Chapman JR. The significance of subclinical rejection and the value of protocol biopsies. Am J Transplant. 2006; 6:2006–2012 [DOI] [PubMed] [Google Scholar]
- 15.Lee DM, Abecassis MM, Friedewald JJ, et al. Kidney graft surveillance biopsy utilization and trends: results from a survey of high-volume transplant centers. Transplant Proc. 2020; 52:3085–3089 [DOI] [PubMed] [Google Scholar]
- 16.Mehta R, Bhusal S, Randhawa P, et al. Short-term adverse effects of early subclinical allograft inflammation in kidney transplant recipients with a rapid steroid withdrawal protocol. Am J Transplant. 2020; 52:3085–3089 [DOI] [PubMed] [Google Scholar]
- 17.Nankivell BJ, Agrawal N, Sharma A, et al. The clinical and pathological significance of borderline T cell-mediated rejection. Am J Transplant. 2019; 19:1452–1463 [DOI] [PubMed] [Google Scholar]
- 18.Loupy A, Vernerey D, Tinel C, et al. Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts. J Am Soc Nephrol. 2015; 26:1721–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gloor JM, Cohen AJ, Lager DJ, et al. Subclinical rejection in tacrolimus-treated renal transplant recipients. Transplantation. 2002; 73:1965–1968 [DOI] [PubMed] [Google Scholar]
- 20.Heilman RL, Devarapalli Y, Chakkera HA, et al. Impact of subclinical inflammation on the development of interstitial fibrosis and tubular atrophy in kidney transplant recipients. Am J Transplant. 2010; 10:563–570 [DOI] [PubMed] [Google Scholar]
- 21.Scholten EM, Rowshani AT, Cremers S, et al. Untreated rejection in 6-month protocol biopsies is not associated with fibrosis in serial biopsies or with loss of graft function. J Am Soc Nephrol. 2006; 17:2622–2632 [DOI] [PubMed] [Google Scholar]
- 22.Anil Kumar MS, Khan S, Ranganna K, et al. Long-term outcome of early steroid withdrawal after kidney transplantation in African American recipients monitored by surveillance biopsy. Am J Transplant. 2008; 8:574–585 [DOI] [PubMed] [Google Scholar]
- 23.Anil Kumar MS, Irfan Saeed M, Ranganna K, et al. Comparison of four different immunosuppression protocols without long-term steroid therapy in kidney recipients monitored by surveillance biopsy: five-year outcomes. Transpl Immunol. 2008; 20:32–42 [DOI] [PubMed] [Google Scholar]
- 24.Friedewald JJ, Kurian SM, Heilman RL, et al. ; Clinical Trials in Organ Transplantation 08 (CTOT-08). Development and clinical validity of a novel blood-based molecular biomarker for subclinical acute rejection following kidney transplant. Am J Transplant. 2019; 19:98–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman W, Mehta R, Jorgensen DR, et al. The impact of early clinical and subclinical T cell-mediated rejection after kidney transplantation. Transplantation. 2019; 103:1457–1467 [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed EF, Rao P, Zhang Z, et al. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. Am J Transplant. 2013; 13:1859–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tait BD, Süsal C, Gebel HM, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013; 95:19–47 [DOI] [PubMed] [Google Scholar]
- 29.Haas M, Sis B, Racusen LC, et al. ; Banff Meeting Report Writing Committee. Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014; 14:272–283 [DOI] [PubMed] [Google Scholar]
- 30.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008; 8:753–760 [DOI] [PubMed] [Google Scholar]
- 31.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018; 18:293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McRae M, Bouchard-Boivin F, Béland S, et al. Impact of the current versus the previous diagnostic threshold on the outcome of patients with borderline changes suspicious for T cell-mediated rejection diagnosed on indication biopsies. Transplantation. 2018; 102:2120–2125 [DOI] [PubMed] [Google Scholar]
- 33.Loupy A, Haas M, Roufosse C, et al. The Banff 2019 Kidney Meeting Report (I): updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am J Transplant. 2020; 20:2318–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannon RB, Matas AJ, Grande J, et al. ; DeKAF Investigators. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. Am J Transplant. 2010; 10:2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faddoul G, Nadkarni GN, Bridges ND, et al. ; CTOT-17 Consortium. Analysis of biomarkers within the initial 2 years posttransplant and 5-year kidney transplant outcomes: results from clinical trials in organ transplantation-17. Transplantation. 2018; 102:673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mengel M, Chang J, Kayser D, et al. The molecular phenotype of 6-week protocol biopsies from human renal allografts: reflections of prior injury but not future course. Am J Transplant. 2011; 11:708–718 [DOI] [PubMed] [Google Scholar]
- 37.Nankivell BJ, P’Ng CH, Chapman JR. Does tubulitis without interstitial inflammation represent borderline acute T cell mediated rejection? Am J Transplant. 2019; 19:132–144 [DOI] [PubMed] [Google Scholar]
- 38.Jackson JA, Kim EJ, Begley B, et al. Urinary chemokines CXCL9 and CXCL10 are noninvasive markers of renal allograft rejection and BK viral infection. Am J Transplant. 2011; 11:2228–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suthanthiran M, Schwartz JE, Ding R, et al. ; Clinical Trials in Organ Transplantation 04 (CTOT-04) Study Investigators. Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N Engl J Med. 2013; 369:20–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreso F, Ibernon M, Gomà M, et al. Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am J Transplant. 2006; 6:747–752 [DOI] [PubMed] [Google Scholar]
- 41.Matas AJ, Helgeson ES, Gaston R, et al. Inflammation in areas of fibrosis: the DeKAF prospective cohort. Am J Transplant. 2020; 20:2509–2521 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


