Abstract
The chronic dysfunction of neuronal cells, both central and peripheral, a characteristic of neurological disorders, may be caused by irreversible damage and cell death. In 2016, more than 276 million cases of neurological disorders were reported worldwide. Moreover, neurological disorders are the second leading cause of death. Generally, the etiology of neurological diseases is not fully understood. Recent studies have related the onset of neurological disorders to viral infections, which may cause neurological symptoms or lead to immune responses that trigger these pathological signs. Currently, this relationship is mostly based on epidemiological data on infections and seroprevalence of patients who present with neurological disorders. The number of studies aiming to elucidate the mechanism of action by which viral infections may directly or indirectly contribute to the development of neurological disorders has been increasing over the years but these studies are still scarce. Comprehending the pathogenesis of these diseases and exploring novel theories may favor the development of new strategies for diagnosis and therapy in the future. Therefore, the objective of the present study was to review the main pieces of evidence for the relationship between viral infection and neurological disorders such as Alzheimer’s disease, Parkinson’s disease, Guillain-Barré syndrome, multiple sclerosis, and epilepsy. Viruses belonging to the families Herpesviridae, Orthomyxoviridae, Flaviviridae, and Retroviridae have been reported to be involved in one or more of these conditions. Also, neurological symptoms and the future impact of infection with SARS-CoV-2, a member of the family Coronaviridae that is responsible for the COVID-19 pandemic that started in late 2019, are reported and discussed.
Introduction
Neurological disorders (NDs) are among the most significant public health challenges in today’s society, and they are mainly associated with the aging of the population [1]. NDs are the leading cause of disability-adjusted life years (DALYs), with approximately 276 million cases [2]. The continuous dysfunction provoked by NDs triggers degeneration and consequent cell death in the nervous system [3]. Although neurological disorders have a multifactorial etiology, most of them have a strong genetic and environmental association [4]. Recently, some studies have also associated NDs such as multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), Alzheimer’s disease (AD), Guillain Barré syndrome (GBS), and epilepsy with viral infections [5–9]. Viral diseases are widely distributed, easily transmitted, and challenging to control. Thus, the hypothesis of viral agents as possible triggers of NDs makes them more impactful [10].
The viral etiology of some neuroinfections is well described in the literature, especially those related to neurotropic viruses such as poliovirus, coxsackievirus, and enterovirus 71 (EV71). However, our understanding of the relationship between other viral infections and the development of neurological diseases is still limited. In addition, current ND challenges include the lack of reliable biomarkers for early diagnosis and effective preventive strategies and treatments [11]. In this context, this work relates NDs, such as AD, PD, GBS, MS, and epilepsy, to viral infections. Moreover, we discuss the possible neurological impact of SARS-CoV-2 infection, which is caused by the new coronavirus responsible for the current COVID-19 pandemic.
The relationship between neurological disorders and infectious etiology
The first report of a central nervous system (CNS) infection with a consequent ND was by Bowery et al. in 1992 [12]. Subsequently, enterovirus (EV) and human herpesvirus (HHV) infections were found to be associated with ALS [13], Japanese encephalitis (JE) virus and Influenza virus with PD [14], herpes simplex virus type 1 (HSV-1) and Chlamydia pneumoniae with AD [15], and Epstein-Barr virus (EBV), varicella-zoster virus (VZV), cytomegalovirus (CMV), HHV-6, and HHV-7 with MS [16]. Although studies have shown that MDs begin in the CNS, the brain-periphery relationship may influence the development and progression of these disorders [17].
These disorders may be caused directly by infection of the CNS by specific pathogens or indirectly, through the host response to the infection. In case of the direct damage, some pathogens can cross the intact blood-brain barrier (BBB), causing severe encephalitis or acute infections that can be fatal or progress to chronic diseases [18]. Also, aging can make the CNS more vulnerable to infectious agents due to changes in the BBB, increased oxidative stress, and less energy production [19]. In the indirect-damage mechanism, various factors may be involved, such as the accumulation of protein aggregates, high levels of oxidative stress, alterations in autophagic mechanisms, synaptopathy, and neuronal destruction [16]. Fig. 1 illustrates the cascade of immune responses produced by the body against infections of the CNS and their deleterious effects.
Fig. 1.
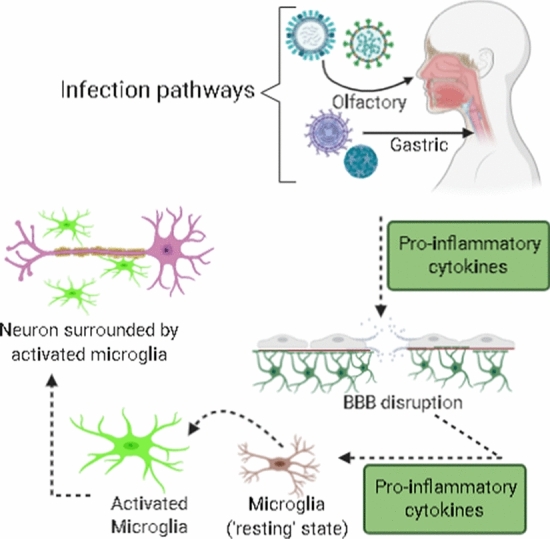
Pathological agents may infect the organism by different pathways, such as olfactory and gastric. These pathogens trigger a cascade of inflammatory responses (increased levels of cytokines, for example) that disrupt the BBB, activate microglia, and lead to a subsequent clustering around neuronal cells, resulting in neuronal damage.
Source: adapted from Limphaibool et al. (2019) [71]
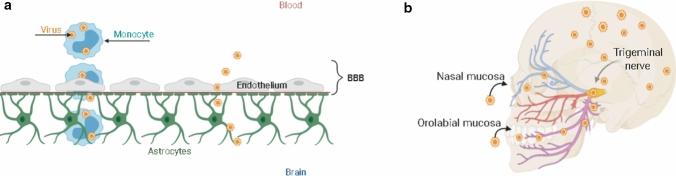
In addition to the direct infection of CNS via blood and the BBB, other possible pathways involve monocyte-macrophage/microglia cells that can cross the BBB or inter- and trans-neuronal transfer in peripheral neurons (Fig. 2A). Human immunodeficiency virus (HIV), for example, crosses the BBB by infecting blood leukocytes and, subsequently, microglia [20]. Although HIV is not capable of attacking neurons directly, this infection provokes an increase in inflammatory cytokines and viral proteins that indirectly harm these cells [16].
Fig. 2.
Mechanisms used by pathological agents to cross the BBB. (a) A direct crossing may be possible when cells of monocyte-macrophage/microglia lineage are infected by the pathogens and carry them through the BBB, reaching the CNS. This mechanism is also called "Trojan horse" because the microorganism eludes the immune system defense by using these cells to move from the bloodstream to the brain. The transport of pathogens to CNS is favored by inflammation, which is typically observed in neurological disorders. During the inflammation process, inflammatory molecules are released, triggering the activation of infected leukocytes. The postcapillary venule is attacked by the infected leukocytes, which encircle the endothelial and parenchyma basement membranes. Next, these cells enter the CNS by crossing the BBB. Another mechanism used by pathological agents is to impair the BBB and reach the CNS directly, using the porous capillaries of the choroid plexus. In various neurological diseases, the BBB is damaged, which favors the entry of pathogens into the brain through the bloodstream. (b) Neurotropic viruses may enter the CNS through retrograde axonal transport. These pathogens infect the peripheral nerve that creates a link from the skin and the mucosa to the sensory, motor, and olfactory neurons. In neuronal cells, viruses can replicate and infect adjacent cells.
Source: adapted from De Chiara et al. (2012) [16]
The neurotropism of some viruses is established through the nerves rooted in mucosa and skin. Infection is dependent on the recognition of receptors in sensory, motor, and olfactory neurons and retrograde axonal transport through axonal microtubules [21]. Certain viruses reach the brain through the olfactory system, which connects peripheral areas and with the CNS. Triplets and vagus nerves may also be an entry point for some viruses in intranasal infections [22]. After entering the CNS, viruses spread from cell to cell by being released in the synaptic cleft or by merging with neighboring neurons. The reinfection of peripheral tissue is possible for some viruses, which travel through anterograde transport and are released into the synaptic cleft [23]. The spread of neurotropic viruses in the CNS is illustrated in Fig. 2B. Several viruses have been associated with NDs, as described below. The effects of viral infections on the pathophysiology of NDs are summarized in Table 1.
Table 1.
Viruses and their neurological impact
| Viruses | Neurological impact | References | |||
|---|---|---|---|---|---|
| Classification | Primary infection/ latency | ||||
| Herpesviridae |
Herpes simplex virus 1 (HSV-1) Subfamily Alphaherpesvirinae |
Epithelial cells of the oral and genital mucosa/ sensory ganglion neurons | AD |
Periodic reactivations of the virus in the CNS – direct cytotoxicity and inflammatory damage in the CNS Formation of amyloid plaques and NFTs ApoE4 factor Oxidative stress |
[7, 42, 49, 50] |
| PD |
Molecular mimicry with α-synuclein promoting its aggregation and consequent neuronal degeneration Increased TNF-α secretion inducing the death of dopaminergic neurons |
[77, 78] | |||
| Epilepsy |
The encephalitis caused by the infection can lead to epilepsy. Inflammatory processes – increased neuronal excitability, contributing to epileptogenesis Neurotropism – damage to brain tissue and neurological sequelae |
[111–113] | |||
| GBS |
Inflammatory nerve injury caused by cross-reactive antibodies against HSV-1 (anti-GQ1b antibodies) Alteration of ganglioside composition on the cell surface of neuronal and glial cells |
[165–168] | |||
|
Cytomegalovirus (CMV) Human herpesvirus 5 (HHV-5) Subfamily Betaherpesvirinae |
Mucosal epithelial cells and leukocytes/ peripheral blood CD14+ monocytes and bone marrow CD34+ cells | AD | Increase of pro-inflammatory cytokine IFN-γ in the CNS and peripheral tissue and association with the formation of NFTs | [55, 56] | |
| PD |
Immunological reactivation Secretion of pro-inflammatory cytokines by dendritic cells Autoimmune response to neuromelanin |
[80, 81] | |||
| Epilepsy | The inflammatory process generated by the activation of microglia triggers the release of cytotoxic substances that lead to cell damage and induce necrosis. | [117] | |||
| GBS |
Expression of an immunogenic GM2-like epitope Autoantibodies against moesin production |
[170, 171] | |||
|
Human herpesvirus 6 (HHV-6) Subfamily Betaherpesvirinae |
B lymphocytes/ monocytes and macrophages, salivary glands, brain and kidneys |
AD | The infection causes a cascade of events, such as decreased autophagy and the stress activation of the endoplasmic reticulum, which may trigger the generation of Aβ, causing tau protein hyperphosphorylation | [59] | |
| PD | Parainfectious cytotoxic changes, immunologically mediated mechanisms, or direct CNS invasion | [79] | |||
| Epilepsy | Tropism for glial cells | [110] | |||
| GBS |
Important antigen-antibody reaction Polyclonal B cell activation Reactivation of a latent infection |
[155, 156, 162, 172] | |||
| MS |
The latency established by HHV-6A in oligodendrocytes may contribute to, or even trigger an autoimmune reaction that leads to myelin impairment. Affecting the repairing process of myelin in the brain by infecting OPCs |
||||
|
Epstein-Barr Virus (EBV) Subfamily Gammaherpesvirinae |
Mucous epithelial cells/ B lymphocytes | PD | Molecular mimicry with α-synuclein promotes its aggregation and consequent neuronal degeneration. | [77] | |
| GBS |
Polyclonal B cell activation Vascular damage: direct invasion of endothelial cells or immune-complex-mediated |
[162, 175] | |||
| MS |
Stimulates the expression of HERVs that contribute to the development of MS EBV replication in CNS chronically activates the immune system, recruiting microglia and astrocytes, which become destructive and neurotoxic. EBV-infected B-cells are not able to protect proteolysis-sensitive immunodominant MOG from the cytotoxic effects of T cells, leading to impaired myelination of CNS nerves and damage to the structural integrity of the myelin sheath. Primary EBV infection induces an increase in BBB permeability. |
[143–146, 148–150] | |||
| Orthomyxoviridae |
Influenza A virus subtype H5N1 (H5N1) |
Respiratory tract Infects the CNS (mice) |
PD |
The direct or indirect inflammatory response in the CNS with degeneration of dopaminergic neurons Neuronal loss in SNpc |
[8, 71] |
| GBS |
Anti-glycolipid antibody production (infection) Autoimmune responses (vaccine) Increases the permeability of BBB by endotoxin Formation of sialic acid-HA complexes that mimic GM-1 |
[176] | |||
| Flaviviridae |
Hepatitis C virus (HCV) |
Peripheral blood lymphocytes and monocytes | AD |
Direct damage to the CNS by activation of neurotoxic cytokines (TNF-α, IL-6) Indirect damage by chronic systemic inflammation that may affect the CNS |
[60, 61] |
| PD | Positive regulation of chemokines with dopaminergic neurotoxicity | [83] | |||
| GBS |
Reactivation of the virus or its enhanced replication Immune complex deposition along the vascular endothelium Anti- MAG antibody production |
[162, 179, 181] | |||
| Dengue virus (DENV) | Dendritic cells, monocytes, and macrophages | Epilepsy | DENV infection may lead to meningitis, encephalitis, and encephalomyelitis. | [119] | |
| GBS |
Pro-inflammatory cytokine production Cross-reactivation of antibodies with endothelial cells (anti-NS1) and platelets |
[182–184] | |||
| Retroviridae |
Human immunodeficiency virus (HIV) |
Dendritic cells, followed by T-helper cell (CD4 + T)/ memory T cells | PD |
Accumulation of α-synuclein in SNpc, presence of HIV in inflammatory infiltrates, glial cells and in the substantia nigra Deregulation of protein levels associated with PD (DJ1 and LRRK2) |
[87, 88] |
| Epilepsy |
Secondary infections of the CNS and metabolic disorders Formation of autoantibodies, causing neuronal death, with increased exocytosis of glutamate and decreased recapture, which leads to the activation of calcium channels and consequent neuronal hyperexcitability |
[125–128] | |||
| GBS |
Direct action on the nerves by neurotropic strains or autoimmune mechanisms Alteration of BBB integrity by Tat, gp120, and Nef Increase of TNFα |
[185, 188, 189] | |||
| Coronaviridae |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) |
Cells in the respiratory tract, most likely type II pneumocytes in the lungs, goblet secretory cells in the nasal passages, and the absorptive enterocytes in the intestines Suggested neurotropism to brain cells (due to high expression of ACE2 receptors in this organ) |
*SARS-CoV-2 infection may trigger encephalitis, seizure (or focal status epilepticus), meningitis, acute cerebrovascular diseases, impaired consciousness, skeletal muscle symptoms, agitation, confusion, and signs of corticospinal tract dysfunction *This infection may trigger immune-mediated processes, which may lead to GBS. *SARS-CoV-2 infection is likely to trigger demyelination similar to MS. *SARS-CoV-2 causes a cytokine storm, which may trigger acute necrotizing hemorrhagic encephalopathy and BBB disruption. |
||
AD, Alzheimer’s disease; PD, Parkinson’s disease; GBS, Guillain-Barré Syndrome; MS, multiple sclerosis; HERVs, human endogenous retrovirus; MOG, myelin oligodendrocyte glycoprotein; OPCs, oligodendrocyte progenitor cells; BBB, blood-brain barrier; CNS, central nervous system; NFTs, neurofibrillary tangles; apoE4, apolipoprotein E4; TNFα, tumor necrosis factor alpha; IFN-γ, interferon gamma; GM-1, gangliosidosis 1; IL-6, interleukin 6; anti-MAG, anti-myelin-associated glycoprotein; SNpc, substantia nigra pars compacta; Tat, transativator of transcription; Nef, negative regulatory factor
*All neurological effects of SARS-CoV-2 infection described in the table are based on isolated cases or studies based on a small group of patients infected. Further investigation must be conducted to clarify the neurological effects of SARS-CoV-2 infection. Also, long-term monitoring of patients is necessary to verify its impact on neuronal function and its possible impact on the development of neurological diseases.
Source: authors
Neurological disorders and viral infections
Alzheimer’s disease (AD)
AD, described for the first time in 1906, is the most common type of dementia, and in approximately 95% of the cases, it occurs after 60 years of age. In young individuals, 13% of cases show an autosomal dominant pattern of inheritance. An increased amount of beta-amyloid (Aβ) is found in the brains of AD patients. This overproduction may be related to the mutation of the genes encoding presenilins I and II (PSEN1 and PSNE2) and amyloid precursor protein (APP). In addition to these mutations, early-onset AD may also be related to mutations in apolipoprotein E (apoE) and tau protein genes [24]. The etiology of late-onset AD is often associated with a complex synergy of factors, such as the susceptibility to multiple genes (the E4 allele of the apoE gene, for example) and environmental factors [25]. ApoE is essential for repairing damage to neurons by redistributing lipids to axons and regenerating Schwann cells, restoring synaptic-dendritic connections [26].
One of the main characteristics of AD is the accumulation of Aβ peptide in the brain. Multiple forms of this peptide are derived from cleavage of the APP, the expression of which increases during cell stress [27]. The homeostasis of the CNS depend on the levels of Aβ in the brain, which assist in vital processes such as synapsis, calcium homeostasis, neurogenesis, the antioxidant system, and metal ion capture [28]. An altered level of Aβ peptide leads to the formation of amyloid fibrils. In a cascade of events, amyloid fibrils trigger amyloid plaques and formation of neurofibrillary tangles (NFTs), causing a loss of synapses and neuronal death [29].
Another factor associated with AD is the tau protein, which contributes to the assembly and stabilization of microtubules [30] and is important in the regulation of plasticity and synaptic function [31]. Under physiological conditions, phosphorylation of tau proteins for binding to microtubules occurs in a balanced way. However, when they are hyperphosphorylated, tau proteins undergo conformational changes leading to the formation of NFTs, destabilization of associated microtubules, synaptic damage, and neurodegeneration [29]. In addition to amyloid plaques and NFTs, the presence of extensive oxidative stress and dysregulation of calcium homeostasis are also characteristic of an AD patient’s brain [32]. Aβ can promote cellular calcium overload, inducing associated oxidative stress and formation of pores in the cell membrane [33]. Oxidative stress can increase Aβ production and tau hyperphosphorylation, promoting the onset and progression of AD [34]. Other events may influence the pathogenesis of AD, such as defective autophagy [35], mitochondrial dysfunction [36], synaptic dysfunction [37], and neuroinflammation [38]. AD causes several types of tissue damage, including brain atrophy, loss of neurons, and amyloid angiopathy [39].
Tests for the presence of microorganisms in the nervous system of patients with AD manifestations have yielded positive results for fungi [40, 41], bacteria [15], and viruses such as CMV, HSV-1, HHV-6, and hepatitis C virus (HCV). Unlike the brains of young patients, postmortem examination of the brains of elderly people with AD has shown them to be positive for HSV-1 DNA [42]. Seropositivity for HSV has already been associated with the development of AD in other studies [43–45]. Reactivation of HSV-1 in the CNS has been suggested to be the main connection between HSV-1 infection and AD development. This reactivation triggers an inflammatory process, causing damage to the cells, along with formation of amyloid plaques and NFTs [7]. The HSV-1 glycoprotein B (gB) is 67% identical to the Aβ peptide. In an in vitro study, gB promoted the development of Aβ fibrils in primary cortical neurons, causing cytotoxicity [46]. Decreased Aβ clearance and the accumulation of amyloid plaques in AD can impair cell autophagy [47]. Neuroblastoma cells infected with HSV-1 also produce hyperphosphorylated tau protein [48]. ApoE seems to have a strong correlation with HSV-1 lip infection in the peripheral nervous system, with the E4 allele being present in 60% of those infected [42]. In mice infected with HSV-1, viral DNA concentrations in the brain were 13.7 times higher in apoE +/+ wild-type mice than in apoE -/- knockout mice. Also, HSV-1 infection induces the expression of cytokines and proinflammatory molecules that can cause oxidative damage [49]. In human neuroblastoma cells infected with HSV-1, experimentally induced oxidative stress has been shown to significantly increase the accumulation of intracellular Aβ, inhibit Aβ secretion, and potentiate the accumulation of autophagic compartments within the cell [50].
Another HHV related to AD is CMV. Studies have demonstrated that individuals with higher levels of CMV IgG have more significant cognitive decline and a higher risk of developing AD [51–53]. Also, 93% of brains with vascular dementia that were examined postmortem were positive for CMV [54]. CMV-specific CD8+ T cells produce increased amounts of proinflammatory IFN-γ and decreased levels of the anti-inflammatory cytokines IL-2 and IL-4, with a potential shift to a proinflammatory cytokine profile in elderly people [55]. Serum levels of CMV-specific IgG have been shown to be significantly associated with NFTs. An increase in IFN-γ was also detected in the cerebrospinal fluid (CSF) of more than 80% of subjects who were positive for CMV [56].
HHV-6 rarely causes serious CNS complications; however, its ability to establish latency in the brain with possible reactivation under conditions of immunosuppression may relate this virus to AD development [57]. HHV-6 has been found in the brain [58] and leukocytes of AD patients, being significantly associated with the development of the disease and the cognitive decline of these individuals [52]. It has been suggested that HHV-6 deregulates autophagy and activates a stress response in the endoplasmic reticulum in various types of cells, particularly in astrocytes. The reduction of autophagy increases the production of Aβ and activates the stress response in the endoplasmic reticulum, promoting hyperphosphorylation of the tau protein [59].
Studies have also suggested a relationship between HCV and AD. It has been proposed that this occurs thorough direct viral infection in the brain or through cerebral or systemic inflammation [60]. In the first hypothesis, HCV can infect monocytes/macrophages, cross the BBB, and provoke the secretion of large amounts of cytokines (TNF-α, IL-6), causing cytotoxicity in the brain tissue. In the second hypothesis, HCV activates the immune system, triggering excessive systemic or local inflammation [61].
Parkinson’s disease (PD)
PD is a neurodegenerative motor disease, initially described by James Parkinson as “paralysis agitans” [62]. Approximately 10 million people are living with PD worldwide. Four percent of PD patients are under the age of 50, and the incidence of this disease increases with age [63]. An epidemiological study based on a North American population has suggested that, by 2030, over 1.2 million people will be living with PD [64]. This disease is characterized by motor changes, cognitive impairment, and autonomic dysfunction [65]. The damage caused by PD is related to dopaminergic neuron degeneration that occurs in the nigrostriatal pathway. The reduction of striatal dopamine modulation is also responsible for disease signs [66]. These alterations happen not only in the substantia nigra (SN) but also in the dorsal motor nucleus of the vagus and peripheral neurons [67].
Lewy bodies are formed mainly by α-synuclein, neurofilament proteins, and ubiquitin. It is suspected that presynaptic α-synuclein is the main protein involved in the formation of these bodies. Genetic or epigenetic factors may be responsible for their appearance in neurons. The PD development is related to the protein aggregates formed inside the nerve cells and their location in the brain. That is, if Lewy bodies are located in the nigrostriatal pathway, they would be related to extrapyramidal manifestations; in autonomic ganglia, postural hypotension; in the limbic cortex, psychosis; and, in the neocortex, cognitive decline [68, 69].
A D620N mutation in vacuolar sorting protein 35 (VPS35) causes subcellular retromer complex dysfunction; therefore, it is believed that it may affect the pathogenesis of PD. An alteration in retromer cargo molecule trafficking, a reduction of cell survival, and alteration of α-synuclein processing were observed when the D620N mutation was present. The retromer complex is also used by viral and bacterial pathogens to aid in their assembly, replication, and movement within the cell and as a mechanism to avoid the destruction that may be triggered by the cell defense machinery [70].
After the H1N1 pandemic in 1918, the number of cases of post-encephalitic parkinsonism and lethargic encephalitis increased. Based on this fact, a “dual-hit hypothesis” was suggested regarding PD pathogenesis. It was proposed that microorganisms may enter the host via the intestinal mucosa and attack the nervous system. These neurotropic agents may infect the substantia nigra pars compacta (SNpc) and trigger neurodegenerative events [71].
Although rare, experimental evidence has shown that some influenza A viruses are neurotropic, moving into the nervous system following systemic infection [72–74]. H5N1 influenza virus was used to infect the CNS of mice. A continuing inflammatory response in the animals’ brains was demonstrated after the viral infection, which may have induced degeneration of dopaminergic neurons [8]. The H1N1 influenza virus does not appear to be neurotropic in mice, suggesting that the peripheral immune response activated after an infection is probably responsible for the secondary inflammation observed in the CNS [75].
HHV infection is also associated with PD development. Elevated serological test values and the presence of serum inflammatory cytokines and α-synuclein support this theory. Moreover, studies suggest a relationship between viral load and the severity of PD symptoms [76]. Scientists have proposed a role of molecular mimicry between HSV-1 (region Ul4222-36) and α-synuclein (αsyn100-114). In the membrane of SNpc dopaminergic neurons, this phenomenon may trigger aggregation of α-synuclein and subsequent neuronal degeneration. A similar mechanism is observed for EBV, with molecular mimicry between a repeat region in latent membrane protein 1 (LMP1) encoded by EBV and the C-terminal region of α-synuclein, inducing its oligomerization [77]. HSV-1 infection may be associated with secretion of TNF-α, which is known to be involved in PD pathogenesis. It has been reported that dopaminergic neurons are very susceptible to TNF-α, which may affect the cells’ plasticity, and that neuronal death can occur in response to TNF-α binding to its receptors [78].
Parkinsonism symptoms were described in a patient who demonstrated HHV-6 infection reactivation after transplantation. Brain injuries may indicate parainfectious cytotoxic changes, direct CNS invasion, or immunologically mediated mechanisms [79]. Immunological reactivation may also be related to the development of PD in CMV infection. Dendritic cells, which preferentially secrete proinflammatory cytokines, are in higher numbers in patients with CMV and PD than in patients with CMV without PD [80]. In addition, the possible immunogens presented by these cells may be derived from dopaminergic neurons, triggering an autoimmune response to neuromelanin [81].
Studies suggest that patients who have previously been infected with HCV are more likely to develop PD. HCV may replicate in the CNS, triggering a higher prevalence of mental illness in chronic HCV patients than in the general population [82]. Dopaminergic neurotoxicity after HCV infection has been observed in co-cultured neuron-glial cells from rats. Also, it has been suggested that HCV infection induces positive regulation of ICAM-1 (intercellular adhesion molecule 1) and RANTES (regulated on activation, normal T expressed, and secreted) chemokines [83]. Tissue inhibitor of metalloproteinases 1 (TIMP-1), which is responsible for neuronal survival, is downregulated by HCV [71]. The entry and replication of this virus in the CNS may be facilitated by the high level of expression of HCV receptors in the brain microvascular endothelium [84]. Despite this fact, no correlation was found between HCV infection and PD development when more than one million patients were studied in the USA [85]. The discrepancy in the results was suggested by the substantial difference in the geographic areas of the studies, considering the prevalence of the infection, pathogenic profile of the genotype, variability of extrahepatic manifestation, and association with comorbidities.
Patients infected with HIV may develop parkinsonian features. This movement disorder may be triggered by a cascade of events caused by the infection, such as basal ganglia dysfunction, BBB alteration, chronic neuroinflammation, and neurodegeneration [86]. Post-mortem autopsies demonstrated signs of HIV in the brain, mostly in inflammatory infiltrates and glial cells, and a higher prevalence of α-synuclein in SNpc [87]. DJ1 regulates the production of reactive oxygen species (ROS) and dopamine transmission in neurons, whereas leucine-rich repeat kinase 2 (LRRK2) mediates neuroinflammation and neuronal damage. Studies suggest that HIV infection may influence DJ1 and LRRK2 levels [88].
Epilepsy
Epilepsy is an ND characterized by the rapid occurrence of epileptic seizures due to abnormal or excessive brain/neuronal activity [89]. An individual can also be diagnosed with epilepsy if he or she experiences an unprovoked or reflexive seizure and has at least a 60% chance of developing another seizure in the next 10 years [90]. Approximately 20% of all epilepsy cases are caused by acute CNS insults, 11% by cerebrovascular accident, 6% by traumatic brain injury, and 4% by infections [91]. Changes associated with post-traumatic epilepsy (PTE) include hemosiderin deposition with an incompletely formed wall of gliosis [92] and persistent BBB disruption. A correlation is also found between late-poststroke seizures and BBB disruption [93]. Post-injury epilepsy develops most commonly in the temporal and frontal lobes [94]. PTE and some infections cause an initial lesion, and if it is outside of the temporal lobes, it may result in seizures due to mesial temporal sclerosis (MTS) [94, 95]. After an injury, the latency time for the development of seizures may vary, suggesting possible variability in mechanisms of epileptogenesis [91]. Surgical specimens from patients with epilepsy show common pathological features that may be relevant to the epileptogenic process, such as astrocytes activated at the BBB, inflammatory cellular infiltrates, extravasation of blood, severe injury, disruption of the BBB with encephalitis, and involvement of frontal or temporal lobes. The majority of these features are associated with inflammatory responses [91, 94].
Inflammation in the CNS may participate in the progression of epileptogenesis as well as in the induction of seizures [96]. The progression of seizures depends on several factors. This cascade of events includes exacerbated generation of inflammatory factors, such as prostaglandin E2 (PGE2), IL-1β, IL-6, and TNF-α, and activation of inflammatory mediators, cyclooxygenase (COX)-2, and nuclear factor kappa B (NF-κB), for example [97]. Depending on its receptor, TNF-α may act as a pro-convulsant via TNF receptor 1 (TNFR1) or an anti-convulsant through TNF receptor 2 (TNFR2) [98]. Similar to other proinflammatory cytokines, such as IL-1β and TNF-α, IL-6 signaling may activate NF-κB transcriptional signaling and induce the synthesis of PGE2 by COX-2. These physiological changes assist in regulating immune and inflammatory responses [99]. The overexpression of TNF-α or IL-6 in mice leads to chronic inflammation in the brain, predisposing to seizures [100].
A great diversity of viruses has been associated with epilepsy [13, 101, 102]. One hypothesis suggests that common childhood viral infections can generate acute and chronic inflammatory processes in the CNS, which increases BBB permeability and neuronal excitability [103]. Acute seizures and epilepsy have been linked to HHV-6 infections, especially in children [104]. The number of HHV-6 infections associated with mesial temporal or temporal lobe epilepsy range from 9.1% to 55.6% [5, 105]. However, many HHV-6 infections are not associated with epilepsy, and the association of this virus with ND is controversial [106–109]. HHV-6 has a tropism for glial cells, and as the nasal cavity is constituted of oligodendrocyte progenitor cells (OPCs), the virus can replicate and cause a significant increase in the production of IL-6, chemokine ligand 1 (CCL-1), and CCL-5 [110].
HVS-1 is the leading agent of viral encephalitis, with an incidence of 2 or 3 cases per million people, per year [111]. Studies suggest that encephalitis caused by HSV-1 replication increases the likelihood of spontaneous seizures and epilepsy by approximately 20%. This fact is due to the involvement of the frontotemporal cortex, including the hippocampus, elevated CSF opening pressure, and signs of cerebral hernia [112, 113]. When viral replication is activated during latency, HSV-1 can ascend through the trigeminal and olfactory nerves to the frontal and temporal lobes, spreading to other regions of the brain [114]. HSV-1 infections trigger an inflammatory response, recruiting activated leukocytes, which, when repeated continuously, may cause brain tissue damage and neurological sequelae [111].
Less commonly, CMV and EBV may also cause nonparaneoplastic autoimmune encephalitis, which has been related to late-onset epilepsy. Antineuronal autoantibodies were detected in 48 of 113 patients with epilepsy and suspected autoimmune encephalitis [115]. The relationship between epilepsy and congenital CMV infection indicates that the 37% of the patients developed epilepsy at approximately 20 months of age [116]. CMV infection may affect the fetus directly via virally encoded gene products that may impair vital cellular processes, such as the cell cycle, cellular proliferation, and apoptosis, or induce inflammatory responses, trigger vascular injury, and promote evasion of host immune responses [117]. Increased expression of late CMV genes has been reported in individuals with intractable epilepsy, in addition to higher levels of CMV-IgG and CMV-IgM, highly sensitive C-reactive protein (Hs-CRP) and IL-6, suggesting increased viral replication and inflammatory responses in these patients [118].
It is known that several arboviruses can cause meningitis, encephalitis, and encephalomyelitis [119]. Verma and Varathanaj [120] reported a case of epilepsia partialis continua associated with dengue virus (DENV) encephalitis. Although seizures occur in approximately 47% of encephalitis cases caused by DENV, it is not possible to establish a causal relationship between encephalitis and the epileptic condition. According to Guabiraba et al. [121], there is currently no specific in vivo model that can demonstrate the relationship of epileptic manifestations and the pathogenesis of DENV infection. Trials were conducted in AG129 mice, which are deficient in interferon (IFN) types I and II and are highly susceptible to DENV infection [122]. When these animals were infected intraperitoneally with a neurotropic strain of DENV-2, 100% paralysis and lethality was demonstrated [123].
The prevalence and incidence of epilepsy and seizures among HIV patients are higher than in the general population. About 5 to 10% of HIV-positive patients in developed countries present with seizures or epilepsy [124]. It is known that HIV can invade neural tissue; however, there is still no proof of the relationship between the damage caused by the virus and seizures. Factors that may be associated with seizures and epilepsy in HIV-positive patients include the course of the disease and the establishment of acquired immunodeficiency syndrome (AIDS), opportunistic infections, and metabolic disorders [125]. Also, HIV infection can induce the formation of autoantibodies, causing neuronal death, with increased glutamate exocytosis and decreased recapture. Glutamate depletion is associated with the activation of calcium channels stimulated by phosphorylation of N-methyl-D-aspartate receptors by kinases arising from the activation of IL-1 receptors, causing neuronal hyperexcitability, with a consequent decrease in the seizure threshold [126–128].
Multiple sclerosis (MS)
MS is an immune-mediated disease in which the myelin sheath of CNS neurons is injured and the communication between the muscles and the brain is progressively interrupted [129]. The International Advisory Committee on Clinical Trials of MS classifies four basic courses for this disease: clinically isolated syndrome, relapsing-remitting, secondary progressive, and primary progressive. The most frequent kind of MS is relapsing-remitting MS (RRMS) [130]. Patients may exhibit cognitive deficits, such as difficulties in processing information and impairment of working memory and attention, as well as balance, locomotion, and fine motor control [131, 132]. Spasticity is a common symptom in MS patients. This condition is characterized by hyperreflexia, spasms, poor muscle tone, and pain, causing severe functional disability, which compromise the quality of life of these patients [133]. The prevalence of MS varies worldwide, reaching 12.8 out of every 100,000 inhabitants in Asia [134], 290 in Canada, 203 in the United Kingdom, 189 in Sweden, and 3.2 in Ecuador [135].
Although the etiology of MS is still uncertain, immunological, genetic, and environmental risk factors have been proposed. Immunological factors that trigger MS are related to T cells and antibodies, which are autoreactive in these patients. Inhibitory molecules, which generally regulate adaptive system activation, are impaired and are not able to suppress uncontrolled immune responses in MS patients. As a consequence, the chronic inflammation process leads to further damage [136]. Family clustering and specific genetic characteristics are being examined as risk factors for MS. While the general population shows a 0.1% risk of recurrence, first-degree relatives of MS index cases have up to a 50-fold greater risk of developing the disease. Moreover, smoking, body mass index, and vitamin D are among the environmental factors that may influence the onset of this disease [137, 138]. Although the etiology of MS is multifactorial, viral infections are mentioned as one of the disease’s environmental risk factors.
When analyzing the relationship between HHV-6 and EBV infections and MS, higher titers of antibodies against these viruses and a higher seroprevalence were found in patients with the disease when compared to healthy paired-control subjects [139]. The first evidence correlating EBV infection and MS was the fact that MS patients’ B lymphocytes carry and transport EBV antigens [140]. Subsequently, other evidence was suggested, such as genetic susceptibility and EBV infection, as a higher risk of MS was found in individuals with infectious mononucleosis (IM) [141, 142]. The indirect effect of EBV on MS onset may be related to the activation of silent human endogenous retrovirus W (HERV-W) [143]. In vitro and in vivo studies demonstrated that the envelope protein (Env) of HERV-W may trigger inflammatory responses and cause cytotoxicity, as well as cell death [144, 145]. Another hypothesis suggested a cascade of events evolving EBV, B cells, T cells, and inflammation processes. In healthy seropositive individuals, the immune system manages to regulate the memory B cells against the latent virus, so there are no further complications. However, in individuals who are genetically predisposed to MS, memory B cells can cross the BBB and trigger an inflammatory response in the CNS and, consequently, germinal-center-like structures. T cells may be activated at this point, and the infected cells, although latent and with limited viral gene expression, may act as antigen-presenting cells. After differentiation, some infected memory B cells can trigger the EBV replicative cycle and the production of virions. In diseases such as MS, microglia and astrocytes are chronically activated, causing neurotoxicity [146].
Myelin oligodendrocyte glycoprotein (MOG) is an essential glycoprotein involved in the myelination process in CNS nerves. MOG is also responsible for ensuring the structural integrity of the myelin sheath [147]. Changes in MOG have been experimentally associated with B cells infected with EBV, which convert the destructive processing of MOG into productive processing. This conversion facilitates the cross-presentation of the pathogenic MOG epitope (residues 40-48) to autoaggressive cytotoxic T cells [148]. Additionally, studies have shown that during primary EBV infection, this virus may induce an increase in BBB permeability, which allows pre-existing polyclonal antibody-producing B cells to penetrate the CNS. This event could explain the lower levels of EBV-specific IgG antibodies in the CNS compared to IgG produced against other viruses [149, 150].
When relating HHV-6A and HHV-6B with MS, HHV-6A was more prevalent in serum and urine samples of patients with MS than HHV-6B [151, 152]. The first murine model of HHV-6-induced brain infection was developed by Reynaud et al. [153]. First, these researchers studied different transgenic mouse lines and their ability to express the receptor for HHV-6, CD46. Further results showed that HHV-6A, but not HHV-6B, triggered the expression of viral transcripts in primary brain glial cultures from CD46-expressing mice. HHV-6B DNA did not persist in the brain, decreasing rapidly after the infection, while HHV-6A DNA levels remained high for up to 9 months. Immunohistological analysis showed the infiltration of lymphocytes in the periventricular region of mice infected with HHV-6A. Moreover, this virus triggered production of proinflammatory chemokines such as CC-chemokine ligand 2 (CCL2), CC-chemokine ligand 5 (CCL5), and C-X-C motif chemokine ligand 10 (CXCL10). A recent study measured IgG reactivity against HHV-6A and HHV-6B immediate-early protein 1 (IE1A and IE1B) and showed a positive association between IgG response against IE1A and an increased risk of developing MS in the future. In contrast, a negative association between the IgG response against IE1B and MS was demonstrated. Therefore, this study supports the role of HHV-6A in the etiology of MS by showing an increase in serological response against the immediate-early protein of this virus [154]. Oligodendrocytes are myelin-producing cells that are targeted by the immune system of patients with MS. The latency established by HHV-6A in oligodendrocytes may contribute, or even trigger, this unwanted autoimmune reaction, which leads to myelin impairment [155]. In patients with MS, in addition to the ongoing destruction of myelin, impairment in myelin repair by differentiating OPC is observed [156].
Guillain-Barré syndrome (GBS)
GBS is characterized by a dysfunction in the peripheral nerve, which suggests that immune and inflammatory mechanisms are involved [157]. Its main clinical manifestations are the absence of reflexes, paresthesia with sensory loss, and motor weakness [158]. Classification of GBS into subtypes depends on the underlying pathology, clinical presentation, and neurophysiological features. The most common subtypes include the following: acute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute axonal motor neuropathy (AMAN), acute motor-sensory axonal polyneuropathy (AMSAN), and Miller-Fisher syndrome (MFS) [159].
In GBS, antibodies and inflammatory cells produced in response to infections cross-react with epitopes on peripheral nerves and roots, leading to demyelination or axonal damage [160]. Macrophages initiate damage to the peripheral nervous system (PNS) by producing and secreting matrix metalloproteinases and nitric oxide. As a consequence, activated T cells release proinflammatory cytokines such as TNF-α [161]. The humoral response is initiated through activation of B cells, and antigen-antibody interactions can activate the complement system, resulting in membrane attack complex (MAC) formation and leading to nerve cell membrane damage and destruction [162]. Multiple antecedents and potentially triggering events have been reported. The association with infections has been established for Campylobacter jejuni, Mycoplasma pneumoniae, Haemophilus influenzae, and the viruses CMV, EBV, influenza A virus, and Zika virus [163]. Some genetic and environmental factors that affect the susceptibility of individuals to the disease have also been described [164].
Cases of patients with a combination of HSV-1 and GBS are suggested by the occurrence of molecular mimicry and high serum anti-GQ1b IgG antibody titers, causing inflammatory nerve damage [165–167]. Infection with HSV-1 could cause a change in the ganglioside composition of neuronal and glial cell surfaces, followed by the activation of autoantibodies in patients with antiganglioside antibodies [168]. Molecular mimicry is also proposed between CMV and GBS, which is the most frequent infectious etiology of GB, described for the first time by Klemola et al. [169]. Patients with CMV who develop GBS have high levels of anti-GM2 antibodies in their CSF and serum. In these people, carbohydrate structures similar to the GM2 ganglioside may induce antiganglioside antibodies [170]. Also, autoantibodies against moesin, which is crucial for myelination, have been demonstrated in 83% of patients with CMV-GBS. This may be due to six consecutive amino acids that are identical in moesin and CMV phosphoprotein 85 [171].
Although studies have associated HHV-6 infection with GBS development, this theory is generally based on minimal observations such as significantly higher antibody titers to HHV-6 in GBS patients compared to control groups [172]. This persistence of HHV-6 antibodies in the serum can be due to a stronger antigen-antibody reaction or to polyclonal B cell activation. Reactivation of latent HHV-6 infection has also been considered, but the influence of HHV-6 in GBS etiology is still inconclusive due to the lack of experimental studies [162]. The neurological involvement of EBV is also unusual, but it should be treated as a post-infection disease due to the abnormal immunological response observed [173]. Grose and Feorino [174] described five cases of GBS with high levels of antibodies to EBV, even in the absence of IM. Multivariate analysis showed that of 154 GBS patients, 10% had serologic evidence of recent EBV infection [6]. It has been suggested that the virus has a predilection for B lymphocytes and that it activates polyclonal B cells with increased production of immunoglobulins [175]. Other studies have suggested that EBV may infect endothelial cells and trigger vascular damage or cause vessel inflammation mediated by the immune complex, which could trigger the development of GBS [162].
The envelope of the influenza A virus consists of a lipid bilayer containing several glycoproteins, such as neuraminidase (NA) and haemagglutinin (HA). Therefore, anti-glycolipid antibodies may be produced during influenza virus infection because of possible molecular mimicry between glycoproteins of influenza viruses and glycolipids localized in human peripheral nerves [176].
Infectious hepatitis has been associated with GBS etiology. The case of a patient with manifested distal paresis of both legs and arms with areflexia and paresthesia was studied by De Klippel and collaborators [177]. Scientists believe the disease onset occurred during the pre-convalescent phase of an acute HCV infection, when the level of liver enzymes was consistently and rapidly normalized and signs of fibrosis were found in this organ. GBS may also occur in patients with chronic HCV infection, albeit rarely [178]. The reactivation of the virus or its intense replication may trigger the development of GBS [179]. A case of severe GBS was related to chronic active HCV infection and mixed cryoglobulinemia (MC) [180]. Other findings, such as immune complex accumulation in the vascular endothelium and vasculitis over the nerve, may explain the relationship between the infection and GBS onset [162]. A few cases of peripheral neuropathy secondary to chronic HCV infection have been described. These cases were often associated with cryoglobulinemia or with anti-myelin-associated-glycoprotein (MAG) antibodies [181].
Scientific studies were performed on the relationship between DENV infection and GBS. The infection may directly influence the disease or trigger postinfectious autoimmune responses that might lead to GBS [171]. Several studies on DENV infection have demonstrated abnormal immune responses, including cytokine and chemokine production, complement activation, and immune cell activation. Shah [182] suggested that proinflammatory cytokines that participate in the immune response in dengue fever might play a causal role in the etiopathogenesis of GBS. This infection may cause the generation of a complex immune response, with high levels of TNFα, IL-2, and IFNγ, as well as an inversion of the CD4:CD8 ratio [183]. Also, autoimmune responses may be involved, mainly in the pathogenesis of the severe phase of dengue. Patients with dengue can produce antibodies that cross-react with platelets and endothelial cells. After DENV infection, antibodies against nonstructural protein 1 (anti-NS1) are generated. Studies suggest these antibodies may influence the cross-reactivity of endothelial cells, which play a crucial role in the development of neurological disease [184].
One of the proposed mechanisms for GBS in HIV-1-infected patients includes a direct action of an HIV-1 neurotropic strain on the nerves. Another theory is based on an autoimmune response, in which abnormal immunoregulation is followed by the formation of antibodies against myelin [185]. HIV can cause direct and indirect neurotoxic effects on the CNS and PNS. The relationship between GBS and the stage of HIV infection is also unclear. The authors characterized the GBS as an indication of early HIV infection or seroconversion [186]. However, GBS has been reported in chronic HIV-1 infection cases or as a complication of immune reconstitution inflammatory syndrome in severely immunocompromised patients [187]. The HIV-1 infection may alter the integrity of the BBB through the action of the viral proteins Tat, gp120, and Nef [188]. In a study using a murine model, exposure to HIV-1 envelope protein gp120 caused swelling and increased TNFα levels in the sciatic nerve trunk. These findings suggest that HIV infection may cause nerve damage [189].
SARS-CoV-2, the virus responsible for COVID-19 pandemic, and its neurological impact
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) is the virus responsible for the disease COVID-19 [190], a global pandemic that started in late 2019 and in a few months has affected 250 countries around the world [191]. Coronaviruses (CoVs) typically cause respiratory disease in humans; however, some studies suggest an association with neurological symptoms. Two different coronaviruses that caused epidemic infections in the past, SARS-CoV, and Middle East respiratory syndrome-associated coronavirus (MERS-CoV), have triggered neurological harm in isolated cases [192]. Patients infected by these viruses have developed neurologic symptoms, such as neuropathy, myopathy, Bickerstaff brainstem encephalitis (BBE), and GBS, two to three weeks after the appearance of typical symptoms [193, 194]. The causality cannot be proven, since these findings were reported in isolated cases and with a small number of patients. On the other hand, some scientists have suggested that the neurological manifestations of MERS might have been neglected and underdiagnosed [193].
In Wuhan, China, a study was performed during the COVID-19 outbreak with 214 patients. The results showed that 36.4% of the patients with severe disease exhibited neurologic manifestations, such as acute cerebrovascular disease, impairment of consciousness, and skeletal muscle symptoms [195]. A study performed in Strasbourg, France, demonstrated that 84% of COVID-19 patients in intensive care units (ICU) who had respiratory difficulties also showed neurological symptoms such as agitation, confusion, and signs of corticospinal tract dysfunction [196]. It has been suggested that these signs of neurological damage might be caused by severe hypoxemia and hypoxia, an inflammatory process triggered by SARS-CoV-2 infection, or by virus infiltration and spread in the brain [197]. The SARS-CoV-2 infection begins with the spike protein S1 binding to the host receptor ACE2 (angiotensin-converting enzyme 2). The human brain expresses ACE2 at a high level, which may allow the virus to invade the CNS [198]. Xiang et al. [199] reported the first confirmed case of encephalitis caused by SARS-CoV-2. The presence of this virus in the CSF of this patient was confirmed by genome sequencing. The first case of meningitis associated with COVID-19 was reported by Moriguchi et al. [200]. Although nasopharyngeal swabs obtained from this patient were negative, the infection was confirmed by viral RNA detection in the spinal fluid.
COVID-19 triggers increased production of inflammatory cells, and along with them, high levels of inflammatory cytokines, which induce immune-mediated processes [201], and this is one of the proposed explanations for GBS symptoms [202]. Sedaghat and Karimi [203] reported the development of GBS in a 65-year-old male COVID-19 patient two weeks after developing cough and fever. In another case study, the first GBS symptoms overlapped with the period of SARS-CoV-2 infection, making investigators unsure about the causal connection between the two [204].
A patient with well-controlled post-encephalitic epilepsy was infected with SARS-CoV-2 and presented with focal status epilepticus in the early stage of the disease. The 78-year-old patient was seizure-free for more than two years, and based on a historical correlation of symptoms, it was suggested that SARS-CoV-2 might have triggered the seizures [205]. The relationship between COVID-19 and epilepsy is still unknown, and conclusions will depend on new reports and updates from clinicians [206].
A study of 90 brain autopsy samples from patients with NDs (mostly MS) and healthy controls showed that 48% of the samples contained human coronavirus RNA [207]. However, further studies should be performed to determine if the presence of virus in human brains is opportunistic or disease-associated. It has been suggested that SARS-CoV-2 infection is likely to trigger demyelination similar to MS. Based on this fact, periodic neurological assessments, such as auditory brainstem responses and neuroimaging, should be carried out on recovered COVID-19 patients to follow up any signs of dysfunction [199]. When comparing COVID-19 with past viral pandemics, a concern about neuropsychiatric sequelae emerges. Previous outbreaks of virus infection have triggered long-term neurodegenerative effects such as encephalopathy, psychosis, demyelinating processes, and neuromuscular dysfunction, weeks, or even months after the patient’s recovery [208]. Therefore, Troyer et al. [209] have also emphasized the need for long-term monitoring of patients who were once infected with SARS-CoV-2. Several neurodegenerative diseases, such as AD, PD, and MS, are related to high levels of cytokines/chemokines and other chronic neuroinflammation effects [210]. In this way, the cytokine storm triggered by COVID-19 and BBB disruption could affect the CNS and cause the onset of these diseases [211, 212]. However, further studies should be conducted to investigate the involvement of SARS-CoV-2 infection in neurodegenerative diseases [211].
Conclusion
In this review, we have discussed a number of studies that relate viral infections to the development of neurological disorders. It is important to consider that viruses are responsible for various epidemics and even pandemics, and some of them can cause irreversible damage to the nervous system. This work demonstrates that considerable attention should be given to the relationship between viral infections and NDs. The inclusion of viruses in the etiology and diagnosis of diseases of the nervous system would have a positive impact on the management and treatment of disabling and potentially lethal complications. New studies that investigate the mechanism of action of the viruses in these pathologies should be encouraged, aiming mainly at the development of novel control and intervention therapies.
Acknowledgements
The authors are grateful to Coordination for the Improvement of Higher Education Personnel (CAPES—Proc. Nos. 88881.362216/2019-01; 88882.448082/2019-01; 88882.448103/2019-01) and National Council for Scientific and Technological Development (CNPq), Brazil, for post-graduate scholarships. Figures 1 and 2 were created with Biorender.com.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
The original online version of this article was revised: Following phrase added to the “acknowledgment” section “Figures 1 and 2 were created with Biorender.com.”
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
1/23/2023
A Correction to this paper has been published: 10.1007/s00705-022-05693-3
References
- 1.Lovrečić L, Maver A, Zadel M, Peterlin B (2013) The role of epigenetics in neurodegenerative diseases. In: Kishore U (ed) Neurodegenerative diseases. IntechOpen, pp 345–365. 10.5772/54744. https://www.intechopen.com/books/neurodegenerative-diseases/the-role-of-epigenetics-in-neurodegenerative-diseases. Accessed 21 Jan 2021
- 2.GBD 2016 Multiple Sclerosis Collaborators Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(3):269–285. doi: 10.1016/S1474-4422(18)30443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown RC, Lockwood AH, Sonawane BR. Neurodegenerative diseases: an overview of environmental risk factors. Environ Health Perspect. 2005;113(9):1250–1256. doi: 10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karatas H, Gurer G, Pinar A, et al. Investigation of HSV-1, HSV-2, CMV, HHV-6 and HHV-8 DNA by real-time PCR in surgical resection materials of epilepsy patients with mesial temporal lobe sclerosis. J Neurol Sci. 2008;264(1–2):151–156. doi: 10.1016/j.jns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs BC, Rothbarth PH, van der Meché FG, et al. The spectrum of antecedent infections in Guillain–Barré syndrome: a case-control study. Neurology. 1998;51(4):1110–1115. doi: 10.1212/wnl.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 7.Itzhaki RF. Herpes and Alzheimer’s disease: subversion in the central nervous system and how it might be halted. J Alzheimers Dis. 2016;54(4):1273–1281. doi: 10.3233/JAD-160607. [DOI] [PubMed] [Google Scholar]
- 8.Jang H, Boltz D, Mcclaren J, et al. Inflammatory effects of highly pathogenic H5N1 influenza virus infection in the CNS of mice. J Neurosci. 2012;32(5):1545–1559. doi: 10.1523/jneurosci.5123-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eldridge R, Osorio D, Amstalden K, et al. Antecedent presentation of neurological phenotypes in the Collaborative Cross reveals four classes with complex sex-dependencies. Sci Rep. 2020;10:7918. doi: 10.1038/s41598-020-64862-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou L, Miranda-Saksena M, Saksena NK. Viruses and neurodegeneration. Virol J. 2013;10:172. doi: 10.1186/1743-422X-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berk C, Paul G, Sabbagh M. Investigational drugs in Alzheimer’s disease: current progress. Expert Opin Investig Drugs. 2014;23(6):837–846. doi: 10.1517/13543784.2014.905542. [DOI] [PubMed] [Google Scholar]
- 12.Bowery NG, Bagetta G, Nisticó G, et al. Intrahippocampal tetanus toxin produces generalized convulsions and neurodegeneration in rats: antagonism by NMDA receptor blockers. Epilepsy Res Suppl. 1992;9:249–256. [PubMed] [Google Scholar]
- 13.Cermelli C, Vinceti M, Beretti F, et al. Risk of sporadic amyotrophic lateral sclerosis associated with seropositivity for herpesviruses and echovirus-7. Eur J Epidemiol. 2003;18(2):123–127. doi: 10.1023/a:1023067728557. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi M, Yamada T. Viral etiology for Parkinson’s disease–a possible role of influenza A virus infection. Jpn J Infect Dis. 1999;52(3):89–98. doi: 10.7883/yoken.52.89. [DOI] [PubMed] [Google Scholar]
- 15.Balin BJ, Gérard HC, Arking EJ, et al. Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Med Microbiol Immunol. 1998;187(1):23–42. doi: 10.1007/s004300050071. [DOI] [PubMed] [Google Scholar]
- 16.De Chiara G, Marcocci ME, Sgarbanti R, et al. Infectious agents and neurodegeneration. Mol Neurobiol. 2012;46(3):614–638. doi: 10.1007/s12035-012-8320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Ben JG, Colin LM, et al. A systemic view of Alzheimer disease—insights from amyloid-β metabolism beyond the brain. Nat Rev Neurol. 2017;13(10):612–623. doi: 10.1038/nrneurol.2017.111. [DOI] [PubMed] [Google Scholar]
- 18.McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol. 2011;1(5):318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattson MP. Infectious agents and age-related neurodegenerative disorders. Ageing Res Rev. 2004;3(1):105–120. doi: 10.1016/j.arr.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strazza M, Pirrone V, Wigdahl B, et al. Breaking down the barrier: the effects of HIV-1 on the blood–brain barrier. Brain Res. 2011;1399:96–115. doi: 10.1016/j.brainres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh MJ, White PJ, Pouton CW. Interaction of viruses with host cell molecular motors. Curr Opin Biotechnol. 2010;21(5):633–639. doi: 10.1016/j.copbio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Matsuda K, Park CH, Sunden Y, et al. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet Pathol. 2004;41(2):101–107. doi: 10.1354/vp.41-2-101. [DOI] [PubMed] [Google Scholar]
- 23.Salinas S, Schiavo G, Kremer EJ. A hitchhiker’s guide to the nervous system: the complex journey of viruses and toxins. Nat Rev Microbiol. 2010;8(9):645–655. doi: 10.1038/nrmicro2395. [DOI] [PubMed] [Google Scholar]
- 24.Lucatelli JF, Barros AC, Maluf SW, et al. Influência genética sobre a doença de Alzheimer de início precoce. Rev Psiq Clín. 2009;36(1):25–30. doi: 10.1590/S0101-60832009000100004. [DOI] [Google Scholar]
- 25.Alonso Vilatela ME, López-López M, Yescas-Gómez P. Genetics of Alzheimer's disease. Arch Med Res. 2012;43(8):622–631. doi: 10.1016/j.arcmed.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Rall SC, Jr, Weisgraber KH, Mahley RW. Human apolipoprotein E. The complete amino acid sequence. J Biol Chem. 1982;257(8):4171–4178. doi: 10.1016/S0021-9258(18)34702-1. [DOI] [PubMed] [Google Scholar]
- 27.Masters CL, Selkoe DJ. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(6):a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cárdenas-Aguayo MDC, Silva-Lucero MDC, Cortes-Ortiz M, et al. Physiological role of amyloid β in neural cells: the cellular trophic activity. In: Heinbockel T, et al., editors. Neurochemistry. London: Intechopen; 2014. pp. 257–281. [Google Scholar]
- 29.De-Paula VJ, Radanovic M, Diniz BS, et al. Alzheimer’s disease. Subcell Biochem. 2012;65:329–352. doi: 10.1007/978-94-007-5416-4_14. [DOI] [PubMed] [Google Scholar]
- 30.Kolarova M, García-Sierra F, Bartos A, et al. Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis. 2012;2012:731526. doi: 10.1155/2012/731526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mondragón-Rodríguez S, Perry G, Zhu X, et al. Phosphorylation of tau protein as the link between oxidative stress, mitochondrial dysfunction, and connectivity failure: implications for Alzheimer’s disease. Oxid Med Cell Longev. 2013;2013:940603. doi: 10.1155/2013/940603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JM, Sun C. Calcium and neurogenesis in Alzheimer’s disease. Front Neurosci. 2010;4:194. doi: 10.3389/fnins.2010.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci USA. 1993;90(2):567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev. 2013;2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boland B, Kumar A, Lee S, et al. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J Neurosci. 2008;28(27):6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Wang W, Li L, et al. (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta. 1842;8:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy PH, Mani G, Park BS et al (2005) Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis. 7(2):103–17; discussion 173–80. 10.3233/jad-2005-7203 [DOI] [PubMed]
- 38.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012;2(1):a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi RH, Nagao T, Gouras GK. Plaque formation and the intraneuronal accumulation of β-amyloid in Alzheimer’s disease. Pathol Int. 2017;67(4):185–193. doi: 10.1111/pin.12520. [DOI] [PubMed] [Google Scholar]
- 40.Alonso R, Pisa D, Marina AI, et al. Fungal infection in patients with Alzheimer’s disease. J Alzheimers Dis. 2014;41(1):301–311. doi: 10.3233/JAD-132681. [DOI] [PubMed] [Google Scholar]
- 41.Pisa D, Alonso R, Rábano A, et al. Different brain regions are infected with fungi in Alzheimer’s disease. Sci Rep. 2015;5:15015. doi: 10.1038/srep15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jamieson GA, Maitland NJ, Wilcock GK, et al. Herpes simplex virus type 1 DNA is present in specific regions of brain from aged people with and without senile dementia of the Alzheimer type. J Pathol. 1992;167(4):365–368. doi: 10.1002/path.1711670403. [DOI] [PubMed] [Google Scholar]
- 43.Letenneur L, Pérès K, Fleury H, et al. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer’s disease: a population-based cohort study. PLoS ONE. 2008;3(11):e3637. doi: 10.1371/journal.pone.0003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.L¨ovheim H, Gilthorpe J, Johansson A, , et al. Herpes simplex infection and the risk of Alzheimer’s disease: a nested case-control study. Alzheimers Dement. 2015;11:587–592. doi: 10.1016/j.jalz.2014.07.157. [DOI] [PubMed] [Google Scholar]
- 45.Mancuso R, Baglio F, Cabinio M, et al. Titers of herpes simplex vírus type 1 antibodies positively correlate with grey matter volumes in Alzheimer’s disease. J Alzheimers Dis. 2014;38(4):741–745. doi: 10.3233/JAD-130977. [DOI] [PubMed] [Google Scholar]
- 46.Cribbs DH, Azizeh BY, Cotman CW, et al. Fibril formation and neurotoxicity by a herpes simplex virus glycoprotein B fragment with homology to the Alzheimer’s A beta peptide. Biochemistry. 2000;39(20):5988–5994. doi: 10.1021/bi000029f. [DOI] [PubMed] [Google Scholar]
- 47.Tallóczy Z, Virgin HW, 4th, Levine B. PKR-dependent autophagic degradation of herpes simplex virus type 1. Autophagy. 2006;2(1):24–29. doi: 10.4161/auto.2176. [DOI] [PubMed] [Google Scholar]
- 48.Wozniak MA, Frost AL, Itzhaki RF. Alzheimer’s disease-specific tau phosphorylation is induced by herpes simplex virus type 1. J Alzheimers Dis. 2009;16(2):341–350. doi: 10.3233/JAD-2009-0963. [DOI] [PubMed] [Google Scholar]
- 49.Valyi-Nagy T, Dermody TS. Role of oxidative damage in the pathogenesis of viral infections of the nervous system. Histol Histopathol. 2005;20(3):957–967. doi: 10.14670/HH-20.957. [DOI] [PubMed] [Google Scholar]
- 50.Santana S, Sastre I, Recuero M, et al. Oxidative stress enhances neurodegeneration markers induced by herpes simplex virus type 1 infection in human neuroblastoma cells. PLoS ONE. 2013;8(10):e75842. doi: 10.1371/journal.pone.0075842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aiello AE, Haan M, Blythe L, et al. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54(7):1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- 52.Carbone I, Lazzarotto T, Ianni M, et al. Herpes virus in Alzheimer’s disease: relation to progression of the disease. Neurobiol Aging. 2014;35:122–129. doi: 10.1016/j.neurobiolaging.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 53.Barnes LL, Capuano AW, Aiello AE, et al. Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J Infect Dis. 2015;211(2):230–237. doi: 10.1093/infdis/jiu437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin WR, Wozniak MA, Wilcock GK, et al. Cytomegalovirus is present in a very high proportion of brains from vascular dementia patients. Neurobiol Dis. 2002;9(1):82–87. doi: 10.1006/nbdi.2001.0465. [DOI] [PubMed] [Google Scholar]
- 55.Almanzar G, Schwaiger S, Jenewein B, et al. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J Virol. 2005;79(6):3675–3683. doi: 10.1128/JVI.79.6.3675-3683.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lurain NS, Hanson BA, Martinson J, et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis. 2013;208(4):564–572. doi: 10.1093/infdis/jit210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao K, Crawford JR, Komaroff AL, et al. Review part 2: Human herpesvirus-6 in central nervous system diseases. J Med Virol. 2010;82(10):1669–1678. doi: 10.1002/jmv.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin WR, Wozniak MA, Cooper RJ, et al. Herpesviruses in brain and Alzheimer’s disease. J Pathol. 2002;197(3):395–402. doi: 10.1002/path.1127. [DOI] [PubMed] [Google Scholar]
- 59.Romeo MA, Gilardini Montani MS, Gaeta A, et al. (2020) HHV-6A infection dysregulates autophagy/UPR interplay increasing beta amyloid production and tau phosphorylation in astrocytoma cells as well as in primary neurons, possible molecular mechanisms linking viral infection to Alzheimer’s disease. Biochim Biophys Acta Mol Basis Dis. 1866;3:165647. doi: 10.1016/j.bbadis.2019.165647. [DOI] [PubMed] [Google Scholar]
- 60.Chiu WC, Tsan YT, Tsai SL et al (2014) Health data analysis in Taiwan (hDATa) Research Group. Hepatitis C viral infection and the risk of dementia. Eur J Neurol 21(8):1068-e59. 10.1111/ene.12317 [DOI] [PubMed]
- 61.Senzolo M, Schiff S, D’Aloiso CM, et al. Neuropsychological alterations in hepatitis C infection: the role of inflammation. World J Gastroenterol. 2011;17(29):3369–3374. doi: 10.3748/wjg.v17.i29.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parkinson J. An essay on the shaking palsy. London: Whittingham and Rowland for Sherwood, Needly and Jones; 1817. [Google Scholar]
- 63.Parkinsons’s Foundation. https://www.parkinson.org/Understanding-Parkinsons/Statistics. Accessed 2 Aug 2020
- 64.Marras C, Beck JC, Bower JH, et al. Parkinson’s Foundation P4 Group. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis. 2018;4:21. doi: 10.1038/s41531-018-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 66.Grosch J, Winkler J, Kohl Z. Early degeneration of both dopaminergic and serotonergic axons—a common mechanism in Parkinson’s disease. Front Cell Neurosci. 2016;10:293. doi: 10.3389/fncel.2016.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braak H, Tredici KD, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 68.Tavares A, Azeredo C. Demência com corpos de Lewy: uma revisão para o psiquiatra. Rev Psiq Clín. 2003;30(1):29–34. doi: 10.1590/S0101-60832003000100004. [DOI] [Google Scholar]
- 69.Mhyre TR, Boyd JT, Hamill RW, et al. Parkinson’s disease. Subcell Biochem. 2012;65:389–455. doi: 10.1007/978-94-007-5416-4_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mohan M, Mellick GD. Role of the VPS35 D620N mutation in Parkinson’s disease. Parkinsonism Related Disord. 2017;36:10–18. doi: 10.1016/j.parkreldis.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Limphaibool N, Iwanowski P, Holstad MJV, et al. Infectious Etiologies of Parkinsonism: pathomechanisms and clinical implications. Front Neurol. 2019;10:652. doi: 10.3389/fneur.2019.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka H, Park CH, Ninomiya A, et al. Neurotropism of the 1997 Hong Kong H5N1 influenza virus in mice. Vet Microbiol. 2003;95(1–2):1–13. doi: 10.1016/s0378-1135(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 73.Klopfleisch R, Werner O, Mundt E, et al. Neurotropism of highly pathogenic avian influenza virus A/chicken/Indonesia/2003 (H5N1) in experimentally infected pigeons (Columbialivia f. domestica) Vet Pathol. 2006;43(4):463–470. doi: 10.1354/vp.43-4-463. [DOI] [PubMed] [Google Scholar]
- 74.Rigoni M, Shinya K, Toffan A, et al. Pneumo- and neurotropism of avian origin Italian highly pathogenic avian influenza H7N1 isolates in experimentally infected mice. Virology. 2007;364(1):28–35. doi: 10.1016/j.virol.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 75.Sadasivan S, Sharp B, Schultz-Cherry S, et al. Synergistic effects of influenza and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can be eliminated by the use of influenza therapeutics: experimental evidence for the multi-hit hypothesis. NPJ Parkinsons Dis. 2017;3:18. doi: 10.1038/s41531-017-0019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bu XL, Wang X, Xiang Y, et al. The association between infectious burden and Parkinson’s disease: a case-control study. Parkinsonism Relat Disord. 2015;21(8):877–881. doi: 10.1016/j.parkreldis.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 77.Woulfe J, Hoogendoorn H, Tarnopolsky M, et al. Monoclonal antibodies against Epstein-Barr virus cross-react with α-synuclein in human brain. Neurology. 2000;55(9):1398–1440. doi: 10.1212/wnl.55.9.1398. [DOI] [PubMed] [Google Scholar]
- 78.Caggiu E, Paulus K, Galleri G, et al. Homologous HSV1 and alpha-synuclein peptides stimulate a T cell response in Parkinson’s disease. J Neuroimmunol. 2017;310:26–31. doi: 10.1016/j.jneuroim.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 79.Cury RG, Lopez WOC. Bilateral striatal lesion due to herpesvirus-6 infection. J Neurol Sci. 2015;358(1–2):538–539. doi: 10.1016/j.jns.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 80.Goldeck D, Maetzler W, Berg D, et al. Altered dendritic cell subset distribution in patients with Parkinson’s disease: impact of cmv serostatus. J Neuroimmunol. 2016;290:60–65. doi: 10.1016/j.jneuroim.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 81.Koutsilieri E, Lutz MB, Scheller C. Autoimmunity, dendritic cells and relevance for Parkinson’s disease. J Neural Transm (Vienna) 2013;120(1):75–81. doi: 10.1007/s00702-012-0842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adinolfi LE, Nevola R, Lus G, et al. Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World J Gastroenterol. 2015;21(8):2269–2280. doi: 10.3748/wjg.v21.i8.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu WY, Kang KH, Chen SL, et al. Hepatitis C virus infection: a risk factor for Parkinson’s disease. J Viral Hepat. 2015;22(10):784–791. doi: 10.1111/jvh.12392. [DOI] [PubMed] [Google Scholar]
- 84.Fletcher NF, Wilson GK, Murray J, et al. Hepatitis C virus infects the endothelial cells of the blood-brain barrier. Gastroenterology. 2012;142(3):634–643.e6. doi: 10.1053/j.gastro.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Golabi P, Otgonsuren M, Sayiner M, et al. The prevalence of parkinson disease among patients with hepatitis C infection. Ann Hepatol. 2017;16(3):342–348. doi: 10.5604/16652681.1235476. [DOI] [PubMed] [Google Scholar]
- 86.Berger JR, Nath A, Greenberg RN, et al. Cerebrovascular changes in the basal ganglia with HIV dementia. Neurology. 2000;4(4):921–926. doi: 10.1212/WNL.54.4.921. [DOI] [PubMed] [Google Scholar]
- 87.Khanlou N, Moore DJ, Chana G, et al. Increased frequency of alpha-synuclein in the substantia nigra in human immunodeficiency virus infection. J Neurovirol. 2009;15(2):131–138. doi: 10.1080/13550280802578075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Puccini JM, Marker DF, Fitzgerald T, et al. Leucine-rich repeat kinase 2 modulates neuroinflammation and neurotoxicity in models of human immunodeficiency virus 1-associated neurocognitive disorders. J Neurosci. 2015;35(13):5271–5283. doi: 10.1523/JNEUROSCI.0650-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Josephson CB, Jetté N. Psychiatric comorbidities in epilepsy. Int Rev Psychiatry. 2017;29(5):409–424. doi: 10.1080/09540261.2017.1302412. [DOI] [PubMed] [Google Scholar]
- 90.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;4:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 91.Klein P, Tyrlikova I. No prevention or cure of epilepsy as yet. Neuropharmacology. 2020;168:107762. doi: 10.1016/j.neuropharm.2019.107762. [DOI] [PubMed] [Google Scholar]
- 92.Messori A, Polonara G, Carle F, et al. Predicting post-traumatic epilepsy with MRI: prospective longitudinal morphologic study in adults. Epilepsia. 2005;46(9):1472–1481. doi: 10.1111/j.1528-1167.2005.34004.x. [DOI] [PubMed] [Google Scholar]
- 93.Gilad R, Lampl Y, Eilam A, et al. SPECT-DTPA as a tool for evaluating the blood-brain barrier in post-stroke seizures. J Neurol. 2012;259(10):2041–2044. doi: 10.1007/s00415-012-6445-2. [DOI] [PubMed] [Google Scholar]
- 94.Klein P, Dingledine R, Aronica E, et al. Commonalities in epileptogenic processes from different acute brain insults: do they translate? Epilepsia. 2018;59(1):37–66. doi: 10.1111/epi.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Del Brutto OH, Engel J, Jr, Eliashiv DS, et al. Update on cysticercosis epileptogenesis: the role of the hippocampus. Curr Neurol Neurosci Rep. 2016;16(1):1. doi: 10.1007/s11910-015-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vezzani A, Fujinami RS, White HS, et al. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131(2):211–234. doi: 10.1007/s00401-015-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paudel YN, Shaikh MF, Shah S, et al. Role of inflammation in epilepsy and neurobehavioral comorbidities: implication for therapy. Eur J Pharmacol. 2018;837:145–155. doi: 10.1016/j.ejphar.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 98.Balosso S, Ravizza T, Aronica E, et al. The dual role of TNF-α and its receptors in seizures. Exp Neurol. 2013;247:267–271. doi: 10.1016/j.expneurol.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 99.Chikuma T, Yoshimoto T, Ohba M, et al. Interleukin-6 induces prostaglandin E(2) synthesis in mouse astrocytes. J Mol Neurosci. 2009;39(1–2):175–184. doi: 10.1007/s12031-009-9187-6. [DOI] [PubMed] [Google Scholar]
- 100.Samland H, Huitron-Resendiz S, Masliah E, et al. Profound increase in sensitivity to glutamatergic- but not cholinergic agonist-induced seizures in transgenic mice with astrocyte production of IL-6. J Neurosci Res. 2003;73(2):176–187. doi: 10.1002/jnr.10635. [DOI] [PubMed] [Google Scholar]
- 101.Singh A, Trevick S. The epidemiology of global epilepsy. Neurol Clin. 2016;34(4):837–847. doi: 10.1016/j.ncl.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 102.Theodore WH. Epilepsy and viral infections. Epilepsy Curr. 2014;14(1 Suppl):35–42. doi: 10.5698/1535-7511-14.s2.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bartolini L, Libbey JE, Ravizza T, et al. Viral triggers and inflammatory mechanisms in pediatric epilepsy. Mol Neurobiol. 2019;56(3):1897–1907. doi: 10.1007/s12035-018-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bartolini L, Piras E, Sullivan K, et al. Detection of HHV-6 and EBV and cytokine levels in saliva from children with seizures: results of a multi-center cross-sectional study. Front Neurol. 2018;9:834. doi: 10.3389/fneur.2018.00834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Niehusmann P, Mittelstaedt T, Bien CG, et al. Presence of human herpes virus 6 DNA exclusively in temporal lobe epilepsy brain tissue of patients with history of encephalitis. Epilepsia. 2010;51(12):2478–2483. doi: 10.1111/j.1528-1167.2010.02741.x. [DOI] [PubMed] [Google Scholar]
- 106.Cuomo L, Trivedi P, Cardillo MR, et al. Human herpesvirus 6 infection in neoplastic and normal brain tissue. J Med Virol. 2001;1:45–51. doi: 10.1002/1096-9071(200101)63:1<45::AID-JMV1006>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 107.Esposito L, Drexler JF, Braganza O, et al. Large-scale analysis of viral nucleic acid spectrum in temporal lobe epilepsy biopsies. Epilepsia. 2015;56(2):234–243. doi: 10.1111/epi.12890. [DOI] [PubMed] [Google Scholar]
- 108.Fotheringham J, Donati D, Akhyani N, et al. Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med. 2007;5:e180. doi: 10.1371/journal.pmed.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luppi M, Barozzi P, Maiorana A, et al. Human herpesvirus-6: a survey of presence and distribution of genomic sequences in normal brain and neuroglial tumors. J Med Virol. 1995;47(1):105–111. doi: 10.1002/jmv.1890470119. [DOI] [PubMed] [Google Scholar]
- 110.Harberts E, Yao K, Wohler JE, et al. Human herpesvirus-6 entry into the central nervous system through the olfactory pathway. Proc Natl Acad Sci USA. 2011;108(33):13734–13739. doi: 10.1073/pnas.1105143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.De Almeida SM, Crippa A, Cruz C, et al. Reactivation of herpes simplex virus-1 following epilepsy surgery. Epilepsy Behav Case Rep. 2015;4:76–78. doi: 10.1016/j.ebcr.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Misra UK, Tan CT, Kalita J. Viral encephalitis and epilepsy. Epilepsia. 2008;49(Suppl 6):13–18. doi: 10.1111/j.1528-1167.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 113.Solomon T, Dung NM, Kneen R, et al. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain. 2002;125(Pt 5):1084–1093. doi: 10.1093/brain/awf116. [DOI] [PubMed] [Google Scholar]
- 114.Jennische E, Eriksson CE, Lange S, et al. The anterior commissure is a pathway for contralateral spread of herpes simplex virus type 1 after olfactory tract infection. J Neurovirol. 2015;21(2):129–147. doi: 10.1007/s13365-014-0312-0. [DOI] [PubMed] [Google Scholar]
- 115.Poulheim F, Esposito L, Elger CE, et al. Large-scale analysis of herpesviridae in epilepsy-patients with signs of autoimmune encephalitis. Seizure. 2017;53:100–102. doi: 10.1016/j.seizure.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki Y, Toribe Y, Mogami Y, et al. Epilepsy in patients with congenital cytomegalovirus infection. Brain Dev. 2008;30(6):420–424. doi: 10.1016/j.braindev.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 117.Schleiss MR. Congenital cytomegalovirus infection: molecular mechanisms mediating viral pathogenesis. Infect Disord Drug Targets. 2011;1(5):449–465. doi: 10.2174/187152611797636721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lei HY, Yang DQ, Li YX, et al. Association between human cytomegalovirus and onset of epilepsy. Int J Clin Exp Med. 2015;8(11):20556–20564. [PMC free article] [PubMed] [Google Scholar]
- 119.Rust RS. Arbovirus Encephalitis. In: Aminoff MJ, Daroff RB, editors. Encyclopedia of the neurological sciences. 1. Amsterdam: Elsevier; 2014. pp. 260–270. [Google Scholar]
- 120.Verma R, Varatharaj A. Epilepsia partialis continua as a manifestation of dengue encephalitis. Epilepsy Behav. 2011;20(2):395–397. doi: 10.1016/j.yebeh.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 121.Guabiraba R, Ryffel B. Dengue virus infection: current concepts in immune mechanisms and lessons from murine models. Immunology. 2014;141(2):143–156. doi: 10.1111/imm.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shresta S, Sharar KL, Prigozhin DM, et al. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80(20):10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johnson AJ, Roehrig JT. New mouse model for dengue virus vaccine testing. J Virol. 1999;73(1):783–786. doi: 10.1128/JVI.73.1.783-786.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kellinghaus C, Engbring C, Kovac S, et al. Frequency of seizures and epilepsy in neurological HIV-infected patients. Seizure. 2008;17(1):27–33. doi: 10.1016/j.seizure.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 125.Ssentongo P. Prevalence and incidence of new-onset seizures and epilepsy in patients with human immunodeficiency virus (HIV): systematic review and meta-analysis. Epilepsy Behav. 2019;93:49–55. doi: 10.1016/j.yebeh.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 126.Devinsky O, Vezzani A, O’Brien TJ, et al. Epilepsy. Nat Rev Dis Primers. 2018;4:18024. doi: 10.1038/nrdp.2018.24. [DOI] [PubMed] [Google Scholar]
- 127.Garg RK. HIV infection and seizures. Postgrad Med J. 1999;75(885):387–390. doi: 10.1136/pgmj.75.885.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vezzani A. Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy Curr. 2014;14(1 Suppl):3–7. doi: 10.5698/1535-7511-14.s2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brownlee WJ, Hardy TA, Fazekas F, et al. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2017;389(10076):1336–1346. doi: 10.1016/S0140-6736(16)30959-X. [DOI] [PubMed] [Google Scholar]
- 130.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ochi H. Cognitive impairment in multiple sclerosis. Brain Nerve. 2014;66(10):1201–1209. doi: 10.11477/mf.1416200011. [DOI] [PubMed] [Google Scholar]
- 132.Tomassini V, Johansen-Berg H, Leonardi L, et al. Preservation of motor skill learning in patients with multiple sclerosis. Mult Scler. 2011;17(1):103–115. doi: 10.1177/1352458510381257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maitin IB, Cruz E. Special considerations and assessment in patients with multiple sclerosis. Phys Med Rehabil Clin N Am. 2018;29(3):473–481. doi: 10.1016/j.pmr.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 134.Eskandarieh S, Heydarpour P, Minagar A, et al. Multiple Sclerosis epidemiology in East Asia, South East Asia and South Asia: a systematic review. Neuroepidemiology. 2016;46(3):209–221. doi: 10.1159/000444019. [DOI] [PubMed] [Google Scholar]
- 135.Gilmour H, Ramage-Morin PL, Wong SL. Multiple sclerosis: prevalence and impact. Health Rep. 2018;29(1):3–8. [PubMed] [Google Scholar]
- 136.Afshar B, Khalifehzadeh-Esfahani Z, Seyfizadeh N, et al. The role of immune regulatory molecules in multiple sclerosis. J Neuroimmunol. 2019;337:577061. doi: 10.1016/j.jneuroim.2019.577061. [DOI] [PubMed] [Google Scholar]
- 137.Sadovnick AD, Risch NJ, Ebers GC. Canadian collaborative project on genetic susceptibility to MS, phase 2: rationale and method. Canadian Collaborative Study Group. Can J Neurol Sci. 1998;25(3):216–221. doi: 10.1017/s0317167100034041. [DOI] [PubMed] [Google Scholar]
- 138.Sadovnick D. The place of environmental factors in multiple sclerosis: genes, environment and the interactions thereof in the etiology of multiple sclerosis. Rev Neurol. 2019;175(10):593–596. doi: 10.1016/j.neurol.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 139.Virtanen JO, Jacobson S. Viruses and multiple sclerosis. CNS Neurol Disord Drug Targets. 2012;11(5):528–544. doi: 10.2174/187152712801661220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kb F, Jhd M, Haire M, et al. Increased tendency to spontaneous in-vitro lymphocyte transformation in clinically active multiple sclerosis. Lancet. 1979;314:715–717. doi: 10.1016/S0140-6736(79)90643-3. [DOI] [PubMed] [Google Scholar]
- 141.Ahmed SI, Aziz K, Gul A, et al. Risk of multiple sclerosis in Epstein–Barr virus infection. Cureus. 2019;11(9):e5699. doi: 10.7759/cureus.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.De Jager PL, Simon KC, Munger KL, et al. Integrating risk factors: HLA-DRB1*1501 and Epstein–Barr virus in multiple sclerosis. Neurology. 2008;70(13 Pt 2):1113–1118. doi: 10.1212/01.wnl.0000294325.63006.f8. [DOI] [PubMed] [Google Scholar]
- 143.Guan Y, Jakimovski D, Ramanathan M, et al. The role of Epstein–Barr virus in multiple sclerosis: from molecular pathophysiology to in vivo imaging. Neural Regen Res. 2019;14(3):373–386. doi: 10.4103/1673-5374.245462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Küry P, Nath A, Créange A, et al. Human endogenous retroviruses in neurological diseases. Trends Mol Med. 2018;24(4):379–394. doi: 10.1016/j.molmed.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grandi N, Tramontano E. Human endogenous retroviruses are ancient acquired elements still shaping innate immune responses. Front Immunol. 2018;9:2039. doi: 10.3389/fimmu.2018.02039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khan G, Hassani A (2019) Epstein–Barr virus in multiple sclerosis. In: Baloyannis SJ (ed) Multiple sclerosis. Intechopen. 10.5772/intechopen.85222. https://www.intechopen.com/books/multiple-sclerosis/epstein-barr-virus-in-multiple-sclerosis
- 147.Linington C, Kaushansky N, Chapple K, Ben-Nun A (2014) Myelin oligodendrocyte glycoprotein (MOG): an archetypal target for demyelinating autoantibodies in the central nervous system. In: Autoantibodies, 3rd edn. Elsvier, pp 617–627. 10.1016/B978-0-444-56378-1.00073-3
- 148.Jagessar SA, Holtman IR, Hofman S, et al. Lymphocryptovirus infection of nonhuman primate B cells converts destructive into productive processing of the pathogenic CD8 T cell epitope in myelin oligodendrocyte glycoprotein. J Immunol. 2016;197(4):1074–1088. doi: 10.4049/jimmunol.1600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ruprecht K, Wildemann B, Jarius S. Low intrathecal antibody production despite high seroprevalence of Epstein–Barr virus in multiple sclerosis: a review of the literature. J Neurol. 2018;265(2):239–252. doi: 10.1007/s00415-017-8656-z. [DOI] [PubMed] [Google Scholar]
- 150.Jarius S, Eichhorn P, Franciotta D, et al. The MRZ reaction as a highly specific marker of multiple sclerosis: re-evaluation and structured review of the literature. J Neurol. 2017;264(3):453–466. doi: 10.1007/s00415-016-8360-4. [DOI] [PubMed] [Google Scholar]
- 151.Akhyani N, Berti R, Brennan MB, et al. Tissue distribution and variant characterization of human herpesvirus (HHV)-6: increased prevalence of HHV-6A in patients with multiple sclerosis. J Infect Dis. 2000;182(5):1321–1325. doi: 10.1086/315893. [DOI] [PubMed] [Google Scholar]
- 152.Leibovitch EC, Brunetto GS, Caruso B, et al. Coinfection of human herpesviruses 6A (HHV-6A) and HHV-6B as demonstrated by novel digital droplet PCR assay. PLoS ONE. 2014;9(3):e92328. doi: 10.1371/journal.pone.0092328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Reynaud JM, Jégou JF, Welsch JC, et al. Human herpesvirus 6A infection in CD46 transgenic mice: viral persistence in the brain and increased production of proinflammatory chemokines via Toll-like receptor 9. J Virol. 2014;88(10):5421–5436. doi: 10.1128/JVI.03763-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Engdahl E, Gustafsson R, Huang J, et al. Increased serological response against human herpesvirus 6A is associated with risk for multiple sclerosis. Front Immunol. 2019;10:2715. doi: 10.3389/fimmu.2019.02715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ahlqvist J, Fotheringham J, Akhyani N, et al. Differential tropism of human herpesvirus 6 (HHV-6) variants and induction of latency by HHV-6A in oligodendrocytes. J Neurovirol. 2005;11(4):384–394. doi: 10.1080/13550280591002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Campbell A, Hogestyn JM, Folts CJ, et al. Expression of the human herpesvirus 6A latency-associated transcript U94A disrupts human oligodendrocyte progenitor migration. Sci Rep. 2017;7(1):3978. doi: 10.1038/s41598-017-04432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McGrogan A, Madle GC, Seaman HE, et al. The epidemiology of Guillain–Barré syndrome worldwide A systematic literature review. Neuroepidemiology. 2008;32(2):150–163. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 158.Ansar V, Valadi N. Guillain–Barré syndrome. Prim Care. 2015;42(2):189–193. doi: 10.1016/j.pop.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 159.Malek E, Salameh J. Guillain–Barre syndrome. Semin Neurol. 2019;39(5):589–595. doi: 10.1055/s-0039-1693005. [DOI] [PubMed] [Google Scholar]
- 160.Leung J, Sejvar JJ, Soares J, et al. Guillain–Barré syndrome and antecedent cytomegalovirus infection, USA 2009–2015. Neurol Sci. 2019;41(4):885–891. doi: 10.1007/s10072-019-04156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tavares AC, Alves CBL, Silva MA et al (2000) Síndrome de Guillain–Barré: revisão de literatura. Cad Bras Med 13(1):2. ISSN 2412-2688
- 162.Rodríguez Y, Rojas M, Pacheco Y, et al. Guillain–Barré syndrome, transverse myelitis and infectious diseases. Cell Mol Immunol. 2018;15(6):547–562. doi: 10.1038/cmi.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kaida K. Guillain–Barré syndrome. Adv Exp Med Biol. 2019;1190:323–331. doi: 10.1007/978-981-32-9636-7_20. [DOI] [PubMed] [Google Scholar]
- 164.Blum S, McCombe PA. Genetics of Guillain–Barré syndrome (GBS) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): current knowledge and future directions. J Peripher Nerv Syst. 2014;19(2):88–103. doi: 10.1111/jns5.12074. [DOI] [PubMed] [Google Scholar]
- 165.Dilena R, Strazzer S, Esposito S, et al. Locked-in-like fulminant infantile Guillain–Barré syndrome associated with herpes simplex virus 1 infection. Muscle Nerve. 2016;53(1):140–143. doi: 10.1002/mus.24908. [DOI] [PubMed] [Google Scholar]
- 166.Yuki N, Susuki K, Odaka M, et al. Overlapping Guillain–Barré syndrome and Bickerstaff’s brainstem encephalitis associated with anti-GQ1b IgG antibody after herpes simplex virus infection. Acta Neurol Scand. 2001;104(1):57–60. doi: 10.1034/j.1600-0404.2001.00288.x. [DOI] [PubMed] [Google Scholar]
- 167.Ang CW, Jacobs BC, Brandenburg AH, et al. Cross-reactive antibodies against GM2 and CMV-infected fibroblasts in Guillain–Barré syndrome. Neurology. 2000;54(7):1453–1458. doi: 10.1212/wnl.54.7.1453. [DOI] [PubMed] [Google Scholar]
- 168.Miyaji K, Furukawa JI, Suzuki Y, et al. Altered gene expression of glycosyltransferases and sialyltransferases and total amount of glycosphingolipids following herpes simplex virus infection. Carbohydr Res. 2016;434:37–43. doi: 10.1016/j.carres.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 169.Klemola E, Weckman N, Haltia K, et al. The Guillain–Barré syndrome associated with acquired cytomegalovirus infection. Acta Med Scand. 1967;181(5):603–607. doi: 10.1111/j.0954-6820.1967.tb07283.x. [DOI] [PubMed] [Google Scholar]
- 170.Irie S, Saito T, Nakamura K, et al. Association of anti-GM2 antibodies in Guillain–Barré syndrome with acute cytomegalovirus infection. J Neuroimmunol. 1996;68(1–2):19–26. doi: 10.1016/0165-5728(96)00059-8. [DOI] [PubMed] [Google Scholar]
- 171.Sawai S, Satoh M, Mori M, et al. Moesin is a possible target molecule for cytomegalovirus-related Guillain–Barré syndrome. Neurology. 2014;83(2):113–117. doi: 10.1212/WNL.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 172.Merelli E, Sola P, Faglioni P, et al. Newest human herpesvirus (HHV-6) in the Guillain–Barré syndrome and other neurological diseases. Acta Neurol Scand. 1992;85(5):334–336. doi: 10.1111/j.1600-0404.1992.tb04054.x. [DOI] [PubMed] [Google Scholar]
- 173.Masajtis-Zagajewska A, Muras K, Mochecka-Thoelke A, et al. Guillain–Barré syndrome in the course of EBV infection after kidney transplantation—a case report. Ann Transplant. 2012;17(3):133–137. doi: 10.12659/aot.883468. [DOI] [PubMed] [Google Scholar]
- 174.Grose C, Feorino P. Epstein–Barr virus and Guillain–Barré syndrome. Lancet. 1972;300(7790):1285–1287. doi: 10.1016/S0140-6736(72)92654-2. [DOI] [PubMed] [Google Scholar]
- 175.Kennedy M, Apostolova M. A rare case of infectious mononucleosis complicated by Guillain–Barre syndrome. Neurol Int. 2013;5(2):20–22. doi: 10.4081/ni.2013.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yamana M, Kuwahara M, Fukumoto Y, et al. Guillain–Barré syndrome and related diseases after influenza virus infection. Neurol Neuroimmunol Neuroinflamm. 2019;6(4):e575. doi: 10.1212/NXI.0000000000000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.De Klippel N, Hautekeete ML, De Keyser J, et al. Guillain–Barré syndrome as the presenting manifestation of hepatitis C infection. Neurology. 1993;43(10):2143. doi: 10.1212/wnl.43.10.2143. [DOI] [PubMed] [Google Scholar]
- 178.Molokwu O, Young BM, Singh M, et al. Reactivation of chronic hepatitis C as a potential trigger for Guillain–Barré syndrome. Cureus. 2019;11(7):e5244. doi: 10.7759/cureus.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lacaille F, Zylberberg H, Hagège H, et al. Hepatitis C associated with Guillain–Barré syndrome. Liver. 1998;18(1):49–51. doi: 10.1111/j.1600-0676.1998.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 180.Chlilek A, Roger C, Muller L, et al. Severe Guillain–Barré syndrome associated with chronic active hepatitis C and mixed cryoglobulinemia: a case report. BMC Infect Dis. 2019;19(1):636. doi: 10.1186/s12879-019-4278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mariotto S, Ferrari S, Monaco S. HCV-related central and peripheral nervous system demyelinating disorders. Inflamm Allergy Drug Targets. 2014;13(5):299–304. doi: 10.2174/1871528113666140908113841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shah I (2007) Dengue presenting as Guillain–Barre syndrome. Dengue Bull. 31:166–8. https://apps.who.int/iris/bitstream/handle/10665/170357/db2007v31p166.pdf?sequence=1&isAllowed=y. Accessed 4 Aug 2020
- 183.Oishi K, Saito M, Mapua CA, et al. Dengue illness: clinical features and pathogenesis. J Infect Chemother. 2007;13(3):125–133. doi: 10.1007/s10156-007-0516-9. [DOI] [PubMed] [Google Scholar]
- 184.Latorre FG, Magalhães GO, Costa ISL et al (2019) Síndrome de Guillain–Barré como complicação da infecção pelo vírus da dengue. Braz J Surg Clin Res 28(1): 25–29. ISSN online: 2317-4404
- 185.Wakerley BR, Yuki N. Infectious and noninfectious triggers in Guillain–Barré syndrome. Expert Rev Clin Immunol. 2013;9(7):627–639. doi: 10.1586/1744666X.2013.811119. [DOI] [PubMed] [Google Scholar]
- 186.Henning F, Bouic P. Increased frequency of Guillain–Barré syndrome in HIV infection: a prospective cohort study. J AIDS Clin Res. 2014;5(8):1–5. doi: 10.4172/2155-6113.1000332. [DOI] [Google Scholar]
- 187.Fantauzzi A, Digiulio MA, Cavallari EN, et al. Guillain Barre syndrome in an HIV-1-infected patient after the beginning of combined antiretroviral therapy: an immune reconstitution inflammatory syndrome? New Microbiol. 2014;37(1):103–107. [PubMed] [Google Scholar]
- 188.Spindler KR, Hsu TH. Viral disruption of the blood-brain barrier. Trends Microbiol. 2012;20(6):282–290. doi: 10.1016/j.tim.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Keswani SC, Polley M, Pardo CA, et al. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003;54(3):287–296. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- 190.World Health Organization (WHO) (2020) Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it Accessed 2 Aug 2020
- 191.Callaway E, Cyranoski D, Mallapaty S, et al. The coronavirus pandemic in five powerful charts. Nature. 2020;579(7800):482–483. doi: 10.1038/d41586-020-00758-2. [DOI] [PubMed] [Google Scholar]
- 192.Matías-Guiu J, Gomez-Pinedo U, Montero-Escribano P, et al. Es esperable que haya cuadros neurológicos por la pandemia de SARS2-CoV? Neurología. 2020;35(3):170–175. doi: 10.1016/j.nrl.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tsai LK, Hsieh ST, Chao CC, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61(11):1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 195.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ogier M, Andéol G, Sagui E, et al. How to detect and track chronic neurologic sequelae of covid-19? Use of auditory brainstem responses and neuroimaging for long-term patient follow-up. Brain Behav Immun Health. 2020;5:100081. doi: 10.1016/j.bbih.2020.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 199.Xiang P, Xu XM, Gao LL, et al. First case of 2019 novel coronavirus disease with encephalitis. ChinaXiv T. 2019;202003:00015. [Google Scholar]
- 200.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sejvar JJ, Baughman AL, Wise M, et al. Population incidence of Guillain–Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36(2):123–133. doi: 10.1159/000324710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sedaghat Z, Karimi N. Guillain–Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;S0967–5868(20):30882–30891. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhao H, Shen D, Zhou H, et al. Guillain–Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vollono C, Rollo E, Romozzi M, et al. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure. 2020;78:109–112. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kuroda N. Epilepsy and COVID-19: associations and important considerations. Epilepsy Behav. 2020;108:107122. doi: 10.1016/j.yebeh.2020.107122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Arbour N, Day R, Newcombe J, et al. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;4(19):8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wilson MP, Jack AS. Coronavirus disease 2019 (COVID-19) in neurology and neurosurgery: a scoping review of the early literature. Clin Neurol Neurosurg. 2020;193:105866. doi: 10.1016/j.clineuro.2020.105866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Frank-Cannon TC, Alto LT, McAlpine FE, et al. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Serrano-Castro PJ, Estivill-Torrús G, Cabezudo-García P, et al. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: a delayed pandemic? Neurologia. 2020;35(4):245–251. doi: 10.1016/j.nrl.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology. 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035.e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]