Abstract
Phaeochromocytomas are catecholamine-producing neuroendocrine tumors that may manifest in many ways, specifically as sustained or paroxysmal hypertension. Data, including data from mental status screening, were prospectively collected from suspected patients. The Hospital Anxiety and Depression Scale was used as a screening tool to identify abnormal mental status. Results showed phaeochromocytoma patients were more likely to experience anxiety and depression. For future phaeochromocytoma treatment, early screening for anxiety and depression should be recommended.
Keywords: anxiety, depression, HADS, phaeochromocytomas
1. Introduction
Phaeochromocytomas are catecholamine-producing neuroendocrine tumors that originate in chromaffin cells in the adrenal medulla or extra-adrenal paraganglia.[1] In the general population, the estimated annual incidence of phaeochromocytoma is 3 per million.[2] However, in the hypertension population, the proportion of phaeochromocytoma is 0.1% to 0.6%.[3] Despite their low prevalence, phaeochromocytomas are associated with a high mortality rate and several complications.[4]
An abnormal mental status has a major influence on cancer patients and could result in the impairment of the immune response, prolonged recovery times, difficulty with symptom control, poor compliance with treatment, and high mortality.[5,6] Many articles have verified that depression and anxiety disorders are more common among individuals affected by other cancers than the general population.[7,8] However, few studies have particularly explored the mental status of phaeochromocytoma patients. The common manifestations of phaeochromocytoma include unpredictable hypertension with an unexpected onset or recurrent events, significantly affecting all domains of patients’ life and resulting in personal, clinical, socioeconomic implications, a diminished quality of life, and more frequent psychological disturbances compared with the general population.
Two reasons may account for the abnormal mental status. First, the extra secretion of catecholamines can cause various clinical symptoms, such as hypertension, light-headedness, syncope, and chronic fatigue. Such “spells” are characteristic manifestations of phaeochromocytomas and start with a sense of shortness of breath, followed by palpitations and a throbbing headache. These “spells” are often accompanied by symptoms of cold peripheries and facial pallor. These symptoms have repercussions for patients’ daily lives and could render patients more vulnerable to experiencing unique psychosocial stressors. Second, catecholamine disruption may cause deficits in the medial frontal cortical regions and amygdala. Dysfunctions in these cortical regions are the basis of anxiety and depression.[5] These symptoms might cause and aggravate patients’ psychological disease. Therefore, it is important to regularly screen and properly manage patients’ psychological disorders.
The objective of this study was to identify the proportion of depression and anxiety in phaeochromocytoma patients. Furthermore, we aimed to explore the factors associated with the risk of depression and anxiety in phaeochromocytoma patients.
2. Methods
2.1. Patients and design
From January 2010 to May 2019, phaeochromocytoma patients were recruited from the department of urological surgery at an academic teaching hospital in China. The academic teaching hospital receives phaeochromocytoma patients from the entire country. For our study, sample size is typically expressed in terms of events per variable (EPV). An EPV of 10 is widely used as the lower limit for developing models that predict a binary outcome.[9] The sampling method used in this study was convenient sampling. The sampling method used in this study was convenient sampling. The diagnosis should be made based on a combination of biochemical tests, imaging localization and biopsy. On the basis of clinical suspicion, 24-hour urinary total metanephrines and catecholamines were used as an initial screening test. The imaging localization of phaeochromocytoma should be performed only after a biochemical diagnosis has been confirmed. At least 1 imaging examination, including computed tomography (CT) and magnetic resonance imaging (MRI), was used for the anatomic localization. The typical computed tomography imaging of phaeochromocytoma patients is shown in Figure 1. After preoperative medical management, the surgeon removed the tumor and performed a pathological examination. The confirmed diagnosis depends on biopsy. The variable morphology of phaeochromocytoma pathological sections reveals chief cells with an abundant granular cytoplasm and large vesicular nuclei and basophilic to amphophilic cells (Fig. 2).[2] For the case–control analysis, we randomly selected healthy volunteers as controls. The control volunteers were matched to the study patients by age and gender in a 2:1 ratio (n = 156). The specific process is shown in Figure 3. Ethical approval was granted by the Ethics Committees of the Third Hospital of Hebei medical university.
Figure 1.
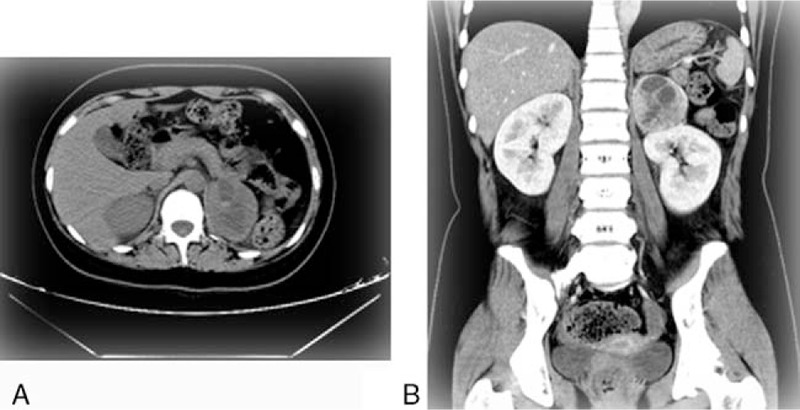
Typical computed tomography imaging of phaeochromocytoma.
Figure 2.
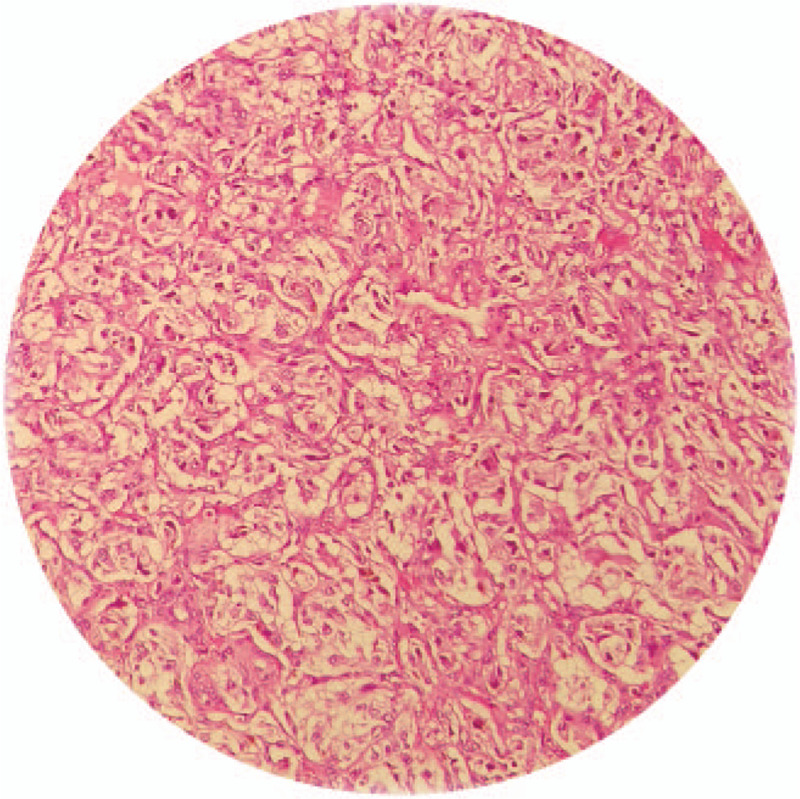
Typical histopathology of phaeochromocytoma.
Figure 3.
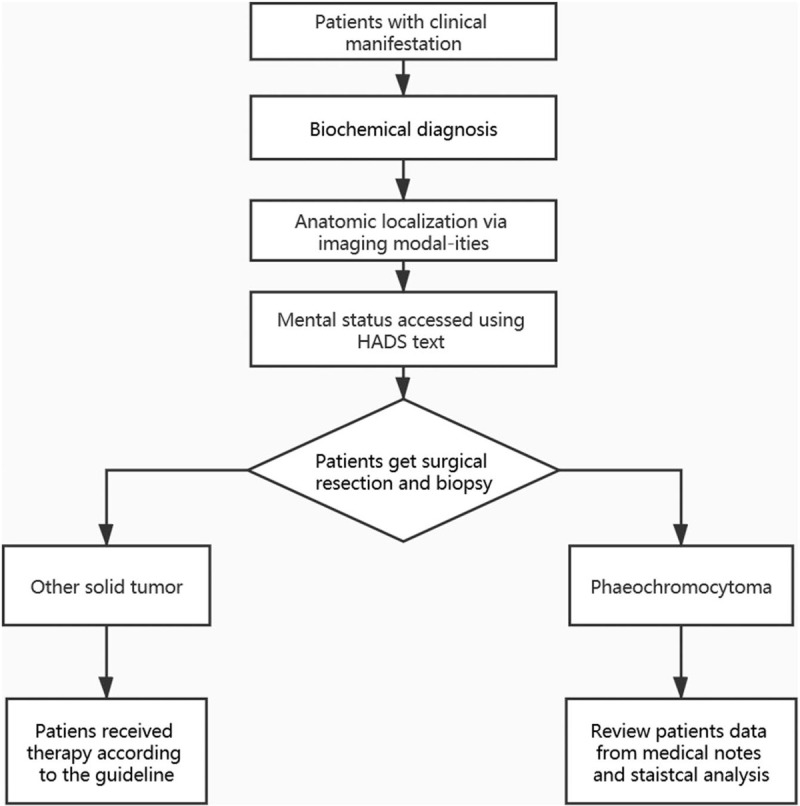
Selection of patient data for the analysis.
The patient inclusion criteria for this study were as follows: patients diagnosed with phaeochromocytoma and the ability to write and read Chinese fluently. The exclusion criteria were as follows: an age <18 years; patients with other active cancers; patients confirmed to have other psychiatric problems; patients with intellectual and/or cognitive impairments or an inability to provide written informed consent; and pregnant and lactating women because these patients own a high risk of depression and anxiety, involving these patients could distort the results.[10]
2.2. Demographics and clinical evaluation
The Chinese-translated pilot-tested version of the Hospital Anxiety and Depression Scale (HADS) (14-item) was used to assess the mental status of the phaeochromocytoma patients and control volunteers.[11] To avoid the effect of the operation on the patients’ mental state, the HADS was performed at the time of admission. All mental status examinations were performed by the same attending psychiatrist. The HADS results were stored in a privacy-protected electronic medical record system. After phaeochromocytoma was confirmed by surgical findings and pathologic results, we reviewed the patient data and analyzed their information. The HADS contains the following two 7-item subscales: HADS Depression and HADS Anxiety. Scores of 8 or above are indicative of a probable disorder, and scores of 7 and below indicate no disorder. Validated HADS in Chinese was used for interviewing our participants.[12] The Chinese translated version of HADS demonstrated satisfactory linguistic equivalence, conceptual equivalence, and scale equivalence (concordance rates at the cutoff of 8 for anxiety and depression subscales were 89.0% and 87.0%, respectively, and at the cutoffs of 11 were 87.0% and 91.0%, respectively) with English version.[12]
The participants (including the phaeochromocytoma patients and control volunteers) provided their sociodemographic information, including age, marital status, socioeconomic status, educational level, gender, and job status. According to the annual household income, socioeconomic status was divided into the following 3 classes: high = earning more than 10,000 Ren Min Bi(RMB), medium = earning 50,001 to 10,000 RMB per year, or low = earning 1 to 5000 RMB per year.
By reviewing the medical records, the following clinical data of the phaeochromocytoma patients were collected: hypertension, diabetes mellitus, cardiovascular events, neurological complications, alcohol use, tobacco use, and duration of symptoms. Hypertension can be paroxysmal hypertension, persistent hypertension, or orthostatic hypotension. On the basis of 2013 ESH/ESC Guidelines for the management of arterial hypertension,[13] the hypertension was categorized into 3 grades, including grade 1 (systolic pressure 140–159 mm Hg; diastolic pressure 90–99 mm Hg), grade 2 (systolic pressure 160–179 mm Hg; diastolic pressure 100–109 mm Hg), and grade 3 (systolic pressure ≥180 mm Hg; diastolic pressure ≥110 mm Hg). Cardiovascular events include arrhythmias, heart failure, baroreflex failure, and ischemic heart disease. Neurological complications included stroke, meningioma, diencephalic epilepsy, migraine, and postural orthostatic tachycardia syndrome (POTS). In the control group, the clinical data were collected by self-reporting.
2.3. Statistical analyses
The research data were statistically analyzed with IBM SPSS for Windows (version 22.0, SPSS Inc., Chicago, IL). The mean, number (N), standard deviation (SD), and percentage (%) were used to describe the phaeochromocytoma patients’ characteristics (independent variables). Chi-squared tests were used to compare the proportion of depression or anxiety between the phaeochromocytoma group and control group.
The occurrences of depression and anxiety were used as the outcomes (dependent variables) of the research. The odds ratio and 95% confidence intervals were used as measures of the associations. The associations between potential prognostic determinants and the outcomes were examined using a univariate logistic regression analysis. Predictors univariably associated with the outcome (P < .05) were included in the multiple-predictor logistic regression model. Our study also retrieved the previous literature. Clinically relevant variables were also included in the multiple-predictor logistic regression model. The Pearson correlation coefficient statistic was used to assess multicollinearity.[14] The overall significance of the model was assessed using the -2 log-likelihood ratio test. We also used the Hosmer–Lemeshow goodness-of-fit Chi-square test to assess the fit of the model. Some diagnostic statistics against predicted values were created to explore the outliers and detect the influential observations by using the estimated values and Pearson and deviation residuals.[14] All reported P-values were 2-tailed, and a P-value under .05 was considered statistically significant.
3. Results
In total, 215 patients diagnosed with phaeochromocytoma were recruited for this study. Final analysis was done on 194 patients, the remaining 21 patients having refused to participate. The response rate was 90.2%. In total, 156 individuals were included in the control group. The demographic and clinical characteristics of the 2 groups are summarized in Table 1. The proportion of depression was 32.5% in the phaeochromocytoma patient group and 5.7% in the control group (P < .001). The proportion of anxiety was 26.8% in the phaeochromocytoma patient group and 4.2% in the control group (P < .001).
Table 1.
Demographic and clinical characteristics of study sample.
| Phaeochromocytoma group (N = 194) | Control group (N = 156) | P | |
| Age, yr | 47.5 ± 7.7 | 48.5 ± 5.9 | .075 |
| Gender, n (%) | |||
| Female | 109 (56.2) | 97 (62.2) | |
| Male | 85 (43.8) | 59 (37.8) | .256 |
| Marital status, n (%) | |||
| Married | 133 (68.6) | 102 (65.4) | |
| Single | 1 (0.5) | 0 | |
| Divorced | 39 (20.1) | 29 (18.6) | |
| Widowed | 21 (10.8) | 25 (16.0) | .421 |
| Education, n (%) | |||
| University | 76 (39,2) | 80 (51.3) | |
| Primary and middle | 93 (47.9) | 56 (35.9) | |
| Illiterate | 25 (12.9) | 20 (12.8) | .055 |
| Job status, n (%) | |||
| Unemployed (%) | 24 (12.4) | 30 (19/2) | |
| Employed | 170 (87.6) | 1126 (80.8) | .077 |
| Socioeconomic status, n (%) | |||
| High | 31 (16.0) | 29 (18.6) | |
| Medium | 88 (45.4) | 70 (44.9) | |
| Low | 75 (38.6) | 57 (36.5) | .798 |
| Tobacco use, n (%) | 12 (6.1) | 9 (5.8) | .817 |
| Alcohol limit, n (%) | 15 (7.7) | 13 (8.3) | .837 |
| Dysarteriotony, n (%) | 130 (67.0) | 42 (26.9) | .000∗ |
| Diabetes mellitus, n (%) | 101 (52.1) | 26 (16.7) | .000∗ |
| Cardiovascular events, n (%) | 24 (12.4) | 0 | .000∗ |
| Neurological complications, n (%) | 152 (78.4) | 0 | .000∗ |
| Duration of symptoms, mo | 14.5 ± 8.2 | NA | |
In the univariable logistic regression analyses, job status (P = .015), gender (P = .008), alcohol use (P = .041), and duration of symptoms (P = .000) were associated with depression at P < .05. Similar to the HADS depression subscale, the duration of symptoms (P = .025), hypertension (P = .034), and cardiovascular events (P = .028) were associated with anxiety at P < .05 (Table 2).
Table 2.
Demographic and clinical factors association with depression and anxiety.
| Depression | Anxiety | ||||||
| N | Present(n = 63) | Absent(n = 131) | P | Present(n = 52) | Absent(n = 142) | P | |
| Demographic characteristics | |||||||
| Age, n (%) | |||||||
| >50 years old | 31 | 13 (20.6) | 18 (13.7) | 10 (19.2) | 21 (14.8) | ||
| ≦50 years old | 163 | 50 (79.4) | 113 (86.3) | .220 | 42 (80.8) | 121 (85.2) | .455 |
| Gender, n (%) | |||||||
| Female | 109 | 44 (69.8) | 65 (49.6) | 27 (51.9) | 82 (57.7) | ||
| Male | 85 | 19 (30.2) | 66 (50.4) | .008∗ | 25 (48.1) | 60 (42.3) | .469 |
| Marital status, n (%) | |||||||
| Married | 133 | 38 (57.1) | 97 (74) | 41 (78.8) | 92 (64.8) | ||
| Single | 1 | 0 | 1 (0.5) | 0 | 1 (0.7) | ||
| Divorced | 39 | 16 (25.4) | 23 (17.6) | 5 (9.6) | 34 (23.9) | ||
| Widowed | 21 | 11 (17.5) | 10 (7.6) | .062 | 6 (11.5) | 15 (10.6) | .146 |
| Education, n (%) | |||||||
| University | 76 | 25 (39.7) | 51 (38.9) | 15 (28.8) | 61 (43) | ||
| Primary and middle | 93 | 26 (41.3) | 67 (51.1) | 30 (57.7) | 63 (44.4) | ||
| Illiterate | 25 | 12 (20.4) | 13 (10.3) | .164 | 7 (13.5) | 18 (12.7) | .186 |
| Job status, n (%) | |||||||
| Unemployed (%) | 24 | 13 (20.6) | 11 (8.4) | 4 (7.7) | 20 (14.1) | ||
| Employed | 170 | 50 (79.4) | 120 (91.6) | .015∗ | 48 (92.3) | 122 (85.9) | .231 |
| Socioeconomic status, n (%) | |||||||
| High | 31 | 15 (23.8) | 16 (12.2) | 12 (23.1) | 19 (13.4) | ||
| Medium | 88 | 28 (44.4) | 60 (45.8) | 24 (46.2) | 64 (45.1) | ||
| Low | 75 | 20 (31.7) | 55 (42) | .093 | 16 (30.8) | 59 (41.5) | .183 |
| Clinical characteristics | |||||||
| Diabetes mellitus, n (%) | 101 | 37 (58.7) | 64 (48.9) | .197 | 23 (44.2) | 78 (54.9) | .186 |
| Blood pressure, n (%) | |||||||
| Normal blood pressure | 64 | 27 (42.9) | 37 (28.2) | 19 (36.5) | 45 (31.7) | ||
| Grade 1 hypertension | 79 | 17 (27) | 62 (47.3) | 27 (51.9) | 52 (36.6) | ||
| Grade 2 hypertension | 16 | 6 (9.5) | 10 (7.6) | 1 (1.9) | 15 (10.6) | ||
| Grade 3 hypertension | 35 | 13 (20.6) | 22 (16.8) | .055 | 5 (9.6) | 30 (21.1) | .034∗ |
| Cardiovascular events, n (%) | 24 | 8 (12.7) | 16 (12.2) | .924 | 2 (3.8) | 22 (15.5) | .028∗ |
| Neurological complications, n (%) | 152 | 50 (79.4) | 102 (77.9) | .812 | 42 (80.8) | 110 (77.5) | .621 |
| Tobacco use, n (%) | 12 | 6 (9.5) | 6 (4.6) | .181 | 4 (7.7) | 8 (5.6) | .598 |
| Alcohol use, n (%) | 14 | 8 (12.7) | 6 ((4.6) | .041 | 4 (7.7) | 10 (7) | .877 |
| Duration of symptoms, n (%) | |||||||
| >1 yr | 39 | 23 (36.5) | 16 (12.2) | 16 (30.8) | 23 (16.2) | ||
| ≦1 yr | 155 | 40 (63.5) | 115 (87.8) | .000∗ | 36 (69.2) | 119 (83.8) | .025∗ |
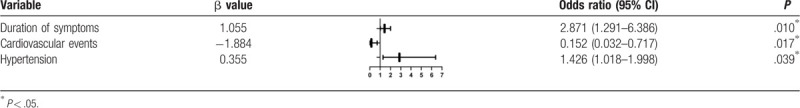
In the multiple-predictor logistic regression analysis, the duration of symptoms was an independent factor associated with depression [odds ratio (OR) = 4.091, confidence interval (CI) 1.347–12.421, P = 0.013] (Table 3). After adjusting for all covariates, the patients with hypertension (OR = 1.426, CI 1.018–1.998, P = .0039), cardiovascular events (OR = 0.152, CI 0.032–0.717, P = .001), and a long duration of symptoms (OR = 2.871, CI 1.291–6.386, P = .010) were more likely to have a diagnosis of anxiety (Table 4).
Table 3.
Logistic regression for variables associated with depression.
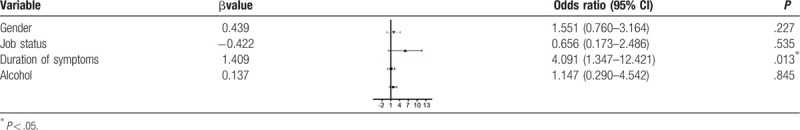
Table 4.
Logistic regression for variables associated with anxiety.
4. Discussion
In this case–control study, we assessed the proportion of anxiety and depression in phaeochromocytoma patients. The HADS is a screening instrument used to identify patients at a high risk of developing depression and anxiety. The results showed that compared with the general adult population, the phaeochromocytoma group had a higher proportion of anxiety and depression. In recent years, increasing interest has been placed on the mental status of cancer patients, and emotional distress has been recognized as the sixth vital sign in cancer care.[15] An abnormal mental status in many solid tumor patients, such as breast cancer and kidney cancer, is increasingly concerning, but limited attention has been paid to phaeochromocytoma.[16,17] Patients’ mental status is fundamental to the development of appropriate interventions because patients with comorbid psychological disorders tend to have severe symptoms, poorer outcomes, and greater use of healthcare resources. Given the frequent co-occurrence of anxiety and depression in phaeochromocytoma patients, paying more attention to their mental status and optimizing the management of this comorbidity are essential.
Phaeochromocytomas are catecholamine-secreting vascular and neuroendocrine tumors. Abnormal catecholamine secretion is the material foundation of mental disease. Catecholamines include norepinephrine, epinephrine, and dopamine. The dysfunction of the noradrenergic system has been confirmed as the principal cause of depression and anxiety disorders.[18] The amygdala is a potential site of noradrenergic/cholinergic interaction. Noradrenergic signaling through α2A receptors in neurons in the amygdala is critical for the regulation of depression and anxiety disorders. The nucleus accumbens (NAc) is a major component of the brain's reward system, and dopamine directly activates the NAc and affects mood. In patients with mood disorders, fluctuations in dopamine levels and dysfunction of the NAc have been observed.[19]
On the basis of these results, the duration of symptoms was an independent risk factor associated with depression and anxiety. A long duration of abnormal symptoms (more than 2 years), including an unexpected onset or recurrent events, significantly affects all aspects of patients’ life and could cause considerable distress. Therefore, for phaeochromocytoma patients, early medical intervention and the prompt achievement of symptom remission could help improve their mental and physical well-being. Hypertension is another factor independently associated with anxiety. Hypertension is the most common symptom of phaeochromocytoma and widely varies. In most cases, hypertension is stable and permanent; however, it is also paroxysmal with wide fluctuations and resistant to treatment.[20] Long-term hypertension can cause extremely serious damage and influence people's lives and is detrimental to physical and mental health. Therefore, in the treatment process, better control of blood pressure should be given particular attention.
This study has several limitations. First, this study had a small sample size, and the current results may not be entirely applicable to other institutions because patient sociodemographic information and clinical characteristics could differ across countries. Second, causality cannot be determined from the logistic regression model. Further longitudinal studies are needed to verify the present research conclusions. Finally, the anxiety and depression symptoms were measured only once, which could affect the accuracy of the research.
5. Conclusion
Phaeochromocytoma patients were at a higher risk of anxiety and depression. The early detection of mental health problems is beneficial for improving treatment outcomes. Phaeochromocytoma care has historically heavily focused on the cure of the disease; however, the psychosocial outcomes of cancer care should not be ignored. Furthermore, it should be recommended that screening for depression and anxiety is included in the standard of care for phaeochromocytoma patients.
Acknowledgments
We thank the subjects for participating in this study.
Author contributions
Conception and design: Chengbai Li, Siming Jia
Administrative support: Chao Zhang, Siming Jia
Provision of study materials or patients: Zhuqing Lei, Qiang Xia
Collection and assembly of data: Zhuqing Lei, Qiang Xia
Data analysis and interpretation: Siming Jia, Qiang Xia, Zhuqing Lei
Manuscript writing: All authors
Final approval of manuscript: All authors
All authors have read and approved the manuscript, and ensure that this is the case.
Conceptualization: Siming jia, Chengbai li.
Formal analysis: Zhuqing Lei.
Methodology: Qiang Xia.
Writing – original draft: yuqing jiang.
Writing – review & editing: yuqing jiang.
Footnotes
Abbreviations: CT = computed tomography, EPV = expressed in terms of events per variable, HADS = Hospital Anxiety and Depression Scale, MRI = magnetic resonance imaging, n = number (), NAc = nucleus accumbens, POTS = postural orthostatic tachycardia syndrome, RMB = Ren Min Bi, SD = standard deviation.
How to cite this article: Jia S, Li C, Lei Z, Xia Q, Jiang Y. Determinants of anxiety and depression among pheochromocytoma patients: a case–control study. Medicine. 2021;100:3(e24335).
YJ and QX contributed equally to this work.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study complied with the Declaration of Helsinki and was approved by Ethics Committees of the Third Hospital of Hebei medical university. Except for illiterate patients, all patients provided written informed consent. Illiterate patients indicate their consent by "making their mark” on the consent form, which were consistent with the local law.
The authors have no interests ethical, legal, and financial conflicts related to the article. All authors read and approved the manuscript to publish.
All of the materials were provided by the Third Hospital of Hebei medical university, and anyone can obtain the materials with appropriate reasons.
The authors declare that they have no conflict of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
P < .05.
Chi-squared test was used.
P < .05.
References
- [1].Whalen RK, Althausen AF, Daniels GH. Extra-adrenal pheochromocytoma. J Urol 1992;147:1–0. [DOI] [PubMed] [Google Scholar]
- [2].Turchini J, Cheung VKY, Tischler AS, et al. Pathology and genetics of phaeochromocytoma and paraganglioma. Histopathology 2018;72:97–105. [DOI] [PubMed] [Google Scholar]
- [3].Anderson GH, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens 1994;12:609–15. [DOI] [PubMed] [Google Scholar]
- [4].Kim JH, Moon H, Noh J, et al. Epidemiology and prognosis of pheochromocytoma/paraganglioma in Korea: a nationwide study based on the National Health Insurance Service. Endocrinol Metab (Seoul) 2020;35:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol 2004;5:617–25. [DOI] [PubMed] [Google Scholar]
- [6].Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med 2010;40:1797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res 2018;20:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen HM, Tsai CM, Wu YC, et al. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br J Cancer 2015;112:438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].van Smeden M, Moons KG, de Groot JA, et al. Sample size for binary logistic prediction models: beyond events per variable criteria. Stat Methods Med Res 2019;28:2455–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nakić Radoš S, Tadinac M, Herman R. Anxiety during pregnancy and postpartum: course, predictors and comorbidity with postpartum depression. Acta Clin Croat 2018;57:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- [12].Leung CM, Ho S, Kan CS, et al. Evaluation of the Chinese version of the Hospital Anxiety and Depression Scale. A cross-cultural perspective. Int J Psychosom 1993;40:29–34. [PubMed] [Google Scholar]
- [13].Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013;34:2159–219. [DOI] [PubMed] [Google Scholar]
- [14].Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods 2009;41:924–36. [DOI] [PubMed] [Google Scholar]
- [15].Bultz BD, Carlson LE. Emotional distress: the sixth vital sign in cancer care. J Clin Oncol 2005;23:6440–1. [DOI] [PubMed] [Google Scholar]
- [16].Jacob L, Kalder M, Kostev K. Incidence of depression and anxiety among women newly diagnosed with breast or genital organ cancer in Germany. Psychooncology 2017;26:1535–40. [DOI] [PubMed] [Google Scholar]
- [17].Vartolomei L, Vartolomei MD, Shariat SF. Bladder cancer: depression, anxiety, and suicidality among the highest-risk oncology patients. Eur Urol Focus 2019;6:1158–61. [DOI] [PubMed] [Google Scholar]
- [18].Mineur YS, Cahuzac EL, Mose TN, et al. Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety- and depression-related behaviors in mice. Neuropsychopharmacology 2018;43:2118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim S, Shou J, Abera S, et al. Sucrose withdrawal induces depression and anxiety-like behavior by Kir2.1 upregulation in the nucleus accumbens. Neuropharmacology 2018;130:10–7. [DOI] [PubMed] [Google Scholar]
- [20].Gunawardane PTK, Grossman A. Phaeochromocytoma and paraganglioma. Adv Exp Med Biol 2017;956:239–59. [DOI] [PubMed] [Google Scholar]


