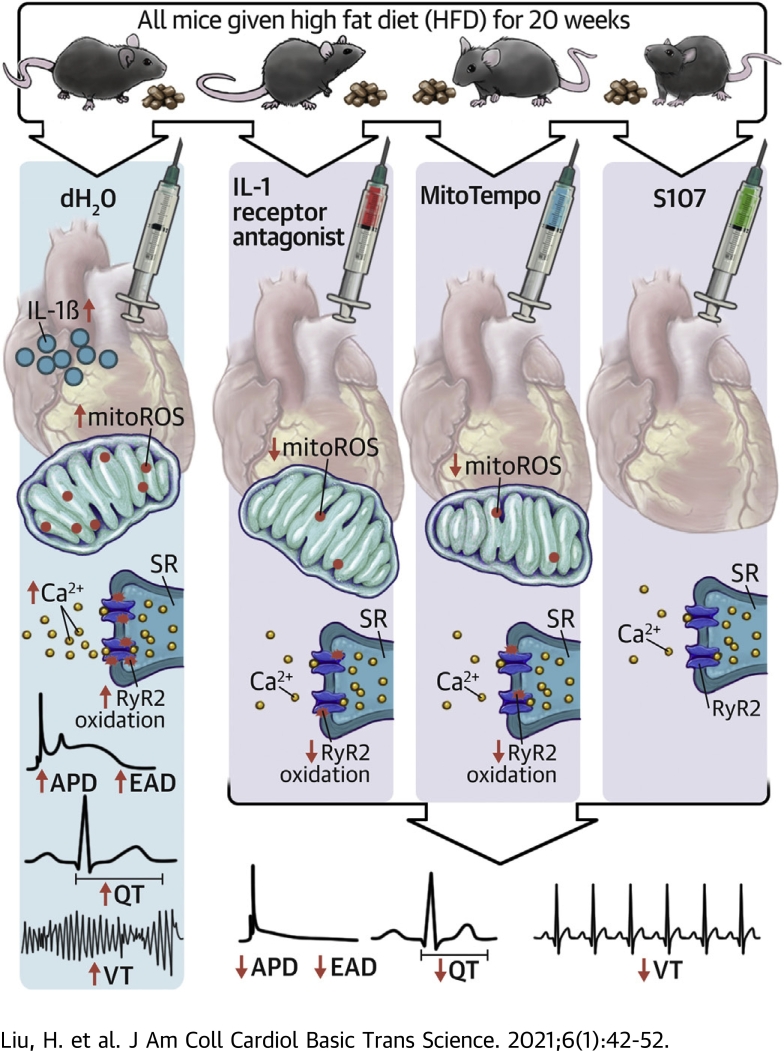
Visual Abstract
Key Words: calcium handling, inflammation, mitochondria, oxidation, sudden cardiac death
Abbreviations and Acronyms: APD, action potential duration; DM, diabetes mellitus; EAD, early afterdepolarization; IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; Ito, transient outward potassium current; mitoROS, mitochondrial reactive oxygen species; RyR2, ryanodine receptor; SR, sarcoplasmic reticulum; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus; VT, ventricular tachycardia
Highlights
-
•
Diabetes-induced arrhythmic risk involved activation of innate immunity, elevation of IL-1β, mitochondrial oxidative stress, SR calcium release channel oxidation, and QT prolongation.
-
•
Diabetes-induced arrhythmic risk could be inhibited by IL-1β antagonism, mitoROS scavenging, and SR calcium release stabilization.
-
•
The relationship of inflammation and arrhythmic risk may account for increased susceptibility of diabetic patients to the effects of COVID-19.
Summary
Diabetes mellitus (DM) is associated with increased arrhythmia. Type 2 DM (T2DM) mice showed prolonged QT interval and increased ventricular arrhythmic inducibility, accompanied by elevated cardiac interleukin (IL)-1β, increased mitochondrial reactive oxygen species (mitoROS), and oxidation of the sarcoplasmic reticulum (SR) Ca2+ release channel (ryanodine receptor 2 [RyR2]). Inhibiting IL-1β and mitoROS reduced RyR2 oxidation and the ventricular arrhythmia in DM. Inhibiting SR Ca2+ leak by stabilizing the oxidized RyR2 channel reversed the diabetic arrhythmic risk. In conclusion, cardiac IL-1β mediated the DM-associated arrhythmia through mitoROS generation that enhances SR Ca2+ leak. The mechanistic link between inflammation and arrhythmias provides new therapeutic options.
More than 400 million people worldwide have diabetes mellitus (DM). In 2016, an estimated 1.6 million deaths were caused directly by DM (1). Roughly 70% of patients with DM die from cardiovascular causes (2), of which around 50% are sudden cardiac deaths (3). The risk of sudden cardiac death in DM is double that of nondiabetic persons (4).
A prolonged QT interval on the surface electrocardiogram is a risk factor for sudden cardiac death (5). Clinical studies demonstrated a high prevalence of QT prolongation in DM patients (6). QT prolongation has been associated with sudden cardiac death in type 2 DM (T2DM), suggesting that electrical remodeling in DM may contribute to ventricular arrhythmias and sudden cardiac death (7, 8, 9). At a cellular level, an increase in the ventricular action potential duration (APD) with a subsequent increase in early afterdepolarizations (EADs) underlies long QT–associated torsades de pointes ventricular arrhythmias (10).
DM is associated with elevated inflammatory cytokines and mitochondrial oxidative stress. A recent publication implicates macrophage activation in the QT prolongation and increased arrhythmic risk in type 1 DM (T1DM) (11). We investigated how innate immune activation may contribute to arrhythmic risk in the more prevalent T2DM.
Methods
Detailed methods are described in the Supplemental Appendix. Briefly, male C57BL/6 mice were fed with high fat diet for a minimal of 20 weeks to induce T2DM. Mice fed with normal chow were used as a control group. The cardiac electrical properties of diabetic mice, including cardiomyocyte APD, EADs, QT interval, and ventricular arrhythmic inducibility, were characterized by patch clamp, optical mapping, surface electrocardiogram, and in vivo electrophysiological test, respectively. The results were compared with control mice. Diabetic mice were then randomized into 3 treatment groups: interleukin (IL)-1 receptor antagonist (IL-1RA), mitochondrial antioxidant (MitoTEMPO), and ryanodine receptor 2 (RyR2) channel stabilizer (S107). At the end of each treatment, alterations on the cardiac electrical properties were evaluated. Mitochondrial reactive oxygen species (mitoROS) was were assessed using MitoSOX stain on isolated cardiomyocytes by flow cytometry. Heart tissue were collected for immunoblotting of IL-1β and oxidized RyR2 channels.
The animal experiments were carried in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee of the University of Minnesota.
Statistical analysis
Continuous data are presented as mean ± SEM when normally distributed. Data that were not normally distributed were logarithmically transformed prior to further statistical analysis. Data were then analyzed using a 2-tailed Student’s t-test or 1-way analysis of variance with Bonferroni post hoc tests for multiple pairwise comparisons. Categorical data are expressed as counts with percentages and compared using a chi-square test. All statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software, San Diego, California). A p value < 0.05 was considered statistically significant.
Results
T2DM causes abnormal Electrocardiograms and increased arrhythmic risk
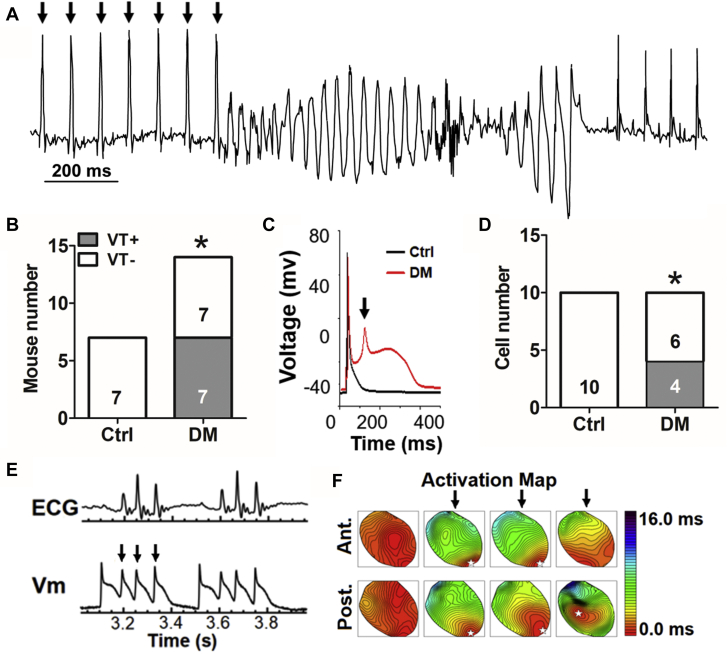
A prolonged QT interval on the surface electrocardiogram is associated with increased arrhythmic risk (12). DM mice had QT intervals on the surface electrocardiogram that were significantly longer compared with the control mice (47 ± 1 ms in control mice vs. 52 ± 1 ms in DM mice; p < 0.001) (Table 1) under comparable heart rate. No other abnormalities on surface electrocardiogram were detected (Supplemental Table 1). Arrhythmia was also examined in conscious mice using telemetry (data not shown). No spontaneous ventricular tachycardia (VT) was observed in the DM mice. Testing for sudden death risk, programmed electrical stimulation revealed that none of the control mice were inducible for VT, whereas half the DM mice had inducible VT (0 of 7 in control mice vs. 7 of 14 in DM mice; p = 0.047) (Figures 1A and 1B). Differences in cardiac contractile function did not explain the increased arrhythmic risk in DM mice (ejection fractions of 54.7 ± 1.4% in control mice vs. 55.5 ± 0.9% in DM mice, n = 6 for each group; p = 0.651). Cardiac chamber size and wall thickness by echocardiography were comparable between the DM and control mice (Supplemental Table 2). Taken together, initial electrophysiological studies demonstrated that DM increased risk markers of sudden cardiac death.
Table 1.
Influence of MT, IL-1RA, and S107 on Action Potential and QT Interval
| Control Mice (n = 14) | DM Mice (n = 32) | DM + IL-1RA Mice (n = 20) | DM + MT Mice (n = 19) | DM + S107 Mice (n = 8) | p Value | |
|---|---|---|---|---|---|---|
| APD90, ms | 61 ± 14 (7)∗ | 411 ± 42 (7) | 105 ± 47 (5)† | 188 ± 66 (9)‡ | 81 ± 42 (8)∗ | <0.001 |
| QT interval, ms | 53 ± 1† | 59 ± 1 | 51 ± 1∗ | 52 ± 1∗ | 51 ± 2† | <0.001 |
| QTc interval, ms | 47 ± 1† | 52 ± 1 | 46 ± 1∗ | 47 ± 1∗ | 47± 2‡ | <0.001 |
| HR, beats/min | 495 ± 15 | 483 ± 8 | 498 ± 8 | 488 ± 10 | 525 ± 26 | 0.515 |
Values are mean ± SEM (n) or mean ± SEM. APD90 was calculated on action potentials without early afterdepolarizations.
APD90 = action potential duration at 90% repolarization; DM = diabetes mellitus; HR = heart rate; IL-1RA, interleukin-1 receptor antagonist; MT = mitoTEMPO.
p < 0.001 vs. DM.
p < 0.01. vs. DM.
p < 0.05 vs. DM.
Figure 1.
VT Induction and EADs in DM Mice
(A) Representative electrocardiogram trace showing induced ventricular tachycardia (VT). Arrows indicate the paced beats. (B) More diabetes mellitus (DM) mice had inducible VT. (C) Representative whole-cell patch-clamp recordings of ventricular action potentials from control (Ctrl) (black) and DM (red) cardiomyocytes showing an early afterdepolarization (EAD). The arrow indicates EAD. (D) More DM cardiomyocytes show EADs. (E) Optical mapping traces of triggered activities (arrows) under slow heart rate by atrioventricular node ablation (n = 2 of 3 DM hearts). (F) The representative activation maps show that EAD or polymorphic VT was caused by triggered activities (arrows). (B, D) The n value is indicated within the bars. ∗p < 0.05. Ant. = anterior wall; Post. = posterior wall; Vm = membrane potential.
T2DM causes APD prolongation and presence of EADs
At the cellular level, cardiac ventricular APD prolongation is known to underlie surface QT interval lengthening and is associated with increased incidence of electrical oscillations before repolarization called EADs (10). EADs are a fundamental mechanism of ventricular arrhythmias and sudden cardiac death (13,14).
Using whole-cell current clamp of isolated cardiomyocytes, we demonstrated APD prolongation (61 ± 14 ms in control mice vs. 411 ± 42 ms in DM mice; p < 0.001) (Table 1) and increased presence of EADs (4 of 10 vs. 0 of 10; p = 0.025) (Figures 1C and 1D) in the DM myocytes compared with the control myocytes. These changes were confirmed by optical mapping on whole hearts. APD measured by optical mapping was 73 ± 3 ms (n = 6) in control and 85 ± 3 ms (n = 6) in DM (p < 0.001). EADs were detected in two-thirds of DM hearts at slow heart rates (Figure 1E and 1F).
DM upregulated cardiac IL-1β and mitoROS
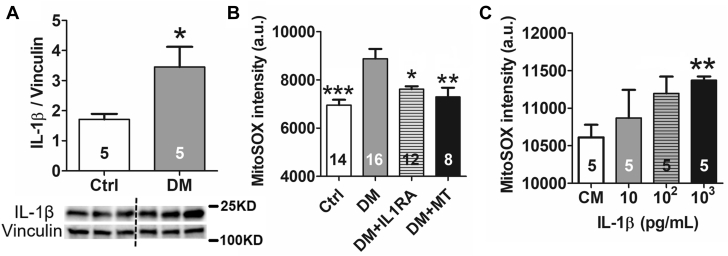
In T2DM mice, cardiac IL-1β protein level was doubled as compared with control mice hearts (1.71 ± 0.18 normalized ratio in control mice vs. 3.45 ± 0.67 in DM mice; p = 0.037) (Figure 2A). IL-1β is known to promote oxidative stress and ROS production (15). We confirmed the increase of mitoROS (6,956 ± 220 arbitrary units electrocardiogram in control mice vs. 9,156 ± 468 arbitrary units in DM mice; p < 0.001) (Figure 2B), which was ameliorated by both IL-1RA (7,618 ± 120 arbitrary units; p = 0.010 vs. DM) and mitoTEMPO (7,298 ± 378 arbitrary units; p = 0.017 vs. DM), a mitochondrial-specific antioxidant. Moreover, adding IL-1β to the cultured cardiomyocytes increased the mitoROS in a dose-dependent manner (Figure 2C). These data implied that elevated IL-1β caused cardiac mitoROS production.
Figure 2.
Increased IL-1β Is Associated With MitoROS in DM
(A) Interleukin (IL)-1β was increased in DM hearts. (B) Mitochondrial reactive oxygen species (mitoROS) was increased in the DM cardiomyocytes. The mitoROS elevation was reversed by a 2-week treatment of IL-1 receptor antagonist (IL-1RA) (3 mg/kg) or mitoTEMPO (MT) (1 mg/kg). (C) IL-1β incubation for 4 h elevated mitoROS level in cultured cardiomyocytes in a dose-dependent manner. Values are mean ± SEM. The n value is indicated within the bars. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. a.u. = arbitrary unit; CM = cardiomyocyte; other abbreviations as in Figure 1.
IL-1β inhibition and mitochondrial antioxidant suppressed arrhythmic risk
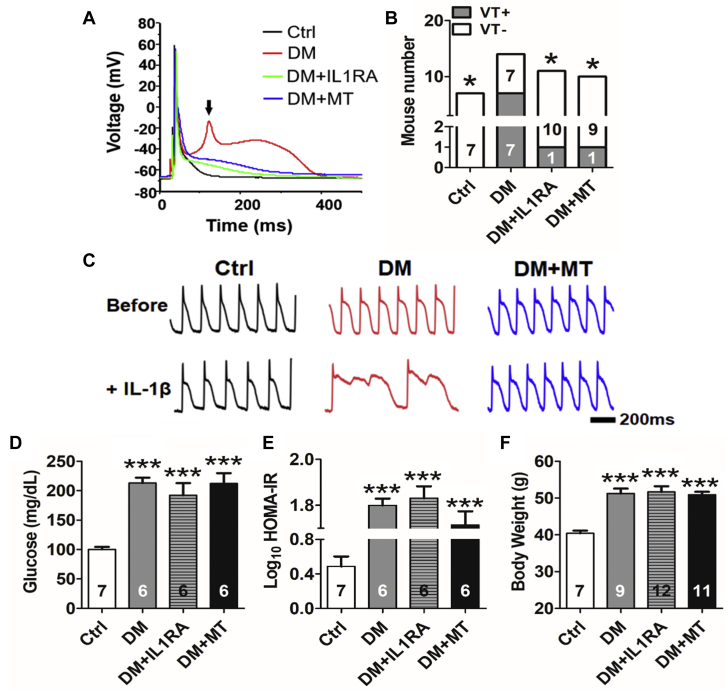
To test directly the roles of IL-1β or mitoROS in DM-associated arrhythmias, we treated DM mice with either IL-1RA (3 mg/kg) or mitoTEMPO (1 mg/kg) for 2 weeks. Both treatments caused substantial reductions in EAD occurrence (Figure 3A), APD (411 ± 42 ms in DM vs. 105 ± 47 ms in DM + IL-1RA; p < 0.01; vs. 188 ± 66 in DM + mitoTEMPO; p = 0.019) (Table 1), QT interval (52 ± 1 ms in DM vs. 46 ± 1 ms in DM + IL-1RA; p < 0.001; vs. 47 ± 1 ms in DM + mitoTEMPO; p < 0.001) (Table 1), and VT inducibility (Figure 3B). VT was induced in 7 of 14 DM mice, and the average VT duration was 1.3 ± 0.4 s. In contrast, only 1 of 11 IL-1RA–treated DM mice (p = 0.042) and 1 of 10 mitoTEMPO-treated DM mice (p = 0.040) had induced VT. The VT duration in these mice was 0.2 s and 0.3 s, respectively. Additionally, in optical mapping (Figure 3C), short-term in vitro perfusion with IL-1β (10 pg/ml for 20 min) prolonged APD and promoted EAD formation in DM mice (n = 3 of 4 hearts) compared with control mice (n = 0 of 4 hearts; p = 0.029), which was abolished by mitoTEMPO (n = 0 of 4 hearts; p = 0.029). Blood glucose level, insulin resistance, and body weight were not altered by mitoTEMPO or IL-1RA (Figures 3D to 3F), suggesting that their antiarrhythmic effect is independent of serum glucose, insulin, or adiposity.
Figure 3.
RyR2 Oxidation and SR Calcium Leak Mediated Arrhythmia in DM Mice
(A) Representative whole-cell patch-clamp recordings of action potentials showing 2-week treatment with IL-1RA (3.0 mg/kg) or MT (1.0 mg/kg) suppressed EAD formation. The arrow indicates EAD. (B) IL-1RA or MT reduced VT inducibility. (C) Representative tracing of action potential duration (APD) by optical mapping showing short-term in vitro perfusion with IL-1β (10 pg/ml for 20 min), prolonged APD, and promoted EAD formation in the DM hearts. MT treatment abolished the arrhythmic effect of IL-1β. (D) Hyperglycemia in DM mice was not affected by 2-week treatment with IL-1RA or MT. (E) HOMA-IR (Homeostatic Model Assessment of Insulin Resistance), an indicator of insulin resistance, and (F) body weight remained unchanged after either treatment. Values are mean ± SEM. (B, D, F) The n value is indicated within the bars. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Scale bar = 200 ms. Abbreviations as in Figures 1 and 2.
Enhanced SR Ca2+ release underlies the effect of DM on arrhythmic risk markers
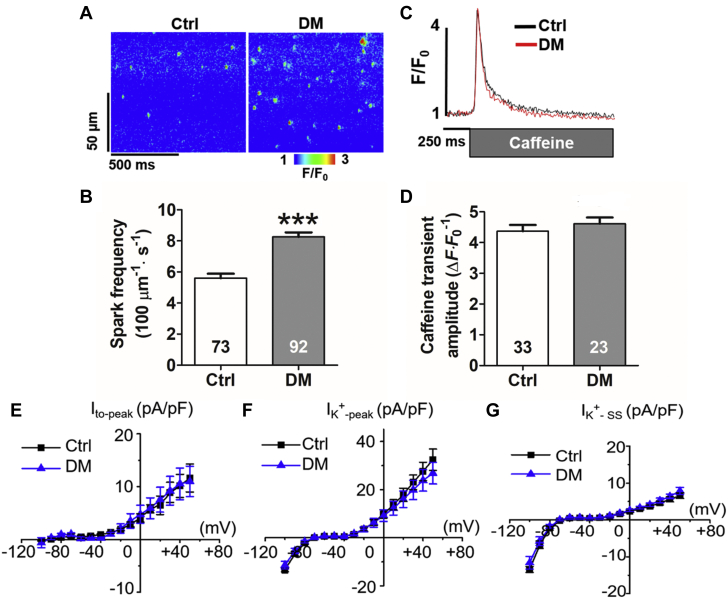
Resting Ca2+ leak from the SR through the RyR has been implicated in slow repolarization, EADs and arrhythmias (16). We confirmed that the RyR was hyperactive using spontaneous Ca2+ sparks as a readout for RyR activity in saponin-permeabilized myocytes isolated from DM mouse hearts (5.6 ± 0.3 sparks [100 μM]–1· s–1 in control mice vs. 8.3 ± 0.3 sparks [100 μM]–1· s–1 in DM mice; p < 0.001) (Figures 4A and 4B). Parallel assessment of caffeine-induced Ca2+ transients amplitude in permeabilized myocytes revealed no difference in SR Ca2+ content between DM and control hearts (p = 0.407) (Figures 4C and 4D). Potassium current is another key contributor to cardiomyocyte repolarization. In rodents, transient outward potassium current (Ito) is a dominant repolarizing current (17). In our study, total potassium peak current density only slightly decreased in DM cardiomyocytes compared with that in control cardiomyocytes, while steady-state current density stayed the same (Figures 4F and 4G). There was almost no change in Ito peak density between these 2 groups (Figure 4E).
Figure 4.
Enhanced Ca2+ Sparks in DM Mice
(A) Representative line-scan images of Ca2+ sparks. (B) Increased frequency of Ca2+ sparks in DM. (C) Representative trace of caffeine-induced Ca2+ transients. (D) Sarcoplasmic reticulum Ca2+ content is unchanged (p = 0.407). (E) Transient outward peak potassium current (Ito-peak), (F) total potassium current (IK+-peak), and (G) steady-state current (IK+-SS), revealed similar current densities between Ctrl (n = 5) and DM (n = 10) mice. Values are mean ± SEM. (B, D) The n value within the bars indicates the total number of cells tested from >3 different mice. ∗∗∗p < 0.001. Abbreviations as in Figures 1 and 2.
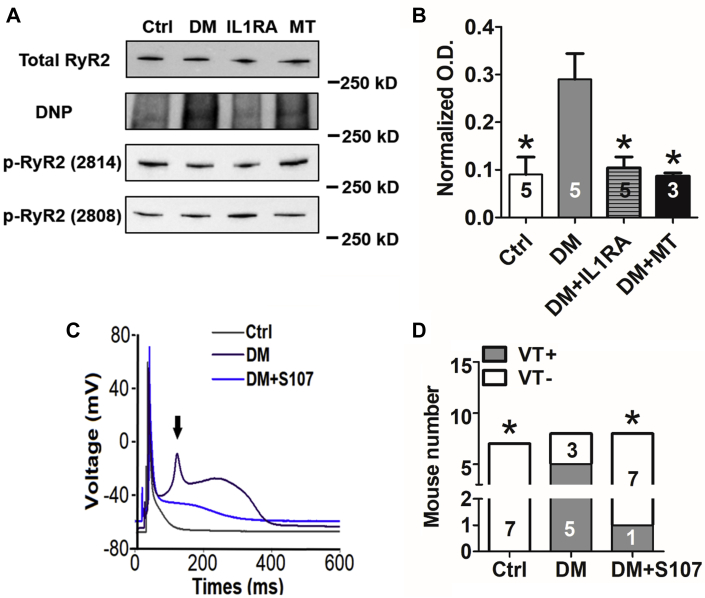
RyR activation by phosphorylation or oxidation leads to increased resting SR Ca2+ release (18, 19, 20, 21). DM significantly enhanced RyR2 oxidation, while RyR2 phosphorylation was not altered (Figures 5A and 5B). Both mitoTEMPO and IL-1RA reduced the RyR2 oxidation (Figures 5A and 5B). RyR2 oxidation destabilizes an interaction with a channel-associated subunit, calstabin, to lead to Ca2+ leak (22). Recently, several drugs, such as S107, have been developed to stabilize the RyR-calstabin interaction and reduce resting SR Ca2+ leak (23, 24, 25). We treated cardiomyocytes immediately after they were isolated from diabetic hearts with the RyR stabilizer S107 (1.0 μM) for 2 h or injected S107 (30 mg/kg) in DM mice for 1 week. We found that S107 was able to ameliorate the APD prolongation in vitro (411 ± 42 ms in DM vs. 81 ± 42 ms in DM + S107; p < 0.001) (Table 1), shorten the QT interval (52 ± 1 ms in DM vs. 47 ± 2 ms in DM + S107; p = 0.005) (Table 1), and suppress EADs and arrhythmic inducibility in DM mice (5 of 8 in DM vs. 1 of 8 in DM + S107; p = 0.039) (Figures 5C and 5D).
Figure 5.
RyR2 Oxidation and SR Calcium Leak Mediated Arrhythmia in DM Mice
(A) Representative Western blots of total ryanodine receptor 2 (RyR2), oxidized (DNP), and phosphorylated RyR2 (s2814 and s2808). RyR2 oxidation was increased in DM and reversed by 2-weeks of IL-1RA (3.0 mg/kg) or MT (1.0 mg/kg) treatment. RyR2 phosphorylation was not changed in DM. (B) Statistical analysis of oxidized RyR2 normalized by total RyR2. (C) Representative whole-cell patch-clamp recordings showing immediate in vitro treatment with S107 (1.0 μM for 2 h) suppressed EADs. The arrow indicates EAD. (D) One-week of S107 (30 mg/kg, subcutaneous) treatment reduced VT inducibility. Values are mean ± SEM. (B to D) The n value is indicated within the bars. ∗p < 0.05. DNP = 2,4-dinitrophenylhydrazone; O.D. = optical density; other abbreviations as in Figures 1 and 2.
These data indicated that DM-associated APD prolongation and arrhythmia was mediated by SR Ca2+ leak from oxidized RyR2 channels.
Discussion
In the present study, we found that DM caused increased arrhythmic risk associated with a prolonged QT interval on the surface electrocardiogram and a corresponding increase in the cardiac APD and increased triggered activity at the cellular level. DM was accompanied by increased IL-1β and mitoROS. Inhibition of IL-1β signaling by IL-1RA or mitochondrial antioxidant ameliorated electrical remodeling and arrhythmic risk. Underlying these electrical changes were alterations in Ca2+ release from oxidized RyR2 channels. Taken together, these findings indicate that DM activates innate immune mechanisms in the heart that link DM to increased arrhythmic risk by modulating mitoROS.
Growing evidence indicates that DM is a low-grade chronic inflammatory state characterized by oversecretion of proinflammatory cytokines in the circulation and in various tissues. The increased systemic and local inflammation are considered key mechanisms underlying the pathogenesis and the progression of DM and diabetic complications (19). Several proarrhythmic cytokines are elevated in DM, including IL-1β, tumor necrosis factor-α, IL-6, and IL-18 (26, 27, 28). Among those proinflammatory mediators, IL-1β lies upstream in the inflammation pathway (29). IL-1β augments tumor necrosis factor-α–mediated inflammation in lung epithelial cells by enhancing receptor expression (30). IL-1β modulates the production of IL-6 and IL-8 in human gingival epithelial cells (31). IL-1β inhibition leads to a significant reduction of C-reactive protein and IL-6 in the patients with myocardial infarction (29,32). A randomized double-blind placebo-controlled clinical trial (i.e., the CANTOS [Canakinumab Anti-Inflammatory Thrombosis Outcomes Study]) showed anti-inflammatory therapy with an IL-1β–specific antibody (canakinumab) significantly reduced cardiovascular events, independently of lipid-level lowering, supporting a role for IL-1β in cardiovascular disease complications. Therefore, we focused on IL-1β in this study (32).
Recently, Monnerat et al. (11) reported the role of innate immunity activation and IL-1β in cardiac electrical remodeling leading to ventricular arrhythmia in T1DM. Our results show that IL-1β antagonism reduced the arrhythmic inducibility by over 80%, suggesting that IL-1β was the most prominent mediator of T2DM-associated arrhythmia. It is reasonable to postulate that IL-1β is the common arrhythmic mechanism shared between T1DM and T2DM. Monnerat et al. (11) observed a decrease in Ito and an increase in calcium sparks as the substrates for QT prolongation and arrhythmia, respectively. Concordantly, we observed increased SR Ca2+ sparks in the diabetic cardiomyocytes. Nevertheless, no alteration in potassium currents were noticed. Enhanced SR Ca2+ leak secondary to oxidative modification likely was sufficient to cause QT and APD prolongation by promoting Na+/Ca2+ exchanger forward mode, thereby slowing repolarization and enabling L-type Ca2+ current reactivation (16). Contrasting data have been reported regarding how DM affects the properties of cardiac ion channels, depending on the DM models, channel activation status, or the parameters being reported (channel expression or function) (20,33). It is possible that the downstream effectors of IL-1β are different between T1DM and T2DM.
More importantly, our study provides a mechanistic linkage between IL-1β–mediated cardiac inflammation and DM-induced arrhythmia. We demonstrated that inhibiting mitoROS had similar effects as IL-1 receptor antagonism and IL-1β–mediated electrophysiological effects could be masked by a mitochondrial targeted antioxidant, implying that mitoROS mediated the effects of IL-1β. It is well known that mitoROS plays a critical role in activating inflammasome and IL-1β (34, 35, 36). Nevertheless, debates exist on how IL-1β and inflammasome interplays with mitoROS (37). Consistent with previous reports (38,39), our results support that IL-1β fosters mitoROS production. This positive feedback between IL-1β and mitoROS would perpetuate the pathological process in DM. The exact mechanism whereby IL-1β regulates mitoROS in DM is yet to be determined.
MitoROS is known to be associated with arrhythmogenesis. For example, in nonischemic heart failure, mitoROS underlies QT prolongation and sudden death, a similar electrical phenotype as in diabetic patients and in our diabetic mice model (40). The putative mechanisms whereby mitoROS may cause arrhythmic risk are myriad and include facilitating focal activity and re-entry, altering cardiac ion currents, promoting cardiac fibrosis, and impairing gap junction function (41,42). RyR2 is the major SR Ca2+ release channel and an important oxidative target in cardiomyocytes (20,43). In aging hearts, mitoROS induces the redox modification of RyR2 causing aberrant Ca2+ handling (44). At the cellular and animal levels, we showed that, in diabetes, the arrhythmic effect of mitoROS was via oxidative modification of RyR2, leading to increased spontaneous SR Ca2+ leak, as S107 alone, a drug that inhibits RyR2 channel–mediated resting Ca2+ leak, reversed the diabetic arrhythmic phenotypes, implying that IL-1β and mitoROS acted predominantly on the RyR2 to mediate their effects. Excessive SR Ca2+ leak through oxidized RyR2 is known to increase free Ca2+ ion transportation into mitochondria causing mitochondrial Ca2+ overload, which subsequently results in more mitoROS production (45). This vicious cycle helps to sustain arrhythmia in DM.
It is possible that our findings may have implications for clinical trial results using sodium-glucose cotransporter-2 inhibitors and glucagon-like peptide-1 agonists to treat DM. Trials have found that these newer glucose-lowering drugs significantly reduce the incidence of major cardiovascular events in diabetic patients and that these cardiovascular benefits are unrelated to their glycemic control effects (46,47). Both treatments have been found to lower the expression and secretion of proinflammatory cytokines, such as IL-6, IL-1β, tumor necrosis factor-α, and C-reactive protein (48). This anti-inflammatory effect may contribute to reduced arrhythmic risk and improved outcomes. Consistent with this idea, blocking IL-1β signaling was antiarrhythmic and independent of the serum glucose level in our study.
Study limitations
We examined neither ROS from other sources nor all ion current components of the cardiac action potential. Nevertheless, mitoTEMPO or S107 alone was sufficient to reverse cardiac electrical remodeling nearly to the control level, suggesting that the impacts from other ROS sources or ion currents are trivial. Second, we did not test the effects of S107 on other cardiac channels. Nevertheless, S107 is reported to bind specifically to RyR (49). Last, our study cannot rule out a potential effect of IL-1α. Nevertheless, a recent investigation on patients with T2DM revealed that the IL-1β concentration is 4 times as high as that of IL-1α (50). Thus, it is reasonable to conclude that IL-1β–mediated inflammation is the main driver in T2DM-associated arrhythmia. Further investigation using a specific IL-1β neutralizing antibody is needed to confirm our findings.
Conclusions
DM results in activation of a cardiac innate immune response associated with increased arrhythmic risk. This arrhythmogenic risk could be inhibited by antagonizing IL-1β, mitoROS, or SR Ca2+ leak. Each of these approaches represents a possible new therapy for inflammation-induced arrhythmic risk. Also, it is possible that our findings of increased cardiac inflammation in diabetes may explain the increased susceptibility of diabetic patients to the cardiac effects of COVID-19.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: DM causes cardiac inflammation that contributes arrhythmic risk.
TRANSLATIONAL OUTLOOK 1: This diabetes-induced arrhythmic risk involves QT prolongation.
TRANSLATIONAL OUTLOOK 2: This diabetes-induced arrhythmic risk can be inhibited by IL-1β antagonism, mitoROS scavenging, and SR calcium release stabilization.
TRANSLATIONAL OUTLOOK 3: The relationship of inflammation and arrhythmic risk may account for increased susceptibility of diabetic patients to the effects of COVID-19.
Author Disclosures
This work was supported by National Heart Lung and Blood Institute Grant Nos. R01HL134791 and R01HL104025. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank E. Papa for the technical assistance on flow cytometry.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For expanded Methods and References sections as well as supplemental tables, please see the online version of this paper.
Appendix
References
- 1.World Health Organization . 2016. Global Report on Diabetes.https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=CDA815B96D56888E110FF243C6040801?sequence=1 Available at: Accessed October 9, 2020. [Google Scholar]
- 2.Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: the Kelly West Award Lecture 2008. Diabetes Care. 2010:442–449. doi: 10.2337/dc09-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker A., Cubbon R. Sudden cardiac death in patients with diabetes mellitus and chronic heart failure. Diab Vasc Dis Res. 2015;12:228–233. doi: 10.1177/1479164115573225. [DOI] [PubMed] [Google Scholar]
- 4.Zaccardi F., Khan H., Laukkanen J.A. Diabetes mellitus and risk of sudden cardiac death: a systematic review and meta-analysis. Int J Cardiol. 2014;177:535–537. doi: 10.1016/j.ijcard.2014.08.105. [DOI] [PubMed] [Google Scholar]
- 5.Straus S.M., Kors J.A., De Bruin M.L. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 6.Veglio M., Chinaglia A., Cavallo-Perin P. QT interval, cardiovascular risk factors and risk of death in diabetes. J Endocrinol Invest. 2004;27:175–181. doi: 10.1007/BF03346265. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso C.R., Salles G.F., Deccache W. Prognostic value of QT interval parameters in type 2 diabetes mellitus: results of a long-term follow-up prospective study. J Diabetes Complications. 2003;17:169–178. doi: 10.1016/s1056-8727(02)00206-4. [DOI] [PubMed] [Google Scholar]
- 8.Cox A.J., Azeem A., Yeboah J. Heart rate-corrected QT interval is an independent predictor of all-cause and cardiovascular mortality in individuals with type 2 diabetes: the Diabetes Heart Study. Diabetes Care. 2014;37:1454–1461. doi: 10.2337/dc13-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linnemann B., Janka H.U. Prolonged QTc interval and elevated heart rate identify the type 2 diabetic patient at high risk for cardiovascular death. The Bremen Diabetes Study. Exp Clin Endocrinol Diabetes. 2003;111:215–222. doi: 10.1055/s-2003-40466. [DOI] [PubMed] [Google Scholar]
- 10.El-Sherif N., Turitto G., Boutjdir M. Acquired long QT syndrome and electrophysiology of torsade de pointes. Arrhythm Electrophysiol Rev. 2019;8:122–130. doi: 10.15420/aer.2019.8.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monnerat G., Alarcón M.L., Vasconcellos L.R. Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat Commun. 2016;7:13344. doi: 10.1038/ncomms13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antoniou C.K., Dilaveris P., Manolakou P. QT prolongation and malignant arrhythmia: how serious a problem? Eur Cardiol. 2017;12:112–120. doi: 10.15420/ecr.2017:16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George A.L., Jr. Molecular and genetic basis of sudden cardiac death. J Clin Invest. 2013;123:75–83. doi: 10.1172/JCI62928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang K.C., Kyle J.W., Makielski J.C., Dudley S.C., Jr. Mechanisms of sudden cardiac death: oxidants and metabolism. Circ Res. 2015;116:1937–1955. doi: 10.1161/CIRCRESAHA.116.304691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D., Elner S.G., Bian Z.M., Till G.O., Petty H.R., Elner V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp Eye Res. 2007;85:462–472. doi: 10.1016/j.exer.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terentyev D., Rees C.M., Li W. Hyperphosphorylation of RyRs underlies triggered activity in transgenic rabbit model of LQT2 syndrome. Circ Res. 2014;115:919–928. doi: 10.1161/CIRCRESAHA.115.305146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotella D., Radicke S., Bortoluzzi A. Impaired glycosylation blocks DPP10 cell surface expression and alters the electrophysiology of Ito channel complex. Pflugers Arch. 2010;460:87–97. doi: 10.1007/s00424-010-0824-2. [DOI] [PubMed] [Google Scholar]
- 18.Yang J., Zhang R., Jiang X. Toll-like receptor 4-induced ryanodine receptor 2 oxidation and sarcoplasmic reticulum Ca2+ leakage promote cardiac contractile dysfunction in sepsis. J Biol Chem. 2018;293:794–807. doi: 10.1074/jbc.M117.812289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peiró C., Lorenzo Ó., Carraro R., Sánchez-Ferrer C.F. IL-1β inhibition in cardiovascular complications associated to diabetes mellitus. Front Pharmacol. 2017;8:363. doi: 10.3389/fphar.2017.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton S., Terentyev D. Proarrhythmic remodeling of calcium homeostasis in cardiac disease; implications for diabetes and obesity. Front Physiol. 2018;9:1517. doi: 10.3389/fphys.2018.01517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Q., Quick A.P., Cao S. Oxidized CaMKII (Ca2+/Calmodulin-Dependent Protein Kinase II) is essential for ventricular arrhythmia in a mouse model of duchenne muscular dystrophy. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.005682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roussel J., Thireau J., Brenner C. Palmitoyl-carnitine increases RyR2 oxidation and sarcoplasmic reticulum Ca2+ leak in cardiomyocytes: Role of adenine nucleotide translocase. Biochim Biophys Acta. 2015;1852:749–758. doi: 10.1016/j.bbadis.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Andersson D.C., Marks A.R. Fixing ryanodine receptor Ca leak - a novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov Today Dis Mech. 2010;7:e151–e157. doi: 10.1016/j.ddmec.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson D.C., Betzenhauser M.J., Reiken S. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shan J., Xie W., Betzenhauser M. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111:708–717. doi: 10.1161/CIRCRESAHA.112.273342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaharieva E., Kamenov Z., Velikova T., Tsakova A., El-Darawish Y., Okamura H. Interleukin-18 serum level is elevated in type 2 diabetes and latent autoimmune diabetes. Endocr Connect. 2018;7:179–185. doi: 10.1530/EC-17-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehman K., Akash M.S.H., Liaqat A., Kamal S., Qadir M.I., Rasul A. Role of interleukin-6 in development of insulin resistance and type 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. 2017;27:229–236. doi: 10.1615/CritRevEukaryotGeneExpr.2017019712. [DOI] [PubMed] [Google Scholar]
- 28.Alzamil H. Elevated serum TNF-α is related to obesity in type 2 diabetes mellitus and is associated with glycemic control and insulin resistance. J Obes. 2020;2020:5076858. doi: 10.1155/2020/5076858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aday A.W., Ridker P.M. Antiinflammatory therapy in clinical care: the CANTOS Trial and beyond. Front Cardiovasc Med. 2018;5:62. doi: 10.3389/fcvm.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saperstein S., Chen L., Oakes D., Pryhuber G., Finkelstein J. IL-1β augments TNF-α-mediated inflammatory responses from lung epithelial cells. J Interferon Cytokine Res. 2009;29:273–284. doi: 10.1089/jir.2008.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eskan M.A., Benakanakere M.R., Rose B.G. Interleukin-1β modulates proinflammatory cytokine production in human epithelial cells. Infect Immun. 2008;76:2080–2089. doi: 10.1128/IAI.01428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridker P.M., Everett B.M., Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 33.Aromolaran A.S., Boutjdir M. Cardiac ion channel regulation in obesity and the metabolic syndrome: relevance to long QT Syndrome and atrial fibrillation. Front Physiol. 2017;8:431. doi: 10.3389/fphys.2017.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Q., Lei Z., Cheng G. Mitochondrial ROS activate interleukin-1β expression in allergic rhinitis. Oncol Lett. 2018;16:3193–3200. doi: 10.3892/ol.2018.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harijith A., Ebenezer D.L., Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352. doi: 10.3389/fphys.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heid M.E., Keyel P.A., Kamga C., Shiva S., Watkins S.C., Salter R.D. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J Immunol. 2013;191:5230–5238. doi: 10.4049/jimmunol.1301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abais J.M., Xia M., Zhang Y., Boini K.M., Li P.L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22:1111–1129. doi: 10.1089/ars.2014.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kowluru R.A., Mohammad G., Santos J.M., Tewari S., Zhong Q. Interleukin-1β and mitochondria damage, and the development of diabetic retinopathy. J Ocul Biol Dis Infor. 2011;4:3–9. doi: 10.1007/s12177-011-9074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ansari M.Y., Khan N.M., Ahmad I., Haqqi T.M. Parkin clearance of dysfunctional mitochondria regulates ROS levels and increases survival of human chondrocytes. Osteoarthritis Cartilage. 2018;26:1087–1097. doi: 10.1016/j.joca.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dey S., DeMazumder D., Sidor A., Foster D.B., O'Rourke B. Mitochondrial ROS drive sudden cardiac death and chronic proteome remodeling in heart failure. Circ Res. 2018;123:356–371. doi: 10.1161/CIRCRESAHA.118.312708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sovari A.A. Cellular and molecular mechanisms of arrhythmia by oxidative stress. Cardiol Res Pract. 2016;2016:9656078. doi: 10.1155/2016/9656078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song J., Yang R., Yang J., Zhou L. Mitochondrial dysfunction-associated arrhythmogenic substrates in diabetes mellitus. Front Physiol. 2018;9:1670. doi: 10.3389/fphys.2018.01670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zima A.V., Mazurek S.R. Functional Impact of ryanodine receptor oxidation on intracellular calcium regulation in the heart. Rev Physiol Biochem Pharmacol. 2016;171:39–62. doi: 10.1007/112_2016_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper L.L., Li W., Lu Y. Redox modification of ryanodine receptors by mitochondria-derived reactive oxygen species contributes to aberrant Ca2+ handling in ageing rabbit hearts. J Physiol. 2013;591:5895–5911. doi: 10.1113/jphysiol.2013.260521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Opbergen C.J.M., den Braven L., Delmar M., van Veen T.A.B. Mitochondrial dysfunction as substrate for arrhythmogenic cardiomyopathy: a search for new disease mechanisms. Front Physiol. 2019;10:1496. doi: 10.3389/fphys.2019.01496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giugliano D., Maiorino M.I., Bellastella G., Chiodini P., Esposito K. Glycemic control, preexisting cardiovascular disease, and risk of major cardiovascular events in patients with type 2 diabetes mellitus: systematic review with meta-analysis of cardiovascular outcome trials and intensive glucose control trials. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Das S.R., Everett B.M., Birtcher K.K. 2018 ACC Expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72:3200–3223. doi: 10.1016/j.jacc.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollack R.M., Donath M.Y., LeRoith D., Leibowitz G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39(Suppl 2):S244–S252. doi: 10.2337/dcS15-3015. [DOI] [PubMed] [Google Scholar]
- 49.Bellinger A.M., Reiken S., Dura M. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc Natl Acad Sci U S A. 2008;105:2198–2202. doi: 10.1073/pnas.0711074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Randeria S.N., Thomson G.J.A., Nell T.A., Roberts T., Pretorius E. Inflammatory cytokines in type 2 diabetes mellitus as facilitators of hypercoagulation and abnormal clot formation. Cardiovasc Diabetol. 2019;18:72. doi: 10.1186/s12933-019-0870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.