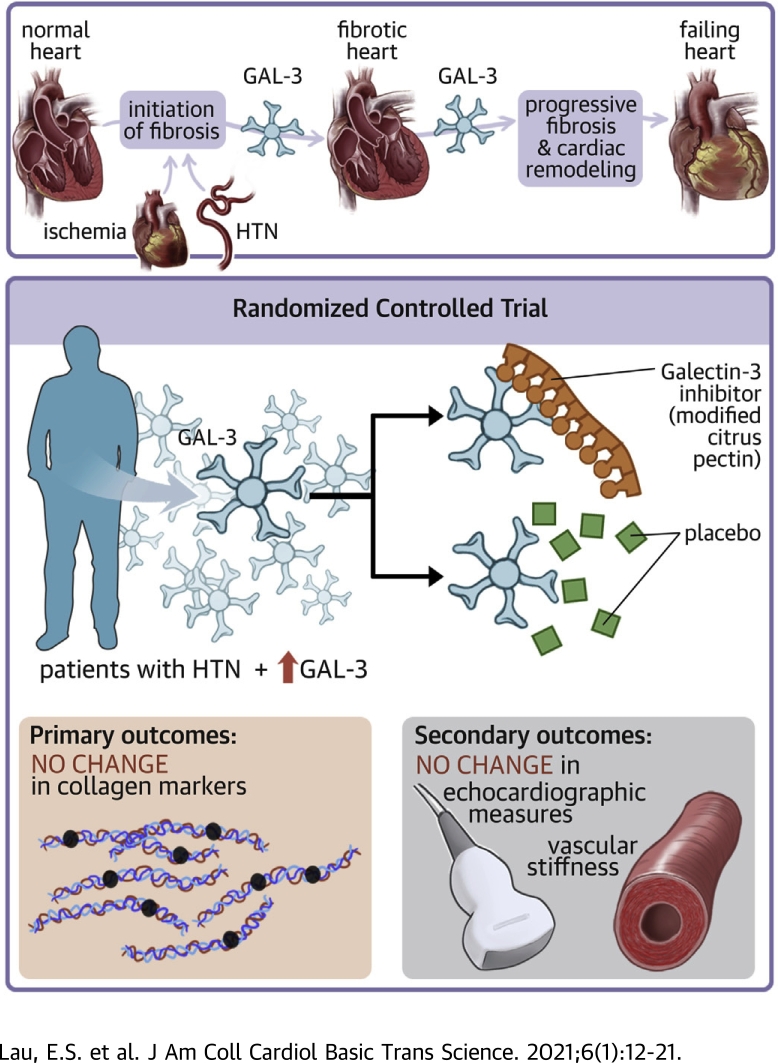
Visual Abstract
Key Words: cardiac fibrosis, galectin-3, heart failure
Abbreviations and Acronyms: AIx, augmentation index; AP, augmentation pressure; CITP, N-terminal telopeptide of type I collagen; eGFR, estimated glomerular filtration rate; Gal-3, galectin-3; HF, heart failure; LV, left ventricular; MCP, modified citrus pectin; MMP, matrix metalloproteinase; PICP, C-terminal propeptide of type I procollagen; PIIINP, N-terminal propeptide of type III procollagen; PWV, pulsed wave velocity
Highlights
-
•
Gal-3 is a beta-galactoside–binding lectin that regulates inflammation and fibrosis and is highly predictive of heart failure events and mortality.
-
•
In a randomized placebo-controlled trial of MCP, Gal-3 inhibition did not influence collagen markers, echocardiographic measures, or vascular function.
-
•
Baseline Gal-3 levels were higher in women compared with men.
-
•
Consistent with previous studies, higher Gal-3 levels were associated with diabetes and reduced GFR.
Summary
We investigated the effect of galectin-3 (Gal-3) inhibition with modified citrus pectin on markers of collagen metabolism in a proof-of-concept randomized placebo-controlled trial of participants with elevated Gal-3 levels and hypertension. Although higher Gal-3 levels were associated with female sex, diabetes, and reduced glomerular filtration rate in cross-sectional analyses, treatment with modified citrus pectin did not change collagen markers. The effect of Gal-3 inhibition among individuals with heart failure warrants further investigation.
Heart failure (HF) is an important public health concern, with a lifetime risk of 1 in 5 for both men and women at 40 years of age (1). HF development is often a clinically inconspicuous process, characterized by progressive cardiac remodeling that is not diagnosed until late in the disease course. Accordingly, most available therapies are implemented during the symptomatic phase of HF when extensive remodeling has already occurred with limited benefit, garnering considerable interest in treatment strategies that target individuals prior to the symptom onset. Indeed, in a prevention trial of patients with asymptomatic left ventricular (LV) dysfunction, treatment with enalapril was associated with a reduction in mortality that was sustained over 12 years (2,3).
Cardiac fibrosis, a pathologic phenomenon caused by numerous conditions including hypertension, ischemia, and aging, contributes to the pathophysiology of HF (4). Galectin-3 (Gal-3) is a beta-galactoside–binding lectin that plays an important role in regulating inflammation and fibrosis. Gal-3 is known to be up-regulated in a number of human fibrotic diseases, including liver cirrhosis (5), pulmonary fibrosis (6), and most recently, cardiac fibrosis (7). We and others have previously shown that elevated circulating Gal-3 levels are associated with incident HF events and mortality in the community (8,9). Most recently, we found that longitudinal changes in Gal-3 were highly predictive of HF independent of baseline Gal-3 levels (10). Among individuals with existing HF, Gal-3 has been approved as a prognostic biomarker of HF and the measurement of Gal-3, alone or in a multimarker strategy, may provide additional risk stratification (11).
Modified citrus pectin (MCP), a soluble dietary fiber found in citrus fruit, is a direct Gal-3 inhibitor that binds to the carbohydrate recognition domain of Gal-3 (12,13). In an animal model of acute kidney injury, MCP was shown to decrease Gal-3 expression and renal fibrosis (14). Although MCP has been tested in human clinical trials of solid tumors and lead intoxication (15, 16, 17), its role in attenuating cardiovascular disease has not been previously investigated. In this context, we conducted a randomized placebo-controlled trial of MCP in patients with hypertension and elevated Gal-3 levels to test our hypothesis that direct inhibition of Gal-3 may reduce subclinical cardiac fibrosis as assessed by biomarkers of collagen metabolism, echocardiographic measures of diastolic function, and vascular stiffness.
Methods
Study sample
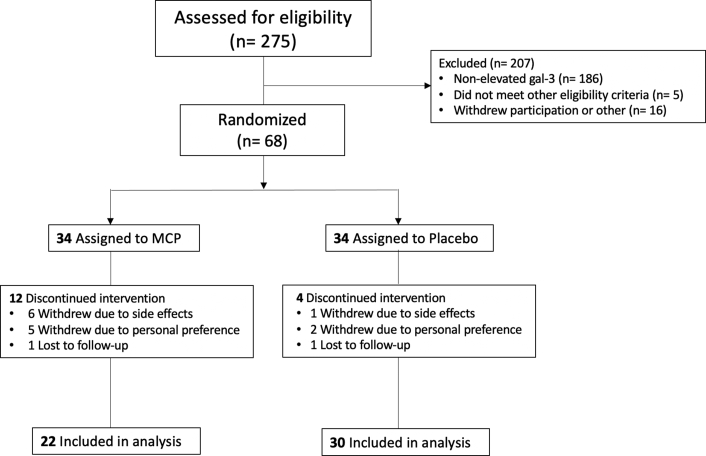
We included individuals 21 to 70 years of age with physician-diagnosed hypertension, who were on stable therapy for at least 3 months, and who had elevated Gal-3 levels. Elevated Gal-3 was defined as ≥50th sex-specific percentile (≥13.1 ng/ml in men, ≥14.3 ng/ml in women) as derived from normative values among participants of the Framingham Heart Study (9). Participants were screened from October 2013 to March 2018. Of this screening sample (n = 275), we excluded participants with nonelevated Gal-3 (n = 186), with renal dysfunction defined as estimated glomerular filtration rate (eGFR) <45 ml/min/1.73 m2 (n = 3), with aldosterone antagonist use (n = 1), with abnormal laboratory values (i.e., hyperkalemia, anemia) (n = 1), and who withdrew participation after initial screening (n = 16). The remaining participants (n = 68) were eligible for randomization and subsequent follow-up as outlined subsequently. The study was performed at the Massachusetts General Hospital and Boston University Medical Center. All participants provided written informed consent, and the study was approved by the appropriate Institutional Review Boards.
Study procedures and ascertainment of outcomes
We randomly assigned 68 participants in double-blind fashion to receive either active MCP or matching placebo at a dose of 4.8 g thrice daily for 6 months in a 1:1 ratio (NCT03349775). MCP and matching placebo were provided by EcoNugenics (Pectasol-C, Santa Rosa, California) (Figure 1). Randomization was done separately for men and women and was performed in blocks of 4 to assure balanced sex representation and group sizes. Investigators and participants were blinded to study assignment.
Figure 1.
CONSORT Diagram
Flowchart showing the number of patients who were screened, randomized into the treatment groups, completed the study, and included in the final analysis. Gal-3 = galectin-3; MCP = modified citrus pectin.
Clinical variables and collagen biomarkers
Medical history, physical examination, and fasting blood samples were obtained at baseline examination prior to randomization and again at final examination after 6 months of medication treatment. Blood serum was separated, aliquoted, and stored at –80°C for further processing. Serum levels of C-terminal propeptide of type I procollagen (PICP) (EIA MicroVue CICP, Quidel Corporation, San Diego, California), N-terminal propeptide of type III procollagen (PIIINP) (RIA, Orion Diagnostica, Espoo, Finland), N-terminal telopeptide of type I collagen (CITP) (RIA, Orion Diagnostica), and matrix metalloproteinase (MMP)-1 (alphaLISA, PerkinElmer, Waltham, Massachusetts) were measured at a core laboratory (18). Assay performance characteristics are summarized in Supplemental Table 1. Measures of renal function (i.e., eGFR and urine albumin-to-creatine ratio) were obtained from fasting blood and urine samples at baseline and final examinations.
Arterial tonometry methods
Supine measurements of arterial stiffness were noninvasively assessed using applanation tonometry or volumetric displacement of the brachial artery using a cuff-based system (SphygmoCor XCEL or SphygmoCor CvMS, AtCor Medical, Naperville, Illinois) at baseline and final examinations (19,20). Central aortic pressure was derived using a generalized transfer function applied to pressure tracings. Pulsed wave analysis included ascertainment of augmentation pressure (AP) (mm Hg) defined as the pressure the reflected wave contributes to systolic blood pressure, augmentation index (AIx) defined as ratio of AP to pulse pressure, and AIx normalized to a heart rate of 75 beats/min. Carotid-femoral pulsed wave velocity (PWV) (m/s) was obtained using electrocardiography-gated pulse waveforms or cuff measurements (20). Pressure tracing and waveform quality control metrics were applied including pre-specified operator index or variability metrics. Intraobserver reproducibility measurements were performed on 16 randomly selected subjects, who underwent 2 separate pulsed wave analysis measurements on the same day. The intraclass correlation coefficient for reported outcome variables was excellent and ranged between 93% and 94%.
Echocardiography
Participants underwent comprehensive transthoracic echocardiography including 2-dimensional pulsed wave and tissue Doppler echocardiography using Vivid 7 and Vivid IQ ultrasonography systems (GE Healthcare, Milwaukee, Wisconsin) at baseline and final examinations. Standard measurements included cardiac dimensions, LV ejection fraction, fractional shortening, and diastolic function assessment as previously described (21). LV mass was calculated as follows: 0.8 (1.04 [LV end-diastolic dimension + LV posterior wall thickness + LV septal wall thickness]3 − [LV end-diastolic dimension3]) + 0.6 (22). Speckle tracking–based analyses of LV global longitudinal strain were performed offline with excellent reproducibility as previously described (2D Cardiac Performance Analysis v1.1, TomTec Imaging Systems; Unterschleißheim, Germany) (23,24). Primary measures obtained included global longitudinal strain of the apical 2- and 4-chamber views. Echocardiography images were analyzed by observers blinded to participant treatment randomization, and measurements were made in triplicate and averaged whenever possible. The interobserver within-subject coefficients of variation for longitudinal strain measures ranged from 3.0% to 4.0% (24).
Statistical analysis
Baseline clinical characteristics are presented by study group assignment as mean ± SD or percentages. Non-normally distributed variables including biomarkers were natural log-transformed due to right-skewed distributions. Galectin-3 values were standardized.
In cross-sectional analyses, we examined the association of Gal-3 with clinical characteristics, arterial stiffness, and renal function among all participants who attended the screening visit (n = 275). We applied a log-normal generalized linear model for continuous outcomes and a logistic model for dichotomous outcomes. We adjusted for age, sex, body mass index, diabetes mellitus, systolic blood pressure, and eGFR for cross-sectional analyses and further adjusted for heart rate in analyses of arterial tonometry measures.
A total of 52 individuals had complete biomarker data at baseline and 6-month follow-up and were included in primary analyses. We examined changes in log-transformed collagen metabolism biomarkers (PICP, PIIINP, CITP, MMP-1, and CITP:MMP-1 ratio) between baseline and final examination in both MCP and placebo treatment groups using paired Student's t-tests. The change from baseline was compared between MCP and placebo groups using unpaired Student's t-tests. In secondary analyses, we used paired Student's t-tests to examine changes in Gal-3, renal function, vascular stiffness, and echocardiographic measures of strain, elastance, and diastolic function in both treatment groups. Group differences were also assessed using unpaired t tests.
For primary analyses comparing between-group differences of collagen biomarkers between MCP and placebo treatment groups, we used a Bonferroni-corrected p value threshold of p = 0.0125 (0.05/4 collagen biomarkers). All other analyses were exploratory and considered statistically significant at a 2-sided p value <0.05. Data analyses were completed in SAS version 9.4 (SAS Institute, Cary, North Carolina), R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria), and RStudio version 1.2.5001 (RStudio, Boston, Massachusetts).
Results
A total of 275 participants attended the screening visit (mean age 55 ± 10 years, 59% women). In addition to hypertension, cardiovascular comorbidities included 24% with diabetes, 53% with obesity (body mass index ≥30 kg/m2), <1% with atrial fibrillation, and 8% who were current smokers (Table 1 for clinical characteristics). The average Gal-3 level was 13.0 ± 4.7 ng/ml in women and 12.2 ± 3.6 ng/ml in men, with 31% of both men and women meeting Gal-3 cutpoint criteria for inclusion in the trial.
Table 1.
Baseline Demographic and Clinical Characteristics
| Screening Sample (n = 275) | Randomized Controlled Trial Sample |
||
|---|---|---|---|
| MCP Group (n = 22) | Placebo Group (n = 30) | ||
| Age, yrs | 55 ± 10 | 57 ± 8 | 54 ± 8 |
| Male | 112 (41) | 11 (50) | 14 (47) |
| Race | |||
| White | 128 (48) | 8 (36) | 18 (60) |
| Black | 108 (41) | 13 (59) | 11 (37) |
| BMI, kg/m2 | 31.0 ± 14.0 | 30.0 ± 5.9 | 33.5 ± 6.5 |
| Obesity | 147 (53) | 9 (41) | 21 (70) |
| Systolic BP, mm Hg | 128 ± 14 | 126 ± 12 | 129 ± 10 |
| Diastolic BP, mm Hg | 80 ± 9 | 78 ± 11 | 81 ± 9 |
| Heart rate, beats/min | 70 ± 3 | 73 ± 18 | 74 ± 11 |
| Hypertension treatment | 373 (99) | 22 (100) | 30 (100) |
| Diabetes mellitus | 65 (24) | 5 (23) | 12 (40) |
| Current smokers | 21 (8) | 6 (27) | 0 (0) |
| Laboratory parameters | |||
| Galectin-3, mg/dl | 12.7 ± 4.3 | 17.9 ± 3.5 | 17.5 ± 4.1 |
| eGFR, ml/min/1.73 m2 | 94.1 ± 18.2 | 92.2 ± 12.9 | 88.3 ± 16.4 |
| Creatinine, mg/dl | 0.87 ± 0.25 | 0.89 ± 0.14 | 0.91 ± 0.17 |
| Hematocrit, % | 41.3 ± 3.4 | 42.5 ± 3.5 | 41.9 ± 3.4 |
| Arterial tonometry parameters | |||
| AP, mm Hg | 11.6 ± 7.1 | 11.7 ± 5.1 | 8.9 ± 5.6 |
| AIx, % | 28.2 ± 11.8 | 29.1 ± 10.8 | 23.9 ± 10.5 |
| PWV, m/s | 8.9 ± 2.2 | 9.3 ± 2.1 | 8.8 ± 1.7 |
Values are mean ± SD or n (%), unless otherwise noted.
AIx = augmentation index; AP = augmentation pressure; BMI = body mass index; BP = blood pressure; eGFR = estimated glomerular filtration rate; MCP = modified citrus pectin; PWV = pulsed wave velocity.
Clinical correlates of Gal-3 in screening sample
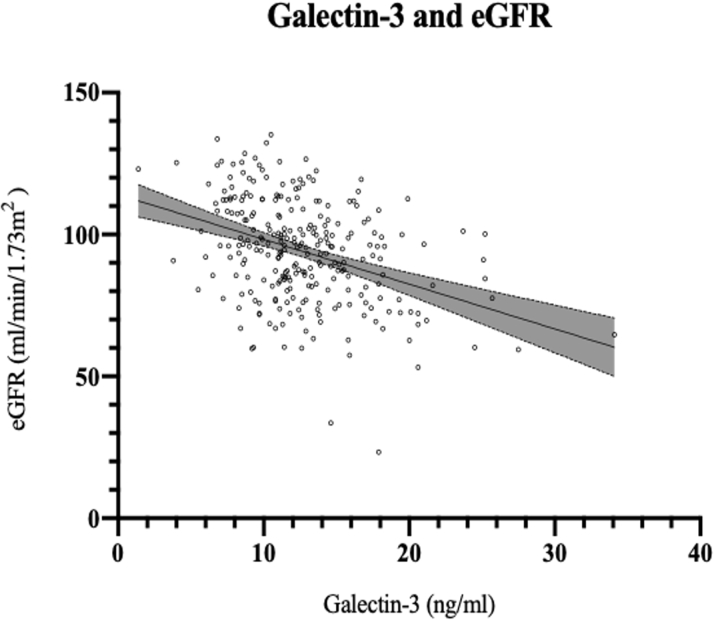
In cross-sectional analyses (n = 275), we examined the association of Gal-3 with clinical characteristics, renal function, arterial stiffness, echocardiographic parameters, and collagen markers (Supplemental Tables 2 and 3). After multivariable adjustment, clinical predictors of circulating Gal-3 levels included presence of diabetes and eGFR. Specifically, the presence of diabetes was associated with a 0.45-SD higher Gal-3 level (β = 0.45, SE = 0.14; p = 0.001), and a 1-SD increase in eGFR was associated with a 0.33-SD lower Gal-3 level (β = –0.33, SE = 0.06; p = 6.02 · 10–7) (Supplemental Table 2 and Figure 2).
Figure 2.
Cross-Sectional Correlation of Galectin-3 With eGFR
Line represents association between Gal-3 and eGFR by univariate linear regression: eGFR =−1.58xGal-3 +114.08. eGFR = estimated glomerular filtration rate.
When examining the association of Gal-3 levels with arterial stiffness, echocardiographic parameters, and collagen markers, we found no significant associations (Supplemental Tables 2 and 3).
Randomized trial of Gal-3 inhibitor MCP versus placebo
A total of 68 participants met enrollment criteria and were randomized to the Gal-3 inhibitor MCP versus matching placebo treatment for a total duration of 6 months. Of randomized participants, 52 completed the final study visit: 22 in the MCP treatment group and 30 in the placebo group (Figure 1). Treatment groups were similar in age and sex (mean age 57 ± 8 years, 50% women for the MCP group; mean age 54 ± 8 years, 53% women for the placebo group), though the placebo group appeared to be heavier (body mass index 33.5 ± 6.5 kg/m2 for the placebo group, 30.0 ± 5.9 kg/m2 for the MCP group). Most common side effects included diarrhea (6 [27%] individuals in the MCP treatment group vs. 7 [23%] individuals in the placebo group), constipation (3 [14%] individuals in the MCP treatment group vs. 8 [27%] individuals in the placebo group), and flatulence (6 [27%] individuals in the MCP treatment group vs. 6 [20%] individuals in the placebo group). Less common side effects included nausea (1 individual in the MCP treatment group) and asthma flare (1 individual in the placebo group).
Effect of Gal-3 inhibition on collagen markers
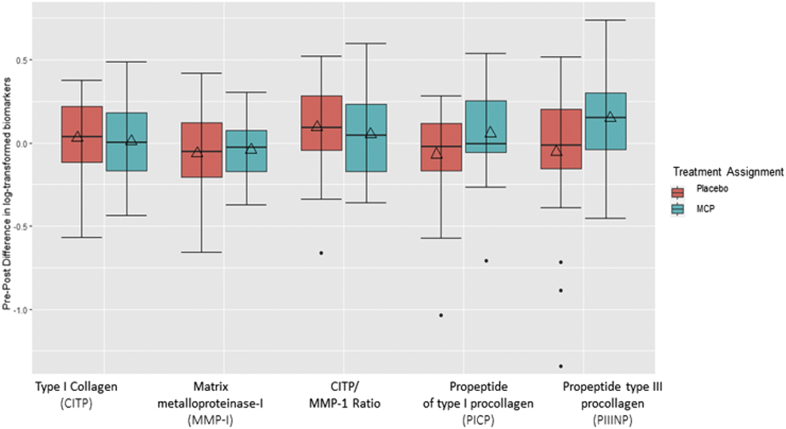
In the primary trial analyses, levels of the collagen metabolism marker PIIINP increased by 0.15 log-transformed units (p = 0.02) after 6 months of treatment with MCP compared with no significant change in the placebo group (p = 0.47) (Table 2, Figure 3). There was a borderline statistically significant between-group difference in the change in PIIINP levels (p = 0.05). There were no other differences comparing baseline with post-treatment collagen biomarkers (PICP, CITP, and MMP-1) after 6 months of treatment regardless of treatment assignment (Table 2).
Table 2.
Effect of Galectin-3 Inhibition on Clinical and Laboratory Parameters, Arterial Tonometry Measures, Collagen Markers, and Echocardiographic Measures
| Placebo |
MCP |
Between-Group Difference |
|||||
|---|---|---|---|---|---|---|---|
| Pre | Post | Difference | Pre | Post | Difference | p Value | |
| Clinical parameters | |||||||
| Body mass index, kg/m2 | 33.5 ± 6.5 | 33.6 ± 6.6 | 0.13 | 30.0 ± 5.9 | 30.3 ± 5.5 | 0.06 | 0.88 |
| Systolic BP, mm Hg | 129 ± 10 | 127 ± 15 | –1.50 | 126 ± 12 | 128 ± 15 | 1.83 | 0.50 |
| Diastolic BP, mm Hg | 81 ± 9 | 78 ± 9 | –2.86 | 78 ± 11 | 81 ± 11 | 1.60 | 0.14 |
| Heart rate, beats/min | 74 ± 11 | 76 ± 12 | 1.21 | 73 ± 18 | 74 ± 11 | 2.70 | 0.74 |
| Laboratory values | |||||||
| Galectin-3, mg/dl | 17.6 ± 4.1 | 17.1 ± 10.3 | –0.48 | 17.9 ± 3.5 | 17.9 ± 6.8 | 0.01 | 0.81 |
| eGFR, ml/min/1.73 m2 | 89.2 ± 16.0 | 89.4 ± 15.1 | 0.29 | 91.7 ± 13.0 | 89.9 ± 14.6 | –1.88 | 0.51 |
| Creatinine, mg/dl | 0.91 ± 0.17 | 0.90 ± 0.20 | –0.01 | 0.89 ± 0.14 | 0.90 ± 0.10 | 0.02 | 0.61 |
| UACR, mg/g | 24.7 ± 44.6 | 18.1 ± 32.2 | –7.84 | 17.7 ± 26.0 | 19.8 ± 32.7 | –0.49 | 0.48 |
| Arterial tonometry measures | |||||||
| AP, mm Hg | 8.90 ± 5.58 | 9.97 ± 7.26 | 1.07 | 11.68 ± 5.10 | 10.59 ± 5.89 | –1.09 | 0.13 |
| AIx, % | 23.86 ± 10.50 | 23.27 ± 14.78 | –0.59 | 29.13 ± 10.876 | 25.86 ± 11.74 | –3.27 | 0.36 |
| PWV, m/s | 8.83 ± 1.71 | 8.82 ± 2.18 | –0.01 | 9.29 ± 2.14 | 8.69 ± 1.88 | –0.54 | 0.32 |
| Collagen markers∗ | |||||||
| PICP, ng/ml | 4.37 ± 0.39 | 4.30 ± 0.30 | –0.07 | 4.29 ± 0.52 | 4.35 ± 0.54 | 0.06 | 0.10 |
| CITP, ng/ml | 1.20 ± 0.34 | 1.23 ± 0.40 | 0.03 | 1.15 ± 0.35 | 1.16 ± 0.41 | 0.01 | 0.73 |
| PIIINP, ng/ml | 1.38 ± 0.40 | 1.32 ± 0.47 | –0.06 | 1.40 ± 0.37 | 1.53 ± 0.33 | 0.15 | 0.054 |
| MMP-1, ng/ml | 2.09 ± 0.72 | 2.03 ± 0.81 | –0.06 | 2.08 ± 0.60 | 2.04 ± 0.52 | –0.04 | 0.76 |
| CITP:MMP-1 ratio | 0.36 ± 0.82 | 0.46 ± 0.88 | –0.09 | 0.32 ± 0.64 | 0.37 ± 0.68 | –0.05 | 0.70 |
| Echocardiography measures | |||||||
| LV end-diastolic dimension, cm | 4.66 ± 0.44 | 4.62 ± 0.48 | –0.04 | 4.52 ± 0.36 | 4.42 ± 0.42 | –0.10 | 0.57 |
| LV wall thickness, cm | 2.03 ± 0.27 | 2.06 ± 0.25 | 0.03 | 1.97 ± 0.26 | 1.93 ± 0.25 | –0.04 | 0.23 |
| LA diameter, cm | 3.79 ± 0.83 | 3.74 ± 0.39 | –0.05 | 3.50 ± 0.49 | 3.62 ± 0.33 | 0.12 | 0.12 |
| LV mass index, g/m2 | 78.8 ± 16.6 | 79.7 ± 17.1 | 0.90 | 76.7 ± 18.1 | 71.2 ± 16.1 | –5.47 | 0.08 |
| Fractional shortening, % | 42.50 ± 7.50 | 41.50 ± 6.17 | –0.93 | 41.80 ± 6.55 | 38.30 ± 6.51 | –3.50 | 0.26 |
| DT, ms | 215.1 ± 34.6 | 223.4 ± 37.6 | 8.27 | 207.9 ± 29.1 | 229.3 ± 32.4 | 21.38 | 0.28 |
| E/A ratio | 1.04 ± 0.29 | 0.99 ± 0.30 | –0.05 | 1.20 ± 0.68 | 0.97 ± 0.27 | –0.24 | 0.20 |
| E/e’ ratio | 8.95 ± 3.54 | 8.86 ± 2.77 | –0.09 | 8.54 ± 2.10 | 8.23 ± 2.70 | –0.31 | 0.74 |
| EndoGLS, %† | –17.50 ± 3.20 | –17.40 ± 3.35 | 0.16 | –18.50 ± 5.65 | –17.80 ± 3.67 | –0.80 | 0.23 |
Values are mean ± SD.
A = late mitral inflow velocity on Doppler; CITP = C-terminal telopeptide of type I collagen; DT = deceleration time; E = early mitral inflow velocity on Doppler; E′ = early mitral annular velocity on tissue Doppler; EndoGLS = endocardial global longitudinal strain; LV = left ventricular; MMP = matrix metalloproteinase; PICP = C-terminal propeptide of type I procollagen; PIIINP = N-terminal propeptide of type III procollagen; UACR = urine albumin-to-creatine ratio; other abbreviations as in Table 1.
Collagen markers are log-transformed.
Average of apical 2 and apical 4 global longitudinal strain.
Figure 3.
Change in Collagen Metabolism Biomarkers in Placebo Versus MCP Groups
Boxes show interquartile ranges and bars represent 25th and 75th percentile values. Dots represent outliers. Squares represent the means. ∗p < 0.05. CITP = C-terminal telopeptide of type I collagen; MCP = modified citrus pectin; MMP = matrix metalloproteinase; PIIINP = N-terminal propeptide of type III procollagen; PICP = C-propeptide of type I procollagen.
Effect of Gal-3 inhibition on secondary endpoints
In secondary analyses, changes in Gal-3, renal function, as quantified by eGFR, creatinine, and urine albumin-to-creatine ratio, and vascular stiffness, as quantified by AP, AIx, and PWV, did not meet statistical significance in either treatment group (Table 2). Although measures of vascular stiffness in the MCP treatment group were lower at the end of the trial compared with unchanged or worse measures in the placebo arm, none of these differences were statistically significant. Echocardiographic parameters of cardiac structure and function are summarized in Table 2. LV mass index was lower after 6 months of MCP therapy (difference = –5.47 g/m2, SE = 2.19 g/m2; p = 0.02), with no change in the placebo group. Similarly, mitral inflow deceleration time increased by 21 ms (difference = 21.38 ms, SE = 8.71 ms; p = 0.02) in the MCP group without significant change in the placebo group. Between-group differences, however, were not significant. Other measures of diastolic function, including mitral E/e′ ratio, as well as cardiac mechanics as measured by global longitudinal strain did not change in either treatment group.
Discussion
We present the results of the first randomized trial of MCP, a direct Gal-3 inhibitor, in human subjects with hypertension and elevated Gal-3 levels for the prevention of subclinical cardiac fibrosis. Our findings are 3-fold. First, baseline Gal-3 levels were significantly higher in women compared with men. Second, in cross-sectional analyses, diabetes and reduced eGFR were independent predictors of Gal-3 level. Third, treatment with MCP compared with placebo did not significantly influence collagen biomarker levels, echocardiographic markers of diastolic function, or arterial stiffness. Taken together, our study confirms previous associations of Gal-3 with diabetes and eGFR but did not find attenuation of subclinical measures of cardiovascular fibrosis with direct Gal-3 inhibition.
In our sample of 275 eligible participants, we confirm previous observations, including significantly higher baseline levels of Gal-3 in women compared with men, and significant cross-sectional association of Gal-3 with diabetes and an inverse association with eGFR. We previously showed that in 2,477 individuals in the community, women had greater increases in Gal-3 over time compared with men (10), and in a study of Gal-3 in 1650 participants with symptomatic HF, female sex was independently predictive of both baseline circulating Gal-3 levels and change in Gal-3 levels at 4 months (25). Interestingly, female sex was not independently associated with Gal-3 levels after multivariable adjustment in our study, highlighting the potential role of comorbidities, including diabetes and chronic kidney disease, in modulating expression of Gal-3 in women preferentially compared with men. Indeed, previous studies have demonstrated that cardiovascular risk factors, including age, diabetes, and body mass index are all associated with both Gal-3 levels and rise in Gal-3 over time in healthy individuals free of HF and in individuals with symptomatic HF (9,25). Renal dysfunction has been most strongly associated with Gal-3 in epidemiologic studies of both healthy individuals and individuals with HF. In prior analyses examining the association of Gal-3 with incident HF events, adjustment for eGFR attenuated the association of Gal-3 with outcomes (9,10,25). We again show a strong inverse relationship between Gal-3 and eGFR, further validating the importance of Gal-3 with kidney function and highlighting the need to consider renal function when evaluating the association of Gal-3 with HF risk.
Beyond clinical risk factors, we further examined the associations of Gal-3 with vascular parameters, echocardiographic measures, and collagen turnover biomarkers but did not find meaningful associations contrary to expectation. Two prior studies found increased arterial stiffness, as measured by PWV, with higher levels of Gal-3 (26,27), and a study of 115 patients presenting to the emergency department with acute dyspnea found that higher Gal-3 levels were significantly associated with echocardiographic markers of LV filling and diastolic function, valvular regurgitation, and right ventricular function (28). We postulate that our neutral results may be related to the restricted sample size or the nature of our study sample, an ostensibly healthy sample with hypertension that has not yet developed echocardiographic evidence of remodeling.
In our primary analysis, we compared differences in collagen turnover markers in 22 individuals randomized to treatment with MCP and 30 randomized to placebo. In contrast to our hypothesis that Gal-3 inhibition would reduce collagen turnover, we did not find significant differences in collagen markers between the active therapy and placebo arms. We observed a borderline statistically significant between-group difference in the change in PIIINP, although this was driven primarily by an increase in PIIINP in the MCP treatment group. Although we did not find changes in markers of collagen metabolism, it is worth describing the motivation for this interventional trial. Circulating biomarkers of collagen metabolism have been well correlated with cardiac fibrosis in hypertensive heart disease and HF (29,30). Prior studies have demonstrated a direct correlation between circulating markers of collagen turnover, histological myocardial fibrosis, and LV chamber stiffness (31). Gal-3 is expressed in activated macrophages with binding sites localized to the myocardial extracellular matrix and cardiac fibroblasts and is thought to promote collagen deposition via activation of cardiac fibroblasts (7). As such, Gal-3 has been previously correlated with markers of collagen metabolism, specifically tissue inhibitor of MMP-1, in healthy and hypertensive subjects (32, 33, 34, 35). Previous studies have found decreases in collagen markers with antihypertensive therapy in patients with hypertension. Collectively, these observations were the driving rationale for this intervention study that interrogated the impact of direct Gal-3 inhibition on circulating markers of collagen metabolism, surrogate markers of cardiac fibrosis. The one borderline finding of increased PIIINP with MCP treatment is surprising. PIIINP, like Gal-3, is a biomarker of cardiac fibrosis and has been associated with HF prognosis. In a study of 63 patients with stable coronary artery disease, elevated serum levels of Gal-3 were associated with cardiac fibrosis assessed by late gadolinium enhancement by cardiac magnetic resonance imaging as well as impaired LV diastolic function, whereas PIIINP was associated with diastolic dysfunction but not late gadolinium enhancement, suggesting that elevated levels of PIIINP may reflect earlier stages of disease progression compared with Gal-3 (36). Although our findings should not be overinterpreted, it warrants further investigation into the relationship between Gal-3 inhibition and PIIINP. Finally, the present investigation sought to modulate the Gal-3 pathway in individuals with elevated Gal-3 levels but who had not yet developed irreversible cardiovascular remodeling or fibrosis. To that end, we enrolled individuals with modest elevations in Gal-3 levels (≥50th sex-specific percentile) based on prior population data (9). It is conceivable that treatment with MCP may have demonstrated greater clinical benefit in individuals with higher baseline Gal-3 levels or more advanced fibrosis.
Finally, we also report neutral results for our exploratory analyses examining the effect of Gal-3 inhibition on vascular function and cardiac diastolic function. Of interest, although the differences in vascular stiffness were not statistically different between the MCP therapy and placebo arms, the absolute reduction in vascular stiffness measures, in particular AP and AIx, were greater in the MCP group. Separately, there was a borderline but nonsignificant difference in LV mass index between the MCP and placebo groups. This result must be cautiously interpreted given its lack of significance but does argue for further exploration.
Study limitations
First, there was significant attrition bias, as premature discontinuation of the study drug was significantly higher in the MCP arm compared with placebo due to gastrointestinal side effects. As such, adherence to treatment at 6 months was 65% in the MCP arm compared with 88% in the placebo group. Second, owing to significant attrition, the sample size was inherently reduced, potentially limiting power to detect meaningful differences between the MCP and placebo groups. Moreover, after premature discontinuation of the study drug, baseline characteristics were no longer balanced between the intervention and placebo arms, potentially confounding interpretation of trial results. Last, owing to variable gastrointestinal MCP absorption related to oral administration, participants were instructed to take MCP a half-hour before or 2 h after food for optimal absorption.
Conclusions
In summary, we present the results of the first proof-of-concept interventional trial of Gal-3 inhibition for the attenuation of cardiac fibrosis in human subjects. We demonstrate higher baseline Gal-3 levels in women compared with men and further validate previous associations of Gal-3 with clinical risk factors including diabetes and reduced eGFR. Gal-3 inhibition via MCP did not influence surrogate measures of cardiac fibrosis, including collagen turnover markers, echocardiographic measures, and vascular function in human subjects at risk for HF. Whether Gal-3 inhibition among individuals with existing HF may meaningfully attenuate cardiac fibrosis and, ultimately, clinical cardiovascular events remains to be studied.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In the first proof-of-concept interventional trial of Gal-3 inhibition in human subjects with hypertension, Gal-3 inhibition with MCP did not influence collagen markers, echocardiographic measures, or vascular function. However, we showed that baseline Gal-3 levels were higher in women compared with men and confirmed previous associations of Gal-3 with diabetes and reduced eGFR.
TRANSLATIONAL OUTLOOK: Future studies are needed to examine whether Gal-3 inhibition can attenuate cardiac fibrosis and cardiovascular events in individuals with HF.
Author Disclosures
This work was supported by National Institutes of Health Grant Nos. NIH-5T32HL094301-07 (to Dr. Lau), R01-HL134893 (to Dr. Ho), R01-HL140224 (to Dr. Ho), and K24-HL153669 (to Dr. Ho) and a Gilead Sciences Research Scholar Award (to Dr. Ho). Dr. Ho has received research grants from Gilead Sciences and Bayer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
Research supplies (modified citrus pectin and placebo) were donated for use in this study by EcoNugenics, Inc.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental figure and tables, please see the online version of this paper.
Appendix
References
- 1.Lloyd-Jones D.M., Larson M.G., Leip E.P. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 2.SOLVD Investigators. Yusuf S., Pitt B., Davis C.E., Hood W.B., Jr., Cohn J.N. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 3.Jong P., Yusuf S., Rousseau M.F., Ahn S.A., Bangdiwala S.I. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. Lancet. 2003;361:1843–1848. doi: 10.1016/S0140-6736(03)13501-5. [DOI] [PubMed] [Google Scholar]
- 4.de Boer R.A., Yu L., van Veldhuisen D.J. Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep. 2010;7:1–8. doi: 10.1007/s11897-010-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson N.C., Mackinnon A.C., Farnworth S.L. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishi Y., Sano H., Kawashima T. Role of galectin-3 in human pulmonary fibrosis. Allergol Int. 2007;56:57–65. doi: 10.2332/allergolint.O-06-449. [DOI] [PubMed] [Google Scholar]
- 7.Sharma U.C., Pokharel S., van Brakel T.J. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 8.de Boer R.A., van Veldhuisen D.J., Gansevoort R.T. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med. 2012;272:55–64. doi: 10.1111/j.1365-2796.2011.02476.x. [DOI] [PubMed] [Google Scholar]
- 9.Ho J.E., Liu C., Lyass A. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghorbani A., Bhambhani V., Christenson R.H. Longitudinal change in galectin-3 and incident cardiovascular outcomes. J Am Coll Cardiol. 2018;72:3246–3254. doi: 10.1016/j.jacc.2018.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow S.L., Maisel A.S., Anand I. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. 2017;135:e1054–e1091. doi: 10.1161/CIR.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 12.Glinsky V.V., Raz A. Modified citrus pectin anti-metastatic properties: one bullet, multiple targets. Carbohydr Res. 2009;344:1788–1791. doi: 10.1016/j.carres.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunning A.P., Bongaerts R.J., Morris V.J. Recognition of galactan components of pectin by galectin-3. FASEB J. 2009;23:415–424. doi: 10.1096/fj.08-106617. [DOI] [PubMed] [Google Scholar]
- 14.Kolatsi-Joannou M., Price K.L., Winyard P.J., Long D.A. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guess B.W., Scholz M.C., Strum S.B., Lam R.Y., Johnson H.J., Jennrich R.I. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: a phase II pilot study. Prostate Cancer Prostatic Dis. 2003;6:301–304. doi: 10.1038/sj.pcan.4500679. [DOI] [PubMed] [Google Scholar]
- 16.Azémar M., Hildenbrand B., Haering B., Heim M.E., Unger C. Clinical benefit in patients with advanced solid tumors treated with modified citrus pectin: a prospective pilot study. Clin Med Insights Oncol. 2007;1 CMO.S285. [Google Scholar]
- 17.Zhao Z.Y., Liang L., Fan X. The role of modified citrus pectin as an effective chelator of lead in children hospitalized with toxic lead levels. Altern Ther Health Med. 2008;14:34–38. [PubMed] [Google Scholar]
- 18.López B., Ravassa S., González A. Myocardial collagen cross-linking is associated with heart failure hospitalization in patients with hypertensive heart failure. J Am Coll Cardiol. 2016;67:251–260. doi: 10.1016/j.jacc.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 19.Butlin M., Qasem A. Large artery stiffness assessment using SphygmoCor technology. Pulse (Basel) 2017;4:180–192. doi: 10.1159/000452448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho J.E., Deeks S.G., Hecht F.M. Initiation of antiretroviral therapy at higher nadir CD4+ T-cell counts is associated with reduced arterial stiffness in HIV-infected individuals. AIDS. 2010;24:1897–1905. doi: 10.1097/QAD.0b013e32833bee44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagueh S.F., Smiseth O.A., Appleton C.P. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Ho J.E., McCabe E.L., Wang T.J. Cardiometabolic traits and systolic mechanics in the community. Circ Heart Fail. 2017;10 doi: 10.1161/CIRCHEARTFAILURE.116.003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng S., McCabe E.L., Larson M.G. Left ventricular mechanical function: clinical correlates, heritability, and association with parental heart failure. Eur J Heart Fail. 2015;17:44–50. doi: 10.1002/ejhf.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng S., Larson M.G., McCabe E.L. Reproducibility of speckle-tracking-based strain measures of left ventricular function in a community-based study. J Am Soc Echocardiogr. 2013;26:1258–1266.e2. doi: 10.1016/j.echo.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anand I.S., Rector T.S., Kuskowski M., Adourian A., Muntendam P., Cohn J.N. Baseline and serial measurements of galectin-3 in patients with heart failure: relationship to prognosis and effect of treatment with valsartan in the Val-HeFT. Eur J Heart Fail. 2013;15:511–518. doi: 10.1093/eurjhf/hfs205. [DOI] [PubMed] [Google Scholar]
- 26.Oikonomou E., Karlis D., Tsalamadris S. Galectin-3 and arterial stiffness in patients with heart failure: a pilot study. Curr Vasc Pharmacol. 2019;17:396–400. doi: 10.2174/1570161116666180703094919. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q., Yin K., Zhu M. Galectin-3 is associated with arterial stiffness among hemodialysis patients. Biomark Med. 2019;13:437–443. doi: 10.2217/bmm-2018-0488. [DOI] [PubMed] [Google Scholar]
- 28.Shah R.V., Chen-Tournoux A.A., Picard M.H., van Kimmenade R.R.J., Januzzi J.L. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12:826–832. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López B., González A., Díez J. Circulating biomarkers of collagen metabolism in cardiac diseases. Circulation. 2010;121:1645–1654. doi: 10.1161/CIRCULATIONAHA.109.912774. [DOI] [PubMed] [Google Scholar]
- 30.Querejeta R., López B., González A. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation. 2004;110:1263–1268. doi: 10.1161/01.CIR.0000140973.60992.9A. [DOI] [PubMed] [Google Scholar]
- 31.Díez J., Querejeta R., López B., González A., Larman M., Martínez Ubago J.L. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed S.H., Clark L.L., Pennington W.R. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- 33.Lindsay M.M., Maxwell P., Dunn F.G. TIMP-1: a marker of left ventricular diastolic dysfunction and fibrosis in hypertension. Hypertension. 2002;40:136–141. doi: 10.1161/01.hyp.0000024573.17293.23. [DOI] [PubMed] [Google Scholar]
- 34.Sundström J., Evans J.C., Benjamin E.J. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham Heart Study. Eur Heart J. 2004;25:1509–1516. doi: 10.1016/j.ehj.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Lin Y.H., Lin L.Y., Wu Y.W. The relationship between serum galectin-3 and serum markers of cardiac extracellular matrix turnover in heart failure patients. Clin Chim Acta. 2009;409:96–99. doi: 10.1016/j.cca.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Lepojärvi E.S., Piira O.-P., Pääkkö E. Serum PINP, PIIINP, galectin-3, and ST2 as surrogates of myocardial fibrosis and echocardiographic left ventricular diastolic filling properties. Front Physiol. 2015;6:200. doi: 10.3389/fphys.2015.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.