Abstract
Global warming, provoked by the greenhouse effect of high levels of atmospheric gases (most notably carbon dioxide and methane), directly threatens human health and survival. Individuals vary in their capacity to tolerate episodes of extreme heat. Because skin is the organ tasked with heat dissipation, it is important for dermatologists to be versed in the physiology of cutaneous heat dissipation and cognizant of clinical settings in which the skin’s thermoregulatory responses may be impaired. When the external temperature is lower than that of the skin, the skin releases internal heat through direct thermal exchange with the environment, a process that is aided by an expansion of cutaneous blood flow and eccrine sweating. Cooling through the evaporation of sweat is effective even when the external temperature exceeds that of skin. Many factors, including environmental and physiological (e.g., age and sex), and pathological (e.g., preexisting illnesses, disorders of eccrine function, and medications) considerations, affect the skin’s capacity to thermoregulate. Identification of individuals at increased risk for heat-related morbidity and mortality will become increasingly important in the care of patients.
Keywords: Anhidrosis, Drugs, Heat illness, Skin blood flow, Sweat glands, Thermoregulation
Introduction: Heat and heat-related illness
More frequent and more severe heat waves, arising from the increasing atmospheric load of greenhouse gases and consequent global warming, constitute one of the major threats to human health in our time (Watts et al., 2019). Morbidity and mortality from heat can be attributed not only to heat stroke and other specific heat-related illnesses (HRIs), but also to a harvesting effect, in which individuals who are chronically ill or near death are prematurely pushed to hospitalization and/or death through the additive stressor of heat (Hajat et al., 2010, Kovats and Hajat, 2008, Lockwood, 2016, Ye et al., 2012). Even small increases in mean annual temperatures lead to more frequent and more prolonged heat waves with potentially devastating lethality (Hanna and Tait, 2015). The 2003 Parisian heat wave, for example, has been estimated to have caused >70,000 deaths (Keller, 2015). Heat waves also can place tremendous stress on health services, as evidenced by a recent heat wave in Japan, which resulted in 18,000 excess hospitalizations (Epstein, 2019). Presently, in the United States and around the world, HRIs are the leading cause of death from extreme weather events (Sherwood and Huber, 2010).
Specific HRIs (Table 1) range from mild and self-limited (e.g., miliaria) to its most severe manifestation (heat stroke), which carries a mortality approaching 80% if not promptly recognized and treated (Wexler, 2002). Mild forms of HRI are treated with removal from the hot environment, rest, and rehydration. Heat-related hyperthermia signifies a rise in the core temperature >37 °C and includes a prodromal phase (heat exhaustion), which can rapidly progress to heat stroke. Heat stroke is characterized by a core temperature of >40 °C with signs and symptoms of central nervous system dysfunction, progressing to multisystem organ failure and, not infrequently, death. Heat stroke is managed by rapid cooling, ideally by cold-water immersion, followed by transport for emergency medical care (Mangus and Canares, 2019, Pryor et al., 2015).
Table 1.
Heat-related illnesses (modified from Mangus and Canares, 2019).
| Mild | |
| Miliaria | Heat rash, prickly heat |
| Heat edema | Distal extremities; 2° vasodilation and vascular pooling |
| Heat cramps | Exercise-induced cramping: predisposed by dehydration, lack of conditioning and/or of acclimatization |
| Heat syncope | Normal core temperature; 2° vasodilation and vascular pooling; must rule out heat exhaustion |
| Heat stress | Normal core temperature but experiencing discomfort in warm environment |
| Moderate | |
| Heat exhaustion | Increase in core temperature (37 °C–40 °C) along with symptoms of thirst, headache, weakness, syncope, vomiting, dizziness. Often associated with dehydration and heavy sweating. Tachycardia and hypotension often present, but no central nervous system signs or symptoms (except mild headache or slight confusion). Without prompt treatment can rapidly progress to heat stroke |
| Severe | |
| Heat stroke | Life-threatening condition with core temperature >40 °C accompanied by central nervous system dysfunction (agitation, confusion, delirium, or coma) in setting of warm environment and/or vigorous exercise |
| Rapid progression to multisystem organ failure with a high mortality (Epstein and Roberts, 2011) |
HRIs are commonly considered in two subgroups, exertional and nonexertional, which facilitates identification of those at greatest risk. Exertional HRIs involve exposure to a warm environment in combination with the internal heat generated from physical exertion. Athletes and outdoor laborers, such as construction and farm workers, are common groups at risk for exertional HRI. Heavy clothing, such as football uniforms, can impair the dissipation of heat through sweating. Poor aerobic conditioning, obesity, and other medical risk factors, as well as certain medications and a lack of acclimatization, also contribute to the risk for exertional HRI (Green et al., 2001). Nonexertional HRI is the form most commonly experienced by the elderly during severe and sustained heat waves. In addition to the factors noted, social factors (e.g., confinement to bed, psychiatric illness, isolation, limited mobility, and lack of air conditioning) are strong predictors of risk for nonexertional HRIs (Hajat et al., 2010, Kenny et al., 2010, Kovats and Hajat, 2008). Infants and small children, who also lack agency, all too often succumb to heat stroke when left unattended in automobiles on warm or sunny days (Mangus and Canares, 2019).
Although dermatologists usually are not on the frontline in the treatment of more severe HRIs, in our warming world it will behoove all physicians to become familiar with the signs and symptoms of these disorders. Moreover, because the skin is the organ tasked with heat dissipation, it is essential that dermatologists understand both the physiology of temperature regulation and its vulnerabilities under physiological and pathological conditions when patients are challenged by a hot environment. This review considers the cutaneous mechanisms involved in heat dissipation and the risk factors for HRI, with particular emphasis on HRIs in which skin failure is involved. Both common skin disorders (e.g., atopic dermatitis and psoriasis) and less common inherited traits (e.g., ichthyoses and ectodermal dysplasias), as well as some medications commonly prescribed by dermatologists, may increase patients’ risk for HRIs. When dermatology patients have other comorbidities (e.g., obesity, diabetes) or disorders that affect the sympathetic nervous system (e.g., Parkinson’s disease or multiple sclerosis), dermatologists can play an important role in educating patients and their primary physicians about patients’ predisposition for an HRI.
The skin and heat dissipation
Maintenance of a constant internal temperature is the essential attribute of mammalian homeothermy. Heat is gained both passively from a warm environment and actively from energy-generative metabolism. Skin dissipates excess internal heat through 1) cutaneous vasodilatation that, by shunting more warm blood from the core to the periphery, facilitates the passive conduction, convection, and radiation of heat to a cooler external environment; and 2) secretion of eccrine sweat on its surface, which cools through evaporation even when the external temperature exceeds that of the skin.
Many physiological and pathological factors, as well as external environmental conditions, can affect the skin’s capacity to dissipate heat. An understanding of these is essential for identifying individuals at increased risk for HRI. Moreover, current and future efforts to mitigate health risks due to global warming will require a comprehensive approach that takes into consideration both social and environmental determinants and these physiologic and pathophysiologic contributors to HRI.
Regulation of cutaneous blood flow in response to hyperthermia
Humans, like many other species, regulate skin blood flow as a first line of defense to maintain thermal neutrality. Although regulation is mediated both centrally and locally, central control, which is signaled though efferent sympathetic nerves, predominates in response to a rise in core temperature (Johnson et al., 2014).
Glabrous and nonglabrous skin differ in their vascular responses to these stimuli. In glabrous (hairless) skin, such as the palms and soles, the effect is solely a relaxation of vascular tone (i.e., release from tonic vasoconstriction under the control of noradrenaline and alpha-adrenergic receptors on vascular smooth muscle; Johnson et al., 2014, Ootsuka and Tanaka, 2015, Romanovsky, 2014). A local abundance of arteriovenous anastomoses can increase blood flow to these regions by several hundred-fold under this relaxation (Johnson et al., 2014), likely accounting for the common occurrence of peripheral edema as an early indicator of heat stress.
Nonglabrous skin responds to central heat stress both by relaxation of vascular tone and active vasodilatation (McAllen and McKinley, 2018). Because of the greater expanse of nonglabrous skin, its active vasodilatation accounts for the majority of increased cutaneous blood flow in response to heat (Johnson et al., 2014). The superficial location of the papillary vascular plexus facilitates the exchange of heat between the skin and the external environment, and the more deeply placed plexus at the dermal-subcutis interface offers a reservoir of larger vessels with a greater capacity for expansion (Johnson et al., 2014). Because heat-induced cutaneous vasodilation can consume as much as 60% of cardia output (Johnson et al., 2016), it is readily apparent how destabilizing heat stress can be for those whose cardiac capacity is already compromised.
The skin also provides feedback via thermal sensors that respond to temperature changes in the external environment. Various transient receptor potential channels are likely candidates for these sensors (Caterina and Pang, 2016). Most of this feedback travels via alternate, sensory neural networks to modify behavioral responses, such as immediate withdrawal from contact with a hot object or removal of excess clothing in a warm environment (Romanovsky, 2014). Local skin feedback also provides input to central temperature centers, modulating their responses. This feedback pathway is largely a secondary and supplemental signal to the central regulatory responses to heat (Romanovsky, 2014). Reductions in thermal sensitivity with aging, as well as the impact of sedative medications and other causes of neurological impairment and/or reduced agency in the elderly, can lead to failure to compensate or correct for a noxious environment and increase the risk of an HRI.
Nitrous oxide, which is produced by many cells in the skin, including keratinocytes, can also alter local vascular reactions (Bruning et al., 2012, Johnson et al., 2016). The cutaneous vasodilation produced by skin heating is comparable with that produced by an overheated core. Consequently, in nonexertional hyperthermia, which is induced by an environment with a temperature that exceeds that of the core, locally signaled expansion of cutaneous blood flow would facilitate the passive absorption of heat and transmission of this heat to the core. In this setting, vasodilatory responses are not only ineffective in dissipating heat, they can be counterproductive by further accelerating heating of the core. Thus, when the temperature of the external environment exceeds that of the core, eccrine sweating provides the only effective means to discharge heat.
Sweat and its regulation in response to hyperthermia
To sweat is human
Although the precise timing of their evolution is not preserved in the fossil record, most evolutionary theorists agree that two distinctive characteristics of human skin—its relatively hairless state and its dense endowment with eccrine glands over its entire surface—evolved in tandem for purposes of thermoregulation (Jablonski, 2006). Whereas eccrine glands of most other primates are largely restricted to the palms and soles (Montagna, 1972), where their secretions aid grasping (Adelman et al., 1975), ancestors of the human lineage had these glands distributed in massive numbers over the entire body surface and their secretory activity was linked to thermoregulatory requirements (Best et al., 2019, Folk and Semken, 1991, Kamberov et al., 2018). Horses and other ungulate species also use sweat for thermoregulation; however, their apocrine gland secretions are far inferior in their ability to evaporate and release heat than is the watery eccrine sweat of humans. A reduction in the number of hair follicles and their further diminution to produce vellus hairs further augmented the cooling efficiency of human sweat (Jablonski, 2006, Kamberov et al., 2018).
Our 1.6 to 4.0 million eccrine glands can generate up to 1.5 to 3 L/h of sweat; if all of that volume evaporates, it can dissipate 1000 to 1700 W of heat (Gagnon and Crandall, 2018, Sato et al., 1989). The qualifier “if it all evaporates” is critical because environmental factors frequently impede the evaporation of sweat. The temperature and vapor pressure of the surrounding air are most critical. The hot and arid climate of the African savannahs, which served as the human birthplace, favored the efficient evaporation of watery eccrine sweat, but these conditions are not nearly as favorable for humans living today in parts of the world with humid climates. Air flow is also important to remove a microclimate of sweat-saturated air directly overlying the skin’s surface and to deliver a less saturated replacement that can accept additional water vapor.
Eccrine sweat physiology
The eccrine gland is composed of a dermal coil, made up of secretory cells and myoepithelial cells that connect to the skin’s surface through an elongated duct. Sweat is formed in the secretory coil through a coordinated interaction of ion channels and transporters that moves water and ions from the blood to the lumen of the secretory coil, producing an isotonic solution of water, sodium, chloride, and potassium (Concepcion et al., 2016, Gagnon and Crandall, 2018, Sato et al., 1989).
Activation of M3R leads to increased cytosolic calcium (Gagnon and Crandall, 2018). Two recently described forms of congenital anhidrosis have provided new insights into the molecular pathway involved (Concepcion et al., 2016). In this scheme, activation of M3R by acetylcholine triggers the release of calcium from the endoplasmic reticulum (Klar et al., 2014). This, in turn, causes an influx of calcium via calcium release-activated calcium (CRAC) channels (Concepcion et al., 2016). This influx then mediates the opening of calcium-activated chloride channels that foster the egress of chloride from the secretory cell into the lumen of the coil. At the same time, to maintain ionic neutrality, potassium channels transport potassium out of the cell. The egress of potassium and chloride activates sodium–potassium-chloride cotransporters, leading to a further influx. Sodium moves into the lumen following the movement of chloride, and water follows in response to the resultant osmotic gradient, likely via aquaporin 5 channels (Inoue, 2016).
The reabsorption of sodium and other ions in the dermal portion of the duct results in a hypotonic secretion at the skin surface (Baker, 2019). Sodium reabsorption is accomplished by amiloride-sensitive Na channels and Na-K-ATPase. Chloride follows through membrane channels (i.e., cystic fibrosis transmembrane conductance regulator). In cystic fibrosis, impairment of these channels leads to elevated sweat chloride, which has served for many years as a diagnostic test for this genetic disorder. The capacity of ductal tissue to reabsorb sodium is dependent on the amount of time the fluid is exposed to ductal cell membranes; thus, sweat sodium increases as sweat rates increase and transit time decreases. Under low sweat rates, the sodium content of sweat ranges between 10 and 20 mM, but it may near 100 mM at maximal rates (Sato et al., 1989).
Sweating must be understood as an evolutionary tradeoff between thermal and fluid homeostasis. In the act of preserving thermal neutrality and protecting the human brain and other tissues from the perils of hyperthermia, copious sweating invariably poses the counter risk of dehydration. Thus, some degree of dehydration, typically hypotonic, is present in most HRIs and is a major contributor to their morbidity and mortality.
Although primarily a dilute salt solution, sweat contains many other compounds, including osmotically active, small molecules such as urea, lactate, glucose, and ammonia, which play roles in stratum corneum hydration and acidification (Elias et al., 2019, Sato et al., 1989). Secretory cells also generate glycoproteins of unknown function that accumulate within the plugged ducts of miliaria and may be involved in the genesis of this disorder. A unique antimicrobial peptide, dermcidin, with broad antimicrobial activity against both gram-positive and gram-negative bacteria, as well as yeasts, is produced by eccrine glands and delivered in sweat (Csosz et al., 2015, Rieg et al., 2005), as are two other antimicrobials, cathelicidin and lactoferrin (Baker, 2019). Proinflammatory cytokines and other proteins with likely protective functions are also delivered to the skin surface in sweat secretions (Csosz et al., 2015, Dai et al., 2013). Many other compounds, including drugs such as griseofulvin and ketoconazole (Sato et al., 1989), have been identified in sweat samples and may be passively delivered to sweat from the circulation, but methodological issues associated with sweat collections complicate the interpretation of these reports (Baker, 2019).
Thermal regulation of sweating
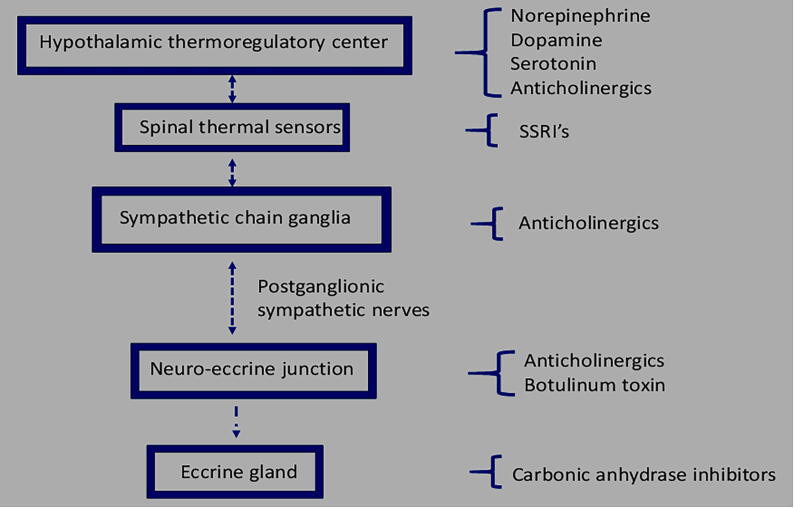
Thermal sensors in the preoptic area and anterior hypothalamus are activated both by an increase in core temperature and input from thermal receptors in the skin and spinal cord in response to warming of the skin (Gagnon and Crandall, 2018, Sato et al., 1989). Impulses travel from there to the thermal sensory sites in the brain stem and spinal cord and onward to the sympathetic ganglia, continuing via postganglionic nerve fibers to the skin and its eccrine glands (Fig. 1). Although both adrenergic and cholinergic fibers enervate eccrine glands, thermal stimuli act predominantly, if not solely, through cholinergic fibers. Acetylcholine, released from sympathetic nerves surrounding the secretory coil, binds to muscarinic-3 receptors (M3R) on the plasma membrane of secretory cells. The released acetylcholine is also degraded by the enzyme acetylcholinesterase such that the sweat response depends on both the quantity of acetylcholine released and the rate of its degradation.
Fig. 1.
Thermoregulatory pathway and sites of action by drugs that affect thermal responses.
Environmental impacts on cutaneous heat dissipation
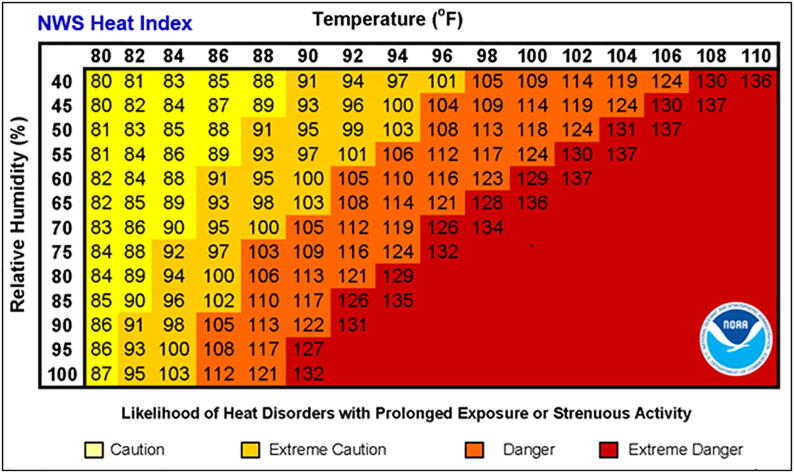
Cutaneous vasodilatation facilitates the dissipation of heat to the environment through conduction, convection, and radiation, but this modality is only effective when the external temperature is lower than that of the core. When the external temperature equals or exceeds that of the core, evaporation of eccrine sweat becomes the only effective means to release excess heat. The outdoor ambient air temperature is measured by a thermometer placed in a shaded site and shielded from air movement. Other commonly reported temperature measurements also take into account additional factors that affect the efficiency of cooling by sweat. The heat index reports the temperature in relation to humidity, approximating the perception of heat by a sedentary person in the shade (Fig. 2). The wet-bulb-globe temperature (WBGT) also measures both ambient air temperature and humidity, as well as air movement and the radiant energy of sunlight, which is dependent on the date, time of day, latitude, and cloud cover. The WBGT is advocated for outdoor workers and athletes as a more precise measure of their risk for an HRI.
Fig. 2.
Heat index as perceived temperature in relation to humidity (reproduced from: https://www.weather.gov/safety/heat-index; accessed April 2, 2020).
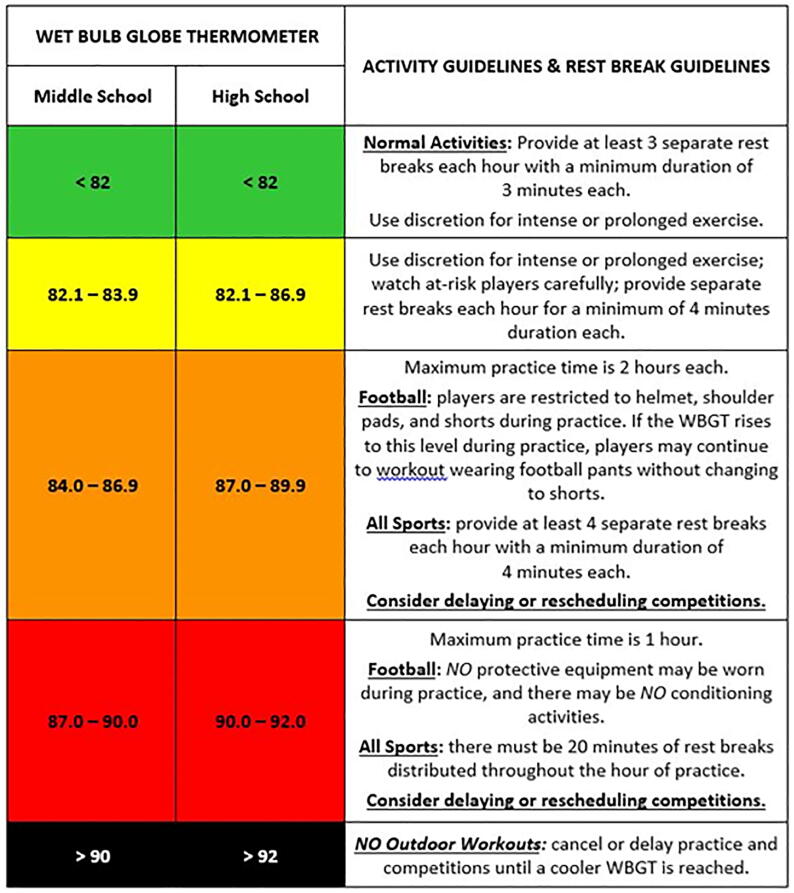
Guidelines prescribing safe activities for student athletes and outdoor workers in relation to WBGT are shown in Fig. 3. (Armstrong et al., 2007) and available at https://www.osha.gov/dts/osta/otm/otm_iii/otm_iii_4.html#heat_hazardassessment (accessed July 13, 2020). Another critical factor in the ability to respond to heat stress is the extent to which sweating is inhibited by clothing, and neither the heat index nor the WBGT correct for this important contributor to many cases of HRI (Budd, 2008, Green et al., 2001).
Fig. 3.
State of Missouri thresholds for wet bulb globe temperature (WBGT). As constructed, the WBGT is always lower than the temperature in direct sunlight. Charts such as the one shown can be used to correlate WBGT readings with the risk for a heat-related illness (reproduced with permission from the Missouri State High School Activities Association).
Physiological, developmental, and sex-related impacts on cutaneous thermoregulation
Aerobic conditioning
With aerobic conditioning, muscles become more efficient, and more metabolic energy is diverted to work with a lesser proportion lost as heat. However, even with optimal aerobic conditioning, the percentage of metabolic energy released as heat is rarely less than 70% (Taylor, 2014). In individuals who are less aerobically fit, heat occupies an even higher percentage of metabolic energy expenditures. Consequently, poor conditioning is a major risk factor for exertional HRI and contributes to the greater heat intolerance of many obese individuals.
Acclimatization
Human physiology adapts on repeated exposures to a hot environment. Thus, in temperate climates, the morbidity and mortality from HRI declines at the end of summer in comparison with the beginning of the warm season. Similarly, visitors to hot climates are more vulnerable to HRI than are residents. These differences in resilience do not seem to have a genetic or racial basis and are best explained by acclimatization (Best et al., 2019, Foster et al., 1971, Taylor, 2014). Physiological adaptations are evident after a week or two of exposure to heat stress (Tyler et al., 2016). Core temperature is lower in acclimatized individuals, allowing for greater thermal gain before exceeding tolerable levels. The basal metabolic rate also slows, resulting in less heat production at rest. Cardiovascular adaptations include expansion of the blood volume, a lower pulse rate, greater stroke volume, and increased cardiac output (Periard et al., 2015, Taylor, 2014). Cutaneous blood flow is also enhanced, with a lower onset threshold and increased sensitivity to thermal stimuli (Periard et al., 2015).
The set point for the onset of sweating is also reduced with acclimatization, permitting evaporative cooling at a lower core temperature. Sweat production becomes more copious, both as a result of central and peripheral autonomic stimulation, as well as enhanced end-organ sensitivity to acetylcholine and eccrine gland hypertrophy (Shin et al., 2016). The sodium content of sweat also declines, not only preserving sodium homeostasis but also rendering the evaporation of sweat more efficient (Buono et al., 2018). These acute adaptations take place even in humid climates where the evaporation of sweat is inhibited and the disadvantages of fluid losses through dripping sweat may predominate. Residents of hot, humid climates demonstrate many of these same physiological adaptations; however, with chronic heat stress in the setting of high humidity, sweating does indeed decrease (Taylor, 2014).
Infants and children
Infants and children are widely recognized as particularly vulnerable to overheating (Falk, 1998, Smith, 2019, Yard et al., 2010). Much of this vulnerability stems from developmental differences in thermoregulation. With a larger surface area to volume ratio, heat dissipation through convection and conduction should, in theory, be more efficient in children. However, when external temperatures exceed internal, this same physiognomy renders them more vulnerable to heat gain. A greater deposition of subcutaneous fat in most infants and young children can insulate and further retard this mechanism for heat dissipation. This greater proportion of body fat also inhibits their capacity to store heat, making them more vulnerable to a rise in core temperature with heat waves. In addition, children have a higher metabolic rate at rest and produce 10% to 15% more metabolic heat during activities (Smith, 2019). Children have a lower cardiac output at a given metabolic rate (Bytomski and Squire, 2003), yet when under thermal stress, they direct a greater proportion of their cardiac output to the skin (Smith, 2019).
A major component of the vulnerability of children to heat stress relates to age-related differences in sweat gland function. At 14 weeks of gestation, eccrine glands first appear on the palms and soles; by 28 weeks, a full complement is present over the skin surface (Mancini and Lawley, 2015). Because eccrine glands only develop during fetal life, the density of glands per unit area is approximately five-fold greater in newborns than in adults (Foster et al., 1971). Eccrine glands begin to function in newborns of approximately 36 weeks of gestational age (Foster et al., 1969), but their sympathetic innervation continues to develop for the 10 weeks or so after birth. Hence, preterm infants do not sweat, and sweating in term neonates in response to thermal stimuli is variable and typically restricted to certain body regions. Sweating in term newborns is generally demonstrable on the forehead and trunk, but often not on the limbs (Foster et al., 1971), and some eccrine glands do not become functional until age 2 years.
Moreover, the volume of sweat produced per gland per minute is much lower in children than in adults (Foster et al., 1971). Infants also have a higher threshold temperature before the onset of sweating—a physiological set point that does not fully mature until sometime after adolescence (Falk, 1998, Smith, 2019). Children’s sweat has a lower sodium content (Smith, 2019), which may be related to their lower sweat flow rates, allowing more time for sodium reabsorption.
This immaturity in eccrine function may not render children more vulnerable to heat stress, because their much higher surface area to volume ratio provides a relatively greater surface for cooling, not only by convection and conduction but also by sweat evaporation (Bergeron, 2015). One could also argue, teleologically, that a higher set point before the onset of sweating and lower sweat volumes offer a selective advantage because children’s low blood volumes render them more vulnerable to dehydration.
Infants and very young children also lack agency to respond to fluid losses from sweating by seeking increased fluid intake, adding to their risks for dehydration in heat. Infants are similarly unable independently to access a cooler environment or remove excess clothing when they overheat. The all too common and tragic car-seat deaths in children left unattended in vehicles are one result of these vulnerabilities (Smith, 2019). However, more capable, older children and adolescents may not fully recognize the importance of rehydration when they exercise in the heat.
Thus, both physiological and psychological developmental factors render infants, children, and adolescents vulnerable to HRIs. Taken together, these factors contribute to the observed 20% increase in neonatal mortality (Basu et al., 2015), a nine-fold increase in sudden infant death syndrome fatalities (Jhun et al., 2017), and the >9000 annual emergency room visits of overheated student athletes during heat waves (Yard et al., 2010).
Aging
The elderly are especially vulnerable to heat-related morbidity and mortality. Eighty percent or more of the excess mortality associated with heat waves occurs in individuals over 60 years of age (Kenny et al., 2010). Social factors (e.g., isolation, poverty, and lack of access to air conditioning), loss of mobility, and reduced agency are widely recognized and critically important indicators of risk for this population (Keller, 2015, Sherwood and Huber, 2010), but some of their increased vulnerability also relates to physiological changes with aging.
Sweat gland function begins to decline in middle age. As early as age 40, eccrine sweat production during exercise is reduced, and it further declines with advancing age (Katayama et al., 1995, Kenney and Hodgson, 1987, Larose et al., 2013, Lee et al., 2014). Moreover, when exposed to a warm environment at rest, older individuals exhibit a higher thermal threshold for the onset of sweating (Kenney and Hodgson, 1987). Sweating is slower to initiate, and the output per gland is lower (Lee et al., 2014). This age-related attenuation of the sweat response appears to localize to the neuro-eccrine unit. Postganglionic sympathetic nerves degenerate with age, and glandular responsiveness to acetylcholine declines and eccrine glands progressively atrophy (Lee et al., 2016). Reduced bioavailability of nitric oxide in aged skin may also account for an age-related reduced cutaneous blood flow in response to heating (Holowatz et al., 2003, Kenney and Hodgson, 1987). Like the decline in sweat function, reduced skin blood flow with heat stress can already be demonstrated in middle-aged adults (Bruning et al., 2012).
Common comorbidities of the elderly, such as diabetes and renal failure, may exacerbate these impaired cutaneous responses. A decline in aerobic power with aging also contributes to their heat intolerance (Kenney and Hodgson, 1987). Similarly, an age-related decline in cardiac output can reduce the capacity to increase cutaneous blood flow during heat stress. A reduced sensibility to thirst and to the discomforts of overheating can be further contributors to the risk of HRIs in the elderly.
Sex differences
Under provocative testing, men and women often exhibit differences in thermoregulation (Kazman et al., 2015, Lisman et al., 2014, Morimoto et al., 1967). Although most of these variances reflect differences in levels of aerobic conditioning and percentages of body fat, women also often have a higher resting core temperature, especially during the luteal phase of their menstrual cycle. Women also typically have a higher body surface area to mass ratio, which facilitates their ability to dissipate heat through conduction and convection when external temperatures are lower than the core, but conversely favors greater core heat gain when external temperatures are higher. Although no clear sex differences in cutaneous blood flow in response to heat have been demonstrated, women’s eccrine capacity is not as robust (Gagnon and Kenny, 2012, Gagnon et al., 2013). Particularly with exercise-induced sweating, the eccrine gland output in women is lower (Gagnon and Kenny, 2012). Paradoxically, although women often demonstrate less efficient heat dissipation on provocative testing and more readily report heat distress, men more often develop serious HRIs, especially heat stroke (Gifford et al., 2019). These sex disparities in morbidity and mortality from heat are observed across all age groups, except in persons age >80 years, where some studies report higher mortality in women (Gifford et al., 2019). The sex differences are greatest in the youngest age groups, which could be due to sex differences in occupational and recreational activities or in their propensity to respond to sensations of overheating and take the necessary steps to alleviate them, such as seeking rest, rehydration, and medical attention.
Pregnancy
A number of studies have demonstrated adverse maternal and fetal outcomes in relation to heat waves (Cil and Cameron, 2017, Kuehn and McCormick, 2017), including increased risk of uterine bleeding in the first trimester and hypertension and eclampsia in the second and third trimesters (Cil and Cameron, 2017). Adverse fetal outcomes in relation to heat exposure during pregnancy include low birthweight, premature birth, and signs of fetal distress (Avalos et al., 2017, Cil and Cameron, 2017, Kuehn and McCormick, 2017, Wells, 2002), as well as stillbirths (Strand et al., 2012). Pregnancy is associated with a higher core temperature and increased metabolic heat production, which may underlie some of these vulnerabilities (Cil and Cameron, 2017).
Stress levels are also higher during heat waves, as evidenced by the linkage of emergency room visits or hospitalization for mental illness (Nitschke et al., 2011) and violent acts—intrapersonal (suicides), interpersonal (domestic violence), and collective (rebellions, wars)—to climatic conditions (Levy et al., 2017, Nitschke et al., 2007, Page et al., 2007). Elevated psychological stress during heat waves may generate higher maternal corticosteroid levels and thereby contribute to these adverse pregnancy outcomes (Cil and Cameron, 2017).
Pathophysiological impacts on cutaneous thermoregulation
A number of preexisting conditions, both dermatologic and nondermatologic, can predispose patients to heat stress and HRIs.
Obesity
Obesity is a significant risk factor for HRI (Flouris et al., 2018, Green et al., 2001). The specific heat capacity of adipose tissue is about half that of lean tissues; hence, fat retards the transfer of heat from the core to the periphery. Moreover, because fat does not hold heat as effectively, less can be stored per unit mass before core temperature rises. Thus, a given exertional workload results in a higher core temperature in obese individuals (Anderson, 1999). Because obese individuals are also often less aerobically fit, a greater proportion of their metabolic energy is directed toward heat production rather than muscular action (Lisman et al., 2014). Other pathophysiological comorbidities of obesity, including increased cardiac strain, hypertension, and diabetes, can further compromise thermoregulatory responses.
Diabetes
Individuals with diabetes face an increased risk of death or hospitalization during heat waves (Schwartz, 2005). This increased vulnerability to heat stress is multifactorial. For example, obesity is a common comorbidity among diabetic individuals, as are cardiac dysfunction and hyperlipidemia, all of which can independently alter the cutaneous thermoregulation of diabetic individuals. The core temperature at which cutaneous vasodilatation is activated is elevated in diabetic individuals, largely attributed to a delay in the active, vasodilatory response to an elevated core temperature (Kenny et al., 2016, Yardley et al., 2013). Thermally-induced skin blood flow in diabetic individuals is also affected by the level of blood glucose control and aerobic conditioning. Most studies have also demonstrated impaired sweat responses in those who are diabetic, particularly in those with neuropathy (Kenny et al., 2016, Yardley et al., 2013). Sweat gland innervation is reduced in relation to poor glucose control (Luo et al., 2012). Hypohidrosis is most evident on the lower body in both type 1 and type 2 diabetic individuals, which is consistent with a neuropathic etiology, as is the observation that axon-reflex-induced sweating is affected more than direct eccrine stimulation (Kihara et al., 1993). However, variations in sweat responses over different regions of the body, with hypohidrosis on the lower body often accompanied by hyperhidrosis on the upper body, render the interpretation of the overall impact on heat dissipation through sweating uncertain. Medications to treat diabetic comorbidities can also negatively affect cutaneous thermoregulatory responses and further augment the thermal vulnerability of diabetic individuals. Those with diabetes appear to be particularly vulnerable to exertional HRIs (Kenny et al., 2016, Yardley et al., 2013).
Hypertension/cardiac insufficiency
Preexisting cardiac failure is a well-established risk factor for disability and death in relation to heat (Balmain et al., 2017). In addition to the destabilizing impact of thermal stress on cardiac performance, thermoregulatory responses to heat may be altered. Although the capacity to sweat does not appear to be affected by heart failure, the ability to increase skin blood flow in response to thermal stress is attenuated (Balmain et al., 2017). Medications to treat heart failure can also affect skin blood flow—particularly beta-blockers, which can attenuate the ability to increase cardiac output as required by cutaneous vasodilatation. Diuretics can also affect cutaneous blood flow by decreasing blood volume. Moreover, even a modest hypovolemia induced by sweating may further reduce cutaneous vasodilation in patients on diuretics.
Congenital disorders with impaired sweating
Ectodermal dysplasias
Hypohidrosis or anhidrosis are present in a number of inherited conditions (Table 2). The ectodermal dysplasias represent a large group of disorders wherein mutations affect the development of skin and its appendages. In some members of this family, the development of eccrine sweat glands is diminished or abolished. In hypohidrotic ectodermal dysplasia, sparse scalp and body hair and abnormal dentition are present in addition to anhidrosis/hypohidrosis. Mutations affecting three genes in the pathway that signals generation of the transcription protein complex, NFkappaB, can produce this phenotype: 1) EDA, encoding the ligand, ectodysplasin, and inherited as an X-linked recessive trait; 2) EDAR, encoding its transmembrane receptor and inherited as an autosomal recessive trait; and 3) EDARADD, encoding the intracellular ligand and inherited as an autosomal dominant. Other mutations in this pathway but more proximal to NFkappaB produce ectodermal dysplasia with immunodeficiency, which can be accompanied by hypohidrosis. Skin biopsies in disorders along this pathway demonstrate reduced-to-absent eccrine glands.
Table 2.
Inherited disorders with hypohidrosis/anhidrosis.
| Disorder | Comments |
|---|---|
| Ectodermal dysplasias (Itin et al., 2017) | |
| Hypohidrotic ED | X-linked, AR and AD inheritance encoding genes EDA, EDAR, and EDARADD for the ligand (ectodysplasin), its transmembrane receptor and its intracellular ligand (respectively), which activates the NEMO/NFkappaB pathway. Mutations result in congenital reduction/absence of eccrine units. |
| Hypohidrotic ED with immunodeficiency | X-linked recessive and AD inheritance encoding |
| NEMO and NFKB1A in the same pathway. Mild hypohidrosis, with reduction/absence of eccrine units. | |
| AEC syndrome: Rapp-Hodgkin | Missense SAM domain of P63. Hypohidrosis in some AR due to WNT10A |
| odonto-onycho-dermal dysplasia | Occasional hypohidrosis |
| Immunodeficiency-myopathy | CRAC-channelopathies. AR due to loss of function |
| Dental dysplasis | Mutations in ORAI1 or STIM1 required for functioning of CRAC channels. Hypohidrosis due to failure to increase intracellular calcium in response to acetylcholine; eccrine glands normal histologically (Concepcion et al., 2016, Lacruz and Feske, 2015) |
| Other multisystem genetic traits | |
| Fabry disease | X-linked due to mutations encoding the lyosomal enzyme alpha-galatosidase A; males usually more severely affected. Protean clinical signs but hypohidrosis often an early manifestation. Secondary to abnormal autonomic innervation or to eccrine gland dysfunction from lysosomal storage (Sahuc et al., 2016) |
| Hypermobile Ehlers-Danlos syndrome | (formerly Type III). Dysautonomia with orthostatic intolerance and other symptoms of dysautonomia. A majority exhibit reduced axon-reflex sweating (Tinkle et al., 2017, De Wandele et al., 2014) |
| Congenital insensitivity to pain with anhidrosis | Hereditary sensory and autonomic neuropathy type 4. |
| AR due to mutations in NTRK1, nerve growth factor receptor. Results in loss of eccrine gland innervation (Wang et al., 2015) | |
| Congenital anhidrosis with kidney damage | Also alacrima, xerostomia, and hypermagnesemia |
| AR due to mutations in gene encoding claudin-10b affecting paracellular Na+ transport in eccrine glands (Klar et al., 2017) | |
| Nonsyndromic genetic traits | |
| Congenital anhidrosis | AR due to mutation in ITPR2 encoding an intracellular Ca++ release channel. Eccrine glands present but do not produce sweat. No other skin abnormalities and serum electrolytes normal (Klar et al., 2014) |
| Other genetic disorders with secondary anhidrosis | |
| Ichthyosis, AD, PSO9 | Common in patients with more severe, generalized disease (e.g., LI/CIE phenotypes); attributed to ductal obstruction by hyperkeratosis. |
ACE = ankyloblepharon-ectodermal dysplasia-clefting syndrome; AD = atopic dermatitis; AR = autosomal recessive; CIE = congenital ichthyosiform erythroderma; CRAC = Ca++ -release-activated Ca++; ED = ectodermal dysplasia; LI = lamellar ichthyosis; PSO = psoriasis.
A few other members of the large ectodermal dysplasia family may also feature hypohidrosis (Table 2). One of these is a severe autosomal recessive trait characterized by combined immunodeficiency, autoimmunity, hypotonia, dental enamel dysplasia, and hypohidrosis (Lacruz and Feske, 2015). This phenotype is caused by loss-of-function mutations in two genes that affect the CRAC-channels pathway, ORAI1 and STIM1, which result in the failure of eccrine gland responsiveness to acetylcholine stimulation (Concepcion et al., 2016). Skin biopsy in this instance reveals a full complement of eccrine glands.
Fabry disease
Hypohidrosis, neuropathic pain, and angiokeratomas can be early features of Fabry disease (Lidove et al., 2012, Sahuc et al., 2016), caused by a deficiency of the lysosomal enzyme alpha-galactosidase A. The clinical manifestations of Fabry disease are broad and variable, which can lead to delays in diagnosis and the initiation of corrective enzyme replacement therapy (Lidove et al., 2012). An X-linked trait, women can be symptomatic depending on the extent of X-inactivation of the normal allele (lyonization). Hypohidrosis/anhidrosis is present in approximately half of affected men and a quarter of women (Orteu et al., 2007). Sweat glands are present, but sweating is impaired due to an accumulation of the enzyme’s substrate within the sweat glands (Wataya-Kaneda, 2016).
Ehlers-Danlos syndrome, hypermobile type
Hypohidrosis due to dysautonomia is a common feature of the hypermobile type of Ehlers-Danlos syndrome (hEDS; Type III; De Wandele et al., 2014, Tinkle et al., 2017). hEDS is an autosomal dominant trait with increased prevalence in women. hEDS is thought to be genetically heterogeneous because an underlying mutation has been identified only in a minority of cases. Although joint hypermobility is the most common sign, skin involvement with changes in texture, extensibility, and atrophic scarring also occur, but to a lesser degree than in other forms of Ehlers-Danlos syndrome (Tinkle et al., 2017). Interestingly, patients with hEDS and greater skin extensibility have more signs of dysautonomia (De Wandele et al., 2014). Cardiac symptoms due to dysautonomia, such as orthostatic intolerance and palpitations, predominate (Hakim et al., 2017). In a study of 39 patients, 65% of patients had reduced axon-mediated sweat responses (De Wandele et al., 2014). Exercise intolerance and postexercise malaise are common symptoms, which may be due in part to impaired thermoregulation (Hakim et al., 2017). Although heat intolerance per se has not been reported, patients with hypohidrosis may not be aware of their condition until they experience an HRI. Small fiber neuropathy with reduced intradermal nerve fiber density may underlie the dysautonomia of hEDS. However, because of common cardiac dysfunction, pain and other symptoms, many hEDS patients are on medications with anticholinergic (blocking the action of the neurotransmitter, acetylcholine) side effects, which could further augment heat intolerance (De Wandele et al., 2014).
Congenital insensitivity to pain with anhidrosis
Congenital insensitivity to pain with anhidrosis is a rare autosomal recessive trait in which the development of eccrine gland innervation is perturbed due to mutations that affect the receptor for nerve growth factor. Growth failure, self-mutilation, chronic infections of bone and joints, and variable mental retardation are additional features of this often-devastating condition. Sweat glands are present but lack sympathetic innervation, predisposing these patients to hyperthermia and heat stroke (Shatzky et al., 2020).
Inborn errors of sweat production
A few kindreds have been described in which congenital anhidrosis is caused by mutations that affect components of the eccrine gland required for sweat production. The CRAC channelopathy form of ectodermal dysplasia is one of these. Another newly described entity is congenital anhidrosis with renal disease and hypermagnesemia (Klar et al., 2017). These patients also exhibit dry eyes and dry mouth. The mutation here affects the tight junction protein claudin 10b, impairing sodium transport in the eccrine gland. Congenital anhidrosis without other systemic manifestations has also been described in one kindred. Here, the mutation perturbs an intracellular channel involved in calcium release from the endoplasmic reticulum (Klar et al., 2014). In all of these instances, sweat glands are present but unable to generate sweat.
Anhidrosis in ichthyosis and other scaling disorders
Finally, anhidrosis or hypohidrosis can be seen as a secondary feature of a number of the inherited disorders of cornification (ichthyoses), particularly those with the lamellar ichthyosis/congenital ichthyosiform erythroderma phenotypes, as well as in the multigenic traits, psoriasis and atopic dermatitis. Although commonly attributed to obstruction of sweat delivery by the hyperkeratosis (Papa and Kligman, 1966), mediators of inflammation, such as histamine, may also inhibit sweat production and delivery (Takahashi et al., 2016, Yamaga et al., 2018). Patients with a large proportion of body surface area involved with an inflammatory dermatitis should be considered at risk for an impaired capacity to sweat and counseled regarding their risk for an HRI.
Acquired disorders with impaired sweating
Neurodegenerative disorders
Neurodegenerative disorders that affect the sympathetic nervous system can be associated with dyshidrosis (Table 3). This is a common feature in Parkinson’s disease, in which hyperhidrosis is most common, but hypohidrosis can also be present, even on different regions of the same individual. Although hypohidrosis in Parkinson’s is usually not symptomatic, it can become clinically relevant with the use of medications that inhibit sweating (Table 4; Skorvanek and Bhatia, 2017). In another closely related disorder, multiple system atrophy, hypohidrosis is a consistent clinical feature, albeit often asymptomatic (Coon et al., 2017). This syndrome features the motor involvement of Parkinson’s disease and/or cerebellar ataxia in combination with autonomic dysfunction, characterized by partial or total anhidrosis, orthostatic hypotension, and bladder dysfunction (Coon et al., 2017). Accumulation of the neuroprotein alpha-synuclein can be demonstrated in skin biopsies of some patients with Parkinson’s disease and less commonly in multiple system atrophy. When present, it is considered highly specific for these disorders (Coon et al., 2017, Haga et al., 2015).
Table 3.
Acquired disorders with generalized hypohidrosis/anhidrosis.
| Disorder | Comments |
|---|---|
| Autonomic neuropathies | |
| Multiple system atrophy | Neurodegenerative disorder with Parkinsonian motor involvement and/or cerebellar ataxia with autonomic failure, including hypohidrosis, orthostatic hypotension, and urinary symptoms (urgency/frequency and/or incontinence). Caused by degeneration of thermoregulatory regions of brain stem and spinal cord, but postganglionic dysfunction can also be present (Coon et al., 2017) |
| Parkinson disease | Dyshidrosis is common clinical feature, especially hyperhidrosis, but also hypohidrosis. Can be regional (e.g., hypohidrosis distally, hyperhidrosis centrally; Skorvanek and Bhatia, 2017). |
| Ross syndrome | Rare syndrome with segmental hypohidrosis and hypopigmentation, often unilateral, often accompanied by tonic pupils and areflexia. May have compensatory hyperhidrosis in uninvolved regions and loss of regulation of cutaneous blood flow. Skin biopsy shows loss of eccrine gland innervation, the segmental distribution suggests involvement of spinal sympathetic centers (Nolano et al., 2006, Sommer et al., 2002). |
| Multiple sclerosis | Impaired sweat response can be demonstrated and related to disease severity (Saari et al., 2008) |
| Autoimmune autonomic | A rare syndrome of dysautonomia with anhidrosis. |
| ganglionopathy | Decreased ganglionic neurotransmission due to autoantibodies to the neuronal nicotinic receptor (Goldstein et al., 2009) |
| Sjögren syndrome | Hypohidrosis/anhidrosis accompanied is some cases by autoantibodies to the nicotinic acetylcholine receptor of the autonomic ganglia, or to the anti-muscarinic M3 receptor and/or infiltration of eccrine glands by T cells. Other cases are attributed to peripheral polyneuropathy. with an autonomic component. Treatment with corticosteroid/other immunosuppressive agents may improve symptoms (Goto et al., 2000, Imrich et al., 2015, Katayama, 2018, Kondo et al., 2009). |
| Infectious diseases | |
| Leprosy | Early involvement of unmyelinated and post-ganglionic autonomic fibers, especially in multibacillary disease. Loss of sweating over distal extremities. Generalized anhidrosis may follow multidrug therapy. Skin biopsy demonstrates loss of innervation and/or involvement of eccrine glands with inflammation and/or atrophy (Carod-Artal, 2018, Facer et al., 1998). |
| HIV and HTLV1 | Both stable HIV patients on combined antiretrovial therapy and HTLV1 patients with myelopathy can experience autonomic dysfunction with sudomotor involvement and hypohidrosis (Carod-Artal, 2018, Chow et al., 2015). |
| Botulism | Clostridium botulinum toxin causes paralysis and dysautonomia. Hypohidrosis is due to blockade of acetylcholine release from neural synapses, as well as insensitivity of eccrine glands to acetylcholine stimulation (Shibasaki et al., 2009). |
| Enterovirus 71 | Linked to polio-like myelitis/encephalitis and hand-foot-mouth disease outbreaks in Southeast Asia. Neurological patients may have autonomic dysfunction with anhidrosis (Carod-Artal, 2018). |
| Scarring disorders | |
| GVH disease | Skin biopsy demonstrates involvement of sweat glands in acute GVH and can progress to scarring with chronic disease (Akosa and Lampert, 1990, Kaminska et al., 2012). |
| Anhidrosis from dermal scaring | Involvement of >40% body surface area in scarring from deep thermal burns associated with heat intolerance (Roskind et al., 1978) |
| Primary disorders of eccrine glands | |
| Acquired idiopathic generalized anhidrosis | Rare syndrome seen most often in Asian males. |
| >25% of the body an- or hypohidrotic in a nonsegmental distribution. Heat may induce symptoms of prickly pain or cholinergic urticaria. Immunoglobulin E may be elevated, and atopic dermatitis sometimes present. Skin biopsy may demonstrate lymphocytic infiltration around eccrine glands, eccrine atrophy, and keratotic plugs in sweat ducts. Failure to induce sweating with injection or iontophoresis of cholinergic agonists. May improve with corticosteroids or other immunosuppressive agents (Munetsugu et al., 2017). | |
| Miliaria profunda | Intraepidermal obstruction of eccrine ducts with papular rash. If extensive can predispose to heat-related illness. May respond to oral retinoids (Lim et al., 2016a, Lim et al., 2016b). |
| Acquired symmetrical hypohidrosis | Nonprogressive, well demarcated patches of anhidrosis without evidence for other causes of anhidrosis. Failure to sweat with cholinergic agonists. Skin biopsy normal (Lim et al., 2016a, Lim et al., 2016b) |
GVH = graft-versus-host; HTLV1 = human T-cell lymphotropic virus type 1.
Table 4.
Drugs with anticholinergic effects (adapted from Duran et al., 2013).
| Drug class | Common examples |
|---|---|
| Anticholinergics | Atropine |
| Scopolamine | |
| Hyoscyamine | |
| Belladona alkaloids | |
| Antitussives | Homatropine |
| Antihistamines | Cyproheptadine |
| Promethazine | |
| Diphenhydramine | |
| Dexchorpheniramine | |
| Hydroxyzine | |
| Chlorpheniramine | |
| Brompheniramine | |
| Carbinoxamine | |
| Pyrilamine | |
| Loratadine | |
| Cetirizine | |
| Fexofenadine | |
| Cimetidine | |
| Ranitidine | |
| Antipruritics | Trimeprazine (alimemazine) |
| SSRI/SSNI antidepressants | Venlafaxine* |
| Trazadone | |
| Fluoxetine | |
| Fluvoxamine | |
| Paroxetine | |
| Citalopram | |
| Mirtazapine | |
| Nefazodone | |
| Tricyclic antidepressants | Amitriptyline |
| Doxepin | |
| Imipramine | |
| Nortriptyline | |
| Chlomipramine | |
| Desipramine | |
| Protriptyline | |
| Trimipramine | |
| Dosulepin | |
| MAO inhibitor antidepressants | Phenelzine |
| Anti-anxiety/insomnia | Chlordiazepoxide |
| Clonazepam | |
| Diazepam | |
| Temazepam | |
| Triazolam | |
| Antipsychotics | Chlozapine |
| Chlorpromazine | |
| Fluphenazine | |
| Thioridazine | |
| Thiothixene | |
| Trifluoperazine† | |
| Perphenazine† | |
| Quetiapine | |
| Olanzapine | |
| Risperidone | |
| Ziprasidone | |
| Haloperidol | |
| Loxapine | |
| Molindone | |
| Promazine | |
| Antiparkinsonians | Benztropine |
| Trihexyphenidyl | |
| Procyclidine | |
| Amantadine | |
| Bromocriptine | |
| Entacapone | |
| Anticonvulsants | Carbamazepine |
| Oxcarbazepine | |
| Bladder antispasmodics | Oxybutynin |
| Tolterodine | |
| Darifenacin | |
| Propantheline | |
| Emepronium | |
| Flavoxate | |
| Gastrointestinal relaxants | Dicyclomine |
| Propantheline | |
| Gastric motility stimulant | Metoclopramide† |
| Antiemetics/antivertigo | Levomepromazine |
| Meclozine | |
| Dimenhydrinate | |
| Promethazine | |
| Domperidone | |
| Prochlorperazine | |
| Muscle relaxants | Orphenadrine |
| Tizanidine | |
| Carisoprodol† | |
| Cyclobenzaprine | |
| Methocarbamol | |
| Baclofen | |
| Inhalants/antibronchospastics | Ipratropium |
MOA = monoamine oxidase; SSRI = selective serotonin reuptake inhibitor; SSNI = serotonin–norepinephrine reuptake inhibitor.
Bold font: high-potency anticholinergic activity; normal font: low-potency anticholinergic activity.
Little to no anticholinergic effect.
Uncertain; high potency in some studies.
Hypohidrosis is also often present in multiple sclerosis, along with other signs and symptoms of autonomic dysfunction, in relation to disease severity (Noronha et al., 1968, Saari et al., 2008). It is attributed to demyelination of central descending pathways in the brain stem and spinal cord (Noronha et al., 1968). Heat is commonly associated with worsening of neurological symptoms in patients with multiple sclerosis. Core hyperthermia has been postulated to be a result of an impaired sweat response under heat stress and could contribute to this deterioration in neurological function (Saari et al., 2008).
Ross syndrome is a rare form of segmental, often unilateral hypohidrosis accompanied by ipsilateral hypopigmentation, tonic pupils, and diminished deep tendon reflexes in its full presentation (Nolano et al., 2006, Panda et al., 2019). Skin biopsy demonstrates a loss of eccrine gland innervation, and the segmental distribution suggests involvement at the level of the spinal sympathetic nerve centers.
Immunologic disorders with hypohidrosis
Immune-mediated neuropathy is the cause of a rare syndrome called autoimmune autonomic ganglionopathy, in which anhidrosis and other symptoms of dysautonomia, such as postural hypotension, are associated with autoantibodies to the nicotinic acetylcholine receptor of ganglionic neurons (Goldstein et al., 2009).
Hypohidrosis is a well-recognized feature of Sjögren syndrome (Katayama et al., 1995) and has been attributed to a sensory polyneuropathy with an autonomic component (Goto et al., 2000, Imrich et al., 2015) and/or to eccrine gland failure (Katayama et al., 1995). Autoantibodies and/or T-cell mediated damage to the eccrine unit have also been implicated in its pathogenesis (de Seze et al., 2004, Iizuka et al., 2015, Katayama, 2018, Mukaino et al., 2016). Sweat testing results suggest that the impaired sweat response of Sjögren syndrome occurs both at the glandular and the neuronal reflex level (Katayama, 2018).
Anhidrosis from epidermal injury and dermal scarring
Destruction of eccrine glands in dermal scarring disorders can lead to anhidrosis with heat intolerance if enough portions of the body are involved. Thus, deep thermal burns affecting ≥40% of the body surface can lead to exertional heat intolerance (Roskind et al., 1978). In graft-versus-host disease, eccrine involvement can be seen during the acute phase with progression to glandular loss in the chronic stage of disease (Akosa and Lampert, 1990, Kaminska et al., 2012). Acute sunburns are also associated with impaired sweating that can persist for several days after the burn (Pandolf et al., 1992).
Acquired anhidrosis, other
Miliaria profunda (i.e., dermal or intraepidermal obstruction of eccrine ducts by keratotic and other debris) can lead to anhidrosis and heat intolerance if the process is sufficiently widespread (Lim et al., 2016a, Lim et al., 2016b, Tey et al., 2015). Similar obstruction is the proposed pathogenesis for the hypohidrosis and pruritus associated with sweating in patients with atopic dermatitis (Haque et al., 2013), as well as other generalized epidermal disorders with inflammation, such as some types of ichthyoses and psoriasis.
Acquired idiopathic generalized anhidrosis (AIGA) is a rare disorder. Most cases have been reported from Japan, where >85% of patients were male. AIGA may represent a heterogenous group that includes cases of sudomotor neuropathy and primary sweat gland failure, as well as a better-defined subgroup called idiopathic pure sudomotor failure (Munetsugu et al., 2017). Idiopathic pure sudomotor failure is characterized by failure of sweat glands to respond to acetylcholine. Although most of the body surface is anhidrotic, the palms/soles, face, and axillae may be spared. Cholinergic urticaria or sensations of prickling or pain often occur with heat stress. An immune-mediated etiology is suggested by the findings of lymphocytic infiltration of the eccrine glands in approximately half of cases, an elevated total serum immunoglobulin E in some, and clinical improvement of sweat gland function with immunosuppressive therapies (e.g., corticosteroids or cyclosporin; Fujita and Hatta, 2013, Munetsugu et al., 2017).
Patients may not be aware they have a disorder of sweat production until they develop an HRI. In one study, a third of soldiers without a known history of predisposing conditions who had experienced signs and symptoms of heat exhaustion or heat stroke were found to have hypohidrosis upon provocative testing (Lim et al., 2016a, Lim et al., 2016b). Most patients had the novel finding of patchy but well-demarcated and symmetrical areas of anhidrosis covering 40% to 50% of the body surface, a condition that the authors termed acquired symmetrical hypohidrosis. The lesions were nonprogressive, and no eccrine pathology was found on skin biopsy. As in AIGA, the anhidrotic regions were insensitive to catechol stimulation, suggesting a postsynaptic defect at the level of the eccrine unit.
Medications that interfere with thermoregulation
Medications can impair an individual’s ability to dissipate heat through a broad range of mechanisms (Cheshire and Fealey, 2008; Fig. 1). Medications may act by impairing the regulation of core temperature and signaling of homeostatic responses at the level of the hypothalamus, downstream at the level of the spinal cord and its sympathetic ganglia, peripherally at the neural-eccrine interface, or directly within the eccrine gland itself (Fig. 1). Others may act through their impacts on cardiac function, cutaneous blood flow, or fluid homeostasis. In contrast, acute drug-induced hyperthermia occurs independently of ambient temperatures and is caused by drugs that act centrally on the hypothalamic-pituitary axis, such as occurs with idiosyncratic reactions to antipsychotics or through toxic doses of seratonergic, sympathomimetic, or anticholinergic drugs (Jamshidi and Dawson, 2019).
Drugs that inhibit sweat production, particularly anticholinergics, or those that alter peripheral vascular responsiveness, such as antihypertensives and diuretics, have the potential to contribute to both exertional and nonexertional HRIs (Kalisch Ellett et al., 2016, Westaway et al., 2015). Centrally active agents, such as amphetamines, anti-Parkinsonian drugs, and antipsychotics, are similarly implicated. Presently, relatively little research has focused on the contribution of such medications to the risk of HRI.
A study of adult patients admitted to the intensive care unit for HRI found that nearly half were taking drugs known to alter thermoregulatory responsiveness, particularly sympathomimetics (amphetamines and cocaine), anticholinergics (benztropine, diphenhydramine and antipsychotics), and diuretics (Levine, 2012). Although the number of patients was relatively small, those using these drugs had a higher morbidity, but no increased mortality. Their mean age was <50 years, which suggests that instances of both exertional and nonexertional HRIs were included. During the 2003 Paris heat wave, the use of psychotropic drugs, particularly antipsychotics and antidepressants, was associated with an increased risk of death in the elderly (Nordon et al., 2009). Of the latter, tricyclic antidepressants were most strongly associated with death, but selective serotonin reuptake inhibitors also were implicated.
An Australian study examined the impact of hospitalization for dehydration or HRI after initiation of medications (Kalisch Ellett et al., 2016). Because the mean age of their patients was 85 years, these were likely cases of nonexertional HRI. Recent initiation of antidepressants, antipsychotics, and anticholinergics, as well as cardiovascular medications, particularly the combination of diuretics and angiotensin-converting enzyme inhibitors, were linked to hospitalizations in this cohort. Heat waves have also been linked to more severe drug reactions, particularly from diuretics and selective serotonin reuptake, angiotensin-converting enzyme, and proton pump inhibitors (Bongers et al., 2020, Michenot et al., 2006, Sommer et al., 2002). In addition to their effects on the thermoregulatory system or, in the case of diuretics and antihypertensives, on the cardiovascular system, some of these medications may also alter adaptation to heat through their central, sedative effects or through inhibition of thirst.
Many drugs that are used for a broad range of indications have anticholinergic effects (Table 4). Most studies on the role of such medications in HRIs examined the impact of smaller categories of anticholinergics, such as antidepressants or antihistamines, but not as the broad aggregate of such agents. Moreover, because individual medications within these groups vary in the strength of their anticholinergic activities, studies on classes of medications may not be sufficiently powered to detect risks associated with the more potent members of a group. Thus, a true assessment of the role of specific drugs in HRI is difficult. Yet, anticholinergic and other medications with thermoregulatory effects are commonly prescribed for elderly patients. For example, an Australian study found that 20% of older patients with diabetes, dementia, or cardiac or respiratory disorders were on ≥5 drugs that affect thermoregulation (Westaway et al., 2015).
The neurotransmitter acetylcholine exerts its effects through muscarinic acetylcholine receptors, of which there are five subtypes. Sweat glands predominantly rely on the M3R subtype, which is also found in the salivary gland, bladder, gastrointestinal smooth muscle, eye, and brain. Thus, M3R anticholinergics can also lead to dry mouth, urinary retention, constipation, blurred vision, and sedation (Cheshire and Fealey, 2008). Most patients are not aware of the antihidrotic effects of these medications, but dry mouth symptoms may provide a clinical proxy (Cheshire and Fealey, 2008). Within therapeutic classes, drugs vary in their affinity for anticholinergic receptors and hence their antisudorific effects (Table 4). When possible, selecting a drug with lower anticholinergic properties for vulnerable patients, such as the elderly during summer months, is prudent.
No studies have examined the overall contribution of medications on HRIs in the pediatric population. Nonetheless, children seem to be particularly sensitive to heat intolerance and hypohidrosis from the antiseizure medications zonisamide (Knudsen et al., 2003) and topiramate (Ben-Zeev et al., 2003, Cerminara et al., 2006). Neither of these drugs are anticholinergics; instead they have been proposed to act more distally by inhibiting the enzyme carbonic anhydrase II within the clear cells of the eccrine secretory coil and apical ductal cells. However, the extent to which inhibition of carbonic anhydrase can limit sweat production is controversial (Moore et al., 2018). Children and adolescents treated with stimulants, such as methylphenidate for attention deficit hyperactivity disorder, may also be vulnerable to exertional hyperthermia (Thoenes, 2011).
Evaluation of patients with suspected hypohidrosis/anhidrosis
Dermatologists may be called on to assess sweat gland function in patients with heat intolerance and/or hypohidrosis. A skin biopsy can provide information on the number and size of eccrine glands, the presence of inflammation, and the density of nerves. Testing for anti-RO and anti-La antibodies and alpha-galactosidase activity can rule out atypical presentations of Sjögren syndrome and Fabry disease, respectively. A qualitative assessment of sweating can be obtained using some variation of the starch-iodine reaction, whereby starch powder in the presence of iodine and water (i.e., sweat) turns purple. Details of this technique and its variations, as well as various methods for quantifying sweat output, are published elsewhere (Baker, 2019, Lim et al., 2016a, Murota, 2016, Sato et al., 1989, Smith et al., 2014).
Another important test of sweat function is the quantitative sudomotor axon reflex test (QSART). In the test, acetylcholine (or a related cholinergic agent) is introduced into the skin by injection or iontophoresis. Quantification of the volume of sweat produced at the injection site provides a measure of eccrine gland responsiveness, whereas that produced more distantly measures postganglionic sympathetic nerve function (For further details on this methodology, see Lee et al., 2014, Low et al., 1992). An abnormal QSART result requires additional testing of the autonomic nervous system (Illigens and Gibbons, 2019).
Heat tolerance testing is employed by military organizations to determine whether members who have suffered exertional heat stroke can safely return to duty (Lisman et al., 2014). Heat tolerance testing entails monitoring of core temperature and heart rate as the individual exercises in a warm environment under moderate humidity. Heat intolerance is defined as a rise in core temperature >38.5 °C and/or a heart rate >150 beats/min under standardized conditions.
Conclusions
This review focuses on the skin’s role in maintaining thermal homeostasis in response to the threat of an overheated core. We consider both physiological states and pathophysiologic conditions in which an individual’s ability to dissipate heat through the skin may be compromised, putting them at risk for an HRI. Dermatologists have an important role to play in the identification of those at increased risk for HRI and in counseling them on strategies to reduce that risk.
Unfortunately, even if optimal efforts to reduce greenhouse gas emissions are enacted, global temperature is anticipated to continue rising for some time before again normalizing. This means that, even at best, HRIs will continue to demand medical attention for years to come. In worst case scenarios (i.e., if global greenhouse gas emissions continue to rise unchecked), substantial parts of the globe may become uninhabitable because environmental conditions will exceed the finite capacity of the human skin to adapt and maintain thermal neutrality (Im et al., 2017, Sherwood and Huber, 2010). In all scenarios, climatic change and its effects on health and food security will place extreme pressures on societies to adapt and respond. Such pressures will likely lead to greater political instability and further mass human migrations. By becoming fully informed about the skin’s vulnerabilities to our changing climates and warming world, dermatologists can join other physicians in advocating for the societal and governmental responses that will be needed to minimize these effects and to develop the resilience required to adapt to those effects that cannot be prevented (Coates et al., 2019, Khalifian and Rosenbach, 2018).
Acknowledgments
Acknowledgments
The author is indebted to Peter M. Elias, M.D., for his careful reading and review of the manuscript, and to Joan Wakefield for her many editorial improvements and administrative assistance in the preparation of the manuscript.
Conflicts of Interest
None.
Funding
None.
Study Approval
N/A.
References
- Adelman S., Taylor C.R., Heglund N.C. Sweating on paws and palms: What is its function? Am J Physiol. 1975;229(5):1400–1402. doi: 10.1152/ajplegacy.1975.229.5.1400. [DOI] [PubMed] [Google Scholar]
- Akosa A.B., Lampert I.A. The sweat gland in graft versus host disease. J Pathol. 1990;161(3):261–266. doi: 10.1002/path.1711610314. [DOI] [PubMed] [Google Scholar]
- Anderson G.S. Human morphology and temperature regulation. Int J Biometeorol. 1999;43(3):99–109. doi: 10.1007/s004840050123. [DOI] [PubMed] [Google Scholar]
- Avalos L.A., Chen H., Li D.K., Basu R. The impact of high apparent temperature on spontaneous preterm delivery: a case-crossover study. Environ Health. 2017;16(1):5. doi: 10.1186/s12940-017-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L.B. Physiology of sweat gland function: the roles of sweating and sweat composition in human health. Temperature (Austin) 2019;6(3):211–259. doi: 10.1080/23328940.2019.1632145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmain B.N., Sabapathy S., Jay O., Adsett J., Stewart G.M., Jayasinghe R. Heart failure and thermoregulatory control: can patients with heart failure handle the heat? J Card Fail. 2017;23(8):621–627. doi: 10.1016/j.cardfail.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Basu R., Pearson D., Sie L., Broadwin R. A case-crossover study of temperature and infant mortality in California. Paediatr Perinat Epidemiol. 2015;29(5):407–415. doi: 10.1111/ppe.12204. [DOI] [PubMed] [Google Scholar]
- Ben-Zeev B., Watemberg N., Augarten A., Brand N., Yahav Y., Efrati O. Oligohydrosis and hyperthermia: pilot study of a novel topiramate adverse effect. J Child Neurol. 2003;18(4):254–257. doi: 10.1177/08830738030180041001. [DOI] [PubMed] [Google Scholar]
- Bergeron M.F. Training and competing in the heat in youth sports: no sweat? Br J Sports Med. 2015;49(13):837–839. doi: 10.1136/bjsports-2015-094662. [DOI] [PubMed] [Google Scholar]
- Best A., Lieberman D.E., Kamilar J.M. Diversity and evolution of human eccrine sweat gland density. J Therm Biol. 2019;84:331–338. doi: 10.1016/j.jtherbio.2019.07.024. [DOI] [PubMed] [Google Scholar]
- Bongers K.S., Salahudeen M.S., Peterson G.M. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9–16. doi: 10.1007/s00228-019-02763-5. [DOI] [PubMed] [Google Scholar]
- Bruning R.S., Santhanam L., Stanhewicz A.E., Smith C.J., Berkowitz D.E., Kenney W.L. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol (1985) 2012;112(12):2019–2026. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd G.M. Wet-bulb globe temperature (WBGT)–Its history and its limitations. J Sci Med Sport. 2008;11(1):20–32. doi: 10.1016/j.jsams.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Buono M.J., Kolding M., Leslie E., Moreno D., Norwood S., Ordille A. Heat acclimation causes a linear decrease in sweat sodium ion concentration. J Therm Biol. 2018;71:237–240. doi: 10.1016/j.jtherbio.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Bytomski J.R., Squire D.L. Heat illness in children. Curr Sports Med Rep. 2003;2(6):320–324. doi: 10.1249/00149619-200312000-00007. [DOI] [PubMed] [Google Scholar]
- Carod-Artal F.J. Infectious diseases causing autonomic dysfunction. Clin Auton Res. 2018;28(1):67–81. doi: 10.1007/s10286-017-0452-4. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Pang Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals (Basel) 2016;9(4) doi: 10.3390/ph9040077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerminara C., Seri S., Bombardieri R., Pinci M., Curatolo P. Hypohidrosis during topiramate treatment: a rare and reversible side effect. Pediatr Neurol. 2006;34(5):392–394. doi: 10.1016/j.pediatrneurol.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Cheshire W.P., Fealey R.D. Drug-induced hyperhidrosis and hypohidrosis: incidence, prevention and management. Drug Saf. 2008;31(2):109–126. doi: 10.2165/00002018-200831020-00002. [DOI] [PubMed] [Google Scholar]
- Chow D., Nakamoto B.K., Sullivan K., Sletten D.M., Fujii S., Umekawa S. Symptoms of autonomic dysfunction in human immunodeficiency virus. Open Forum Infect Dis. 2015;2(3) doi: 10.1093/ofid/ofv103. ofv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cil G., Cameron T.A. Potential climate change health risks from increases in heat waves: abnormal birth outcomes and adverse maternal health conditions. Risk Anal. 2017;37(11):2066–2079. doi: 10.1111/risa.12767. [DOI] [PubMed] [Google Scholar]
- Coates S.J., Davis M.D.P., Andersen L.K. Temperature and humidity affect the incidence of hand, foot, and mouth disease: a systematic review of the literature - a report from the International Society of Dermatology Climate Change Committee. Int J Dermatol. 2019;58(4):388–399. doi: 10.1111/ijd.14188. [DOI] [PubMed] [Google Scholar]
- Concepcion A.R., Vaeth M., Wagner L.E., 2nd, Eckstein M., Hecht L., Yang J. Store-operated Ca2+ entry regulates Ca2+-activated chloride channels and eccrine sweat gland function. J Clin Invest. 2016;126(11):4303–4318. doi: 10.1172/JCI89056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon E.A., Fealey R.D., Sletten D.M., Mandrekar J.N., Benarroch E.E., Sandroni P. Anhidrosis in multiple system atrophy involves pre- and postganglionic sudomotor dysfunction. Mov Disord. 2017;32(3):397–404. doi: 10.1002/mds.26864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csosz E., Emri G., Kallo G., Tsaprailis G., Tozser J. Highly abundant defense proteins in human sweat as revealed by targeted proteomics and label-free quantification mass spectrometry. J Eur Acad Dermatol Venereol. 2015;29(10):2024–2031. doi: 10.1111/jdv.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Okazaki H., Hanakawa Y., Murakami M., Tohyama M., Shirakata Y. Eccrine sweat contains IL-1alpha, IL-1beta and IL-31 and activates epidermal keratinocytes as a danger signal. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0067666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Seze J., Dubucquoi S., Fauchais A.L., Hachulla E., Matthias T., Lefranc D. Autoantibodies against alpha-fodrin in Sjogren's syndrome with neurological manifestations. J Rheumatol. 2004;31(3):500–503. [PubMed] [Google Scholar]
- De Wandele I., Rombaut L., Leybaert L., Van de Borne P., De Backer T., Malfait F. Dysautonomia and its underlying mechanisms in the hypermobility type of Ehlers-Danlos syndrome. Semin Arthritis Rheum. 2014;44(1):93–100. doi: 10.1016/j.semarthrit.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Duran C.E., Acermai M., Vander Stichele R.H. Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol. 2013;69:1485–1496. doi: 10.1007/s00228-013-1499-3. [DOI] [PubMed] [Google Scholar]
- Elias P.M., Wakefield J.S., Man M.Q. Moisturizers versus current and next-generation barrier repair therapy for the management of atopic dermatitis. Skin Pharmacol Physiol. 2019;32(1):1–7. doi: 10.1159/000493641. [DOI] [PubMed] [Google Scholar]
- Epstein K. Climate change isn’t an intangible future risk. It’s here now, and it’s killing us. Washington Post. 2019 [Google Scholar]
- Epstein Y., Roberts W.O. The pathopysiology of heat stroke: an integrative view of the final common pathway. Scand J Med Sci Sports. 2011;21(6):742–748. doi: 10.1111/j.1600-0838.2011.01333.x. [DOI] [PubMed] [Google Scholar]
- Facer P., Mathur R., Pandya S.S., Ladiwala U., Singhal B.S., Anand P. Correlation of quantitative tests of nerve and target organ dysfunction with skin immunohistology in leprosy. Brain. 1998;121(Pt 12):2239–2247. doi: 10.1093/brain/121.12.2239. [DOI] [PubMed] [Google Scholar]
- Falk B. Effects of thermal stress during rest and exercise in the paediatric population. Sports Med. 1998;25(4):221–240. doi: 10.2165/00007256-199825040-00002. [DOI] [PubMed] [Google Scholar]
- Flouris A.D., McGinn R., Poirier M.P., Louie J.C., Ioannou L.G., Tsoutsoubi L. Screening criteria for increased susceptibility to heat stress during work or leisure in hot environments in healthy individuals aged 31–70 years. Temperature (Austin) 2018;5(1):86–99. doi: 10.1080/23328940.2017.1381800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk G.E., Jr, Semken H.A., Jr. The evolution of sweat glands. Int J Biometeorol. 1991;35(3):180–186. doi: 10.1007/BF01049065. [DOI] [PubMed] [Google Scholar]
- Foster K.G., Hey E.N., Katz G. The response of the sweat glands of the newborn baby to thermal stimuli and to intradermal acetylcholine. J Physiol. 1969;203(1):13–29. doi: 10.1113/jphysiol.1969.sp008846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K.G., Hey E.N., O'Connell B. Sweat function in babies with defects of the central nervous system. Arch Dis Child. 1971;46(248):444–451. doi: 10.1136/adc.46.248.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K., Hatta K. Acquired generalized anhidrosis: review of the literature and report of a case with lymphocytic hidradenitis and sialadenitis successfully treated with cyclosporine. Dermatology. 2013;227(3):270–277. doi: 10.1159/000355332. [DOI] [PubMed] [Google Scholar]
- Gagnon D., Crandall C.G. Sweating as a heat loss thermoeffector. Handb Clin Neurol. 2018;156:211–232. doi: 10.1016/B978-0-444-63912-7.00013-8. [DOI] [PubMed] [Google Scholar]
- Gagnon D., Crandall C.G., Kenny G.P. Sex differences in postsynaptic sweating and cutaneous vasodilation. J Appl Physiol (1985) 2013;114(3):394–401. doi: 10.1152/japplphysiol.00877.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon D., Kenny G.P. Sex differences in thermoeffector responses during exercise at fixed requirements for heat loss. J Appl Physiol (1985) 2012;113(5):746–757. doi: 10.1152/japplphysiol.00637.2012. [DOI] [PubMed] [Google Scholar]
- Gifford R.M., Todisco T., Stacey M., Fujisawa T., Allerhand M., Woods D.R. Risk of heat illness in men and women: a systematic review and meta-analysis. Environ Res. 2019;171:24–35. doi: 10.1016/j.envres.2018.10.020. [DOI] [PubMed] [Google Scholar]
- Goldstein D.S., Holmes C., Imrich R. Clinical laboratory evaluation of autoimmune autonomic ganglionopathy: preliminary observations. Auton Neurosci. 2009;146(1–2):18–21. doi: 10.1016/j.autneu.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H., Matsuo H., Fukudome T., Shibuya N., Ohnishi A., Nakamura H. Chronic autonomic neuropathy in a patient with primary Sjogren's syndrome. J Neurol Neurosurg Psychiatry. 2000;69(1):135. doi: 10.1136/jnnp.69.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H., Gilbert J., James R., Byard R.W. An analysis of factors contributing to a series of deaths caused by exposure to high environmental temperatures. Am J Forensic Med Pathol. 2001;22(2):196–199. doi: 10.1097/00000433-200106000-00018. [DOI] [PubMed] [Google Scholar]
- Haga R., Sugimoto K., Nishijima H., Miki Y., Suzuki C., Wakabayashi K. Clinical utility of skin biopsy in differentiating between Parkinson's disease and multiple system atrophy. Parkinsons Dis. 2015;2015 doi: 10.1155/2015/167038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat S., O'Connor M., Kosatsky T. Health effects of hot weather: from awareness of risk factors to effective health protection. Lancet. 2010;375(9717):856–863. doi: 10.1016/S0140-6736(09)61711-6. [DOI] [PubMed] [Google Scholar]
- Hakim A., O'Callaghan C., De Wandele I., Stiles L., Pocinki A., Rowe P. Cardiovascular autonomic dysfunction in Ehlers-Danlos syndrome-Hypermobile type. Am J Med Genet C Semin Med Genet. 2017;175(1):168–174. doi: 10.1002/ajmg.c.31543. [DOI] [PubMed] [Google Scholar]
- Hanna E.G., Tait P.W. Limitations to thermoregulation and acclimatization challenge human adaptation to global warming. Int J Environ Res Public Health. 2015;12(7):8034–8074. doi: 10.3390/ijerph120708034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M.S., Hailu T., Pritchett E., Cusack C.A., Allen H.B. The oldest new finding in atopic dermatitis: subclinical miliaria as an origin. JAMA Dermatol. 2013;149(4):436–438. doi: 10.1001/2013.jamadermatol.109. [DOI] [PubMed] [Google Scholar]
- Holowatz L.A., Houghton B.L., Wong B.J., Wilkins B.W., Harding A.W., Kenney W.L. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284(5):H1662–H1667. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- Iizuka M., Tsuboi H., Asashima H., Hirota T., Kondo Y., Matsui M. M3 muscarinic acetylcholine receptor reactive IL-17 producing T cells promotes development of Sjogren's syndrome like sialadenitis. Mod Rheumatol. 2015;25(1):158–160. doi: 10.3109/14397595.2014.884683. [DOI] [PubMed] [Google Scholar]
- Illigens B.M.W., Gibbons C.H. Autonomic testing, methods and techniques. Handb Clin Neurol. 2019;160:419–433. doi: 10.1016/B978-0-444-64032-1.00028-X. [DOI] [PubMed] [Google Scholar]
- Im E.S., Pal J.S., Eltahir E.A.B. Deadly heat waves projected in the densely populated agricultural regions of South Asia. Sci Adv. 2017;3(8) doi: 10.1126/sciadv.1603322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imrich R., Alevizos I., Bebris L., Goldstein D.S., Holmes C.S., Illei G.G. Predominant glandular cholinergic dysautonomia in patients with primary Sjogren's syndrome. Arthritis Rheumatol. 2015;67(5):1345–1352. doi: 10.1002/art.39044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue R. New findings on the mechanism of perspiration including aquaporin-5 water channel. Curr Probl Dermatol. 2016;51:11–21. doi: 10.1159/000446754. [DOI] [PubMed] [Google Scholar]
- Itin P., Bree A., Sybert V.P. Elsevier; Bolognia: 2017. Ectodermal dysplasias; pp. 1046–1056. [Google Scholar]
- Jablonski N.G. University of California Press; Berkeley: 2006. Skin: a natural history. [Google Scholar]
- Jamshidi N., Dawson A. The hot patient: acute drug-induced hyperthermia. Aust Prescr. 2019;42(1):24–28. doi: 10.18773/austprescr.2019.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhun I., Mata D.A., Nordio F., Lee M., Schwartz J., Zanobetti A. Ambient temperature and sudden infant death syndrome in the United States. Epidemiology. 2017;28(5):728–734. doi: 10.1097/EDE.0000000000000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.M., Minson C.T., Kellogg D.L., Jr. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol. 2014;4(1):33–89. doi: 10.1002/cphy.c130015. [DOI] [PubMed] [Google Scholar]
- Johnson R.S., Titze J., Weller R. Cutaneous control of blood pressure. Curr Opin Nephrol Hypertens. 2016;25(1):11–15. doi: 10.1097/MNH.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch Ellett L.M., Pratt N.L., Le Blanc V.T., Westaway K., Roughead E.E. Increased risk of hospital admission for dehydration or heat-related illness after initiation of medicines: a sequence symmetry analysis. J Clin Pharm Ther. 2016;41(5):503–507. doi: 10.1111/jcpt.12418. [DOI] [PubMed] [Google Scholar]
- Kamberov Y.G., Guhan S.M., DeMarchis A., Jiang J., Wright S.S., Morgan B.A. Comparative evidence for the independent evolution of hair and sweat gland traits in primates. J Hum Evol. 2018;125:99–105. doi: 10.1016/j.jhevol.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska E.C., Larson R.A., Petronic-Rosic V. Amelanocytic anhidrotic alopecia areata-like phenotype after allogeneic hematopoietic cell transplant. Arch Dermatol. 2012;148(8):931–934. doi: 10.1001/archdermatol.2012.335. [DOI] [PubMed] [Google Scholar]
- Katayama I. Dry skin manifestations in Sjogren syndrome and atopic dermatitis related to aberrant sudomotor function in inflammatory allergic skin diseases. Allergol Int. 2018;67(4):448–454. doi: 10.1016/j.alit.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Katayama I., Yokozeki H., Nishioka K. Impaired sweating as an exocrine manifestation in Sjogren's syndrome. Br J Dermatol. 1995;133(5):716–720. doi: 10.1111/j.1365-2133.1995.tb02744.x. [DOI] [PubMed] [Google Scholar]
- Kazman J.B., Purvis D.L., Heled Y., Lisman P., Atias D., Van Arsdale S. Women and exertional heat illness: Identification of gender specific risk factors. US Army Med Dep J. 2015;Apr–Jun:58–66. [PubMed] [Google Scholar]
- Keller R.C. University of Chicago Press; Chicago: 2015. Fatal isolation, the devastating Paris heat wave of 2003. [Google Scholar]
- Kenney W.L., Hodgson J.L. Heat tolerance, thermoregulation and ageing. Sports Med. 1987;4(6):446–456. doi: 10.2165/00007256-198704060-00004. [DOI] [PubMed] [Google Scholar]
- Kenny G.P., Sigal R.J., McGinn R. Body temperature regulation in diabetes. Temperature (Austin) 2016;3(1):119–145. doi: 10.1080/23328940.2015.1131506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny G.P., Yardley J., Brown C., Sigal R.J., Jay O. Heat stress in older individuals and patients with common chronic diseases. CMAJ. 2010;182(10):1053–1060. doi: 10.1503/cmaj.081050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifian S., Rosenbach M. Dermatology, climate change, and the perils of attacks on expertise. J Am Acad Dermatol. 2018;79(2):397–399. doi: 10.1016/j.jaad.2018.02.054. [DOI] [PubMed] [Google Scholar]
- Kihara M., Opfer-Gehrking T.L., Low P.A. Comparison of directly stimulated with axon-reflex-mediated sudomotor responses in human subjects and in patients with diabetes. Muscle Nerve. 1993;16(6):655–660. doi: 10.1002/mus.880160612. [DOI] [PubMed] [Google Scholar]
- Klar J., Hisatsune C., Baig S.M., Tariq M., Johansson A.C., Rasool M. Abolished InsP3R2 function inhibits sweat secretion in both humans and mice. J Clin Invest. 2014;124(11):4773–4780. doi: 10.1172/JCI70720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar J., Piontek J., Milatz S., Tariq M., Jameel M., Breiderhoff T. Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. PLoS Genet. 2017;13(7) doi: 10.1371/journal.pgen.1006897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen J.F., Thambi L.R., Kapcala L.P., Racoosin J.A. Oligohydrosis and fever in pediatric patients treated with zonisamide. Pediatr Neurol. 2003;28(3):184–189. doi: 10.1016/s0887-8994(02)00511-8. [DOI] [PubMed] [Google Scholar]
- Kondo T., Inoue H., Usui T., Mimori T., Tomimoto H., Vernino S. Autoimmune autonomic ganglionopathy with Sjogren's syndrome: significance of ganglionic acetylcholine receptor antibody and therapeutic approach. Auton Neurosci. 2009;146(1–2):33–35. doi: 10.1016/j.autneu.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Kovats R.S., Hajat S. Heat stress and public health: a critical review. Annu Rev Public Health. 2008;29:41–55. doi: 10.1146/annurev.publhealth.29.020907.090843. [DOI] [PubMed] [Google Scholar]
- Kuehn L., McCormick S. Heat exposure and maternal health in the face of climate change. Int J Environ Res Public Health. 2017;14(8) doi: 10.3390/ijerph14080853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz R.S., Feske S. Diseases caused by mutations in ORAI1 and STIM1. Ann N Y Acad Sci. 2015;1356:45–79. doi: 10.1111/nyas.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larose J., Boulay P., Sigal R.J., Wright H.E., Kenny G.P. Age-related decrements in heat dissipation during physical activity occur as early as the age of 40. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.B., Kim J.H., Murota H. Perspiration functions in different ethnic, age, and sex populations: modification of sudomotor function. Curr Probl Dermatol. 2016;51:109–119. doi: 10.1159/000447370. [DOI] [PubMed] [Google Scholar]
- Lee J.B., Lee I.H., Shin Y.O., Min Y.K., Yang H.M. Age- and sex-related differences in sudomotor function evaluated by the quantitative sudomotor axon reflex test (QSART) in healthy humans. Clin Exp Pharmacol Physiol. 2014;41(6):392–399. doi: 10.1111/1440-1681.12234. [DOI] [PubMed] [Google Scholar]
- Levy B.S., Sidel V.W., Patz J.A. Climate change and collective violence. Annu Rev Public Health. 2017;38:241–257. doi: 10.1146/annurev-publhealth-031816-044232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., LoVecchio F., Ruha A.-M., Chu G., Roque P. Influence of drug use on morbidity and mortality in heatstroke. J Med Toxicol. 2012;8:252–257. doi: 10.1007/s13181-012-0222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidove O., Kaminsky P., Hachulla E., Leguy-Seguin V., Lavigne C., Marie I. Fabry disease 'The New Great Imposter': results of the French Observatoire in Internal Medicine Departments (FIMeD) Clin Genet. 2012;81(6):571–577. doi: 10.1111/j.1399-0004.2011.01718.x. [DOI] [PubMed] [Google Scholar]
- Lim J.H., Choo W., Chang J.H., Tey H.L., Chong W.S. Application of iodinated starch powder using an atomizer spray gun - a new and effective tool to evaluate hypohidrosis. Skin Res Technol. 2016;22(3):370–374. doi: 10.1111/srt.12275. [DOI] [PubMed] [Google Scholar]
- Lim J.H., Kok W.L., Bin Ali N., Chong W.S., Tey H.L. Hypohidrosis in individuals with exertional heat injury: a prospective open cohort study. Dermatology. 2016;232(1):50–56. doi: 10.1159/000439108. [DOI] [PubMed] [Google Scholar]
- Lisman P., Kazman J.B., O'Connor F.G., Heled Y., Deuster P.A. Heat tolerance testing: association between heat intolerance and anthropometric and fitness measurements. Mil Med. 2014;179(11):1339–1346. doi: 10.7205/MILMED-D-14-00169. [DOI] [PubMed] [Google Scholar]
- Lockwood A.H. MIT Press; Cambridge, MA: 2016. Heat advisory, protecting health on a warming planet. [Google Scholar]
- Low P.A., Opfer-Gehrking T.L., Kihara M. In vivo studies on receptor pharmacology of the human eccrine sweat gland. Clin Auton Res. 1992;2(1):29–34. doi: 10.1007/BF01824208. [DOI] [PubMed] [Google Scholar]
- Luo K.R., Chao C.C., Hsieh P.C., Lue J.H., Hsieh S.T. Effect of glycemic control on sudomotor denervation in type 2 diabetes. Diabetes Care. 2012;35(3):612–616. doi: 10.2337/dc11-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini A.J., Lawley L. Structure and function of newborn skin. In: Eichenfield L.F., Frieden I.J., editors. Neonatal and infant dermatology. Elsevier; London: 2015. pp. 14–23. [Google Scholar]
- Mangus C.W., Canares T.L. Heat-related illness in children in an era of extreme temperatures. Pediatr Rev. 2019;40(3):97–107. doi: 10.1542/pir.2017-0322. [DOI] [PubMed] [Google Scholar]
- McAllen R.M., McKinley M.J. Efferent thermoregulatory pathways regulating cutaneous blood flow and sweating. Handb Clin Neurol. 2018;156:305–316. doi: 10.1016/B978-0-444-63912-7.00018-7. [DOI] [PubMed] [Google Scholar]
- Michenot F., Sommet A., Bagheri H., Lapeyre-Mestre M., Montastruc J.L. French Network of PharmacoVigilance Centres. Adverse drug reactions in patients older than 70 years during the heat wave occurred in France in summer 2003: A study from the French PharmacoVigilance Database. Pharmacoepidemiol Drug Saf. 2006;15(10):735–740. doi: 10.1002/pds.1284. [DOI] [PubMed] [Google Scholar]
- Montagna W. The skin of nonhuman primates. Amer Zoologist. 1972;12(1):109–124. [Google Scholar]
- Moore J., Northway S., Wells N., Woolf E., Buono M.J. Local inhibition of carbonic anhydrase does not decrease sweat rate. J Basic Clin Physiol Pharmacol. 2018;30(1):47–50. doi: 10.1515/jbcpp-2018-0039. [DOI] [PubMed] [Google Scholar]
- Morimoto T., Slabochova Z., Naman K., Sargent F. Sex differences in physiological reactions to thermal stress. Physiology. 1967;22(3):526–532. doi: 10.1152/jappl.1967.22.3.526. [DOI] [PubMed] [Google Scholar]
- Mukaino A., Nakane S., Higuchi O., Nakamura H., Miyagi T., Shiroma K. Insights from the ganglionic acetylcholine receptor autoantibodies in patients with Sjogren's syndrome. Mod Rheumatol. 2016;26(5):708–715. doi: 10.3109/14397595.2016.1147404. [DOI] [PubMed] [Google Scholar]
- Munetsugu T., Fujimoto T., Oshima Y., Sano K., Murota H., Satoh T. Revised guideline for the diagnosis and treatment of acquired idiopathic generalized anhidrosis in Japan. J Dermatol. 2017;44(4):394–400. doi: 10.1111/1346-8138.13649. [DOI] [PubMed] [Google Scholar]
- Murota H. Old and new approaches for assessing sweating. In: Yokozeki H., Murota H., Katayama I., editors. Perspiration research. Karger; Basel, Swizterland: 2016. pp. 22–29. [Google Scholar]
- Nitschke M., Tucker G.R., Bi P. Morbidity and mortality during heatwaves in metropolitan Adelaide. Med J Aust. 2007;187(11–12):662–665. doi: 10.5694/j.1326-5377.2007.tb01466.x. [DOI] [PubMed] [Google Scholar]
- Nitschke M., Tucker G.R., Hansen A.L., Williams S., Zhang Y., Bi P. Impact of two recent extreme heat episodes on morbidity and mortality in Adelaide, South Australia: a case-series analysis. Environ Health. 2011;10:42. doi: 10.1186/1476-069X-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolano M., Provitera V., Perretti A., Stancanelli A., Saltalamacchia A.M., Donadio V. Ross syndrome: a rare or a misknown disorder of thermoregulation? A skin innervation study on 12 subjects. Brain. 2006;129(Pt 8):2119–2131. doi: 10.1093/brain/awl175. [DOI] [PubMed] [Google Scholar]
- Nordon C., Martin-Latry K., de Roquefeuil L., Latry P., Begaud B., Falissard B. Risk of death related to psychotropic drug use in older people during the European 2003 heatwave: a population-based case-control study. Am J Geriatr Psychiatry. 2009;17(12):1059–1067. doi: 10.1097/JGP.0b013e3181b7ef6e. [DOI] [PubMed] [Google Scholar]
- Noronha M.J., Vas C.J., Aziz H. Autonomic dysfunction (sweating responses) in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1968;31(1):19–22. doi: 10.1136/jnnp.31.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ootsuka Y., Tanaka M. Control of cutaneous blood flow by central nervous system. Temperature (Austin) 2015;2(3):392–405. doi: 10.1080/23328940.2015.1069437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orteu C.H., Jansen T., Lidove O., Jaussaud R., Hughes D.A., Pintos-Morell G. Fabry disease and the skin: data from FOS, the Fabry outcome survey. Br J Dermatol. 2007;157(2):331–337. doi: 10.1111/j.1365-2133.2007.08002.x. [DOI] [PubMed] [Google Scholar]
- Page L.A., Hajat S., Kovats R.S. Relationship between daily suicide counts and temperature in England and Wales. Br J Psychiatry. 2007;191:106–112. doi: 10.1192/bjp.bp.106.031948. [DOI] [PubMed] [Google Scholar]
- Panda S., Verma D., Budania A., Bharti J.N., Sharma R.K. Clinical and laboratory correlates of selective autonomic dysfunction due to Ross syndrome. J Family Med Prim Care. 2019;8(4):1500–1503. doi: 10.4103/jfmpc.jfmpc_151_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolf K.B., Gange R.W., Latzka W.A., Blank I.H., Kraning K.K., 2nd, Gonzalez R.R. Human thermoregulatory responses during heat exposure after artificially induced sunburn. Am J Physiol. 1992;262(4 Pt 2):R610–R616. doi: 10.1152/ajpregu.1992.262.4.R610. [DOI] [PubMed] [Google Scholar]
- Papa C.M., Kligman A.M. Mechanisms of eccrine anidrosis. I. High level blockade. J Invest Dermatol. 1966;47(1):1–9. doi: 10.1038/jid.1966.93. [DOI] [PubMed] [Google Scholar]
- Periard J.D., Racinais S., Sawka M.N. Adaptations and mechanisms of human heat acclimation: applications for competitive athletes and sports. Scand J Med Sci Sports. 2015;25(Suppl 1):20–38. doi: 10.1111/sms.12408. [DOI] [PubMed] [Google Scholar]
- Pryor R.R., Roth R.N., Suyama J., Hostler D. Exertional heat illness: emerging concepts and advances in prehospital care. Prehosp Disaster Med. 2015;30(3):297–305. doi: 10.1017/S1049023X15004628. [DOI] [PubMed] [Google Scholar]
- Rieg S., Steffen H., Seeber S., Humeny A., Kalbacher H., Dietz K. Deficiency of dermcidin-derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. J Immunol. 2005;174(12):8003–8010. doi: 10.4049/jimmunol.174.12.8003. [DOI] [PubMed] [Google Scholar]
- Romanovsky A.A. Skin temperature: its role in thermoregulation. Acta Physiol (Oxf) 2014;210(3):498–507. doi: 10.1111/apha.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskind J.L., Petrofsky J., Lind A.R., Paletta F.X. Quantitation of thermoregulatory impairment in patients with healed burns. Ann Plast Surg. 1978;1(2):172–176. doi: 10.1097/00000637-197803000-00007. [DOI] [PubMed] [Google Scholar]
- Saari A., Tolonen U., Paakko E., Suominen K., Pyhtinen J., Sotaniemi K.A. Sympathetic skin responses in multiple sclerosis. Acta Neurol Scand. 2008;118(4):226–231. doi: 10.1111/j.1600-0404.2008.01003.x. [DOI] [PubMed] [Google Scholar]
- Sahuc P., Chiche L., Dussol B., Pouget J., Franques J. Sudoscan as a noninvasive tool to assess sudomotor dysfunction in patients with Fabry disease: results from a case-control study. Ther Clin Risk Manag. 2016;12:135–138. doi: 10.2147/TCRM.S99241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Kang W.H., Saga K., Sato K.T. Biology of sweat glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol. 1989;20(4):537–563. doi: 10.1016/s0190-9622(89)70063-3. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Who is sensitive to extremes of temperature? A case-only analysis. Epidemiology. 2005;16(1):67–72. doi: 10.1097/01.ede.0000147114.25957.71. [DOI] [PubMed] [Google Scholar]
- Shibasaki M., Davis S.L., Cui J., Low D.A., Keller D.M., Crandall C.G. Botulinum toxin abolishes sweating via impaired sweat gland responsiveness to exogenous acetylcholine. Br J Dermatol. 2009;161(4):757–761. doi: 10.1111/j.1365-2133.2009.09248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatzky S., Moses S., Levy J., Pinsk V., Hershkovitz E., Herzog L. Congenital insensitivity to pain with anhidrosis (CIPA) in Israeli-Bedouins: genetic heterogeneity, novel mutations in the TRKA/NGF receptor gene, clinical finds, and results of nerve conduction studies. Am J Med Genet. 2020;92(5):353–360. doi: 10.1002/1096-8628(20000619)92:5<353::aid-ajmg12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Sherwood S.C., Huber M. An adaptability limit to climate change due to heat stress. Proc Natl Acad Sci U S A. 2010;107(21):9552–9555. doi: 10.1073/pnas.0913352107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.O., Lee J.B., Kim J.H. Seasonal acclimation in sudomotor function evaluated by QSART in healthy humans. Korean J Physiol Pharmacol. 2016;20(5):499–505. doi: 10.4196/kjpp.2016.20.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorvanek M., Bhatia K.P. The skin and Parkinson's disease: review of clinical, diagnostic, and therapeutic issues. Mov Disord Clin Pract. 2017;4(1):21–31. doi: 10.1002/mdc3.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.G., Lessard M., Reyna S., Doudova M., Singleton J.R. The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complications. 2014;28(4):511–516. doi: 10.1016/j.jdiacomp.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.J. Pediatric thermoregulation: considerations in the face of global climate change. Nutrients. 2019;11(9) doi: 10.3390/nu11092010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C., Lindenlaub T., Zillikens D., Toyka K.V., Naumann M. Selective loss of cholinergic sudomotor fibers causes anhidrosis in Ross syndrome. Ann Neurol. 2002;52(2):247–250. doi: 10.1002/ana.10256. [DOI] [PubMed] [Google Scholar]
- Strand L.B., Barnett A.G., Tong S. Maternal exposure to ambient temperature and the risks of preterm birth and stillbirth in Brisbane, Australia. Am J Epidemiol. 2012;175(2):99–107. doi: 10.1093/aje/kwr404. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Tani S., Murota H., Katayama I. Histamine modulates sweating and affects clinical manifestations of atopic dermatitis. Curr Probl Dermatol. 2016;51:50–56. doi: 10.1159/000446758. [DOI] [PubMed] [Google Scholar]
- Taylor N.A. Human heat adaptation. Compr Physiol. 2014;4(1):325–365. doi: 10.1002/cphy.c130022. [DOI] [PubMed] [Google Scholar]
- Tey H.L., Tay E.Y., Cao T. In vivo imaging of miliaria profunda using high-definition optical coherence tomography: diagnosis, pathogenesis, and treatment. JAMA Dermatol. 2015;151(3):346–348. doi: 10.1001/jamadermatol.2014.3612. [DOI] [PubMed] [Google Scholar]
- Thoenes M.M. Heat-related illness risk with methylphenidate use. J Pediatr Health Care. 2011;25(2):127–132. doi: 10.1016/j.pedhc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Tinkle B., Castori M., Berglund B., Cohen H., Grahame R., Kazkaz H. Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): clinical description and natural history. Am J Med Genet C Semin Med Genet. 2017;175(1):48–69. doi: 10.1002/ajmg.c.31538. [DOI] [PubMed] [Google Scholar]
- Tyler C.J., Reeve T., Hodges G.J., Cheung S.S. The effects of heat adaptation on physiology, perception and exercise performance in the heat: a meta-analysis. Sports Med. 2016;46(11):1699–1724. doi: 10.1007/s40279-016-0538-5. [DOI] [PubMed] [Google Scholar]
- Wang Q., Guo S., Duan G., Xiang G., Ying Y., Zhang Y. Novel and novel de novo mutations in NTRK1 associated with congenital insensitivity to pain with anhidrosis: a case report. Medicine (Baltimore) 2015;94(19) doi: 10.1097/MD.0000000000000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wataya-Kaneda M. Genetic disorders with dyshidrosis: ectodermal dysplasia, incontinentia pigmenti, Fabry disease, and congenital insensitivity to pain with anhidrosis. Curr Probl Dermatol. 2016;51:42–49. doi: 10.1159/000446757. [DOI] [PubMed] [Google Scholar]
- Watts N., Amann M., Arnell N., Ayeb-Karlsson S., Belesova K., Boykoff M. The 2019 report of The Lancet Countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate. Lancet. 2019;394(10211):1836–1878. doi: 10.1016/S0140-6736(19)32596-6. [DOI] [PubMed] [Google Scholar]
- Wells J.C. Thermal environment and human birth weight. J Theor Biol. 2002;214(3):413–425. doi: 10.1006/jtbi.2001.2465. [DOI] [PubMed] [Google Scholar]
- Westaway K., Frank O., Husband A., McClure A., Shute R., Edwards S. Medicines can affect thermoregulation and accentuate the risk of dehydration and heat-related illness during hot weather. J Clin Pharm Ther. 2015;40(4):363–367. doi: 10.1111/jcpt.12294. [DOI] [PubMed] [Google Scholar]
- Wexler R.K. Evaluation and treatment of heat-related illnesses. Am Fam Physician. 2002;65(11):2307–2314. [PubMed] [Google Scholar]
- Yamaga K., Murota H., Tamura A., Miyata H., Ohmi M., Kikuta J. Claudin-3 loss causes leakage of sweat from the sweat gland to contribute to the pathogenesis of atopic dermatitis. J Invest Dermatol. 2018;138(6):1279–1287. doi: 10.1016/j.jid.2017.11.040. [DOI] [PubMed] [Google Scholar]
- Yard E.E., Gilchrist J., Haileyesus T., Murphy M., Collins C., McIlvain N. Heat illness among high school athletes–United States, 2005–2009. J Safety Res. 2010;41(6):471–474. doi: 10.1016/j.jsr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Yardley J.E., Stapleton J.M., Sigal R.J., Kenny G.P. Do heat events pose a greater health risk for individuals with type 2 diabetes? Diabetes Technol Ther. 2013;15(6):520–529. doi: 10.1089/dia.2012.0324. [DOI] [PubMed] [Google Scholar]
- Ye X., Wolff R., Yu W., Vaneckova P., Pan X., Tong S. Ambient temperature and morbidity: a review of epidemiological evidence. Environ Health Perspect. 2012;120(1):19–28. doi: 10.1289/ehp.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]