Abstract
Children are known to disproportionately bear the health impacts of climate change, particularly children living in impoverished areas. Owing to their developing physiology and immature metabolism, distinct exposure behaviors, and reliance on adults for care and protection, children are uniquely susceptible to the adverse effects of our warming planet. Herein, we summarize the known impacts of climate change on pediatric skin health, including its effects on atopic dermatitis, vector-borne and other infectious diseases, nutritional deficiencies, and psychodermatoses.
Keywords: Climate change, Pediatric dermatology, Atopic dermatitis, Infestations, Nutritional deficiencies, Psychodermatology
Introduction
Our continually warming planet and corresponding changes in regional climates have created and will continue to create unique and worsening health risks to the global community. As summarized by the Lancet countdown on health and climate change, if the world’s current rate of fossil fuel combustion continues unabated, children born today will experience adverse health impacts secondary to climate change across all ages and stages of life (Watts et al., 2019). Children are known to disproportionately bear the health impacts of climate change, particularly children living in impoverished areas (Ahdoot et al., 2015). Owing to their developing physiology, immature metabolism, and distinct exposure behaviors, children are uniquely susceptible to adverse environmental changes. For example, children are more susceptible to heat-related illness secondary to immature thermoregulatory mechanisms and, in neonates, infants, and toddlers, the inability to independently replace fluid losses (for a comprehensive review of heat related illness, see Williams’ “Global warming, heat-related illnesses and the dermatologist” in this issue).
Such physiologic and behavioral risk factors are also compounded by children’s reliance on adults for care and protection, limiting their agency in making decisions that may protect their own health (e.g., evacuating in advance of a hurricane, living in an area with less ambient pollution). As a result, children are at greater risk of disease secondary to meteorological events, such as increased heat exposure and flooding, as well as vector-borne illnesses and natural disasters (Ahdoot et al., 2015). Herein, we summarize the known impacts of climate change on pediatric skin disease.
Atopic dermatitis
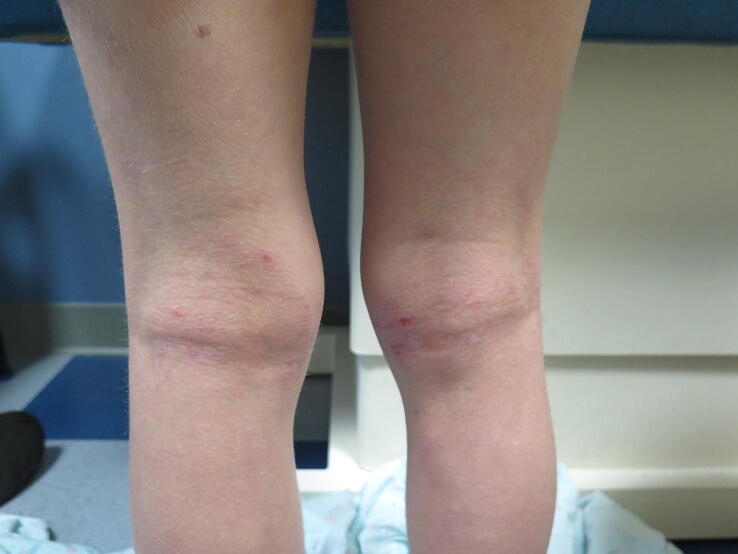
Atopic dermatitis (AD) is a chronic, pruritic dermatitis characterized by weeping erythema with associated scale of the cheeks and extensor extremities in infancy, typically transforming with age into a chronic, dry, and lichenified dermatitis in a flexural distribution (Fig. 1). The pathophysiology of AD is determined by a complex interplay of genetic, immunologic, and environmental factors. The global burden of AD increased in the 20th century, affecting approximately 5% to 20% of children worldwide, with variations in prevalence across countries and regions of the world (Nguyen et al., 2019, Williams et al., 1999).
Fig. 1.
Erythematous, scaly patches present within the popliteal fossae in a young child with atopic dermatitis.
This variation has been suggested to be secondary to local environmental factors that may impact the burden of atopic disease (Williams et al., 1999). Many studies have investigated which environmental exposures contribute to the development or exacerbation of AD, often providing mixed results. This may be because AD is best regarded as a heterogeneous disorder with varied molecular endotypes that may translate into distinct clinical subgroups with unique risk factors and prognoses (Czarnowicki et al., 2019, Paternoster et al., 2018). As such, the effects of environmental variables, such as meteorological conditions and air pollution, may affect different subsets of patients with AD in distinct ways (Ahn, 2014).
Air pollutants are airborne gaseous or particulate substances that adversely affect human health. They can be generated from natural phenomena, including wildfires and volcano ash, or from anthropogenic sources, such as motor vehicles and power plants (Ahn, 2014). Volatile organic compounds, particulate matter (PMx) of varying diameters (0.1, 2.5, or 10 μm), nitrogen oxide compounds (NOx), and sulfur oxide compounds (SOx) represent major classes of air pollution that may negatively affect those with AD and other allergic diseases, depending on their concentration and length of exposure (Ahn, 2014). In particular, PM can be coated with polycyclic aromatic hydrocarbons (PAHs) that are lipophilic and readily able to penetrate the skin. These particles may subsequently induce oxidative stress and activate pathways relevant to AD (Araviiskaia et al., 2019, Hidaka et al., 2017, Krutmann et al., 2014). Ozone and other secondary pollutants that occur as a result of photochemical reactions involving the aforementioned (primary) pollutants and sunlight may also be relevant to the development of AD (Krutmann et al., 2014).
Myriad studies suggest a link between increased air pollution and increased incidence or severity of AD. A prospective, population-based study using multivariate survey logistic regression models found that in the United States, nitrogen dioxide (NO2), sulfate (SO3), and sulfur dioxide (SO2) were associated with an increased prevalence of AD, whereas nitrate (NO3), organic carbon, and PM2.5 levels were associated with a greater likelihood of more severe AD (Kathuria and Silverberg, 2016). A retrospective, time-series study from Shanghai similarly found that SO2, NO2, and PM10 levels positively correlated with outpatient clinic visits for AD (Li et al., 2016). A retrospective, population-based study in Minsk, Belarus, also found that higher mean levels of seven outdoor air pollutants, including CO, NO2, formaldehyde, and lead, were associated with an increased incidence of infantile AD (Belugina et al., 2018). Additionally, a longitudinal study in Korea found that elevated levels of volatile organic compounds and PM10 aggravated symptoms of AD, and NO2 exposure was positively associated with AD in a German study (Kim et al., 2013, Morgenstern et al., 2008). This latter report also identified an increased risk of AD in children who lived closer (<50 m) to a main road, suggesting that traffic-related pollution promotes the development of AD (Morgenstern et al., 2008). Intriguingly, individual studies have also found that prenatal exposure to specific traffic-related air pollutants may also influence the development of AD via epigenetic changes or direct effects of maternal exposure on the neonate’s immune system (Ahn, 2014, Huang et al., 2015, Lu et al., 2017). Growing evidence supports the hypothesis that air pollution adversely influences the incidence and severity of AD; this is comprehensively reviewed elsewhere (see Roberts’ “Air pollution and skin disorders” in this issue; Ahn, 2014).
Temperature, humidity, ultraviolet exposure, and precipitation are a subset of meteorological factors that may influence AD. A prospective, population-based study of the prevalence of AD in the United States found that lower temperatures, ultraviolet exposure, humidity, increased indoor heating, and increased precipitation were associated with an increased prevalence of AD (Silverberg et al., 2013). Additionally, in a Korean cohort, daily increased temperature and relative humidity were found to be associated with decreased AD symptomatology, whereas increased rainfall and diurnal temperature range exacerbated symptoms of AD (Kim et al., 2017). Increased humidity and increased daily temperatures have been found to be protective against exacerbations of AD in other populations as well, although additional studies have demonstrated the opposite effect of these on AD prevalence (Guo et al., 2019, Kathuria and Silverberg, 2016, Li et al., 2016, Silverberg et al., 2013) Taken together, it appears that climate variables likely interact with air pollutants to modify AD prevalence or severity (Belugina et al., 2018, Guo et al., 2019) For instance, AD prevalence has been shown to be increased in geographic areas with elevated levels of organic carbon, SO3, SO2, and PM2.5 in the meteorological context of greater heat, humidity, and precipitation (Kathuria and Silverberg, 2016). Similarly, a study from Beijing, China, showed that increased levels of atmospheric NO2, SO2, PM2.5, and PM10 positively correlated with increased outpatient and emergency department visits for AD; this association was enhanced at higher temperatures (Guo et al., 2019).
The pathophysiologic mechanism by which air pollution influences AD is multifactorial and remains incompletely understood. Compounds such as ozone likely induce skin damage and inflammatory dermatoses via oxidative stress and the production of free radicals on the skin surface (Araviiskaia et al., 2019, Koohgoli et al., 2017). Two specific molecular signaling pathways have also been proposed as central to the association between airborne pollution and AD. The pregnane X receptor (PXR) is a transcription factor that regulates the expression of proteins involved in the detoxification and elimination of xenobiotic compounds (foreign chemical substances—typically manmade—identified in organisms or environments where they are not naturally found). PXR is upregulated in the skin in response to compounds including PAHs; when overexpressed in mouse models, it induces skin barrier defects (dry, scaly skin) and increased transepidermal water loss (Elentner et al., 2018). This is due, in part, to skewing of the cutaneous immune milieu towards a Th2/Th17 phenotype, as well as induction of the aryl hydrocarbon receptor (Ahr) gene. In support of the role of PXR signaling in the development of AD in humans, the transcription of PXR is altered and that of its downstream targets is increased in lesional skin of patients with AD (Elentner et al., 2018).
A second pathway that links air pollution to AD involves activation of AhR by these environmental pollutants, most notably PAHs. Like PXR, AhR is a transcription factor that upregulates expression of other proteins involved in detoxification and xenobiotic metabolism (Hidaka et al., 2017, Oetjen et al., 2018). In a transgenic mouse model, AhR also appears to upregulate a subset of proinflammatory genes that skew immune responses toward a Th2 phenotype and are relevant to AD, including Tslp and Il33. Furthermore, AhR overexpression in this murine model upregulated expression of the Artn gene; its product, artemin, induces epidermal hyperproliferation and alloknesis. Mice transgenic for AhR exhibited phenotypic similarities to AD: increased transepidermal water loss, more frequent scratching behavior, and skin that histologically showed an inflammatory infiltrate with accompanying hyperkeratosis and acanthosis (Hidaka et al., 2017). In a nontransgenic mouse model, chronic application of diesel exhaust particles (including PAHs) similarly induced the expression of AD-related genes in an AhR-dependent manner (Hidaka et al., 2017). Importantly, this molecular signaling pathway appears to be retained in humans; both AhR activation and artemin expression are increased in lesional skin of AD (Hidaka et al., 2017). Taken together, these findings provide a plausible mechanism by which exposure to airborne pollution increases susceptibility to AD.
Aeroallergens such as pollen may also contribute to the burden of AD. Pollen levels positively correlate with other atopic diseases, such as asthma exacerbations (Schmier and Ebi, 2009), and early evidence suggests that pollen may affect AD as well. Specifically, a German cohort study found that cutaneous exposure to birch pollen directly correlated with worsening subjective patient assessment of AD, higher SCORAD scores, and increased pruritus (Fölster-Holst et al., 2015). Given that annual pollen seasons have been documented to begin earlier worldwide and elevated temperature and CO2 levels are thought to increase pollen production (Sheffield et al., 2011), pollen exposure may become an increasingly important risk factor for AD exacerbations. Taken together, these findings suggest that, in a rapidly warming world with concomitant environmental changes including wildfires that occur with greater frequency and severity, increased aeroallergen and pollen burden, and fossil fuel use that continues unabated, the health care burden of AD is likely to increase (Watts et al., 2019, Whitman et al., 2019).
Food scarcity and nutritional deficiencies
Climate change will have dramatic impacts on established agricultural practices and the cultures of many regions of the world. Although specific effects are impossible to predict, multiple workgroups have noted that land and water resources are currently being utilized at unprecedented and unsustainable rates, regardless of the looming threat of climate change. A report from the United Nations Intergovernmental Panel on Climate Change (Shukla et al., 2019) noted that >500 million people live in areas undergoing desertification. Many regions are losing arable soil at rates 10 to 100 times faster than soil is forming. Climate change will add to this dire situation with unpredictable weather, including storms, floods, drought, and extremes in temperature, which would dramatically affect local agrarian practices and the ability to successfully grow food.
Moreover, evidence suggests that higher atmospheric levels of carbon dioxide reduce the content of protein, minerals (e.g., iron and zinc), and vitamins (e.g., riboflavin and thiamine) in food crops grown under these conditions (Smith and Myers, 2018, Smith and Myers, 2019). Therefore, the pace of global environmental changes may be faster than the ability of agricultural systems to adapt, leading to food shortages and less nourishing food (Dhankher and Foyer, 2018, Zhang et al., 2018). This disproportionately affect children, who rely on macro- and micronutrients for proper growth and development. In areas struck by food shortages, migration will also increase, with health impacts as described later. Desperation-induced migration has already occurred as a result of climate change: droughts in El Salvador, Guatemala, and Honduras between 2010 and 2015 were responsible for a five-fold increase in migrants at the Southern U.S. border during that time (Flavelle, 2019).
A variety of skin disorders are associated with malnutrition and food shortages. The two classical syndromes of severe acute malnutrition are marasmus and kwashiorkor; these both have characteristic clinical and dermatologic features (Bhutta et al., 2017). Presently, they account for at least 10% of all deaths in children age <5 years worldwide. Marasmus (wasting syndrome) results from total caloric insufficiency and is typically seen in young children and babies who exhibit low weight for height and a reduced mid-upper arm circumference. These children appear emaciated and weak with shrunken extremities and buttocks. Redundant skin folds develop due to the loss of subcutaneous fat, and their heads often appear large relative to their very thin bodies. In addition to displaying irritability, marasmatic children are often bradycardic, with hypothermia and hypotension. The skin of children with marasmus is typically thin and dry, and their hair is sparse, brittle, and easily extracted.
In contrast, kwashiorkor (edematous malnutrition, secondary to relative protein deficiency in relation to calorie intake) classically exhibits symmetric peripheral pitting edema that begins in the most dependent areas and gradually progresses as malnutrition persists. This edema can be so severe so as to obscure corresponding growth failure (Liu et al., 2001). The abdomen is often protuberant due to hepatomegaly from fatty infiltration of the liver and dilated intestinal loops. The cutaneous changes of kwashiorkor are characteristic and striking, often evocatively described as “flaking paint” or “crazy pavement” (Tierney et al., 2010). The skin is thin, dry, and peeling, with areas of hyperkeratosis and hyperpigmentation. Individual lesions may weep, and a secondary infection or yeast overgrowth is also common. Children with kwashiorkor also have hair that is dry, dull, lighter in color, and easily extractable (Liu et al., 2001, Tierney et al., 2010). In some patients, periodic restoration of diet induces a return of hair color, which can lead to the development of alternating bands of color, termed the “flag sign,” in the hair. A full discussion of the evaluation and treatment of severe acute malnutrition is beyond the scope of this article, but the World Health Organization has published standards for the assessment and management of acute malnutrition (Ashworth, 2003, United Nations Children's Fund, 2007).
Alterations in food supply or limitation in the variety of foods available can lead to deficiencies in individual vitamins or other nutrients, with specific nutritional deficiencies expected to become more prevalent as a result of climate change (Smith and Myers, 2019).
The cutaneous manifestations of key nutrient deficiencies are summarized in Table 1 (DiBaise and Tarleton, 2019, Golden, 1991, Green and Datta Mitra, 2017, Kirkland and Meyer-Ficca, 2018, Lonsdale, 2018, Lopez et al., 2016, Saedisomeolia and Ashoori, 2018).
Table 1.
Dermatologic and other clinical findings associated with select nutrient deficiencies.
| Vitamin | Mucocutaneous signs of deficiency | Other clinical signs | Comments/references |
|---|---|---|---|
| Zinc | Eczematous or psoriasiform patches and plaques in a periorificial, acral, and anogenital distribution, ± bullae; glossitis | Secondary infection; yeast overgrowth; alopecia; diarrhea | (Golden, 1991) |
| Thiamine (B1) | Glossitis | Beriberi (wet or dry); neurologic changes; cardiovascular dysfunction; edema | (Lonsdale, 2018) |
| Riboflavin (B2) | Glossitis; cheilitis; stomatitis; seborrheic dermatitis-like rash | Normochromic normocytic anemia | (Saedisomeolia and Ashoori, 2018) |
| Niacin (B3) | Photosensitive pigmented dermatitis; “Casal’s necklace” | Diarrhea; dementia; glossitis; neurologic symptoms | Nixtamalization (alkaline treatment) of corn/sorghum enhances niacin availability (Kirkland and Meyer-Ficca, 2018) |
| Pyridoxine (B6) | Stomatitis; glossitis; cheilosis; seborrheic dermatitis-like rash | Neurologic symptoms; neuropathy; seizures; microcytic anemia | (DiBaise and Tarleton, 2019) |
| Iron | Atrophic glossitis; pruritus; hair loss; koilonychia; xerosis; pallor | Microcytic hypochromic anemia; tachycardia; fatigue; exercise intolerance; restless leg syndrome; beeturia | (Lopez et al., 2016) |
| Folate (B9) | Pallor; jaundice; oral ulcers | Megaloblastic anemia; fatigue; irritability; neurocognitive changes | Can develop rapidly (Green and Datta Mitra, 2017) |
Climate refugees, infections, and infestations
Climate change has increased the frequency of extreme weather events, such as droughts, floods, and heat waves. These events contribute to overcrowding in refugee camps and limit access to safe water, food, and routine medical care. Such unsanitary conditions have a disproportionate impact on children due to their immature physiology, different behaviors, and dependence on caregivers, promoting a variety of skin diseases including infections and infestations (Sheffield and Landrigan, 2011). Those of particular importance to pediatric health are reviewed herein (see Kwak et al.’s “Mass migration and climate change” in this issue).
Scabies is an intensely pruritic skin condition characterized by papules, burrows, and crusted nodules typically present in the interdigital spaces, waist, wrists, and axillae. Scabies is caused by the mite Sarcoptes scabiei var. hominis. It is spread by direct person-to person contact and as such is more prevalent among children and in resource-poor settings, such as refugee camps after climate disasters. Scabies was a leading cause of morbidity after the 2010 floods in Baluchistan, Pakistan, accounting for 19% of patient visits after the disaster (World Health Organization, 2010). In 2015, the greatest disease burden from scabies worldwide was seen in East Asia, Southeast Asia, Oceania, and tropical Latin America, regions of the world among the most at risk for climate-related disasters (Karimkhani et al., 2017). Although not a life-threatening condition, complications of untreated or severe scabies infection include postscabetic itching, impetigo, and sepsis (Thomas et al., 2020). In resource-poor settings, the complications of secondarily infected scabies also include acute poststreptococcal glomerulonephritis and chronic kidney disease (Karimkhani et al., 2017, Whitehall et al., 2013). Similarly, pediculosis capitis (head lice infestation) is common after climate-related disasters. Severe flooding in Paraguay in 2014 displaced approximately 240,000 people into improvised, overcrowded shelters with poor sanitation. Head lice was the most common skin condition diagnosed by dermatologists after this event, affecting 36% of pediatric patients (Moreno et al., 2016).
Wet environments after floods and hurricanes have also been linked to superficial dermatophyte infections. Tinea corporis was the most common skin infection in patients seen by dermatologists in Indonesia after the 2004 tsunami, likely due to submersion in water, hot and humid weather, and a lack of sanitary conditions (Lee et al., 2006). Mobile medical workers also reported frequent outbreaks of tinea corporis and capitis in pediatric patients in Louisiana after Hurricane Katrina in 2005, which has been attributed in part to overcrowding and limited access to hot water (Madrid et al., 2008). Skin infections with methicillin-resistant Staphylococcus aureus were also seen after Hurricane Katrina, with reported cases in 30 pediatric and adult refugees in an evacuee facility in Dallas, Texas (Centers for Disease Control and Prevention, 2005).
Climate-related disasters may also cause outbreaks of disease in children via direct contact with environmental pathogens. Leptospirosis is a zoonotic infection caused by spirochetes of the genus Leptospira; it is transmitted via contact with soil or water contaminated by urine or other bodily fluids from infected mammals (Zaki and Shanbag, 2010). The eruption of leptospirosis is characterized by erythematous macules, papules, urticaria, and petechiae, often accompanied by extracutaneous symptoms that may include fevers, chills, myalgias, meningitis, uveitis, and multiorgan system dysfunction (Bandino et al., 2015). In 2005, the northern suburbs of Mumbai, India, experienced its heaviest rainfall event in 90 years, which led to severe flooding and a leptospirosis outbreak affecting 27 children admitted to a local hospital (Zaki and Shanbag, 2010). Risk exposures for these children included playing in flood water, wading through flood water to go to school, or flood water entering their homes.
Buruli ulcer is an infection cause by Mycobacterium ulcerans, transmitted from an unclear aquatic environmental source. Its earliest manifestation is an insect bite–like papule or nodule, which progresses into a plaque with eventual necrosis and ulceration of the dermis and subcutaneous adipose tissue. Complications can include severe scarring and limb contractures. Notably, there is a close relationship between rainfall patterns and Buruli ulcer, with increased diagnoses after periods of heavy rainfall (Combe et al., 2017). Children age 5 to 15 years are most affected in both incidence and severity in endemic countries of West Africa (Yotsu et al., 2015; see Bandino’s “An expanding abscess after a flooding disaster” in this issue).
Increasing temperatures have also been associated with an expanded geographic range of arboviral diseases, including dengue fever, Chikungunya, and Zika virus (Stanberry et al., 2018). Zika virus emerged in Latin America and the Caribbean between 2014 and 2016 during a period of severe drought and unusually high temperatures (Muñoz et al., 2016). The virus most commonly manifests in the skin as a nonspecific morbilliform eruption. Importantly, congenital Zika virus infection has been associated with microcephaly and other birth defects, with significant long-term developmental impacts on afflicted children (Anderko et al., 2020; see Coates and Norton’s “The effects of climate change on infectious diseases with cutaneous manifestations” in this issue).
Natural disasters, children’s health, and psychodermatology
Of those at risk of disaster-related psychologic harm, children are among the most vulnerable. This stems in part from their immature and developing physiology, reliance on adult caregivers for security and protection, and prolonged disruptions to their community after weather-related catastrophes (Ahdoot et al., 2015). Importantly, the consequences of natural disasters or subsequent displacement may expose children to posttraumatic stress disorder and toxic stress, described as a “strong, frequent, or prolonged activation of the body’s stress response systems” without the protection of stable and supportive relationships (Ahdoot et al., 2015, Murray, 2018, Scheeringa and Zeanah, 2008, Shonkoff et al., 2012). Although a complete discussion of childhood toxic stress and its effects on measures of pediatric and subsequent adult health is beyond the scope of this paper, a few pertinent insights about this phenomenon are worth noting. Adverse childhood events trigger endocrinologic stress responses that, if prolonged, can result in permanent changes in neurologic development and function. This in turn may promote subsequent unhealthy behaviors that negatively affect the skin (i.e., tobacco use, drug abuse, obesity) and directly increase the risk of disease in childhood and adulthood, even in the absence of these maladaptive behaviors (Felitti et al., 1998, Oh et al., 2018, Shonkoff et al., 2012). Importantly, those exposed to toxic stress also manifest immunologic alterations and elevated inflammatory markers that may predispose to a variety of inflammatory and autoimmune diseases, with implications for cutaneous and overall health (Barnthouse and Jones, 2019, Felitti et al., 1998, Oh et al., 2018, Shonkoff et al., 2012).
Although a direct link between toxic stress and dermatologic disease has not been explored, to the authors’ knowledge, accompanying psychologic distress is known to adversely affect the skin. Specifically, psychosocial stress has been associated with the onset and severity of a variety of dermatologic conditions in children and adults, including AD, acne, psoriasis, vitiligo, and chronic urticaria (Gupta and Gupta, 1996, Gupta and Gupta, 2003, Manolache et al., 2009). The psychological stress associated with natural disasters, such as tsunamis and earthquakes, has been documented to cause flares of AD, psoriasis, and urticaria; this effect appears compounded by subsequent unhygienic living conditions, lack of access to health care, and physical loss of medications in the disaster’s aftermath (Bandino et al., 2015, Kodama et al., 1999, Lee et al., 2006, Stewart and Goodman, 1989). As exposure to floods, heatwaves, wildfires, drought, and storm-related disasters has increased in specific regions of the world in the context of climate change—a trend that is predicted to continue with time (Watts et al., 2019)—it is probable that the burden of cutaneous disease influenced by psychological stress will also escalate.
Conclusion
Children are uniquely susceptible to the adverse effects of our warming planet, with important implications for the cutaneous health of this population. Identifying conditions that are more frequently or severely affected by climate change allows dermatologists to anticipate corresponding impacts on their practices and most effectively care for these patients. Moreover, the visible impact of climate change on childhood skin conditions serves as an important reminder that now is the time to advocate for societal changes to mitigate the impacts of global warming, protecting the health of children now and in the future.
Financial disclosures
None.
Funding
None.
Study Approval
N/A.
References
- Ahdoot S., Pacheco S.E., Council on Environmental Health Global climate change and children’s health. Pediatrics. 2015;136:e1468–e1484. doi: 10.1542/peds.2015-3233. [DOI] [PubMed] [Google Scholar]
- Ahn K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol. 2014;134:993–999. doi: 10.1016/j.jaci.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Anderko L., Chalupka S., Du M., Hauptman M. Climate changes reproductive and children’s health: a review of risks, exposures, and impacts. Pediatr Res. 2020;87(2):414–419. doi: 10.1038/s41390-019-0654-7. [DOI] [PubMed] [Google Scholar]
- Araviiskaia E., Berardesca E., Bieber T., Gontijo G., Sanchez Viera M., Marrot L. The impact of airborne pollution on skin. J Eur Acad Dermatol Venereol. 2019;33:1496–1505. doi: 10.1111/jdv.15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth A. World Health Organization; Geneva, Switzerland: 2003. Guidelines for the inpatient treatment of severely malnourished children. [Google Scholar]
- Bandino J.P., Hang A., Norton S.A. The infectious and noninfectious dermatological consequences of flooding: a field manual for the responding provider. Am J Clin Dermatol. 2015;16:399–424. doi: 10.1007/s40257-015-0138-4. [DOI] [PubMed] [Google Scholar]
- Barnthouse M., Jones B.L. The impact of environmental chronic and toxic stress on asthma. Clin Rev Allergy Immunol. 2019;57:427–438. doi: 10.1007/s12016-019-08736-x. [DOI] [PubMed] [Google Scholar]
- Belugina I.N., Yagovdik N.Z., Belugina O.S., Belugin S.N. Outdoor environment, ozone, radionuclide-associated aerosols and incidences of infantile eczema in Minsk, Belarus. J Eur Acad Dermatol Venereol. 2018;32:1977–1985. doi: 10.1111/jdv.15063. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Berkley J.A., Bandsma R.H.J., Kerac M., Trehan I., Briend A. Severe childhood malnutrition. Nat Rev Dis Primer. 2017;3:17067. doi: 10.1038/nrdp.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Infectious disease and dermatologic conditions in evacuees and rescue workers after Hurricane Katrina–multiple states, August-September, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:961–964. [PubMed] [Google Scholar]
- Combe M., Velvin C.J., Morris A., Garchitorena A., Carolan K., Sanhueza D. Global and local environmental changes as drivers of Buruli ulcer emergence. Emerg Microbes Infect. 2017;6(4) doi: 10.1038/emi.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnowicki T., He H., Krueger J.G., Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1–11. doi: 10.1016/j.jaci.2018.10.032. [DOI] [PubMed] [Google Scholar]
- Dhankher O.P., Foyer C.H. Climate resilient crops for improving global food security and safety: climate resilient crops for improving global food security and safety. Plant Cell Environ. 2018;41:877–884. doi: 10.1111/pce.13207. [DOI] [PubMed] [Google Scholar]
- DiBaise M., Tarleton S.M. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2019;34:490–503. doi: 10.1002/ncp.10321. [DOI] [PubMed] [Google Scholar]
- Elentner A., Schmuth M., Yannoutsos N., Eichmann T.O., Gruber R., Radner F.P.W. Epidermal overexpression of xenobiotic receptor PXR impairs the epidermal barrier and triggers Th2 immune response. J Invest Dermatol. 2018;138:109–120. doi: 10.1016/j.jid.2017.07.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Flavelle C. Climate change threatens the world’s food supply, United Nations warns. New York Times; August 8, 2019.
- Fölster-Holst R., Galecka J., Weißmantel S., Dickschat U., Rippke F., Bohnsack K. Birch pollen influence the severity of atopic eczema - prospective clinical cohort pilot study and ex vivo penetration study. Clin Cosmet Investig Dermatol. 2015;8:539–548. doi: 10.2147/CCID.S81700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden M.H.N. The nature of nutritional deficiency in relation to growth failure and poverty. Acta Paediatr. 1991;80:95–110. doi: 10.1111/j.1651-2227.1991.tb12012.x. [DOI] [PubMed] [Google Scholar]
- Green R., Datta Mitra A. Megaloblastic anemias. Med Clin North Am. 2017;101:297–317. doi: 10.1016/j.mcna.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Guo Q., Xiong X., Liang F., Tian L., Liu W., Wang Z. The interactive effects between air pollution and meteorological factors on the hospital outpatient visits for atopic dermatitis in Beijing, China: a time-series analysis. J Eur Acad Dermatol Venereol. 2019;33:2362–2370. doi: 10.1111/jdv.15820. [DOI] [PubMed] [Google Scholar]
- Gupta M.A., Gupta A.K. Psychiatric and psychological co-morbidity in patients with dermatologic disorders: epidemiology and management. Am J Clin Dermatol. 2003;4:833–842. doi: 10.2165/00128071-200304120-00003. [DOI] [PubMed] [Google Scholar]
- Gupta M.A., Gupta A.K. Psychodermatology: an update. J Am Acad Dermatol. 1996;34:1030–1046. doi: 10.1016/s0190-9622(96)90284-4. [DOI] [PubMed] [Google Scholar]
- Hidaka T., Ogawa E., Kobayashi E.H., Suzuki T., Funayama R., Nagashima T. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat Immunol. 2017;18:64–73. doi: 10.1038/ni.3614. [DOI] [PubMed] [Google Scholar]
- Huang C.C., Wen H.J., Chen P.C., Chiang T.L., Lin S.J., Guo Y.L. Prenatal air pollutant exposure and occurrence of atopic dermatitis. Br J Dermatol. 2015;173:981–988. doi: 10.1111/bjd.14039. [DOI] [PubMed] [Google Scholar]
- Karimkhani C., Colombara D.V., Drucker A.M., Norton S.A., Hay R., Engelman D. The global burden of scabies: a cross-sectional analysis from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1247–1254. doi: 10.1016/S1473-3099(17)30483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria P., Silverberg J.I. Association of pollution and climate with atopic eczema in U.S. children. Pediatr Allergy Immunol. 2016;27:478–485. doi: 10.1111/pai.12543. [DOI] [PubMed] [Google Scholar]
- Kim J., Kim E.H., Oh I., Jung K., Han Y., Cheong H.K. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol. 2013;132 doi: 10.1016/j.jaci.2013.04.019. 495–8.e1. [DOI] [PubMed] [Google Scholar]
- Kim Y.M., Kim J., Han Y., Jeon B.H., Cheong H.K., Ahn K. Short-term effects of weather and air pollution on atopic dermatitis symptoms in children: a panel study in Korea. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JB, Meyer-Ficca ML. Niacin. In: Advances in food and nutrition research. Amsterdam: Elsevier; 2018. pp. 83–149. [DOI] [PubMed]
- Kodama A., Horikawa T., Suzuki T., Ajiki W., Takashima T., Harada S. Effect of stress on atopic dermatitis: investigation in patients after the great Hanshin earthquake. J Allergy Clin Immunol. 1999;104:173–176. doi: 10.1016/s0091-6749(99)70130-2. [DOI] [PubMed] [Google Scholar]
- Koohgoli R., Hudson L., Naidoo K., Wilkinson S., Chavan B., Birch-Machin M.A. Bad air gets under your skin. Exp Dermatol. 2017;26:384–387. doi: 10.1111/exd.13257. [DOI] [PubMed] [Google Scholar]
- Krutmann J., Liu W., Li L., Pan X., Crawford M., Sore G. Pollution and skin: from epidemiological and mechanistic studies to clinical implications. J Dermatol Sci. 2014;76:163–168. doi: 10.1016/j.jdermsci.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Choi C., Eun H., Kwon O. Skin problems after a tsunami. J Eur Acad Dermatol Venereol. 2006;20(7):860–863. doi: 10.1111/j.1468-3083.2006.01666.x. [DOI] [PubMed] [Google Scholar]
- Li Q., Yang Y., Chen R., Kan H., Song W., Tan J. Ambient air pollution, meteorological factors and outpatient visits for eczema in Shanghai, China: a time-series analysis. Int J Environ Res Public Health. 2016;13:1106. doi: 10.3390/ijerph13111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Howard R.M., Mancini A.J., Weston W.L., Paller A.S., Drolet B.A. Kwashiorkor in the United States: fad diets, perceived and true milk allergy, and nutritional ignorance. Arch Dermatol. 2001;137:630–636. [PubMed] [Google Scholar]
- Lonsdale D. Thiamin. In: Advances in food and nutrition research. Amsterdam: Elsevier; 2018. pp. 1–56. [DOI] [PubMed]
- Lopez A., Cacoub P., Macdougall I.C., Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–916. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]
- Lu C., Deng L., Ou C., Yuan H., Chen X., Deng Q. Preconceptional and perinatal exposure to traffic-related air pollution and eczema in preschool children. J Dermatol Sci. 2017;85:85–95. doi: 10.1016/j.jdermsci.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Madrid P.A., Sinclair H., Bankston A.Q., Overholt S., Brito A., Domnitz R., Grant R. Building integrated mental health and medical programs for vulnerable populations post-disaster: connecting children and families to a medical home. Prehospital Disaster Med. 2008;23:314–321. doi: 10.1017/s1049023x0000594x. [DOI] [PubMed] [Google Scholar]
- Manolache L., Petrescu-Seceleanu D., Benea V. Correlation of stressful events with onset of vitiligo in children. J Eur Acad Dermatol Venereol. 2009;23:187–188. doi: 10.1111/j.1468-3083.2008.02764.x. [DOI] [PubMed] [Google Scholar]
- Moreno T., Rodríguez L., Salgueiro L., Riveros R., Mancía S., Narváez D. Skin diseases in children living in shelters in flooded areas. Pediatría Asunción. 2016;43:39–44. [Google Scholar]
- Morgenstern V., Zutavern A., Cyrys J., Brockow I., Koletzko S., Krämer U. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008;177:1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- Muñoz Á.G., Thomson M.C., Goddard L., Aldighieri S. Analyzing climate variations at multiple timescales can guide Zika virus response measures. Gigascience. 2016;5(1):1–6. doi: 10.1186/s13742-016-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.S. Toxic stress and child refugees. J Spec Pediatr Nurs. 2018;23 doi: 10.1111/jspn.12200. [DOI] [PubMed] [Google Scholar]
- Nguyen G.H., Andersen L.K., Davis M.D.P. Climate change and atopic dermatitis: is there a link? Int J Dermatol. 2019;58:279–282. doi: 10.1111/ijd.14016. [DOI] [PubMed] [Google Scholar]
- Oetjen L.K., Trier A.M., Kim B.S. PXR: A new player in atopic dermatitis. J Invest Dermatol. 2018;138:8–10. doi: 10.1016/j.jid.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Oh D.L., Jerman P., Silvério Marques S., Koita K., Purewal Boparai S.K., Burke Harris N. Systematic review of pediatric health outcomes associated with childhood adversity. BMC Pediatr. 2018;18:83. doi: 10.1186/s12887-018-1037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternoster L., Savenije O.E.M., Heron J., Evans D.M., Vonk J.M., Brunekreef B. Identification of atopic dermatitis subgroups in children from 2 longitudinal birth cohorts. J Allergy Clin Immunol. 2018;141:964–971. doi: 10.1016/j.jaci.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedisomeolia A., Ashoori M. Advances in food and nutrition research. Elsevier; Amsterdam: 2018. Riboflavin in human health: a review of current evidences; pp. 57–81. [DOI] [PubMed] [Google Scholar]
- Scheeringa M.S., Zeanah C.H. Reconsideration of harm’s way: onsets and comorbidity patterns of disorders in preschool children and their caregivers following Hurricane Katrina. J Clin Child Adolesc Psychol. 2008;37:508–518. doi: 10.1080/15374410802148178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmier J.K., Ebi K.L. The impact of climate change and aeroallergens on children’s health. Allergy Asthma Proc. 2009;30:229–237. doi: 10.2500/aap.2009.30.3229. [DOI] [PubMed] [Google Scholar]
- Sheffield P.E., Landrigan P.J. Global climate change and children’s health: threats and strategies for prevention. Environ Health Perspect. 2011;119:291–298. doi: 10.1289/ehp.1002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield P.E., Weinberger K.R., Kinney P.L. Climate change, aeroallergens, and pediatric allergic disease. Mt Sinai J Med J Transl Pers Med. 2011;78:78–84. doi: 10.1002/msj.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff J.P., Garner A.S., The Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, and Section on Developmental and Behavioral Pediatrics The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- Shukla PR, Skea J, Slade R, van Diemen R, Haughey E, Malley J, et al. (eds.). Technical summary. In: Climate change and land: An IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. Geneva, Switzerland: Intergovernmental Panel on Climate Change; 2019.
- Silverberg J.I., Hanifin J., Simpson E.L. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752–1759. doi: 10.1038/jid.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.R., Myers S.S. Global health implications of nutrient changes in rice under high atmospheric carbon dioxide. GeoHealth. 2019;3(7):190–200. doi: 10.1029/2019GH000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.R., Myers S.S. Impact of anthropogenic CO2 emissions on global human nutrition. Nat Clim Change. 2018;8:834–839. [Google Scholar]
- Stanberry L.R., Thomson M.C., James W. Prioritizing the needs of children in a changing climate. PLoS Med. 2018;15(7) doi: 10.1371/journal.pmed.1002627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.H., Goodman M.M. Earthquake urticaria. Cutis. 1989;43:340. [PubMed] [Google Scholar]
- Thomas C., Coates S.J., Engelman D., Chosidow O., Chang A.Y. Ectoparasites: Scabies. J Am Acad Dermatol. 2020;82(3):533–548. doi: 10.1016/j.jaad.2019.05.109. [DOI] [PubMed] [Google Scholar]
- Tierney E.P., Sage R.J., Shwayder T. Kwashiorkor from a severe dietary restriction in an 8-month infant in suburban Detroit, Michigan: case report and review of the literature. Int J Dermatol. 2010;49:500–506. doi: 10.1111/j.1365-4632.2010.04253.x. [DOI] [PubMed] [Google Scholar]
- United Nations Children's Fund . United Nations Children's Fund; Geneva, Switzerland: 2007. Community-based management of severe acute malnutrition: A joint statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children’s Fund. [Google Scholar]
- Watts N., Amann M., Arnell N., Ayeb-Karlsson S., Belesova K., Boykoff M. The 2019 report of the Lancet Countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate. Lancet. 2019;394:1836–1878. doi: 10.1016/S0140-6736(19)32596-6. [DOI] [PubMed] [Google Scholar]
- Whitehall J., Kuzulugil D., Sheldrick K., Wood A. Burden of paediatric pyoderma and scabies in North West Queensland: scabies burden. J Paediatr Child Health. 2013;49:141–143. doi: 10.1111/jpc.12095. [DOI] [PubMed] [Google Scholar]
- Whitman E., Parisien M.A., Thompson D.K., Flannigan M.D. Short-interval wildfire and drought overwhelm boreal forest resilience. Sci Rep. 2019;9:18796. doi: 10.1038/s41598-019-55036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Floods in Pakistan [Internet]. 2010 [cited 2020 March 8]. Available from: https://www-who-int.offcampus.lib.washington.edu/hac/crises/pak/sitreps/15august2010/en/.
- Williams H., Robertson C., Stewart A., Aït-Khaled N., Anabwani G., Anderson R. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125–138. doi: 10.1016/s0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]
- Yotsu R.R., Murase C., Sugawara M., Suzuki K., Nakanaga K., Ishii N. Revisiting Buruli ulcer. J Dermatol. 2015;42:1033–1041. doi: 10.1111/1346-8138.13049. [DOI] [PubMed] [Google Scholar]
- Zaki S.A., Shanbag P. Clinical manifestations of dengue and leptospirosis in children in Mumbai: an observational study. Infection. 2010;38:285–291. doi: 10.1007/s15010-010-0030-3. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li Y., Zhu J.K. Developing naturally stress-resistant crops for a sustainable agriculture. Nat Plants. 2018;4:989–996. doi: 10.1038/s41477-018-0309-4. [DOI] [PubMed] [Google Scholar]