Supplemental Digital Content is available in the text
Keywords: beta-blockers, prognosis, secondary prevention, ST-elevation myocardial infarction
Abstract
The use of beta-blockers (BB) in the context of ST-segment elevation myocardial infarction (STEMI) was a universal practice in the pre-reperfusion era. Since then, evidence of their use for secondary prevention after STEMI is scarce. Our aim is to determine treatment results associated with BB therapy after a STEMI at 1-year follow-up in a contemporary nationwide cohort.
A prospective analysis involving 49 national centers, including patients admitted with STEMI, enrolled between October 2010 and September 2019 was conducted. The primary outcome was defined as the composite of all-cause mortality or hospital re-admission for a cardiovascular (CV) cause in the first year after STEMI. The patients were distributed into 2 groups, depending on whether they received therapy with BB at hospital discharge or not (BB and NB group, respectively).
A total of 3145 patients were included in the analysis, of which 2526 (80.3%) in the BB group. A total of 12.2% of patients reached the primary outcome. Regarding the univariate Cox regression analysis, the BB group presented lower mortality or re-admission for CV cause at 1-year follow-up [hazard ratio (HR) 0.69, confidence interval (CI) 95% 0.55–0.87, P = .001]. However, after adjustment for significant covariates, this association was lost (HR 0.73, CI 95% 0.51–1.04, P = .081). In patients with preserved (HR 0.73, CI 95% 0.51–1.04, P = .081) and mid-range (HR 1.01, CI 95% 0.64–1.61, P = .959) left ventricular ejection fraction (LVEF), the primary outcome was similar between the 2 groups, while in patients with reduced LVEF, the BB group presented a better prognosis, with fewer patients reaching the primary outcome (HR 0.431, CI 95% 0.262–0.703, P = .001).
BB universal therapy after STEMI has not proved useful, but it seems to be beneficial in patients with reduced LVEF.
1. Introduction
The use of beta-blockers (BB) in the context of ST-segment elevation myocardial infarction (STEMI) was consolidated in the 1980s, after its use was associated with lower mortality, cardiac arrest, and re-infarction.[1–4] However, in the reperfusion era, evidence of BB in the postacute myocardial infarction (AMI) context is scarce. In the main multicentre randomized trials performed, such as CAPRICORN[5] and COMMIT,[6] about half of the patients received reperfusion therapy and in the case of CAPRICORN, only patients with left ventricular dysfunction were included, so the results cannot be universally applied.
There is solid evidence showing that in the context of left ventricular dysfunction after AMI, BB reduce the frequency of all-cause and cardiovascular (CV) mortality and recurrent AMI.[5] However, little is known about the usefulness of BB in patients with preserved left ventricular ejection fraction (LVEF), who receive immediate reperfusion and revascularization, treated with evidence-based therapies, such as potent dual antiplatelet therapy, high-dose angiotensin-converting-enzyme inhibitor (ACEi), or angiotensin II receptor blockers (ARB) and effective statins. Some studies have suggested that the post-AMI BB prescription in patients with preserved LVEF did not lead to increased survival.[7–9] However, the current guidelines of the European Society of Cardiology (ESC) for the management of STEMI still state that BB should be considered (class of recommendation IIa) intravenously at the time of presentation in patients undergoing primary percutaneous coronary intervention (PCI) without contraindications, as well as routine oral treatment during hospital stay and continuance thereafter.[10]
The aim was to determine whether the use of BB at discharge after STEMI would translate into better outcomes at 1-year follow-up in a contemporary cohort.
2. Methods
2.1. Data source
The Portuguese Registry of Acute Coronary Syndromes (ProACS) is a continuous, prospective observational registry promoted by the Portuguese Society of Cardiology.[11] All cardiology departments of Portuguese hospitals are invited to participate in the registry. The inclusion of patients started in 2002 and continues to the present day. All centers are requested to consecutively include hospitalized patients with the diagnosis of acute coronary syndrome (ACS): unstable angina (UA), non-ST elevation acute myocardial infarction (NSTEMI), and STEMI. The diagnosis of ACS was based on a combination of clinical presentation, electrocardiogram (ECG), and myocardial necrosis biomarkers. The information collected included demographic data, baseline characteristics of the patient, laboratorial progression, clinical progression, therapies administered, percutaneous intervention data, discharge data, and postdischarge follow-up (6-month and 1 year). The data is centralized at the National Center of Data Collection in Cardiology in Coimbra, in a database that includes all reported patients, whose identification remains anonymous. The ProACS has been approved by the Portuguese Data Protection Authority (n° 3140/2010) and is registered on the clinicaltrials.gov platform (NCT 01642329).
Since its creation, a total of 49 centers have participated in the registry, 11 of which are university hospitals, 12 hospitals with cardiac surgery, and 25 with cardiac catheterization laboratories. The last data published from the registry in 2018 represented a total of 45,141 registries in 15 years of existence.[11] The diagnosis of hospital discharge was recorded using the International Classification of Diseases, Tenth revision (ICD-10).[12]
2.2. Study population
All patients admitted to ProACS hospitals for STEMI (ICD-10 code I21.0-I21.3)[1] between October 1st 2010 to September 4th 2019, aged 18 years-old or older at the time of admission and discharged under optimal secondary prevention therapy (ie, a combination of BB, ACEi or ARB, statin and antithrombotic therapy) were eligible for the study. We excluded patients with an ACS diagnosis other than STEMI; previously known heart failure (HF) or previous AMI; no information on LVEF during hospitalization; stay <24 hours or death during hospitalization; no information on follow-up postdischarge and no information on the use of BB after discharge (Fig. 1).
Figure 1.
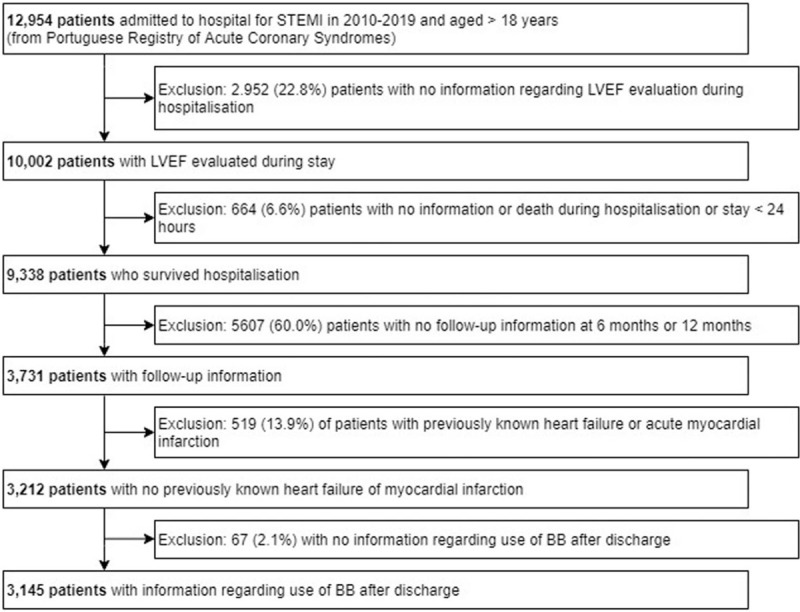
Patient selection flowchart. BB = beta-blocker, LVEF = left ventricular ejection fraction, STEMI = ST-elevation myocardial infarction, LVEF = left ventricular ejection fraction, BB = beta-blocker.
2.3. Data analysis
The patients were divided into 2 groups: patients discharged with BB (BB group) and patients discharged without BB (NB group). LVEF groups were defined in accordance with the most recent ESC HF guidelines.[13]
Three outcomes were evaluated: the primary outcome, composite of all-cause mortality or hospital re-admission for a CV cause; and 2 separate secondary outcomes,
-
(1)
all-cause mortality and
-
(2)
hospital re-admission for a cardiovascular (CV) cause.
Patients were followed up until one year after hospital discharge and events were recorded until that time. Re-admission for a CV cause was defined as re-admission with any of the following diagnoses: supraventricular or ventricular arrhythmias, HF, ACS, stroke, recurrence of angina, or need for urgent revascularization during the follow-up period. All patients included in the analysis have survived hospitalization, and all completed 1 year of follow-up. Patients were only censured if an event, death, or hospital admission (the first of the 2) occurred during the 1-year follow-up period. The follow-up period started after hospital discharge.
2.4. Statistical analysis
Both the global population and the 2 groups were characterized regarding categorical variables using absolute frequencies and relative frequencies; for continuous variables, the central tendency of the data was characterized by sample mean or median; for this type of variables, the data dispersion was characterized by the standard deviation, in the case of the sample mean, or by the interquartile range (IQR), in the case of median. The normal distribution was evaluated from histograms, P-P plots, and Kolmogorov-Smirnov test.
Since there was an interest in assessing the impact of BB's administration at the time of discharge until the occurrence of adverse events after 1 year, only those patients who were discharged or transferred and who were followed-up at 1 year were selected. The time for the occurrence of adverse events within 1 year has been set from the date of admission.
A comparison was made between 2 groups: those discharged with BB (BB group) and those discharged without BB (NB group). For the categorical variables, the comparison was made using the chi-square test or Fisher exact test, when the assumptions of the former were not met. The continuous variables were compared concerning the means using the T test for independent samples and regarding the medians using the nonparametric Mann-Whitney test. The survival functions of the 2 groups to be compared were estimated using the Kaplan-Meier method and represented in the form of Kaplan-Meier curves. The survival functions of the 2 groups were compared using the Log Rank test, for the 3 endpoints at 1 year: combined all-cause mortality or CV re-admission, CV re-admission, and all-cause mortality. To know if LVEF was a modifier of the effect of medication at discharge with BB, the analyses were repeated with Kaplan-Meier and with Log Rank test separately for LVEF ≥50%, LVEF between 40% and 49%, and LVEF < 40%. Additionally, for each of the 3 endpoints, the Cox regression models were adjusted, first including only the BB variable at discharge (univariable model); then including BB, LVEF categorized into 3 categories, and the interaction term between these 2 variables (a way of testing whether the BB effect at discharge is different according to the LVEF category). Finally, the multivariable Cox regression models were adjusted for potential confounders (demographic, electrocardiographic, presentation, multivessel disease, and stenosis ≥50%, among others). In the Cox model, the Wald test was considered, the stepwise forward variable selection method with the likelihood ratio test and the effect of the variables was evaluated estimating HR and its confidence interval.
All statistical analysis was performed at the 5% significance level. In the case of the separate analysis for the 3 groups of LVEF, the log-rank test was applied to a significance level adjusted by the Bonferroni method, 5%/3 = 1.7%. The analysis was conducted using IBM SPSS Statistics, software, version 26.0. The authors had full access to the data and take full responsibility for its integrity.
3. Results
A total of 3145 patients were included in the study (Fig. 2), involving 3145 patient-years. The mean age was 64 ± 14 years, 76.3% were male and 21.1% were overweight. Of all surviving STEMI patients, a total of 80.3% (n = 2526) were discharged with BB.
Figure 2.
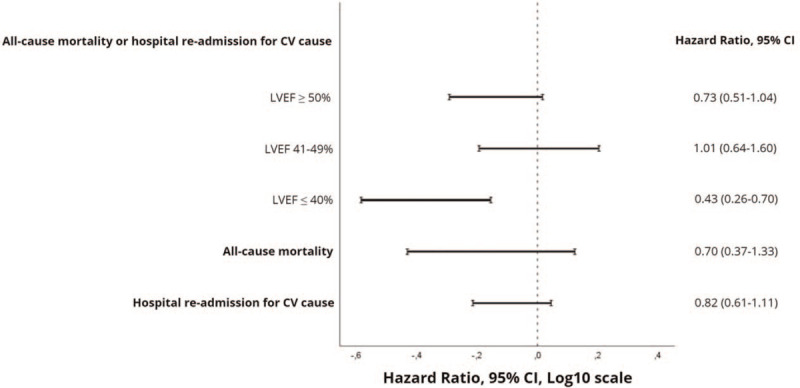
Forest plot showing risk ratios of primary and secondary outcomes in patients discharged with vs without β-blocker. The primary result was stratified according to left ventricular ejection fraction group. CI = confidence interval, CV = cardiovascular, LVEF = left ventricular ejection fraction.
There were baseline differences between the 2 groups of patients (Table 1): The patients in the NB group were older (mean age 68 ± 14 vs 62 ± 14, P < .001), had a lower mean body mass index (26.5 ± 4.4 vs 27.3 ± 4.3, P < .001) and a higher proportion of women (26.7% vs 22.9%, P = .05), compared with patients in the BB group. Regarding the time from symptom onset to the first medical contact, the NB group registered a higher median time [160; IQR 83–381 vs 139; IQR 74–270 minutes, P < .001]. Regarding comorbidities and risk factors, valvular disease (2.0% vs 0.6%, P = .002), peripheral vascular disease (3.7% vs 1.7%, P = .001), chronic kidney failure (3.8% vs 1.6%, P < .001), chronic obstructive pulmonary disease (COPD) (8.4% vs 1.9%, P < .001) and dementia (3.9% vs 1.8%, P = .001) were more common in the NB group. Inferior STEMI was more common in the NB group (65.3% vs 48.7%, P < .001) in contrast with previous STEMI, which was more prevalent in BB group (50.6% vs 33.9%, P < .001). At admission, patients in the NB group had a lower heart rate (73 ± 21 vs 78 ± 19, P < .001) with a much higher number of patients with mean heart rate <60 bpm (26.5% vs 11.7%, <.001), as well as a lower mean systolic blood pressure (130 ± 30 vs 139 ± 29, P < .001) and a higher number of patients with systolic blood pressure <90 mm Hg (8.1% vs 3.0%, P < .001). Patients in the same group were more commonly admitted with cardiogenic shock (3.6% vs 1.4%, P < .001) and had commonly more left bundle branch block (2.1% vs 1.1%, P = .04) and right bundle branch block (7.0% vs 3.9%, P < .001).
Table 1.
Characteristics of the study population.
| Variable | All patients (n = 3145) | B-blocker group (n = 2526) | No β-blocker group (n = 619) | P value |
| Demographics | ||||
| Male gender | 2401 (76.3%) | 1947 (77.1%) | 454 (73.3%) | .05 |
| Age (yrs, SD) | 64 ± 14 | 62 ± 14 | 68 ± 14 | <.001 |
| BMI (Kg/m2. SD) | 27.1 ± 4.3 | 27.3 ± 4.3 | 26.5 ± 4.4 | <.001 |
| BMI ≥30 | 575 (21.1%) | 479 (22.0%) | 96 (17.7%) | .03 |
| Medical history | ||||
| Active Smoker | 1218 (38.8%) | 985 (39.1%) | 233 (37.6%) | .52 |
| Arterial hypertension | 1856 (60.0%) | 1502 (60.4%) | 354 (58.3%) | .34 |
| Diabetes Mellitus | 687 (22.3%) | 547 (22.1%) | 140 (23.2%) | .56 |
| Dyslipidemia | 1474 (49.3%) | 1203 (49.9%) | 271 (47.1%) | .24 |
| Family history of CAD | 240 (8.3%) | 208 (8.9%) | 32 (5.8%) | .02 |
| Valvular heart disease | 28 (0.9%) | 16 (0.6%) | 12 (2.0%) | .002 |
| Previous stroke/TIA | 168 (5.3%) | 128 (5.1%) | 40 (6.5%) | .17 |
| Peripheral artery disease | 65 (2.1%) | 42 (1.7%) | 23 (3.7%) | .001 |
| Chronic kidney disease | 63 (2.0%) | 40 (1.6%) | 23 (3.8%) | <.001 |
| Neoplasia | 125 (4.0%) | 94 (3.8%) | 31 (5.1%) | .12 |
| COPD | 98 (3.2%) | 47 (1.9%) | 51 (8.4%) | <.001 |
| Clinical and analytical data at admission | ||||
| Heart rate (bpm, SD) | 77 ± 19 | 78 ± 19 | 73 ± 21 | <.001 |
| Systolic blood pressure (mmHg, SD) | 137 ± 29 | 130 ± 30 | 139 ± 29 | <.001 |
| Killip-Kimball class ≥II | 284 (9.1%) | 208 (8.3%) | 76 (12.3%) | .002 |
| Complete bundle branch block | 257 (8.2%) | 174 (6.9%) | 83 (13.5%) | <.001 |
| Haemoglobin (g/dL, SD) | 14.1 ± 1.8 | 14.2 ± 1.8 | 13.8 ± 1.9 | <.001 |
| BNP (pg/mL, IQR) | 131.5 (45–308) | 121 (42–293) | 162.5 (76–397) | <.001 |
| LVEF (%, SD) | 54 ± 12 | 53 ± 12 | 55 ± 12 | .01 |
Regarding analytical variables, both hemoglobin at admission (13.8 ± 1.9 vs 14.2 ± 1.8 g/dL, P < .001) and minimum hemoglobin during stay (12.3 ± 2.0 vs 12.9 ± 1.8 g/dL, P < .001) were lower in the NB group, in contrast to median brain natriuretic peptides levels which were higher (162.5; IQR 76–397 vs 121; IQR 42–293 pg/mL, P < .001). The same was observed with total serum cholesterol (181 ± 42 vs 192 ± 45 mg/dL, P < .001) and low-density lipoprotein cholesterol (113 ± 37 vs 125 ± 40 mg/dL, P < .001).
When previous medication use was considered, there were no significant differences between groups, except for the previous use of BB (6.7% vs 9.6%, P = .02) and the previous use of aspirin (10.3% vs 13.4%, P = .03) which were less common in the NB group. Drug use during stay was also different between groups (Table 2). Unfractionated heparin was used less often in the NB group (23.8% vs 34.4%, P < .001), as well as BB (34.0% vs 93.3%, P < .001), ACEi (77.3% vs 88.6%, P < .001) and statins (95.8% vs 97.5%, P = .03). On the other side, ivabradine (8.6% vs 2.9%, P < .001), diuretics (31.6% vs 21.6%, P < .001), amiodarone (9.6% vs 6.4%, P = .01) and other antiarrhythmic drugs (2.3% vs 0.7%, P < .001) were used more commonly in the NB group, as well as inotropic agents (9.9% vs 3.5%, P < .001). Regarding medication at discharge (Table 2), ACEi (67.9% vs. 83.6%, P < .001) and statins (93.5% vs 97.6%, P < .001) were less commonly prescribed in the NB group, in contrast to calcium-channel blockers (6.6% vs 4.5%, P = .03), ivabradine (11.1% vs 2.9%, P < .001), diuretics (26.0% vs 19.4%, P < .001) and amiodarone (6.6% vs 2.5%, P < .001) which were more commonly prescribed at discharge in the same group.
Table 2.
Cardiovascular drug use during hospital stay and at discharge.
| Variable | All patients (n = 3145) | B-blocker group (n = 2526) | No β-blocker group (n = 619) | P value |
| In-hospital | ||||
| Antiplatelet agent | 3110 (98.9%) | 2497 (98.9%) | 613 (99.0%) | .76 |
| Unfractionated heparin | 1013 (32.3%) | 866 (34.4%) | 147 (23.8%) | <.001 |
| Enoxaparin | 1519 (48.4%) | 1208 (47.9%) | 311 (50.3%) | .29 |
| Fondaparinux | 257 (8.2%) | 178 (7.1%) | 79 (12.8%) | <.001 |
| B-blocker | 2564 (81.7%) | 2354 (93.3%) | 210 (34.0%) | <.001 |
| ACEi | 2713 (87.6%) | 2235 (88.6%) | 478 (77.3%) | <.001 |
| ARB | 53 (1.7%) | 44 (1.8%) | 9 (1.5%) | .61 |
| Statin | 3054 (97.1%) | 2461 (97.5%) | 593 (95.8%) | .03 |
| Ivabradine | 125 (4.0%) | 72 (2.9%) | 53 (8.6%) | <.001 |
| MRA | 370 (11.8%) | 284 (11.3%) | 86 (13.9%) | .07 |
| Diuretic | 739 (23.6%) | 544 (21.6%) | 195 (31.6%) | <.001 |
| Amiodarone | 219 (7.0%) | 160 (6.4%) | 59 (9.6%) | .01 |
| Inotropic or vasopressor | 149 (4.8%) | 88 (3.5%) | 61 (9.9%) | <.001 |
| At discharge | ||||
| Aspirin | 3058 (97.2%) | 2479 (98.1%) | 579 (93.5%) | <.001 |
| Clopidogrel | 2598 (82.7%) | 2122 (84.2%) | 476 (76.9%) | <.001 |
| B-blocker | 2526 (80.3%) | 2526 (100.0%) | 0 (0.0%) | <.001 |
| ACEi | 2529 (80.5%) | 2109 (83.6%) | 420 (67.9%) | <.001 |
| ARB | 169 (5.4%) | 141 (5.6%) | 28 (4.5%) | 0.29 |
| Statin | 3043 (96.8%) | 2464 (97.6%) | 579 (93.5%) | <.001 |
| CCB | 154 (4.9%) | 113 (4.5%) | 41 (6.6%) | 0.03 |
| Ivabradine | 143 (4.6%) | 74 (2.9%) | 69 (11.1%) | <.001 |
| MRA | 318 (10.1%) | 253 (10.0%) | 65 (10.5%) | 0.74 |
| Diuretic | 649 (20.7%) | 488 (19.4%) | 161 (26.0%) | <.001 |
| Amiodarone | 104 (3.3%) | 63 (2.5%) | 41 (6.6%) | <.001 |
The information regarding the characteristics of revascularization therapy is presented in Table 3. A total of 81.4% (n = 2561) patients underwent revascularization therapy with primary PCI performed in 91.6% (n = 2345) and fibrinolysis in 8.4% (n = 216). There were no differences in median time from symptom onset to reperfusion between groups (249.5; IQR 171–369 vs 245; IQR 172–386 minutes, P = .67). In contrast, the median door-to-balloon time was inferior in the NB group (43; IQR 18–113 vs 65; IQR 21–142 minutes, P < .001). Left main >50% stenosis was more common in the NB group (3.6% vs 2.0%, P = .04) as well as right coronary artery >50% stenosis (65.1% vs 55.3%, P < .001). Conversely, left anterior descending artery >50% stenosis was more common in the BB group (68.7% vs 52.1%, P < .001). Multivessel disease (2 or 3 vessels with >50% stenosis) was more common in the BB group (44.6% vs 38.1%, P = .01). The left anterior descending artery was the most common culprit artery in the BB group (49.0% vs 31.9%, P < .001) in contrast to the NB group, where right coronary artery was the dominant culprit artery (50.6% vs 35.8%, P < .001). Coronary angioplasty was more commonly performed in the BB group (89.3% vs 77.9%, P < .001).
Table 3.
Reperfusion therapy and coronary angiography characteristics.
| Variable | All patients (n = 3145) | B-blocker group (n = 2526) | No β-blocker group (n = 619) | P value |
| Reperfusion therapy | 2561 (81.4%) | 2105 (83.3%) | 456 (73.7%) | <.001 |
| Fibrinolysis | 216 (8.4%) | 173 (8.2%) | 43 (9.4%) | .39 |
| Primary PCI | 2345 (91.6%) | 1932 (91.8%) | 413 (90.6%) | .39 |
| Pre-hospital fibrinolysis | 15 (6.9%) | 9 (5.2%) | 6 (14.0%) | .09 |
| Access to emergency angioplasty | ||||
| Admission in hospital without catheterization laboratory | 1239 (40.6%) | 1031 (42.0%) | 208 (34.6%) | <.001 |
| Time from hospital admission to balloon inflation (minutes, IQR) | 60 (20–139) | 65 (21–142) | 43 (18–113) | <.001 |
| Time from hospital admission to balloon inflation ≥90 minutes | 890 (38.5%) | 773 (40.6%) | 117 (29.0%) | <.001 |
| Stenosis ≥50% | ||||
| LM | 56 (2.3%) | 39 (2.0%) | 17 (3.6%) | .04 |
| LAD | 1811 (65.6%) | 1546 (68.7%) | 265 (52.1%) | <.001 |
| Cx | 943 (36.1%) | 774 (36.8%) | 169 (33.3%) | .14 |
| RCA | 1549 (57.1%) | 1213 (55.3%) | 336 (65.1%) | <.001 |
| N° of vessels with stenosis ≥50% | ||||
| 1 vessel | 1427 (58.2%) | 1137 (57.8%) | 290 (59.9%) | .39 |
| 2 vessels | 620 (25.3%) | 511 (26.0%) | 109 (22.5%) | .12 |
| 3 vessels | 350 (14.3%) | 285 (14.5%) | 65 (13.4%) | .55 |
| Multivessel disease | 1133 (43.3%) | 942 (44.6%) | 191 (38.1%) | .01 |
| Culprit artery | ||||
| LM | 7 (0.3%) | 5 (0.2%) | 2 (0.4%) | .62 |
| LAD | 1227 (45.8%) | 1068 (49.0%) | 159 (31.9%) | <.001 |
| Cx | 338 (12.6%) | 271 (12.4%) | 67 (13.5%) | .53 |
| RCA | 1032 (38.5%) | 780 (35.8%) | 252 (50.6%) | <.001 |
| Not identified | 68 (2.5%) | 51 (2.3%) | 17 (3.4%) | .17 |
| Coronary Angioplasty | 2736 (87.1%) | 2254 (89.3%) | 482 (77.9%) | <.001 |
The need for noninvasive ventilation was more frequent in the NB group (2.7% vs 0.5%, P < .001), as well as need for temporary transvenous pacemaker (8.6% vs 1.6%, P < .001). However, the need for invasive ventilation was not different between groups (2.3% vs 2.0%, P = .66). Most of the adverse events considered during hospitalization were more frequent in the NB group. HF was significantly more common (20.1% vs 9.8%, P < .001), as well as shock (7.3% vs 1.7%, P < .001), atrial fibrillation (8.4% vs 4.1%, P < .001), atrioventricular (AV) block (12.1% vs 2.3%, P < .001), stroke (1.3% vs 0.4%, P = .01) and major bleeding (3.9% vs 1.0%, P < .001). However, the incidence of cardiac arrest was not different between groups (4.9% vs 4.5%, P = .68). Detailed information on other interventions and complications during hospital stay is described in Table 4.
Table 4.
Other interventions and complications during hospital stay.
| Variable | All patients (n = 3145) | B-blocker group (n = 2526) | No β-blocker group (n = 619) | P value |
| Invasive mechanical ventilation | 64 (2.0%) | 50 (2.0%) | 14 (2.3%) | .66 |
| Non-invasive mechanical ventilation | 30 (1.0%) | 13 (0.5%) | 17 (2.7%) | <.001 |
| Temporary transvenous pacemaker | 93 (3.0%) | 40 (1.6%) | 53 (8.6%) | <.001 |
| Reinfarction | 26 (0.8%) | 20 (0.8%) | 6 (1.0%) | .66 |
| Heart failure | 372 (11.8%) | 248 (9.8%) | 124 (20.1%) | <.001 |
| Shock | 87 (2.8%) | 42 (1.7%) | 45 (7.3%) | <.001 |
| Atrial fibrillation | 156 (5.0%) | 104 (4.1%) | 52 (8.4%) | <.001 |
| AMI-related mechanical complication | 6 (0.2%) | 2 (0.1%) | 4 (0.6%) | .02 |
| AV block | 133 (4.2%) | 58 (2.3%) | 75 (12.1%) | <.001 |
| Sustained VT | 66 (2.1%) | 47 (1.9%) | 19 (3.1%) | .06 |
| Aborted cardiac arrest | 143 (4.5%) | 113 (4.5%) | 30 (4.9%) | .68 |
| Stroke | 18 (0.6%) | 10 (0.4%) | 8 (1.3%) | .01 |
| Major bleeding | 49 (1.6%) | 25 (1.0%) | 24 (3.9%) | <.001 |
The mean LVEF was slightly higher in the NB group (55 ± 12% vs 53 ± 12%, P = .01). There was no significant difference between groups concerning preserved LVEF (66.1% vs 64.2%, P = .37), mildly reduced LVEF (22.9% vs 22.8%, P = .96), moderately reduced LVEF (8.9% vs 10.5%, P = .24) and severely reduced LVEF (2.1% vs 2.5%, P = .57).
3.1. Long-term outcomes
Our primary outcome, the cumulative incidence of the composite of all-cause mortality or hospital re-admission for a CV cause, was recorded in 12.2% of patients, which means a total of 1 death or re-hospitalization for every 8 patient-years in the study. The unadjusted 1-year all-cause mortality or re-admission for a CV cause was lower in the BB group (HR 0.690, CI 95% 0.550–0.865, P = .001). However, this finding was not consistent after a multivariate analysis (HR 0.731, CI 95% 0.510–1.043, P = .08). As a significant interaction between LVEF and BB was found, the impact of the use of BB on LVEF stratified discharge interval was plotted. The use of BB did not result in a reduced primary outcome rate in patients with LVEF >50% (HR 0.731, CI 95% 0.510–1.043, P = .08) or in patients with an LVEF between 41% and 49% (HR 1.012, CI 95% 0.644–1.609, P = .96). However, in patients with a LVEF ≤40%, the use of BB was associated with a reduced incidence of the primary endpoint of all-cause mortality or re-hospitalization at 1 year (HR 0.431, CI 95% 0.262–0.703, P = .001). The results are shown in Figure 2. Univariate and multivariate analyses are depicted in Table 5 and the Kaplan-Meier curves stratified by the LVEF group can be seen in Figure 3.
Table 5.
Cox survival analysis of the STEMI cohort (primary endpoint: composite of all-cause mortality or hospital re-admission for a CV cause at 1 year).
| Univariate model | Multivariate model | |||
| Variable | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value |
| BB at discharge | 0.690 (0.550–0.865) | .001 | 0.73 (0.51–1.05) | .08 |
| LVEF 40%–49% (vs LVEF ≥50%) | 1.20 (0.73–1.98) | .48 | ||
| LVEF <40% (vs LVEF ≥50%) | 2.29 (1.37–3.83) | .002 | ||
| Valvular heart disease | 3.579 (1.909–6.707) | <.001 | 3.16 (1.61–6.21) | .001 |
| Neoplasia | 2.465 (1.725–3.523) | <.001 | 2.07 (1.41–3.04) | <.001 |
| Heart rate > 100 bpm | 1.781 (1.234–2.325) | <.001 | 1.45 (1.08–1.94) | .01 |
| Killip-Kimball class at admission ≥II | 2.475 (1.918–3.194) | <.001 | 1.64 (1.21–2.22) | .002 |
| Complete right bundle branch block | 2.018 (1.412–2.884) | <.001 | 1.62 (1.10–2.38) | .01 |
| Nitrate at discharge | 2.106 (1.630–2.721) | <.001 | 1.55 (1.15–2.09) | .004 |
| Diuretic at discharge | 2.835 (2.312–3.476) | <.001 | 1.57 (1.21–2.04) | .001 |
| Multivessel disease | 2.021 (1.583–2.493) | <.001 | 0.57 (0.39–0.84) | .004 |
| Stroke during stay | 3.324 (1.484–7.447) | .002 | 2.78 (1.20–6.46) | .02 |
| Mechanical complication during stay | 5.134 (3.256–7.378) | 3.90 (1.40–10.88) | .009 | |
| Other variables | ||||
| Female gender (vs male) | 1.421 (1.143–1.767) | .001 | ||
| Arterial hypertension | 1.274 (1.030–1.576) | .03 | ||
| Diabetes | 1.325 (1.058–1.659) | .01 | ||
| Previous stroke/TIA | 1.821 (1.292–2.566) | <.001 | ||
| Chest pain as predominant symptom | 0.432 (0.310–0.602) | <.001 | ||
| Sinus rhythm | 0.614 (0.438–0.862) | <.001 | ||
| Normal duration QRS | 0.557 (0.416–0.747) | <.001 | ||
| Atrial fibrillation | 1.693 (1.159–2.472) | .01 | ||
| Haemoglobin at admission, per g/L | 0.861 (0.816–0.908) | <.001 | ||
| Diuretic during stay | 2.962 (2.422–3.622) | <.001 | ||
| Amiodarone during stay | 1.769 (1.293–2.422) | <.001 | ||
| Inotropic during stay | 2.394 (1.712–3.347) | <.001 | ||
| Aspirin at discharge | 0.444 (0.286–0.690) | <.001 | ||
| Statin at discharge | 0.581 (0.366–0.921) | .02 | ||
| Amiodarone at discharge | 2.024 (1.328–3.085) | <.001 | ||
| Re-infarction during stay | 2.235 (1.058–4.720) | .03 | ||
| HF during stay | 2.442 (1.932–3.087) | <.001 | ||
Figure 3.
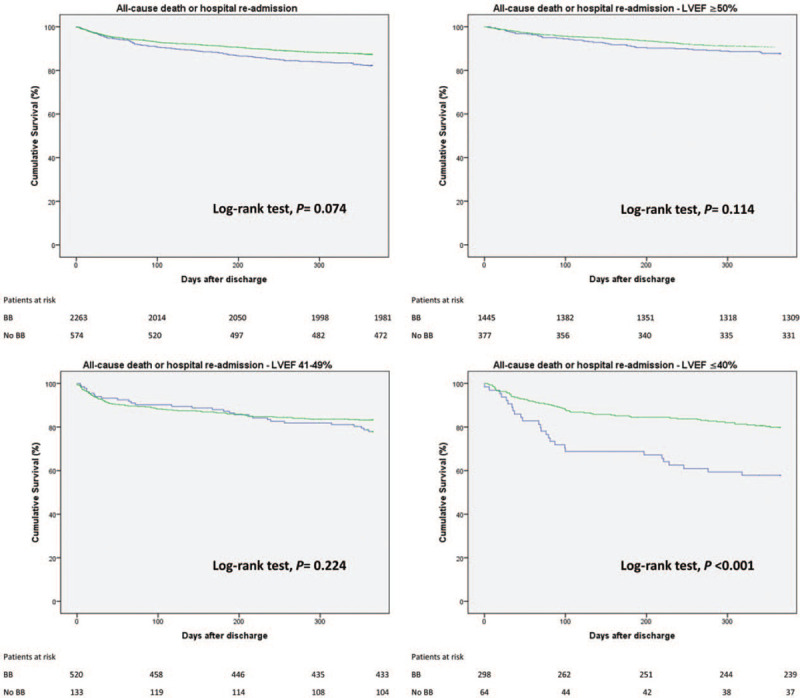
Kaplan-Meier curves depict cumulative survival free of the primary composite outcome of all-cause mortality or hospital re-admission in patients discharged with or without β-blocker. Analysis was stratified according to left ventricular ejection fraction group. The green curve represents the BB group; The blue curve, the NB group.
Regarding all-cause mortality, a total of 4.6% patients died within 1 year, resulting in a total of 1 death for every 22 patient-years. The unadjusted 1-year mortality rate was lower in the BB group (HR 0.499, CI 95% 0.353–0.705, P < .001). After adjustment for other variables described, BB at discharge did not lead to a significant reduction in 1-year mortality (HR 0.70, CI 95% 0.37–1.33, P = .28).
Regarding re-hospitalization for CV cause, the cumulative incidence of the outcome was 9.3%, which means a total of 1 re-hospitalization for every 11 patient-years. The unadjusted 1-year re-admission was tendentially lower in the BB group (HR 0.783, CI 95% 0.599–1.024, P = .07). After adjustment for other variables, BB at discharge was not significantly associated with reduced re-admissions within 1 year (HR 0.82, CI 95% 0.61–1.11, P = .20).
As patients in the NB group had a worse clinical profile than patients in the BB group, we performed a multivariable analysis, adjusted for specific clinically relevant variables that were significantly different between groups (age, valvular disease, previous peripheral vascular disease, chronic kidney disease, COPD, inferior STEMI, Killip-Kimball class >1, HF during hospitalization, shock during hospitalization, atrial fibrillation, mechanical complication, and AV block). This adjusted analysis showed no reduction in the primary outcome of death or re-hospitalization at 1 year (HR 0.80, 95% CI 0.63–1.02, P = .06). When analysis was stratified by LVEF, the use of BB was not associated with reduced mortality or re-hospitalization at 1 year in patients with LVEF ≥50% (HR 0.822, 95% CI 0.583–1.158, P = .26) and LVEF between 41 and 49% (HR 0.893, 95% CI 0.567–1.407, P = .63). However, in patients with LVEF ≤40%, the use of BB was associated with reduced mortality or re-hospitalization at 1 year (HR 0.444, 95% CI 0.278–0.710, P = .001), in line with previous results of the main analysis.
3.2. Patients with reduced LVEF
Patients discharged without BB were older (mean age 71 ± 14 vs 65 ± 14 years, P = .001), had a higher prevalence of chronic kidney disease (7.8% vs 1.6%, P = .01) and had a higher admission serum creatinine (1.2 ± 0.5 vs 1.1 ± 1.2 mg/dL, P = .001), were less frequently treated with a BB (47.1% vs 94.2%, P < .001) or an ACEi/ARB (66.2% vs 92.3%, P < .001) during the index admission and were more frequently given diuretics (74.6% vs 59.0%, P = .02) and inotropic agents (25.4% vs 8.4%, P < .001) during the hospitalization. Additionally, these patients were also less frequently submitted to revascularization (63.2% vs 75.6%, P = 0.03). Also, the complications during the admission were more frequent in the group discharged without a BB, such as acute HF (45.6% vs 24.7%, P < .001), shock (25.0% vs 4.9%, P < .001), and complete AV block (8.8% vs 2.7%, P = .03) (see Table S1, Supplemental Content, which shows in detail univariate analysis of patients with reduced LVEF).
In order to probe for an indication bias in this group of patients, we performed further analyses. Adjusted Cox regression models showed that in this group of patients the prescription of a BB at discharge was still associated with a lower hazard of all-cause death/hospital re-admission at 1 year of follow-up (HR 0.502, 95% CI 0.310–0.812, P = .01). The same protective effect of BB in these patients was also observed regarding both secondary outcomes: all-cause death (HR 0.431, 95% CI 0.221–0.844, P = .01) and hospital re-admission (HR 0.470, 95% CI 0.259–0.853, P = .01) at 1 year of follow-up (see Table S2, Supplemental Content, which gives a detailed overview of survival analysis of patients with reduced LVEF). We ran a multivariable Fine- Gray regression to assess competing risk of all-cause mortality and hospital re-admission at 1 year of follow-up, which also showed that the use of beta-blocker therapy after discharge was not significantly associated with lower all-cause mortality/hospital re-admission at 1 year of follow-up (HR 0.779, 95% CI 0.546–1.111, P = 0.168) (see Table S3, Supplemental Content, which shows in detail the Multivariate Fine-Gray competing risk regression for primary outcome, with and without interaction with LVEF).
4. Discussion
In this observational study, it was sought to determine whether the use of BB at discharge after a STEMI would lead to better results at 1-year follow-up in a contemporary cohort. It was found that the use of BB after a STEMI was associated with a lower incidence of the composite endpoint of all-cause mortality or 1-year hospital re-admission in patients with LVEF ≤40%, after an adjustment for confounding variables. The findings were consistent with previous studies.[5,14–19] In contrast, in patients with an LVEF >40% no benefit was found with the use of BB after STEMI.
After a STEMI event, BB therapy may exert its beneficial effect by inhibiting over-stimulation of CV β-adrenergic receptors, caused by high levels of catecholamine.[20] Through this mechanism, intracellular levels of cyclic adenosine monophosphate and calcium are lowered, leading to reduced cardiac contractility, systemic arterial pressure and heart rate, key determinants of myocardial oxygen consumption.[21] This reduction in myocardial oxygen demand is the basis of its anti-ischemic activity in areas of myocardium threatened by the interruption of coronary flow.[22] Moreover, by slowing the heart rate and lowering blood pressure left ventricular compliance is improved. With the prolongation of diastole also comes a better perfusion of the ischemic myocardium, particularly in the subendocardium, limiting infarct size.[23,24] In addition, BB may attenuate the increase in endothelial shear, platelet aggregation and blood viscosity, reducing the probability of new coronary plaque rupture or thrombosis.[25,26] BB are also associated with decreased risk of ventricular fibrillation and sudden cardiac death by specific effects that may include lengthening of the effective ventricular refractory period, suppression of triggered activity and automaticity and attenuation of electrophysiological heterogeneity.[27]
In the prefibrinolytic period, BB administration during and after a STEMI event was highly associated with lower mortality, cardiac arrest, and reinfarction.[1–4,28,29] When both fibrinolysis and antiplatelet therapy started to be the mainstay of STEMI treatment, the use of BB in this context led to less convincing benefits. In the landmark TIMI II-B study,[30] the use of metoprolol IV within 2 hours of initiation of the recombinant tissue-type plasminogen activator was not associated with reduced mortality within 1 year. In the large COMMIT trial, IV followed by oral metoprolol was not followed by a reduction in mortality at 4 weeks and was also associated with an increase in cardiogenic shock, especially on the first day after admission.[6]
The current ESC guidelines for the management of STEMI suggest the use of intravenous BB at the time of presentation in patients undergoing primary PCI without contraindications, as well as routine oral treatment during hospital stay and its continuation thereafter.[10] The same recommendation is given by the American College of Cardiology Foundation/American Heart Association STEMI guidelines, which advocate the initiation of oral BB in the first 24 hours and continuation during hospitalization and discharge.[31] However, the evidence on which these guidelines were based is not always clear on the benefit of BB in STEMI. In the METOCARD-CNIC trial,[32] which included 270 previous STEMI patients treated with primary PCI, the primary outcome was the infarct size evaluated by cardiac magnetic resonance imaging (MRI). Very early administration of intravenous metoprolol at the time of diagnosis and oral metoprolol in the first 24 hours reduced infarct size and increased LVEF. However, this strategy did not significantly reduce major adverse cardiovascular events at 2 years. Also, in the EARLY-BAMI trial,[33] 683 patients with STEMI within 12 hours of onset were randomized to either receive intravenous metoprolol or placebo and oral metoprolol thereafter within 12 hours. In this trial, this strategy did not result in reduced infarct size measured by cardiac magnetic resonance or lower levels of release of cardiac biomarkers. Similar findings were reported in the GRACE registry, where early the use of BB in STEMI was associated with increased hospital mortality.[34]
In the case of mid and long-term use of BB, most of the supporting data comes from trials performed in the pre-reperfusion era, as evidenced by the systematic review by Freemantle et al.[35] More recent data has shown inconsistent results, pointing however to a strong interaction with LVEF.[7,36–40]
In fact, there is increasing evidence challenging the usefulness of BB after STEMI. In a 2014 meta-analysis with more than 100,000 patients, it was shown that BB had no benefits in terms of mortality, conferring only a reduction in short-term recurrent myocardial infarction or angina at the expense of HF, cardiogenic shock, and drug discontinuation.[41] These results were also supported by 2 recent studies.[8,9] However, some subgroups may benefit from the use of BB. In 5628 consecutive patients admitted with STEMI and treated with emergency PCI, the use of BB was associated with a lower mortality risk in an analysis of subgroups of high-risk patients, such as those with GRACE score ≥121, symptomatic HF, or LVEF < 40%.
We now live in the era of early reperfusion therapy. In the specific case of Portugal, in 2016, the reperfusion rate in STEMI increased to 84%, mainly through primary PCI.[11] In addition, evidence-based therapies, at discharge followed the increase in primary PCI, with high prescription of dual antiplatelet therapy (88%), ACEi/ARB (85%), and statins (96%). In-hospital mortality after STEMI developed favorably with an incidence of 2.5% in 2016, which corresponds to a reduction of 65% comparing to 2002. The same was observed with 6-month mortality, which was set at 5.2%. This effect can be mostly explained by better compliance with the guidelines and the very high resource to revascularization therapy, mainly primary PCI. However, the time from symptom onset to revascularization still remains high, which is explained by the delays in centers without interventional cardiology, as inter-hospital transport still remains a problem.[11,42]
As in our study the use of BB at discharge did not result in better outcomes in the majority of the STEMI population, unnecessary prescriptions should make us think about the potential side effects of this class of drugs. A negative chronotropic effect is expected with the use of BB, and as such can lead to symptomatic bradycardia or AV block. Acute BB withdrawal can also lead to significant morbidity and even mortality.[43] Other noncardiac side effects can occur with the use of BB, such as increased airway resistance with drugs such as short-acting propanolol.[44] In the recent BLOCK COPD trial, in which moderate to severe patients without cardiovascular indication for the use of BB were randomized to either receive placebo or extended-release metoprolol, patients in the BB arm had a greater risk of severe exacerbation, very severe exacerbation and death and therefore the trial was discontinued.[45] A review published in 2013 stated that in patients with moderate to severe peripheral artery disease, the use of BB was not associated with a reduction of time or distance to claudication or difference in calf blood flow, vascular resistance or skin temperature. However, the trials analyzed were more than 20 years old, were small, and of poor quality.[46] The ESC guidelines on the diagnosis and treatment of peripheral arterial diseases do not contraindicate the use of BB in patients with lower extremity artery disease, but state that they should be used with caution in chronic limb-threatening ischemia.[47] Hyperkalaemia is also a noticeable adverse effect of the use of BB, which is more common when the patient has associated conditions, such as end-stage kidney disease and insulin deficiency.[48] The issues of depression, fatigue, and sexual dysfunction were addressed in a 2002 review of 15 trials involving more than 35,000 patients, which found that although depressive symptoms did not increase significantly, there was a small increase in the risk of fatigue and sexual dysfunction.[49]
As the efficacy of BB in patients with preserved or even mid-range LVEF is questioned, the impact of BB prescription on these patients should be well thought out. There is a need for proper selection of patients with STEMI who would benefit from BB therapy to decrease health care costs, increase patient compliance, and reduce the incidence of BB's side effects. To answer this question, there are 2 major ongoing trials, predominantly including patients without left ventricular dysfunction, REDUCE-SWEDEHEART, and BETAMI, which aim to determine whether long-term treatment with oral BB is associated with clinical benefit.
4.1. Study limitations
Our study has some important limitations. First, our study lacks specific data on BB and dosage, which may affect outcomes, as this class of drugs is heterogeneous.[19,43] Second, how long the use of BB was continued after patient discharge is not known. Previous studies reported an important rate of discontinuation of BB after AMI, ranging from 9.5% to 76% in the first year,[50,51] so the prescription of BB at discharge may not represent a long-term use of BB. Third, the selection bias is a known consequence of the lack of randomization of observational registry data. Even after adjusting our analysis to a multitude of variables that could impact BB selection, the influence of unmeasured variables as prescription bias cannot be excluded. Fourth, adverse events after discharge were confirmed by the principal investigator of each hospital and it was not always possible to determine the concrete cause of death, so we could only select all-cause mortality as one of the endpoints of our study. However, we were able to ensure that re-hospitalization was of cardiovascular cause. Finally, the follow-up was performed for 12 months, which can be a short follow-up period to draw conclusions on the long-term BB benefit after a STEMI.
5. Conclusion
Among patients hospitalized with STEMI, the use of BB was not universally associated with lower all-cause mortality or hospital re-admission at 1-year follow-up. In fact, effectiveness of the use of BB was only observed in patients with reduced LVEF. This result supports the previous literature, according to which the routine prescription of BB might not be beneficial in patients with preserved or mid-ranged LVEF after STEMI.
Acknowledgments
The authors would like to thank all the staff of the catheterization laboratories and clinical research coordinators of all participating centers. We would like to thank Adriana Belo from the Centro Nacional de Coleção de Dados em Cardiologia (CNCDC) for the statistical support.
Author contributions
Conceptualization: João Ferreira, Rui Baptista, Sílvia Monteiro.
Data curation: Rui Baptista, Sílvia Monteiro, Lino Gonçalves.
Investigation: João Ferreira, Rui Baptista, Sílvia Monteiro, Lino Gonçalves.
Methodology: João Ferreira, Sílvia Monteiro.
Project administration: João Ferreira, Sílvia Monteiro, Lino Gonçalves.
Supervision: Rui Baptista, Sílvia Monteiro, Lino Gonçalves.
Writing – original draft: João Ferreira.
Writing – review & editing: João Ferreira, Rui Baptista, Sílvia Monteiro, Lino Gonçalves.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ACEi = angiotensin-converting-enzyme inhibitor, ACS = acute coronary syndrome, AMI = acute myocardial infarction, ARB = angiotensin II receptor blocker, AV = atrioventricular, BB = beta-blockers, CI = confidence interval , COPD = chronic obstructive pulmonary disease, CV = cardiovascular, ECG = electrocardiogram, ESC = European Society of Cardiology , HF = heart failure, HR = hazard ratio, IQR = interquartile range, LVEF = left ventricular ejection fraction, PCI = percutaneous coronary intervention , ProACS = Portuguese Registry of Acute Coronary Syndromes, STEMI = ST-segment elevation myocardial infarction.
How to cite this article: Ferreira JA, Baptista RM, Monteiro SR, Gonçalves LM. Usefulness of universal beta-blocker therapy in patients after ST-elevation myocardial infarction. Medicine. 2021;100:3(e23987).
The authors have no funding and conflicts of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
BMI = body mass index, BNP = basal natriuretic peptide, Bpm = beats per minute, CAD = coronary artery disease, COPD = Chronic obstructive pulmonary disease, IQR = interquartile range, LDL = low-density lipoprotein, LVEF = left ventricular ejection fraction, SD = standard deviation, TIA = transient ischemic attack.
ACEi = angiotensin-converting-enzyme inhibitor, ARB = angiotensin II receptor blockers, CCB = calcium channel blocker, MRA = mineralocorticoid receptor antagonist.
Cx = circumflex artery, IQR = interquartile range, LAD = left anterior descending artery, LM = left main, PCI = percutaneous coronary intervention, RCA = right coronary artery.
AMI = acute myocardial infarction, AV = atrioventricular, VT = ventricular tachycardia.
BB = beta-blocker, HF = heart failure, HF = heart failure, LVEF = left ventricular ejection fraction, RCA = right coronary artery.
References
- [1].Yusuf S, Ramsdale D, Peto R, et al. Early intravenous atenolol treatment in suspected acute myocardial infarction. Preliminary report of a randomised trial. Lancet 1980;2:273–6. [DOI] [PubMed] [Google Scholar]
- [2].Hjalmarson A, Herlitz J, Holmberg S, et al. The Göteborg metoprolol trial. Effects on mortality and morbidity in acute myocardial infarction. Circulation 1983;67(6 Pt 2):I26–32. [PubMed] [Google Scholar]
- [3].Sleight P. Use of beta adrenoceptor blockade during and after acute myocardial infarction. Annu Rev Med 1986;37:415–25. [DOI] [PubMed] [Google Scholar]
- [4].Metoprolol in acute myocardial infarction (MIAMI). A randomised placebo-controlled international trial. The MIAMI Trial Research Group. Eur Heart J 1985;6:199–226. [PubMed] [Google Scholar]
- [5].Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385–90. [DOI] [PubMed] [Google Scholar]
- [6].Chen ZM, Pan HC, Chen YP, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1622–32. [DOI] [PubMed] [Google Scholar]
- [7].Dondo TB, Hall M, West RM, et al. β-blockers and mortality after acute myocardial infarction in patients without heart failure or ventricular dysfunction. J Am Coll Cardiol 2017;69:2710–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hong J, Barry AR. Long-term beta-blocker therapy after myocardial infarction in the reperfusion era: a systematic review. Pharmacotherapy 2018;38:546–54. [DOI] [PubMed] [Google Scholar]
- [9].Goldberger JJ, Bonow RO, Cuffe M, et al. Effect of beta-blocker dose on survival after acute myocardial infarction. J Am Coll Cardiol 2015;66:1431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. [DOI] [PubMed] [Google Scholar]
- [11].Timóteo AT, Mimoso J. em nome dos investigadores do Registo Nacional de Síndromes Coronárias Agudas. Portuguese Registry of Acute Coronary Syndromes (ProACS): 15 years of a continuous and prospective registry. Rev Port Cardiol 2018;37:563–73. [DOI] [PubMed] [Google Scholar]
- [12].World Health Organization. International statistical classification of diseases and related health problems, 10th revision (ICD-10). World Heal Organ 2016;1:332–45. [Google Scholar]
- [13].Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- [14].Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA 2000;283:1295–302. [DOI] [PubMed] [Google Scholar]
- [15].Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651–8. [DOI] [PubMed] [Google Scholar]
- [16].Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002;106:2194–9. [DOI] [PubMed] [Google Scholar]
- [17].The cardiac insufficiency bisoprolol study, II, (CIBIS-II): a randomised, trial. Lancet 1999;353:9–13. [PubMed] [Google Scholar]
- [18].Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215–25. [DOI] [PubMed] [Google Scholar]
- [19].Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003;362:7–13. [DOI] [PubMed] [Google Scholar]
- [20].Zheng M, Zhu W, Han Q, et al. Emerging concepts and therapeutic implications of beta-adrenergic receptor subtype signaling. Pharmacol Ther 2005;108:257–68. [DOI] [PubMed] [Google Scholar]
- [21].Lubbe WF, Podzuweit T, Opie LH. Potential arrhythmogenic role of cyclic adenosine monophosphate (AMP) and cytosolic calcium overload: implications for prophylactic effects of beta-blockers in myocardial infarction and proarrhythmic effects of phosphodiesterase inhibitors. J Am Coll Cardiol 1992;19:1622–33. [DOI] [PubMed] [Google Scholar]
- [22].Kopecky SL. Effect of beta blockers, particularly carvedilol, on reducing the risk of events after acute myocardial infarction. Am J Cardiol 2006;98:1115–9. [DOI] [PubMed] [Google Scholar]
- [23].Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mechanisms and management. Ann Intern Med 1992;117:502–10. [DOI] [PubMed] [Google Scholar]
- [24].Basu S, Senior R, Raval U, et al. Beneficial effects of intravenous and oral carvedilol treatment in acute myocardial infarction. A placebo-controlled, randomized trial. Circulation 1997;96:183–91. [DOI] [PubMed] [Google Scholar]
- [25].Borrello F, Beahan M, Klein L, et al. Reappraisal of beta-blocker therapy in the acute and chronic post-myocardial infarction period. Rev Cardiovasc Med 2003;4: Suppl 3: S13–24. [PubMed] [Google Scholar]
- [26].Frishman WH, Chang CM. Beta-adrenergic blockade in the prevention of myocardial infarction: a new theory. J Hypertens Suppl 1991;9:S31–4. [DOI] [PubMed] [Google Scholar]
- [27].Grandi E, Ripplinger CM. Antiarrhythmic mechanisms of beta blocker therapy. Pharmacol Res 2019;146:104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].A randomized trial of propranolol in patients with acute myocardial, infarction., I., Mortality, results. JAMA 1982;247:1707–14. [DOI] [PubMed] [Google Scholar]
- [29].Norwegian Multicenter Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med 1981;304:801–7. [DOI] [PubMed] [Google Scholar]
- [30].Roberts R, Rogers WJ, Mueller HS, et al. Immediate versus deferred beta-blockade following thrombolytic therapy in patients with acute myocardial infarction. Results of the Thrombolysis in Myocardial Infarction (TIMI) II-B Study. Circulation 1991;83:422–37. [DOI] [PubMed] [Google Scholar]
- [31].O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:e362–425. [DOI] [PubMed] [Google Scholar]
- [32].Ibanez B, Macaya C, Sánchez-Brunete V, et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation 2013;128:1495–503. [DOI] [PubMed] [Google Scholar]
- [33].Roolvink V, Ibanez B, Ottervanger JP, et al. Early intravenous beta-blockers in patients with ST-segment elevation myocardial infarction before primary percutaneous coronary intervention. J Am Coll Cardiol 2016;67:2705–15. [DOI] [PubMed] [Google Scholar]
- [34].Park KL, Goldberg RJ, Anderson FA, et al. Beta-blocker use in ST-segment elevation myocardial infarction in the reperfusion era (GRACE). Am J Med 2014;127:503–11. [DOI] [PubMed] [Google Scholar]
- [35].Freemantle N, Cleland J, Young P, et al. beta Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ 1999;318:1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yang JH, Hahn JY, Song YB, et al. Association of beta-blocker therapy at discharge with clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardiovasc Interv 2014;7:592–601. [DOI] [PubMed] [Google Scholar]
- [37].Huang BT, Huang FY, Zuo ZL, et al. Meta-analysis of relation between oral (-blocker therapy and outcomes in patients with acute myocardial infarction who underwent percutaneous coronary intervention. Am J Cardiol 2015;115:1529–38. [DOI] [PubMed] [Google Scholar]
- [38].Misumida N, Harjai K, Kernis S, et al. Does oral beta-blocker therapy improve long-term survival in st-segment elevation myocardial infarction with preserved systolic function? A meta-analysis. J Cardiovasc Pharmacol Ther 2016;21:280–5. [DOI] [PubMed] [Google Scholar]
- [39].Raposeiras-Roubín S, Abu-Assi E, Redondo-Diéguez A, et al. Prognostic benefit of beta-blockers after acute coronary syndrome with preserved systolic function. still relevant today? Rev Esp Cardiol (Engl Ed) 2015;68:585–91. [DOI] [PubMed] [Google Scholar]
- [40].Puymirat E, Riant E, Aissaoui N, et al. β blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ 2016;354:i4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bangalore S, Makani H, Radford M, et al. Clinical outcomes with β-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med 2014;127:939–53. [DOI] [PubMed] [Google Scholar]
- [42].Pereira H, Pinto FJ, Calé R, et al. The stent for life initiative: factors predicting system delay in patients with st-segment elevation myocardial infarction. Rev Port Cardiol 2018;37:681–90. [DOI] [PubMed] [Google Scholar]
- [43].Krukemyer JJ, Boudoulas H, Binkley PF, et al. Comparison of hypersensitivity to adrenergic stimulation after abrupt withdrawal of propranolol and nadolol: influence of half-life differences. Am Heart J 1990;120:572–9. [DOI] [PubMed] [Google Scholar]
- [44].Beumer HM, Hardonk HJ. Effects of beta-adrenergic blocking drugs on ventilatory function in asthmatics. Eur J Clin Pharmacol 1972;5:77–80. [Google Scholar]
- [45].Dransfield MT, Voelker H, Bhatt SP, et al. Metoprolol for the prevention of acute exacerbations of COPD. N Engl J Med 2019;381:2304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Paravastu SC, Mendonca DA, Da Silva A. Beta blockers for peripheral arterial disease. Cochrane Database Syst Rev 2013;2013:CD005508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763–816. [DOI] [PubMed] [Google Scholar]
- [48].Hawboldt J, McGrath D. Possible metoprolol-induced hyperkalemia. J Pharm Pract 2006;19:320–5. [Google Scholar]
- [49].Ko DT, Hebert PR, Coffey CS, et al. Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 2002;288:351–7. [DOI] [PubMed] [Google Scholar]
- [50].Kramer JM, Hammill B, Anstrom KJ, et al. National evaluation of adherence to beta-blocker therapy for 1 year after acute myocardial infarction in patients with commercial health insurance. Am Heart J 2006;152:454.e1–4548. [DOI] [PubMed] [Google Scholar]
- [51].Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation 2008;117:1028–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


