Abstract
The objectives of this article are to understand the effects of stressors (nonsteroidal antiinflammatory drug, exercise, and pregnancy) and components in the diet, specifically prebiotics and probiotics, on intestinal barrier function. Stressors generally reduce barrier function, and these effects can be reversed by supplements such as zinc or glutamine that are among the substances that enhance the barrier. Other dietary factors in the diet that improve the barrier are vitamins A and D, tryptophan, cysteine, and fiber; by contrast, ethanol, fructose, and dietary emulsifiers increase permeability. Effects of prebiotics on barrier function are modest; on the other hand, probiotics exert direct and indirect antagonism of pathogens, and there are documented effects of diverse probiotic species, especially combination agents, on barrier function in vitro, in vivo in animal studies, and in human randomized controlled trials conducted in response to stress or disease. Clinical observations of benefits with combination probiotics in inflammatory diseases have simultaneously not appraised effects on intestinal permeability. In summary, probiotics and synbiotics enhance intestinal barrier function in response to stressor or disease states. Future studies should address the changes in barrier function and microbiota concomitant with assessment of clinical outcomes.
INTRODUCTION
The objectives of this article are to understand the effects of stressors [nonsteroidal antiinflammatory drugs (NSAIDs), exercise, and pregnancy] and components in the diet, specifically prebiotics and probiotics, on intestinal barrier function. As a prelude to addressing those objectives, it is relevant to briefly review components of the intestinal barrier and its defense and to introduce the measurements of intestinal barrier function that are commonly used to assess the deleterious and potentially protective effects of environmental factors of interest.
COMPONENTS OF THE INTESTINAL BARRIER AND DEFENSE FACTORS
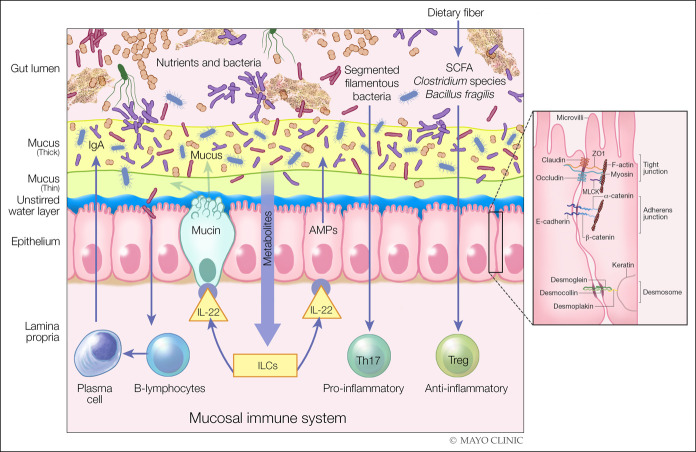
There are several components of the intestinal barrier (1), as illustrated in Figure 1 (2,3). In the lumen, there is degradation of bacteria and antigens by bile, gastric acid, and pancreatic juice. Commensal bacteria inhibit the colonization of pathogens by production of antimicrobial substances. The microclimate includes the unstirred water layer, glycocalyx, and mucus layer preventing bacterial adhesion by immunoglobulin (Ig) A secretion. Epithelial cells are connected by junctional complexes that not only have the ability to transport luminal content but also react to noxious stimuli by secretion of chloride and antimicrobial peptides. The lamina propria includes innate and acquired immunity cells secreting Ig and cytokines.
Figure 1.
Components of intestinal barrier. Components in the diet and specific immune mechanisms are involved in maintaining the integrity of the barrier, for example, through the production of short-chain fatty acids (SCFAs) by the gut microbiota. The SCFA are used by the colonic epithelium as a source of energy and can, independently, induce immune tolerance through T regulatory (Treg) cells. Other metabolites in diet can activate innate lymphoid cells to produce IL-22, which, in turn, can enhance the production of mucin and antimicrobial peptides (AMPs) by the intestinal epithelium to fortify gut barrier function. Plasma cells can also produce and secrete sIgA into the intestinal mucus layer to protect the host from the luminal contents of the intestinal tract. The intercellular space is sealed by the tight junction, which is a component of the apical junctional complex, the key elements being the zona occludens (ZO) and zona adherens, each made up of different components. Myosin light chain kinase (MLCK) is associated with the perijunctional actomyosin ring, and desmosomes reinforce the barrier. In general, diffusion through claudins and occludin is energy independent, whereas ZO-1 facilitates exchange energy-dependent mechanisms.(2,3) Left panel is reproduced from Camilleri M, Lyle BJ, Madsen KL, Sonnenburg J, Verbeke K, Wu GD. Role for diet in normal gut barrier function: Developing guidance within the framework of food labeling regulations. Am J Physiol 2019;317:G17-G39, which is published under a CC-BY license. Right panel is reprinted by permission from Springer Nature, Nature Reviews Immunology. Intestinal mucosal barrier function in health and disease. Turner JN. 2009;9:799–809.
These and other structures present diverse defensive factors in the barrier. These defensive barriers include the mucus layers (4). Mucin 2 is the most abundant mucus protein secreted by goblet cells. There is an inner layer of mucus that is firmly adhered to the epithelium; in this inner layer, bacteria are sparse, and peptides with protective, antibacterial functions are secreted by epithelial Paneth cells and lamina propria plasma cells. In the thicker and loosely adherent outer layer of mucus, bacteria and their products are abundant, but these bacteria do not access the epithelium. This outer layer of mucus is thicker in the colon than that in the small intestine where it may reach 800 μM (4), which is considerable given that the height of the entire villus ranges from 500–1,600 μM.
Beneath the inner mucus layer, there is the unstirred water layer. Normal intestinal absorption of nutrients requires efficient luminal mixing to deliver solute to the brush border. In the absence of such mixing, which is facilitated by villus contractility, the buildup of thick unstirred layers over the mucosa has the potential to markedly retard absorption of rapidly transported compounds. However, in the normal human jejunal mucosa, it has been estimated that the unstirred water layer is 35–48 μM wide (5,6), suggesting it is unlikely that this layer constitutes a rate-limiting step in absorption of rapidly transported compounds other than lipophilic molecules before the latter undergo micellar solubilization by bile. On the other hand, in the absence of villus contractility and stirring, as might occur in patients with celiac disease who have villus atrophy, the unstirred layer may be far thicker (average 170 µM) and could potentially contribute to malabsorption (7).
Beneath the unstirred water layer, there are the epithelium and its tight junction complexes, and myofibroblasts immediately beneath the epithelial basement membrane. The myofibroblasts play a role in closure of the interepithelial tight junctions and the paracellular space. The epithelial layer is the subject of many research studies of permeability, and there is regional variation. The most permeable region of the gastrointestinal tract is, paradoxically, the gall bladder, where estimates of pore radius of 12–40Å have been reported (8). In the small intestine, the pore size increases from 4 to 5Å at the villus tip to more than 20Å at the base of the crypt. The colon is less permeable than the small intestine, and in monolayers of colonic epithelial cells, the pore size radius is estimated at 4.3–4.5Å (9).
Intestinal epithelial cells also express transmembrane mucins that are attached with glycolipids to the apical surface, forming a glycocalyx that extends up to 1 μM from the cell membrane into the lumen. Transmembrane mucin MUC17 is an integral part of the glycocalyx because it covers the brush border membrane of small intestinal enterocytes and the apical membranes of colonocytes (10). MUC17 presents an extended O-glycosylated mucin domain into the intestinal lumen such that the mucin molecule adopts the bottle-brush-rod-like shape with a total length of approximately 0.8 µM (11), while being anchored to the apical membrane domain by an interaction with the scaffolding protein PDZK1 (12).
In addition, in the epithelial cell layer (13), the Paneth cells produce secretions that contain antibacterial peptides, defensins, lysozyme, TNFα, phospholipase A2, and a secreted scavenger receptor cysteine-rich protein that is deleted in malignant brain tumors 1. The lamina propria harbors cells (innate lymphoid cells and plasma cells) with immunoregulatory functions such as synthesis and secretion of antimicrobial proteins and secretory IgA (sIgA) molecules that provide additional chemical and physical defense functions in the epithelium. It has also been demonstrated that antigen passage from the diet, commensal flora, and potential pathogens through goblet cells or goblet cell-associated antigen passages can promote the development of regulatory T cells, mediated in part by intestinal dendritic cells, and provides another level of defense in the small bowel and the colon (14,15). This immune-mediated protective role of goblet cells complements the protective role played by goblet cells through the secretion of mucus (16). Finally, the endocrine and enteric nervous systems induce intestinal propulsive motility to move any potentially injurious agent or substance in the lumen from establishing a foothold in the intestinal mucosa.
APPROACHES TO MEASURE INTESTINAL PERMEABILITY
There are 3 main approaches to measure intestinal permeability, as described extensively elsewhere (2). Overall, the assessment of the entire barrier function seems to provide more comprehensive assessment of the overall barrier integrity or “leakiness.”
The first approach involves urine excretion of probe molecules in vivo or the appearance in serum of biomarkers such as bacterial lipopolysaccharide (LPS). Factors that impact the measured excretion of these probe molecule include the molecular size of the probe molecule (17), the concentration gradient of the probe molecule across the barrier, the barrier function, contact time, location and transit of the probe molecule, the length and surface area of the gut, and digestion or bacterial degradation of the molecule (18).
Among the sugar probe molecules, sucrose, mannitol, and lactulose are extensively degraded by colonic bacteria in contrast to sucralose (19). This observation suggested that sucralose would be ideal for measuring colonic permeability. However, several studies documented sucralose excretion during the first 2 hours after oral administration in both children and adults, suggesting small intestinal rather than exclusive colonic absorption (20).
Significant confounders with the use of these saccharides include the potential for the osmotic load to alter intestinal transit and, therefore, impact the site of the intestine assessed based on timed urine collections. Thus, the development of HPLC mass spectrophotometric assays has advanced the field by reducing the amount of lactulose administered as the permeability probe molecule from 10 to 1 g (21). Similarly, the introduction of 13C-mannitol as a probe addressed the confounding caused by mannitol (22), which is present in many foods and dermatological preparations and was identified in baseline measurements before administration of the sugars for the test.
A final pitfall relates to the significance of ratio measurements that may be erratic because of the relatively small mass of the disaccharide (e.g., lactulose and sucralose) compared with the monosaccharide (e.g., mannitol or rhamnose), which is actually absorbed and excreted after oral administration. For example, the median fractional urine recoveries of lactulose in children from Peru or Zambia with environmental enteropathy were 0.15% and 0.03%, respectively (23); thus, a very small change in the percent of lactulose excretion has a potentially large impact on the disaccharide to monosaccharide excretion ratios.
These methods assess the entire barrier functions of the intestine, and the timing of urine excretion of the probe molecules reflects the region of the gastrointestinal tract that is being assessed. Thus, urine collections from 0 to 2 hours generally reflect small intestinal permeability (21,24), whereas from 8- to 24-hour urine collections reflect colonic permeability (21).
Other in vivo measurements involve the assay of circulating levels of markers of mucosal damage such as increase in serum I-FABP (intestinal fatty acid binding protein), serum zonulin, and serum LPS. These markers may be most relevant in conditions that are associated with mucosal damage such as celiac disease, conditions associated with significant stress such as endurance exercise, or chronic liver diseases typically in association with portal hypertension, as reviewed elsewhere (25). However, it is unclear whether such markers of mucosal damage are sufficiently sensitive to identify more subtle levels of alterations in barrier integrity or the potential improvement in barrier function in association with prebiotics or probiotics.
The second approach to measure mucosal permeability involves in vitro measurement using cell lines or human biopsies. These measurements include the transepithelial passage of probe molecules, transepithelial electrical resistance (TEER), or expression of diverse tight junction proteins (such as claudins, occludins, and zonula occludens) documented histologically (26). It is important to note that the estimated molecular diameters of typical probe molecules, such as dextran 4 kDA or bacterial endotoxins, in these in vitro measurements are 30 or 45.7–62.8Å, respectively. The tissue preparation assessed consists predominantly of an epithelial layer without several other components of the epithelial barrier or components that are relevant for the passage of the absorbed molecule into the portal circulation, such as the permeability of end capillaries or the neurohormonal mechanisms (27,28) that may alter vascular functions (2).
The third approach to measure mucosal permeability involves in vivo endoscopic measurements using confocal endomicroscopy (that identifies increased gaps in the intestinal epithelium through the visualization during endoscopy of the passage of fluoroscein administered intravenously) and mucosal impedance measured with a catheter having with two 360° circumferential sensors placed 2 mm apart; the catheter is inserted through the biopsy channel of the endoscope, and measurements are obtained in all quadrants of the duodenum with a decompressed lumen after all fluid is aspirated (29,30). The sensors are connected to an impedance voltage transducer using thin wires, running through the length of the catheter. The voltage (V) generated by the transducer produces 10 μA current at a frequency of 2 kHz, and the resistance in current (I) flow between the electrodes provides impedance measurement is expressed in ohms (R = V/I). These measurements are generally not applied in large studies.
EFFECTS OF STRESSORS ON HUMAN INTESTINAL PERMEABILITY
Diseases associated with mucosal inflammation or ulceration such as celiac or inflammatory bowel diseases clearly alter intestinal barrier function. It is relevant to note that some environmental stressors also result in dysfunction of the gut mucosal barrier, as reviewed elsewhere (2). Endurance exercise as observed in marathon runners or in biking challenges is associated with positive fecal occult blood or bloody diarrhea, increased intestinal permeability (saccharide tests), or intestinal mucosal damage (increased serum intestinal fatty acid binding protein [I-FABP]). Similarly, NSAIDs induce overt enteropathy including ulceration or diaphragm disease, but more subtle effects are alterations in barrier function as measured by 51CrEDTA or saccharide probes (31,32), and this can be reversed with zinc supplements (33). A third stressor, pregnancy with or without obesity, has been associated with elevated serological markers of increased permeability (e.g., LPS and zonulin). Extensive burns are also associated with increased intestinal permeability (measured by urine saccharide excretion) and intestinal damage (measured by plasma diamine oxidase), and enteral glutamine treatment reduces these markers of increased permeability (34,35). Other forms of stress on intestinal barrier function occur in extraintestinal diseases, as in chronic liver diseases such as nonalcoholic fatty liver disease (36).
EFFECTS OF NUTRIENTS AND SUPPLEMENTS ON INTESTINAL PERMEABILITY OR BARRIER INTEGRITY
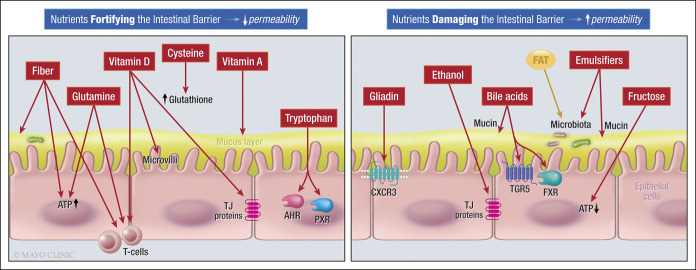
A recent literature review (37) has identified dietary factors (Figure 2) that decrease barrier integrity or increase intestinal permeability (e.g., emulsifiers, surfactants, and alcohol), and effects of these dietary items in disease states such as metabolic syndrome, liver disease or colitis are documented as examples of barrier dysfunction in the multifactorial diseases. On the other hand, other dietary factors enhance the barrier (e.g., fiber, short chain fatty acids, glutamine, and vitamin D) (37).
Figure 2.
Dietary components that impact the intestinal barrier function (37).
EFFECTS OF PREBIOTICS, PROBIOTICS, AND SYNBIOTICS ON INTESTINAL PERMEABILITY
Prebiotics
Prebiotics are nondigestible dietary components that have beneficial effects for the host through effects on colonic bacterial activity. In obese adults, the prebiotic galactooligosaccharide reduced postaspirin excretion of saccharide markers, that is, the sucralose:lactulose ratios and sucralose excretion alone (38). On the other hand, there were only marginal effects of prebiotic (8 g of oligofructose-enriched inulin p.o./d) compared with placebo (3.3 g maltodextrin p.o./d) on intestinal permeability (lactulose:mannitol ratio, P = 0.076) of children with type 1 diabetes mellitus. The prebiotic arm was associated with a significant increase in the relative abundance of Bifidobacterium at 3 months; however, this was no longer present after a 3-month washout period (39). Similarly, there was no significant decrease in intestinal permeability with enteral supplementation of a prebiotic mixture of nonhuman milk galactooligosaccharides, fructooligosaccharides, and acidic oligosaccharides compared with placebo maltodextrin in preterm infants with a gestational age younger than 32 weeks and/or birth weight <1,500 g who were being fed breast milk or mixed breast milk/formula feeding between days 3 and 30 of life (40). A fourth experience showed that prebiotic ingestion did not improve gastrointestinal barrier function (measured by the lactulose:mannitol ratio) in patients with burn during 3 weeks after the burn incident (41).
Nonsugar prebiotics are being studied for their potential to enhance the epithelial barrier function, such as soy protein hydrolysates (42). The protective effects of pretreatment with 6 soy hydrolysates on calcium ionophore A23187-induced reduction in TEER, Lucifer yellow flux, and tight junction gene expression were studied in T84 cells. After exposure to barrier disruptors (A23187, mellitin, and deoxynivalenol) that work through different intracellular pathways, one of the 6 hydrolysates protected the epithelial cells from a decrease in TEER induced by A23187 and mellitin (but not disruption by DON), and increasing claudin-1 and decreasing claudin-2 expression. These promising observations suggest that specific soy hydrolysates may be designed to strengthen the epithelial barrier.
Synbiotics
Synbiotics are combinations of specific probiotic strain(s) with the prebiotics that feed them (43). Studies have been conducted with synbiotics in healthy male subjects who were participating in physical activity (44). There were no significant changes in intestinal permeability measured by the lactulose:mannitol ratio after 3 weeks of administration of 1 of 2 regimens: a synbiotic supplement (Gut Balance) including multiple probiotic organisms including several Lactobacillus and Bifidobacterium species, plus 2 prebiotics (bovine whey–derived lactoferrin and immunoglobulins with acacia gum), or the single prebiotic, acacia gum. However, the synbiotics decreased by approximately 50% (90% CI, 20%–68%) the circulating levels of an inflammatory cytokine interleukin (IL)-16 compared with the single prebiotic alone.
A double-blind, parallel-group, randomized, controlled trial in 20 adults who were administered indomethacin to increase intestinal permeability were compared with 2-week treatment of the synbiotic Ecologic 825 with a control supplement (maltodextrin). Ecologic 825 contained 1.5 × 1010 CFU multispecies probiotic mixture [Bifidobacterium bifidum (W23), Bifidobacterium lactis (W51), B. lactis (W52), Lactobacillus acidophilus (W22), Lactobacillus casei (W56), Lactobacillus paracasei (W20), Lactobacillus plantarum (W62), Lactobacillus salivarius (W24), and Lactococcus lactis] plus the prebiotic fructooligosaccharide 10 g/d. Urinary sugars and ratios, plasma zonulin, cytokines and chemokines, and GI symptom scores were not significantly different between the 2 treatments (45).
Probiotics
Probiotics are live microorganisms available in dietary sources and may exert beneficial effects on the host, such as maintaining homeostasis in gut mucosa by enhancing integrity of gut barrier, increasing the production of butyrate, and strengthening the tight junction proteins (e.g., occludin and claudin 3). The ability of probiotics to alter intestinal barrier and microbiome has been recently reviewed (46) and is summarized in this study. First, in a randomized, crossover study in 7 healthy subjects, intraduodenal administration of 1012 L. plantarum cells increased duodenal expression of zona occludens 1 (but not occludin) and increased toll-like receptor (TLR)-2 signaling compared with a control buffer (47). In a second study, the probiotic Lactobacillus GG had a significant effect on gastric but not on intestinal mucosal barrier alterations induced by indomethacin in humans (48).
Probiotics boost host immunity through 1 or more mechanisms of action (49), ranging from production of organic acids to reduce intestinal pH, production of enzymes, secretion of mucin, influencing immune and other host cells directly (e.g. decreasing inflammation and inducing phagocytosis and antibody responses), and cross-feeding other commensal microbes, resulting in stabilization of the commensals and production of antimicrobial components.
Specifically, probiotics can antagonize pathogens by direct or indirect actions as documented by Bron et al. (46): the direct mechanisms are as follows: First, bacteria compete with enteric pathogens by competition for carbohydrate substrates depending on the diet; second, bacteria such as Lactobacillus salivarius UCC118 produces a bacteriocin in vivo, which protects mice against foodborne infection by Listeria monocytogenes; third, Bacteroides species type VI secretion system (T6SS) results in the exporting of antibacterial proteins; and fourth, probiotics inhibit colonization of pathogens by competition for common receptors of adhesion to epithelial cells (46). Several cell surface structures are involved in mediating the host–probiotic relationship, including pili, mucin-binding protein, TLR ligands, lipotechoic acid, exopolysaccharides, and surface layer–associated proteins (49).
There are also indirect mechanisms whereby probiotics antagonize pathogens. These include the following: First, recognition of microbe-associated molecular patterns by host pattern recognition receptors such as TLRs and nucleotide-binding oligomerization domain-like receptors, which activate immune defenses and protect against infection. A second indirect mechanism is the TLR signaling that induces expression of defensins (in enterocytes) and antimicrobial factors (in Paneth cells); third, nucleotide-binding oligomerization domain 2 recognition of bacterial peptidoglycan induces expression by Paneth cells of cryptdins (which are disulfide-rich cationic antimicrobial peptides that are defensins active against many Gram-negative and Gram-positive bacteria, fungi, and enveloped viruses). A fourth indirect mechanism results from the sensing of commensal microbes, which stimulates lymphoid cells to secrete IL-22; the latter signals increase expression of the mucin and antimicrobials, including Reg3 proteins, which are mainly expressed throughout the small intestine and modulate host defense process through bactericidal activity. Finally, segmented filamentous bacteria in the ileum stimulate maturation of B- and T-cells increasing sIgA and T helper (Th17) cell differentiation and increasing inflammatory cytokines and IL-22.
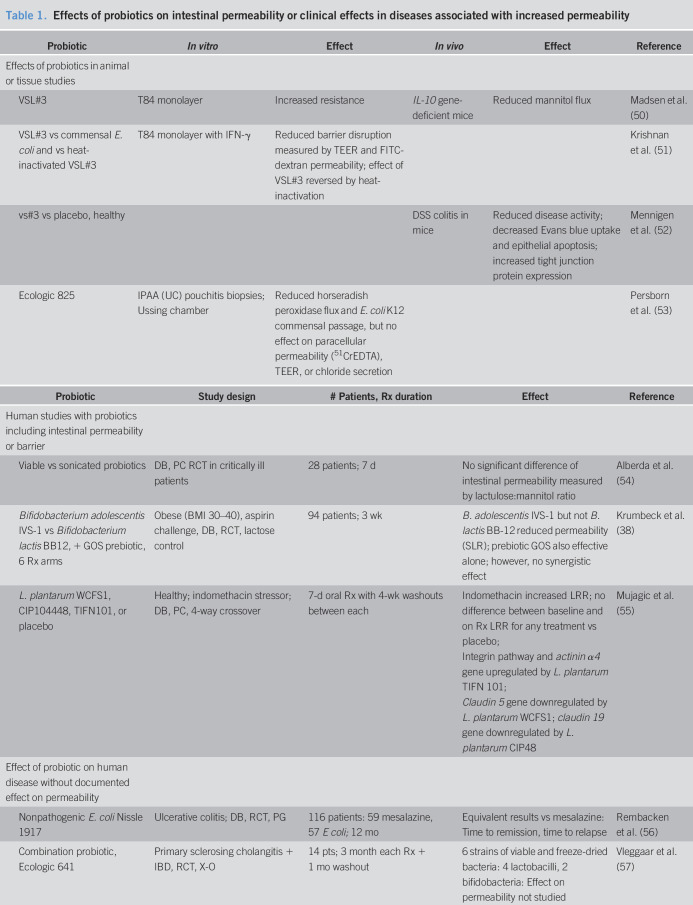
The evidence that probiotics alter intestinal permeability is equivocal, and examples from the literature are summarized in Table 1 (38,50–58). One of the diseases that present increased mucosal permeability is pouchitis. There are several potential mechanisms resulting in the change in permeability: one of these mechanisms is the effect of fecal protease from patients with active pouchitis; these fecal proteases have been shown to activate PAR2 receptors, resulting in disruption of the epithelial barrier and increasing permeability as shown by the increased fluorescein isothiocyanate–dextran flux in CaCo2 monolayers. Pouchitis also compromises tight junction proteins such as ZO-1 and occludin. The proteases in fecal supernatants from patients with pouchitis have been shown to cleave PAR2 and PAR4 (but not PAR1) in contrast to the fecal supernatants obtained from healthy controls or patients with normal pouch (59). This significant disruption of mucosal permeability in response to inflammatory bowel disease or stressors such as NSAIDs provides the basis for evaluating the effects of probiotics on intestinal permeability as exemplified by several articles in the published literature (Table 1 (38,50–58)). Table 1 summarizes the effects of probiotics on cellular monolayers in vitro or in animal models of disease, and the effects on intestinal barrier function in vivo in humans based on placebo-controlled trials.
Table 1.
Effects of probiotics on intestinal permeability or clinical effects in diseases associated with increased permeability
| Probiotic | In vitro | Effect | In vivo | Effect | Reference |
| Effects of probiotics in animal or tissue studies | |||||
| VSL#3 | T84 monolayer | Increased resistance | IL-10 gene-deficient mice | Reduced mannitol flux | Madsen et al. (50) |
| VSL#3 vs commensal E. coli and vs heat-inactivated VSL#3 | T84 monolayer with IFN-γ | Reduced barrier disruption measured by TEER and FITC-dextran permeability; effect of VSL#3 reversed by heat-inactivation | Krishnan et al. (51) | ||
| vs#3 vs placebo, healthy | DSS colitis in mice | Reduced disease activity; decreased Evans blue uptake and epithelial apoptosis; increased tight junction protein expression | Mennigen et al. (52) | ||
| Ecologic 825 | IPAA (UC) pouchitis biopsies; Ussing chamber | Reduced horseradish peroxidase flux and E. coli K12 commensal passage, but no effect on paracellular permeability (51CrEDTA), TEER, or chloride secretion | Persborn et al. (53) |
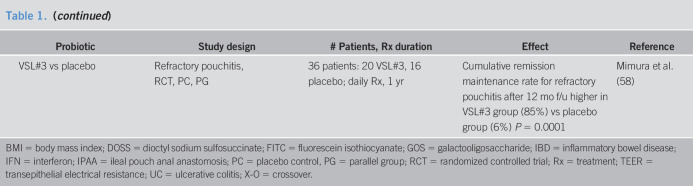
| Probiotic | Study design | # Patients, Rx duration | Effect | Reference |
| Human studies with probiotics including intestinal permeability or barrier | ||||
| Viable vs sonicated probiotics | DB, PC RCT in critically ill patients | 28 patients; 7 d | No significant difference of intestinal permeability measured by lactulose:mannitol ratio | Alberda et al. (54) |
| Bifidobacterium adolescentis IVS-1 vs Bifidobacterium lactis BB12, + GOS prebiotic, 6 Rx arms | Obese (BMI 30–40), aspirin challenge, DB, RCT, lactose control | 94 patients; 3 wk | B. adolescentis IVS-1 but not B. lactis BB-12 reduced permeability (SLR); prebiotic GOS also effective alone; however, no synergistic effect | Krumbeck et al. (38) |
| L. plantarum WCFS1, CIP104448, TIFN101, or placebo | Healthy; indomethacin stressor; DB, PC, 4-way crossover | 7-d oral Rx with 4-wk washouts between each | Indomethacin increased LRR; no difference between baseline and on Rx LRR for any treatment vs placebo; Integrin pathway and actinin α4 gene upregulated by L. plantarum TIFN 101; Claudin 5 gene downregulated by L. plantarum WCFS1; claudin 19 gene downregulated by L. plantarum CIP48 |
Mujagic et al. (55) |
| Effect of probiotic on human disease without documented effect on permeability | ||||
| Nonpathogenic E. coli Nissle 1917 | Ulcerative colitis; DB, RCT, PG | 116 patients: 59 mesalazine, 57 E coli; 12 mo | Equivalent results vs mesalazine: Time to remission, time to relapse | Rembacken et al. (56) |
| Combination probiotic, Ecologic 641 | Primary sclerosing cholangitis + IBD, RCT, X-O | 14 pts; 3 month each Rx + 1 mo washout | 6 strains of viable and freeze-dried bacteria: 4 lactobacilli, 2 bifidobacteria: Effect on permeability not studied | Vleggaar et al. (57) |
| VSL#3 vs placebo | Refractory pouchitis, RCT, PC, PG | 36 patients: 20 VSL#3, 16 placebo; daily Rx, 1 yr | Cumulative remission maintenance rate for refractory pouchitis after 12 mo f/u higher in VSL#3 group (85%) vs placebo group (6%) P = 0.0001 | Mimura et al. (58) |
BMI = body mass index; DOSS = dioctyl sodium sulfosuccinate; FITC = fluorescein isothiocyanate; GOS = galactooligosaccharide; IBD = inflammatory bowel disease; IFN = interferon; IPAA = ileal pouch anal anastomosis; PC = placebo control, PG = parallel group; RCT = randomized controlled trial; Rx = treatment; TEER = transepithelial electrical resistance; UC = ulcerative colitis; X-O = crossover.
Clinical trials with probiotics support the efficacy in suppressing inflammation that was believed to be attributable to alteration in barrier function (Table 1). However, the absence of formal measurement of barrier function or permeability in those trials did not permit a conclusive statement regarding the role of reduced permeability showing beneficial effects. In addition, a Cochrane systematic review and meta-analysis evaluated the effects of probiotics for pouchitis and demonstrated that, compared with placebo, Lactobacillus GG did not result in clinical improvement at 12 weeks, nor did Bifidobacterium longum protect patients from further episodes of acute pouchitis at 6 months. By contrast, a specific formulation of VSL#3 was superior to placebo in maintaining clinical remission at 9–12 months of follow-up (60). These results from clinical trials seem to be consistent with experimental data obtained from in vitro or in vivo studies, as summarized in Table 1 (38,50–58). Although probiotics and prebiotics have been proposed in the treatment and prevention of patients with obesity-related nonalcoholic fatty liver disease, their therapeutic use is not supported by high-quality clinical studies (61).
CONCLUSIONS
There is continued need for a validated method to measure permeability in large studies, in well-phenotyped states of health, disorder, or disease in vivo to complement the valuable information obtained from in vitro studies obtained with human samples such as biopsies and fecal supernatants. It is important to appreciate the recommendation for caution in attributing disease states to the leaky gut (25). It is also still relevant to note (62) that altered permeability may be an epiphenomenon; any inflammatory process may impair barrier integrity, and other luminal and systemic factors such as dietary components, bile acids, allergens, stress, and physical activity can independently influence barrier function. Moreover, experimental animal models have shown that impaired barrier function (e.g., genetically determined defects in barrier components) do not, in isolation, lead to the emergence of a disease phenotype. It is also still not convincingly demonstrated that interventions that restore or improve barrier function in humans can alter the natural history of disease.
Although there is evidence that dietary components may increase or decrease permeability and that effects of prebiotics, synbiotics, and probiotics on intestinal barrier function are promising, the evidence of efficacy and benefit in disease state is limited. The role of restoration of intestinal permeability in mediating beneficial treatment effects is still incompletely understood. Nevertheless, the safety of these approaches and the direct and indirect mechanisms whereby probiotics can counter pathogens (46) argue for further research, particularly using probiotics and synbiotics in disease states, and for further documentation of the role of restoration of the barrier function in mediating the associated benefits.
ACKNOWLEDGEMENT
I thank Mrs Cindy Stanislav for excellent secretarial assistance.
CONFLICTS OF INTEREST
Guarantor of the article: Michael Camilleri, MD.
Specific author contributions: M. Camilleri conceived the idea for this article, collected the data, and drafted and finalized the manuscript.
Financial support: M. Camilleri is supported by grant R01-DK115950 from National Institutes of Health for permeability studies in bile acid diarrhea and by a research grant from ILSI North America to develop optimal methods to study human intestinal permeability.
Potential competing interests: M. Camilleri has submitted a patent application entitled “Methods and Materials for Assessing Intestinal Permeability,” Publication number: 20190145953.
References
- 1.Keita AV, Soderholm JD. The intestinal barrier and its regulation by neuroimmune factors. Neurogastroenterol Motil 2010;22:718–33. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Lyle BJ, Madsen KL, et al. Role for diet in normal gut barrier function: Developing guidance within the framework of food labeling regulations. Am J Physiol 2019;317:G17–G39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009;9:799–809. [DOI] [PubMed] [Google Scholar]
- 4.Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020;0:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strocchi A, Levitt MD. A reappraisal of the magnitude and implications of the intestinal unstirred layer. Gastroenterology 1991;101:843–7. [DOI] [PubMed] [Google Scholar]
- 6.Levitt MD, Strocchi A, Levitt DG. Human jejunal unstirred layer: Evidence for extremely efficient luminal stirring. Am J Physiol 1992;262:G593–G596. [DOI] [PubMed] [Google Scholar]
- 7.Strocchi A, Corazza G, Furne J, et al. Measurements of the jejunal unstirred layer in normal subjects and patients with celiac disease. Am J Physiol 1996;270:G487–G491. [DOI] [PubMed] [Google Scholar]
- 8.Schultz SG. The role of paracellular pathways in isotonic fluid transport. Yale J Biol Med 1977;50:99–113. [PMC free article] [PubMed] [Google Scholar]
- 9.Watson CJ, Rowland M, Warhurst G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am J Physiol Cell Physiol 2001;281:C388–C397. [DOI] [PubMed] [Google Scholar]
- 10.Schneider H, Pelaseyed T, Svensson F, et al. Study of mucin turnover in the small intestine by in vivo labeling. Sci Rep 2018;8:5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelaseyed T, Gustafsson JK, Gustafsson IJ, et al. Carbachol-induced MUC17 endocytosis is concomitant with NHE3 internalization and CFTR membrane recruitment in enterocytes. Am J Physiol Cell Physiol 2013;305:C457–C467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malmberg EK, Pelaseyed T, Petersson AC, et al. The C-terminus of the transmembrane mucin MUC17 binds to the scaffold protein PDZK1 that stably localizes it to the enterocyte apical membrane in the small intestine. Biochem J 2008;410:283–9. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff SC, Barbara G, Buurman W, et al. Intestinal permeability--a new target for disease prevention and therapy. BMC Gastroenterol 2014;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDole JR, Wheeler LW, McDonald KG, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012;483:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoop KA, McDonald KG, McCrate S, et al. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol 2015;8:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knoop KA, Newberry RD. Goblet cells: Multifaceted players in immunity at mucosal surfaces. Mucosal Immunol 2018;11:1551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: An overview. Gastroenterology 1995;108:1566–81. [DOI] [PubMed] [Google Scholar]
- 18.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut 2006;55:1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology 1998;114:83–92. [DOI] [PubMed] [Google Scholar]
- 20.McOmber ME, Ou CN, Shulman RJ. Effects of timing, sex, and age on site-specific gastrointestinal permeability testing in children and adults. J Pediatr Gastroenterol Nutr 2010;50:269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camilleri M, Nadeau A, Lamsam J, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil 2010;22:e15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grover M, Camilleri M, Hines J, et al. 13C mannitol as a novel biomarker for measurement of intestinal permeability. Neurogastroenterol Motil 2016;28:1114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faubion WA, Camilleri M, Murray JA, et al. Improving the detection of environmental enteric dysfunction: A lactulose, rhamnose assay of intestinal permeability in children aged under 5 years exposed to poor sanitation and hygiene. BMJ Glob Health 2016;1:e000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musa MA, Kabir M, Hossain MI, et al. Measurement of intestinal permeability using lactulose and mannitol with conventional five hours and shortened two hours urine collection by two different methods: HPAE-PAD and LC-MSMS. PLoS One 2019;14:e0220397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camilleri M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019;68:1516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gecse K, Róka R, Ferrier L, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: A colonic lumenal factor impairing colonic permeability and sensitivity. Gut 2008;57:591–9. [DOI] [PubMed] [Google Scholar]
- 27.Hayden UL, Carey HV. Neural control of intestinal ion transport and paracellular permeability is altered by nutritional status. Am J Physiol Regul Integr Comp Physiol 2000;278:R1589–R1594. [DOI] [PubMed] [Google Scholar]
- 28.Phillips T, Phillips T, Neutra M. Macromolecules can pass through occluding junctions of rat ileal epithelium during cholinergic stimulation. Cell Tissue Res 1987;247:547–54. [DOI] [PubMed] [Google Scholar]
- 29.Fritscher-Ravens A, Schuppan D, Ellrichmann M, et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 2014;147:1012–20.e4. [DOI] [PubMed] [Google Scholar]
- 30.Peters SA, Edogawa S, Sundt WJ, et al. Constipation-predominant irritable bowel syndrome females have normal colonic barrier and secretory function. Am J Gastroenterol 2017;112:913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjarnason I, Scarpignato C, Holmgren E, et al. Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology 2018;154:500–14. [DOI] [PubMed] [Google Scholar]
- 32.Moore A, Bjarnason I, Cryer B, et al. Evidence for endoscopic ulcers as meaningful surrogate endpoint for clinically significant upper gastrointestinal harm. Clin Gastroenterol Hepatol 2009;7:1156–63. [DOI] [PubMed] [Google Scholar]
- 33.Mahmood A, FitzGerald AJ, Marchbank T, et al. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut 2007;56:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou YP, Jiang ZM, Sun YH, et al. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: A randomized, double-blind, controlled clinical trial. J Parenter Enteral Nutr 2003;27:241–5. [DOI] [PubMed] [Google Scholar]
- 35.Peng X, Yan H, You Z, et al. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 2004;30:135–9. [DOI] [PubMed] [Google Scholar]
- 36.Cui Y, Wang Q, Chang R, et al. Intestinal barrier function–non-alcoholic fatty liver disease: Interactions and possible role of gut microbiota. J Agric Food Chem 2019;67:2754–62. [DOI] [PubMed] [Google Scholar]
- 37.Khoshbin K, Camilleri M. Effects of dietary components on intestinal permeability in health and disease. Am J Physiol Gastrointest Liver Physiol 2020;319:G589–G608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krumbeck JA, Rasmussen HE, Hutkins RW, et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho J, Nicolucci AC, Virtanen H, et al. Effect of prebiotic on microbiota, intestinal permeability, and glycemic control in children with type 1 diabetes. J Clin Endocrinol Metab 2019;104:4427–40. [DOI] [PubMed] [Google Scholar]
- 40.Westerbeek EAM, van den Berg A, Lafeber HN, et al. The effect of enteral supplementation of a prebiotic mixture of non-human milk galacto-, fructo- and acidic oligosaccharides on intestinal permeability in preterm infants. Br J Nutr 2011;105:268–74. [DOI] [PubMed] [Google Scholar]
- 41.Olguin F, Araya M, Hirsch S, et al. Prebiotic ingestion does not improve gastrointestinal barrier function in burn patients. Burns 2005;31:482–8. [DOI] [PubMed] [Google Scholar]
- 42.Kiewiet MBG, González Rodríguez MI, Dekkers R, et al. The epithelial barrier-protecting properties of a soy hydrolysate. Food Funct 2018;9:4164–72. [DOI] [PubMed] [Google Scholar]
- 43.Schrezenmeir J, de Vrese M. Probiotics, prebiotics, and synbiotics—Approaching a definition. Am J Clin Nutr 2001;73:361s–364s. [DOI] [PubMed] [Google Scholar]
- 44.West NP, Pyne DB, Cripps AW, et al. Gut balance, a synbiotic supplement, increases fecal Lactobacillus paracasei but has little effect on immunity in healthy physically active individuals. Gut Microbes 2012;3:221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilms E, Gerritsen J, Smidt H, et al. Effects of supplementation of the synbiotic Ecologic® 825/FOS P6 on intestinal barrier function in healthy humans: A randomized controlled trial. PLoS One 2016;11:e0167775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bron PA, Kleerebezem M, Brummer R-J, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 2017;117:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karczewski J, Troost FJ, Konings I, et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol 2010;298:G851–G859. [DOI] [PubMed] [Google Scholar]
- 48.Gotteland M, Cruchet S, Verbeke S. Effect of Lactobacillus ingestion on the gastrointestinal mucosal barrier alterations induced by indometacin in humans. Aliment Pharmacol Ther 2001;15:11–7. [DOI] [PubMed] [Google Scholar]
- 49.Ashaolu TJ. Immune boosting functional foods and their mechanisms: A critical evaluation of probiotics and prebiotics. Biomed Pharmacother 2020;130:110625. [DOI] [PubMed] [Google Scholar]
- 50.Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 2001;121:580–91. [DOI] [PubMed] [Google Scholar]
- 51.Krishnan M, Penrose HM, Shah NN, et al. VSL#3 probiotic stimulates T-cell protein tyrosine phosphatase-mediated recovery of IFN-γ-induced intestinal epithelial barrier defects. Inflamm Bowel Dis 2016;22:2811–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mennigen R, Nolte K, Rijcken E, et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 2009;296:G1140–G1149. [DOI] [PubMed] [Google Scholar]
- 53.Persborn M, Gerritsen J, Wallon C, et al. The effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis. Aliment Pharmacol Ther 2013;38:772–83. [DOI] [PubMed] [Google Scholar]
- 54.Alberda C, Gramlich L, Meddings J, et al. Effects of probiotic therapy in critically ill patients: A randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2007;85:816–23. [DOI] [PubMed] [Google Scholar]
- 55.Mujagic Z, de Vos P, Boekschoten MV, et al. The effects of Lactobacillus plantarum on small intestinal barrier function and mucosal gene transcription; a randomized double-blind placebo controlled trial. Sci Rep 2017;7:40128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rembacken BJ, Snelling AM, Hawkey PM, et al. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: A randomised trial. Lancet 1999;354:635–9. [DOI] [PubMed] [Google Scholar]
- 57.Vleggaar FP, Monkelbaan JF, van Erpecum KJ. Probiotics in primary sclerosing cholangitis: A randomized placebo-controlled crossover pilot study. Eur J Gastroenterol Hepatol 2008;20:688–92. [DOI] [PubMed] [Google Scholar]
- 58.Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 2004;53:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffman S, Cohen NA, Carroll IM, et al. Faecal proteases from pouchitis patients activate protease activating receptor-2 to disrupt the epithelial barrier. J Crohns Colitis 2019;13:1558–68. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen N, Zhang B, Holubar SD, et al. Treatment and prevention of pouchitis after ileal pouch-anal anastomosis for chronic ulcerative colitis. Cochrane Database Syst Rev 2019;5(5):CD001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tarantino G, Finelli C. Systematic review on intervention with prebiotics/probiotics in patients with obesity-related nonalcoholic fatty liver disease. Future Microbiol 2015;10:889–902. [DOI] [PubMed] [Google Scholar]
- 62.Odenwald MA, Turner JR. Intestinal permeability defects: Is it time to treat? Clin Gastroenterol Hepatol 2013;11:1075–83. [DOI] [PMC free article] [PubMed] [Google Scholar]