Supplemental Digital Content is available in the text.
Keywords: anticoagulation, coronavirus, critical care, direct-acting oral anticoagulants, severe acute respiratory syndrome coronavirus, venous thromboembolism
Abstract
Objectives:
Practices regarding anticoagulation use in coronavirus disease 2019 focus primarily on its efficacy in the critically ill without a clear understanding of when to begin anticoagulation. We sought to understand the association of preinfection daily oral anticoagulation use and the short-term mortality of patients hospitalized with coronavirus disease 2019.
Design:
Retrospective chart review.
Setting:
Large health system with high coronavirus disease 2019 prevalence.
Patients:
Patients 60 years or older admitted to the hospital with positive coronavirus disease 2019 polymerase chain reaction test.
Interventions:
We compared both those on warfarin and those on a direct oral anticoagulant prior to admission and throughout disease course with those who were never exposed to an oral anticoagulant.
Results:
Our primary outcome was inhospital mortality at 21 days from the first coronavirus disease 2019 test ordered. Patients in the direct oral anticoagulant group (n = 104) were found to have significantly lower 21-day all-cause in hospital mortality than patients in the control group (n = 894) both prior to adjustment (14.4% vs 23.8%; odds ratio, 0.57 [0.29–0.92]; p = 0.03) and after controlling for age, gender, and comorbidities (odds ratio, 0.44 [0.20–0.90]; p = 0.033). Patients on warfarin (n = 28) were found to have an elevated unadjusted mortality rate of 32% versus 23.8% in the control group (odds ratio, 1.51 [0.64–3.31]; p = 0.31). After adjustment, a reduction in mortality was observed but not found to be statistically significant (odds ratio, 0.29 [0.02–1.62]; p = 0.24). There was no statistical difference noted in the number of bleeding events in each group.
Conclusions:
In this retrospective cohort study evaluating oral anticoagulant use among patients with coronavirus disease 2019, we found that patients who are on daily oral anticoagulation at the time of infection and throughout their disease course had significantly lower risk of all-cause mortality at 21 days. Validation of these findings should be performed on population-based levels. While research regarding anticoagulation algorithms is ongoing, we believe these results support future randomized control trials to understand the efficacy and risk of the use of early oral anticoagulation.
Current evidence suggests that the pathophysiology of the novel coronavirus of 2019 (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) resides in the inflammatory state that the virus provokes. There is subsequent development of microthrombi, leading to capillary occlusion and the destruction of organ systems—most notably in the lungs with a unique type of Acute Respiratory Distress Syndrome characteristic of coronavirus disease 2019 (COVID-19) (1–6). Microthrombi appear to predispose patients to an increased risk of typical thromboembolic pathologies (i.e., deep venous thromboses, pulmonary emboli, strokes, myocardial infarction, etc.) as disease progression leads to hypercoagulation and clot development (4). Virchow’s triad names three elements that predispose to thrombosis: a hypercoagulable state, stasis, and endothelial injury. SARS-CoV-2 has been shown to contribute to both a hypercoagulable state (7) and endothelial injury (1, 2, 8).
Early studies have demonstrated that SARS-CoV-2 is associated with an increased risk of venous thromboembolism (VTE) in critically ill patients (9, 10). A more recent study noted a 16% prevalence of thromboembolic events in all hospitalized patients infected with SARS-CoV-2, and notably, the all-cause mortality was 24.5% higher in those with thrombotic events (11).
To date, studies have primarily focused on the use of therapeutic anticoagulation and its potential benefit in patients who are critically ill—with no data to support therapeutic anticoagulant doses for patients who are early in the course of disease (4). Although anticoagulation treatment algorithms are highly variable, it has become increasingly accepted to therapeutically anticoagulate critically ill COVID-19 patients in order to prevent VTE (3, 12, 13). These anticoagulation practices lack robust prospective data (14) and, as of yet, are not standard of care according to National Institutes of Health treatment guidelines (15). Nonetheless, there is increasing evidence to suggest that critically ill COVID-19 patients benefit from therapeutic anticoagulation. Notably, those on daily preinfection anticoagulation were excluded from the analysis (16). Current data are therefore insufficient to recommend therapeutic doses of anticoagulation for COVID-19 patients who are not critically ill or who lack another indication for therapeutic doses of anticoagulation, such as atrial fibrillation or VTE (4, 15).
If SARS-CoV-2 infection creates an environment that promotes microthrombi and capillary occlusion, patients who are receiving treatment to protect against clot formation with daily anticoagulation at the time of their SARS-CoV-2 infection should be protected from this mechanism of injury compared with other patients. While there is some suggestion that there is a physiologic mechanism that direct anticoagulants will protect against the infectivity of SARS-CoV-2 (17), early therapeutic anticoagulation may protect against the morbidity and mortality caused by prothrombotic state. We are not aware of any study that seeks to understand the value of premorbid therapeutic anticoagulation and how it may affect progression of disease.
In our large, 500-bed community teaching hospital (that surged to 700 beds to meet pandemic needs), situated in a state that experienced high COVID-19 prevalence, it was anecdotally noted that patients who were on oral anticoagulation medication at the time of COVID-19 infection seemed to become less ill than their counterparts or were simply absent from the typical flow seen in the emergency department and inpatient units. These clinical observations, coupled with the increasing evidence of thrombotic complications during disease progression, prompted us to hypothesize that daily anticoagulation therapy before and during the course of COVID-19 infection may confer protection from the disease’s morbidity and mortality.
MATERIALS AND METHODS
To understand the relationship between daily preinfection anticoagulation and mortality from COVID-19, we reviewed the charts of all SARS-CoV-2 RNA polymerase chain reaction (PCR)-positive COVID-19 patients age 60 or older from March 1, 2020, to May 13, 2020, in a large health system in a state with high COVID-19 prevalence. Initially, we noted a trend in our large community teaching hospital and therefore expanded to include data from the entire health system. The health system includes five different hospitals. Of the three that have cared for the majority of COVID-19 patients, one is a large academic medical center, one is a large community teaching hospital, and the third is a smaller community hospital. All are located within commuting distance to New York City. This retrospective study received expedited Institutional Review Board (IRB) approval at Yale New Haven Health-Bridgeport Hospital (IRB Number 052001).
Study Design and Participant Inclusion
We conducted a retrospective chart review of all PCR-positive COVID-19 patients age 60 or older who were admitted to a hospital within the health system. We chose to focus on patients 60 and older as they are at increased risk for severe COVID-19 and in an effort to reduce the variability between control and study groups. In order to assess 21-day mortality, all patients in the study had a SARS-CoV-2 RNA PCR performed before April 22, 2020 (n = 1,132). Mortality was assessed from the date of the first RNA PCR test undertaken during hospitalization, even if that first test was negative. If a later RNA PCR test demonstrated disease, we assumed the first test to be falsely negative (18) and a better marker of illness onset compared with the date of eventual positive RNA-PCR result. Those who had history of oral anticoagulation therapy with either a direct oral anticoagulant (DOAC) (apixaban, rivaroxaban, dabigatran) or warfarin were separated from the control group. Data retrieval could not differentiate between patients on oral anticoagulation prior to infection compared with those prescribed oral anticoagulation during hospitalization for COVID-19. Therefore, a chart review of all patients with exposure to oral anticoagulation was performed (n = 238) (Fig. 1).
Figure 1.
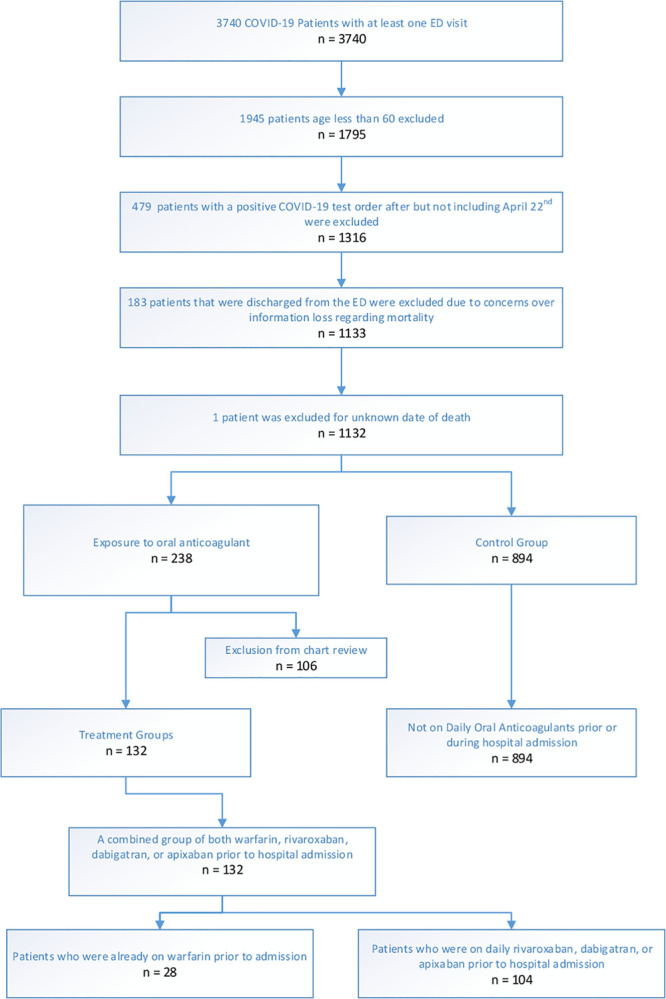
Patient inclusion & exclusion flow chart. COVID-19 = coronavirus disease 2019, ED = emergency department.
There were exclusions from chart review (n = 106): patients who were hospitalized for noninfectious conditions just prior to COVID-19 infection were excluded (n = 2, 0 deaths). Patients who were briefly started on oral anticoagulation during their hospital course but then had the anticoagulation discontinued due to a complication were also excluded (n = 7, 0 deaths). Patients who were not chronically on oral anticoagulation with a DOAC or warfarin but were discharged or expired while taking an oral anticoagulant during the course of hospital admission for COVID-19 were also excluded from the analysis (n = 93; eight deaths). Four patients not otherwise mentioned above were also excluded because they did not meet inclusion criteria for either the study groups or the control group: one patient that was on fondaparinux at the time of admission (alive); another patient that was not initially on anticoagulation but discharged on enoxaparin (alive); a patient previously prescribed warfarin but clearly identified as nonadherent with medication and with subtherapeutic international normalized ratio (INR) upon diagnosis of COVID-19 (deceased); and one patient whose chart review had conflicting documentation of whether they were on apixaban or warfarin (deceased). Of note, if the chart did not explicitly mention lack of compliance even if blood testing revealed a subtherapeutic anticoagulant effect at the time of admission, the patient was included in the appropriate group.
Ultimately, the final analysis included three comparison groups. The study groups include patients that were on a DOAC (n = 104) and patients on warfarin (n = 28). The control group included people with no exposure to oral anticoagulation prior to or during their hospital course (n = 894). Because the focus of this analysis is oral anticoagulation, we did not exclude patients on varying doses of heparin in the control group. During the study period, our health system’s practice for the treatment of all COVID-19 patients incorporated a protocol for varying doses of heparin/low-molecular-weight heparin based on level of d-dimer and clinical evidence of thromboembolism. (Specifically, if d-dimer < 5 mg/L prophylactic doses were given, if d-dimer > 5 mg/L intermediate doses were given and if confirmed thromboembolic process, therapeutic doses were given.) We assumed that other than the continuation of their baseline oral anticoagulant, patients in all three groups received similar care based on system-wide protocols for COVID-19 treatment, which remained unchanged with regards to anticoagulant use during the study period (those with oxygen < 94% were treated with hydroxychloroquine and tocilizumab; the critically ill were additionally treated with methylprednisolone and some with convalescent plasma).
We searched the electronic medical record for the primary outcome of all-cause mortality for the two study groups and the control group. Outcome was assessed at 21 days from the date of the first order for a COVID-19 RNA-PCR test. As a secondary outcome, bleeding events were reviewed and accounted for using International Classification of Diseases, 10th Revision (ICD-10) codes that include major bleeding events.
Analysis
Demographics of the two study groups (DOAC only and warfarin only) were collected and then compared with the control group (Table 1). Historical comorbid diseases were found through ICD-10 search within patient encounter problem lists. Diagnoses reviewed included diseases that would raise risk concerns for both COVID-19 and major bleeding events: coronary artery disease, congestive heart failure, atrial fibrillation, hypertension, diabetes, chronic kidney disease, cancer, gastric bleeding, and liver disease. Confounders were identified using consideration for both adjusted associations and unadjusted associations, with an aim toward identification of associations likely to modify mortality and anticoagulation utilization. Associations between anticoagulant use and mortality were assessed using a multivariable logistic regression model weighted by inverse propensity score (Appendix 1, http://links.lww.com/CCX/A485). Propensity scores were found by using multivariable gradient boosting machine models to estimate the probability of a patient having been placed on the medication under evaluation, and each covariate was evaluated individually for balance across treatment groups. Final logistic regression models containing the same covariates were then weighted by inverse probability of treatment by using this propensity score in order to achieve double robustness and mitigate potential confounding by indication (19).
TABLE 1.
Study Population Characteristics (n = 1,027)
| Study Population: | Warfarin | Direct Oral Anticoagulant | Control |
|---|---|---|---|
| Number of patients (%) | 28 (2.7) | 104 (10.1) | 894 (87.1) |
| Age | |||
| Mean (p) | 81.6 (0.01) | 78.3 (0.02) | 76.1 (NA) |
| Median (interquartile range) | 85 (69.5–100.5) | 79 (71.5–86.5) | 75 (67–85) |
| Gender, n (%; p) | |||
| Male | 13 (46.4; 1) | 67 (64.4; < 0.001) | 407 (45.5; NA) |
| Female | 15 (53.6; 1) | 37 (35.6; < 0.001) | 487 (54.5; NA) |
| Body mass index | |||
| Mean (p) | 28.7 (0.64) | 29.3 (0.03) | 28 (NA) |
| Median | 27.1 | 28.6 | 27.0 |
| Race/ethnicity, n (%; p) | |||
| White | 18 (64.3; 0.44) | 72 (69.2; 0.01) | 500 (55.9; NA) |
| Hispanic | 4 (14.3; 1) | 6 (5.8; 0.003) | 145 (16.2; NA) |
| Black | 6 (21.4; 1) | 21 (20.2; 0.80) | 196 (21.9; NA) |
| Other/unknown | 0 (0.0; 1) | 4 (3.9; 1) | 32 (3.6; NA) |
| Asian | 0 (0.0; 1) | 1 (1.0; 1) | 17 (1.9; NA) |
| Native American | 0 (0.0; 1) | 0 (0.0; 1) | 1 (0.1; NA) |
| Comorbid diagnosis, n (%; p) | |||
| Coronary artery disease | 12 (42.9; 0.007) | 47 (45.2; < 0.001) | 172 (19.2; NA) |
| Congestive heart failure | 14 (50; < 0.001) | 56 (53.9; < 0.001) | 178 (19.9; NA) |
| Atrial fibrillation | 24 (85.7; < 0.001) | 76 (73.1; < 0.001) | 116 (13.0; NA) |
| Hypertension | 25 (89.3; 0.08) | 88 (84.6; 0.009) | 649 (72.6; NA) |
| Diabetes | 15 (53.6; 0.12) | 47 (45.2; 0.14) | 335 (37.5; NA) |
| Chronic kidney disease | 6 (21.4; 1) | 35 (33.7; 0.009) | 194 (21.7; NA) |
| Cancer | 7 (25; 0.81) | 30 (28.9; 0.108) | 195 (21.8; NA) |
| Gastric bleed | 4 (14.3; 0.55) | 19 (18.3; 0.04) | 99 (11.1; NA) |
| Liver disease | 5 (17.9; 0.17) | 8 (7.7; 0.85) | 79 (8.8; NA) |
NA = not available.
RESULTS
Characteristics of the Study Subjects
Baseline demographics and medical conditions that have been identified as a risk for severe COVID-19 or severe bleeding complications were reviewed and compared between the study groups and the control group. All p values were adjusted for multiple comparisons. Patients in the DOAC group (n = 104) were found to be older (mean age 78.3 vs 76.1; p = 0.02), more likely to be male (64.4% vs 45.5%; p < 0.001), had a higher body mass index (mean: 29.3 vs 28.0; p = 0.03), and more likely to have a diagnosis of coronary artery disease (45.2% vs 19.2%; p < 0.001), congestive heart failure (53.9% vs 19.9%; p < 0.001), atrial fibrillation (73.1% vs 13.0%; p < 0.001), hypertension (84.6% vs 72.6%; p < 0.01), diabetes (45.2% vs 37.5%; p = 0.14), chronic kidney disease (33.7% vs 21.7%; p < 0.01), cancer (28.9% vs 21.8%; p = 0.11), and gastric bleeding (18.3% vs 11.1%; p = 0.04), as compared with the control group (n = 894), although not all of these differences were statistically significant. Patients in the warfarin group (n = 28) were found to be older (mean age 81.6 vs 76.1; p = 0.01), similarly male (46.4% vs 45.5%; p = 1), had similar body mass index (mean: 28.7 vs 28.0; p = 0.64), but were more likely to have a diagnosis coronary artery disease (42.9% vs 19.2%; p < 0.01), congestive heart failure (50% vs 19.9%; p < 0.001), atrial fibrillation (85.7% vs 13.0%; p < 0.001), hypertension (89.3% vs 72.6%; p = 0.08), diabetes (53.6% vs 37.5%; p = 0.12), and liver disease (17.9% vs 8.8%; p = 0.17) as compared with the control, although not all differences reach significance (Table 1).
Main Results
In this high-risk population over 60 years old with multiple comorbidities, a total of 236 of 1,026 patients died, an overall mortality rate of 23.0% assessed at 21 days from when a clinician first ordered a COVID-19 RNA PCR test. The control group, those without exposure to an oral anticoagulant prior to or during their hospitalization for COVID-19, had a gross all-cause mortality outcome of 213 of 894 deaths with an overall mortality rate of 23.8% at 21 days from first ordered test. Those that were on daily oral anticoagulation at the time of COVID-19 infection with a DOAC had 15 of 104 deaths with an overall mortality rate of 14.4% at 21 days with a crude odds ratio (OR) of 0.54 (95% CI, 0.29–0.92; p = 0.03) prior to adjustment. Those on daily warfarin at the time of COVID-19 infection had nine of 28 deaths with an overall mortality rate of 32.1% at 21 days with a crude OR of 1.51 (95% CI, 0.64–3.31; p = 0.31). When comparing DOAC only and the control, there is an absolute reduction of 9.4% at 21 days without adjusting for confounding variables such as age, gender, and comorbidities.
After statistical analysis with propensity score weighted multivariable logistic regression controlling for age, gender, and confounding variables, patients on a DOAC were found to be significantly less likely to die (OR, 0.44; 95% CI, 0.20–0.90; p = 0.033) relative to those in the control group (Table 2). Patients on warfarin (n = 28) had a trend toward reduced mortality after adjustment, however, those findings were not statistically significant (OR, 0.29; 95% CI, 0.02–1.62; p = 0.24) (Table 3). Of note, patients on warfarin were not optimally therapeutic upon admission to the hospital. Chart review revealed that 21 of 28 were not therapeutic with 10 subtherapeutic (three expired) and 11 supratherapeutic (four expired).
TABLE 2.
Outcomes of Multivariable Logistic Regression Comparing Direct Oral Anticoagulant Versus Control Group for All-Cause 21-Day Mortality After Inverse Propensity Weighting
| Variable | OR (2.5–97.5%) | p |
|---|---|---|
| Direct oral anticoagulant vs control | 0.44 (0.20–0.90) | 0.033 |
| Age (per 10 yr) | 1.88 (1.68–2.09) | < 0.001 |
| log(body mass index) | 2.77 (1.31–5.93) | 0.008 |
| Gender: male | 1.87 (1.33–2.64) | < 0.001 |
| Race: Black | 0.78 (0.50–1.21) | 0.280 |
| Race: unknown/other | 0.67 (0.32–1.35) | 0.267 |
| Ethnicity: Hispanic/Latinx | 0.92 (0.43–1.92) | 0.821 |
| Ethnicity: unknown/other | 0.82 (0.16–2.98) | 0.780 |
| Coronary artery disease | 0.97 (0.63–1.47) | 0.875 |
| Congestive heart failure | 1.49 (0.98–2.27) | 0.062 |
| Atrial fibrillation | 1.49 (0.99–2.24) | 0.057 |
| Hypertension | 0.98 (0.64–1.50) | 0.910 |
| Chronic kidney disease | 1.21 (0.82–1.80) | 0.336 |
OR = odds ratio.
TABLE 3.
Outcomes of Multivariable Logistic Regression Comparing Warfarin Versus Control Group for All-Cause 21-Day Mortality After Inverse Propensity Weighting
| Variable | OR (2.5–97.5%) | p |
|---|---|---|
| Warfarin vs control | 0.29 (0.02–1.62) | 0.237 |
| Age (per 10 yr) | 1.87 (1.67–2.09) | < 0.001 |
| log(body mass index) | 2.55 (1.20–5.48) | 0.016 |
| Gender: male | 1.96 (1.38–2.81) | 0.000 |
| Race: Black | 0.84 (0.53–1.31) | 0.448 |
| Race: unknown/other | 0.65 (0.31–1.32) | 0.245 |
| Ethnicity: Hispanic/Latinx | 1.01 (0.47–2.14) | 0.980 |
| Ethnicity: unknown/other | 0.88 (0.18–3.17) | 0.863 |
| Coronary artery disease | 0.97 (0.62–1.51) | 0.905 |
| Congestive heart failure | 1.65 (1.06–2.55) | 0.026 |
| Atrial fibrillation | 1.54 (0.98–2.39) | 0.056 |
| Hypertension | 0.94 (0.62–1.45) | 0.779 |
| Chronic kidney disease | 0.96 (0.63–1.46) | 0.852 |
OR = odds ratio.
No statistical difference was noted for the prevalence of bleeding events as captured by ICD-10 encounter diagnosis search. Overall, the control group experienced 46 bleeding events (5.1%). The warfarin group had two (7.1%; p = 0.65) and the DOAC group had six (5.8%; p = 0.67) (Table 4).
TABLE 4.
Bleeding Events
| Group | Any Bleeding Event, n (%) | No Bleeding Event, n | p (vs Control) |
|---|---|---|---|
| Warfarin | 2 (7.1) | 26 | 0.65 |
| Direct oral anticoagulant | 6 (5.8) | 98 | 0.67 |
| Control | 46 (5.1) | 848 | Not available |
DISCUSSION
This retrospective chart review found that use of a DOAC for a chronic medical condition at the time of infection with the novel coronavirus 2019 to be associated with lower mortality compared with matched patients. This finding suggests the potential benefit of early anticoagulation to reduce morbidity and mortality in COVID-19.
Patients on daily oral anticoagulation tend to be older and to have more comorbidities, an association that was seen in both of our study groups when compared with the control. Both of our study groups were thus presumed higher risk for severe COVID-19 (7, 20, 21). However, our study found the opposite, suggesting that preinfection anticoagulation should be considered when estimating the prognosis of older, at-risk adults infected with COVID-19 and discussing their goals of care.
Although these results are highly encouraging, we note several important limitations to our study. We included only those patients that required hospitalization and those who had a positive SARS-CoV-2 RNA-PCR, a test limited by high false-negative rates (18). Additionally, in an effort to capture a clear understanding of mortality with a sufficient sample size, we elected to use ages 60 years old and greater and a 21-day in hospital mortality. We believe this captures the at risk age group and is a sufficient time period to establish clear trends, but it provides a limited perspective on mortality.
Interpretation of our study results could be limited due to the large number of exclusions, in particular, those started on anticoagulation during their admission for COVID-19. We made this choice because we sought to understand the association between preinfection oral anticoagulant use and its continuation throughout course of illness and the prognosis of COVID-19. Overall, this group of cases—those whose use of a DOAC or warfarin coincided with their admission for COVID-19—had a much lower overall mortality than that of our control group. Analysis of this group and its mortality rate is misleading, however, because of selection bias: these patients had to be well enough to take an oral medication, and many were given this therapy in the setting of a thrombotic complication or a new diagnosis of atrial fibrillation.
Of note, we did not find statistical benefit to being on warfarin, although after logistic regression, we noted a nonsignificant trend toward protection. The overall study group size was small and given the labile nature of warfarin dosing and management, it is possible that the noted variations in INR also contributed to the lack of significant protection. Additionally, given the more readily reversible nature of warfarin, patients who are taking it (as opposed to a DOAC) may have risk factors that contribute to more severe overall illness and therefore may bias them to more severe disease from COVID-19.
We did not find a statistical difference in bleeding events between the groups. This may have been limited by the documentation of ICD-10 codes in the large sample size. Minor bleeding events may not have been added to the problem list if it did not alter the course of treatment. We believe this would have similarly been observed across groups.
An important factor to note is that the control group is not naive to anticoagulation. The control group did receive varying doses of heparin based on our health system’s COVID-19 treatment algorithm. Although not able to fully control for otherwise similar treatment for the three groups, the sensitivity analyses utilizing E-value calculations for the DOAC group (22) indicate that potential unmeasured confounders would need to collectively have a risk ratio in the opposite direction of the observed effect of at least 2.08 in order to explain away the observed improvement in mortality of the DOAC group. This may suggest one of two things: the mortality benefit we observed is secondary to something intrinsic to a DOAC (17) or that a steady state therapeutic anticoagulation from the onset of, or early in, the disease is what confers improved prognosis.
Use of anticoagulation to prevent VTE during the later stages of COVID-19 has become an increasingly accepted practice, but there are, to our knowledge, no current evidence-based recommendations for the use of therapeutic anticoagulation in the early stages of illness (3, 4). There are an increasing number of registered clinical trials evaluating various dosing of anticoagulation, mostly evaluating heparin or low molecular weight heparin with fewer that specifically plan to assess the value of a DOAC as a treatment for COVID-19 (23). There has been ongoing discussion on the potential utility, value, and risk of anticoagulating patients early on in the infection (3, 4, 24, 25).
CONCLUSIONS
This study advances the hypothesis that morbidity and mortality in COVID-19 may be related to an early microthrombotic state and suggests that steady state anticoagulation prior to and throughout disease course may improve prognosis. Retrospective, population-based studies of extended care facilities and hospitals with high burdens of COVID-19 cases should be performed to validate our findings. Given that elevated d-dimer at the time of admission is associated with increased mortality and rising levels precede multiple organ dysfunction (often noted around day 4 of hospitalization) (4, 26), we believe that the above results provide sufficient evidence to warrant randomized control trials evaluating the use of oral anticoagulants at the time of suspected diagnosis and/or upon hospitalization. Such trials are essential if we are to understand the true efficacy and risk of oral anticoagulants in the treatment of COVID-19. Although this study did not note a significant difference in bleeding events, increased use of oral anticoagulation will require monitoring of bleeding complications. A large cohort study from New York City also showed no significant difference in bleeding events for patients with COVID-19 who were on therapeutic anticoagulation compared with those who were not (16). In general, major bleeding manifestations with COVID-19 have remained low despite the coagulopathy (4, 25). Despite the abundance of safety data for anticoagulation, new studies in the context of COVID-19 are vital. Data are evolving regarding the pathologic mechanisms of SARS-CoV-2 and the known interactions between anticoagulants and anti-viral medications that are currently under investigation (3, 8, 14, 27).
Even in the presence of effective vaccination, the mitigation of COVID-19’s morbidity and mortality is vital for our patients and our public health. Despite its limitations, our observations suggest that high-risk patients on a DOAC prior to SARS-CoV-2 infection and throughout the course of disease experience an improved prognosis. Further study will be required to understand if this improved prognosis would also be observed in patients, high-risk or otherwise, that are started on therapeutic oral anticoagulation after virus exposure or upon symptom onset.
ACKNOWLEDGMENTS
We thank you to Evelyn Colon, Karen Hutchinson, MD, and Jill Comerford for their support with the Institutional Review Board process. We also greatly appreciate those who were available for review of statistical methods, specifically Wei Teng, Joint Data Analytics Team, Yale New Haven Health. We dedicate this work to our families, grateful for their unwavering support during this pandemic. We also dedicate this work to those who taught us to be clinicians and to learn from our patients, specifically to the memory of Dr. Lorna Breen—mentor, colleague, and friend.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
Dr. Harrison, Mr. Forte, and Dr. Buscher conceived the study and its design, had full access to the data, and take responsibility for the integrity of the data and the design. Mrs. Forte and Patel retrieved the data. Dr. Harrison, Mrs. Forte, and Chess analyzed the data with statistical report compiled by Mr. Chess. Chart review was performed by Dr. Harrison, Mr. Forte, Drs. Buscher, Moylan, and Werdmann. Dr. Harrison drafted the article with significant revisions from Dr. Mize. Dr. Harrison, Mr. Forte, Dr. Buscher, Mr. Chess, Drs. Mize, Werdmann, and Ferrigno contributed to both data interpretation and article revisions.
The authors have disclosed that they do not have any potential conflicts of interest.
This work was performed at Yale New Haven Health System.
REFERENCES
- 1.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020; 395:1417–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020; 383:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikdeli B, Madhavan MV, Jimenez D, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020; 75:2950–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020; 135:2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gralinski LE, Sheahan TP, Morrison TE, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018; 9:e01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020; 220:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020; 180:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan IH, Savarimuthu S, Leung MST, et al. The need to manage the risk of thromboembolism in COVID-19 patients. J Vasc Surg. 2020; 72:799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020; 191:145–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms J, Tacquard C, Severac F, et al. ; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020; 46:1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA. 2020; 324:799–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020; 18:1094–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song J, Wang G, Zhang W, et al. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Military Med Res. 2020; 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreuziger LB, Lee A, Garcia D, et al. COVID-19 and VTE/Anticoagulation: Frequently Asked Questions. 2020, American Society of Hematology; Available at: https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation. Accessed August 13, 2020 [Google Scholar]
- 15.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines: Antithrombotic Therapy in Patients with COVID-19. 2020National Institutes of Health; Available at: https://www.covid19treatmentguidelines.nih.gov/antithrombotic-therapy/. Accessed August 13, 2020 [PubMed] [Google Scholar]
- 16.Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020; 76:122–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du L, Kao RY, Zhou Y, et al. Cleavage of spike protein of SARS coronavirus by protease factor Xa is associated with viral infectivity. Biochem Biophys Res Commun. 2007; 359:174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucirka LM, Lauer SA, Laeyendecker O, et al. Variation in false-negative rate of reverse transcriptase polymerase chain reaction–based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 2020; 173:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funk MJ, Westreich D, Wiesen C, et al. Doubly robust estimation of causal effects. Am J Epidemiol. 2011; 173:761–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.COVID-NET. A Weekly Summary of U.S. COVID-19 Hospitalization Data. Centers for Disease Control and Prevention; 2020. Available at: https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html. Accessed August 13, 2020 [Google Scholar]
- 21.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020; 395:1763–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med. 2017; 167:268–274 [DOI] [PubMed] [Google Scholar]
- 23.ClinicalTrials.gov; NIH; U.S. National. Library of Medicine. 2020. Available at: https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=anticoagulation&cntry=&state=&city=&dist=&Search=Search. Accessed August 13, 2020
- 24.Rezaie S. REBEL Cast Ep81: COVID-19, Thrombosis, and Anticoagulation. REBEL EM blog. 2020 Available at: https://rebelem.com/rebel-cast-ep81-covid-19-thrombosis-and-anticoagulation/. Accessed August 13, 2020.
- 25.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020; 136:489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020; 18:844–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.University of Liverpool. Covid-19 Drug Interactions. 2020. Available at: https://covid19-druginteractions.org/. Accessed August 13, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


