Abstract
Background
Climate change is broadly affecting human health, with grave concern that continued warming of the earth’s atmosphere will result is serious harm. Since the mid-20th century, skin cancer incidence rates have risen at an alarming rate worldwide.
Objective
This review examines the relationship between climate change and cutaneous carcinogenesis.
Methods
A literature review used the National Institutes of Health databases (PubMed and Medline), the Surveillance, Epidemiology, and End Results and International Agency for Research on Cancer registries, and published reports by federal and international agencies and consortia, including the Australian Institute of Health and Welfare, Climate and Clean Air Coalition, U.S. Environmental Protection Agency, Intergovernmental Panel on Climate Change, National Aeronautics and Space Administration, National Oceanic and Atmospheric Administration, United Nations Environment Programme, World Health Organization, and World Meteorological Organization.
Results
Skin cancer risk is determined by multiple factors, with exposure to ultraviolet radiation being the most important. Strong circumstantial evidence supports the hypothesis that factors related to climate change, including stratospheric ozone depletion, global warming, and ambient air pollution, have likely contributed to the increasing incidence of cutaneous malignancy globally and will continue to impose a negative on influence skin cancer incidence for many decades to come.
Conclusion
Because much of the data are based on animal studies and computer simulations, establishing a direct and definitive link remains challenging. More epidemiologic studies are needed to prove causality in skin cancer, but the evidence for overall harm to human health as a direct result of climate change is clear. Global action to mitigate these negative impacts to humans and the environment is imperative.
Keywords: Skin cancer, Melanoma, Nonmelanoma skin cancer, Climate change, Global warming, Air pollution
Introduction
As we face growing evidence of the adverse impact of climate change on human and environmental health, it is paramount to consider how climate specifically affects the incidence of cutaneous malignancy, one of the most common and potentially serious dermatologic diagnoses. This review examines the specific relationship and causality of increasing global skin cancer rates with increased ultraviolet radiation (UVR) resulting from stratospheric ozone depletion (SOD), the carcinogenic and behavioral effects of heat due to global warming, and air pollution (AP) from fossil-fuel combustion.
Epidemic of skin cancer
Skin cancer represents the world’s most common cancer, and incidence rates increased substantially in the latter part of the 20th century, with the highest incidence observed in fair-skinned populations (Garbe and Leiter, 2009, Lomas et al., 2012). Nonmelanoma skin carcinoma (NMSC), specifically keratinocyte carcinomas (basal cell carcinoma [BCC] and squamous cell carcinoma [SCC]), is well accepted as accounting for the majority of cutaneous malignancies, but establishing the true incidence has been difficult owing to the lack of inclusion in many national cancer registries (Lomas et al., 2012, Rogers et al., 2015). Despite this limitation, temporal trends in NMSC incidence demonstrate an alarming increase in the United States since the 1980s (Table 1). Likewise, rates of NMSC show an upward global trend. Based on cancer statistics for Nordic countries (Nordic Cancer Registries data), the incidence of NMSC in Scandinavia more than doubled since 1960, while a 4-fold increase is reported for Australia (International Agency for Research on Cancer, 2020, Perera et al., 2015).
Table 1.
Estimated number of new cases of nonmelanoma skin carcinoma in the United States from 1983 to 2012 (Miller and Weinstock, 1994, Rogers et al., 2010, Rogers et al., 2015, Scotto et al., 1983).
| Year | New cases of nonmelanoma skin carcinoma |
|---|---|
| 1983 | 400,000–500,000 |
| 1992 | 900,000–1, 200,000 |
| 2006 | 3,500,000 |
| 2012 | 5,300,000 |
Of greater concern is the rapid increase in the incidence rate of cutaneous malignant melanoma (CMM; Erdmann et al., 2013, Garbe and Leiter, 2009, Godar, 2011, Matthews et al., 2017). New cases in the United States rose 3-fold over the last 4 decades (Fig. 1; National Cancer Institute, 2020). Globally, CMM incidence increased on average 4% to 5% annually (Godar, 2011). Extremely high rates of skin cancer in Australia and New Zealand give these nations the unenviable designation of having the greatest incidence of cutaneous malignancy in the world (Erdmann et al., 2013). The dramatic increase in skin cancer incidence over recent decades (Table 2, Table 3) is in part due to a perilous combination of fair-skinned residents, subtropical latitude, and a sun-centric culture that emphasizes outdoor recreation (Australian Institute of Health and Welfare, 2016). However, many authors have argued that the CMM epidemic is artificially amplified by increased screening and biopsies, clinicians’ improved diagnostic ability, and an evolution in histologic criteria. These factors undoubtedly contribute, but the increasing CMM incidence is independent of socioeconomics and tumor thickness, validating the concept that the increasing trend is not the sole function of enhanced detection (Apalla et al., 2017).
Fig. 1.
Surveillance, Epidemiology, and End Results 9 and 13 data for new cutaneous malignant melanoma cases per 100,000 people (all races, males and females, age-adjusted) in the United States from 1975–2016 (National Cancer Institute).
Table 2.
Estimated incidence of nonmelanoma skin carcinoma per 100,000 person-years in Australia from 1985–2011 (Perera et al., 2015).
| Date | Incidence |
|---|---|
| 1985 | 555 |
| 2011 | 2448 |
Table 3.
Estimated incidence of cutaneous malignant melanoma per 100,000 person-years in Australia from 1982–2016 (Australian Institute of Health and Welfare, 2016).
| Date | Incidence |
|---|---|
| 1982 | 27 |
| 2016 | 49 |
The overall burden of skin cancer is considerable. Worldwide, almost 126,000 deaths due to skin cancer occurred in 2018 (Ferlay et al., 2019). Perhaps more profound are the 2015 rates for global age-standardized disability-adjusted life years for CMM: 27 for men and 19 for women (Karimkhani et al., 2017). The impact on quality of life resulting from esthetic outcomes, functional morbidity, and psychological burden stemming from the diagnosis and treatment of cutaneous malignancy is also significant and equally important to acknowledge (Răducu et al., 2020, Sampogna et al., 2019). Beyond these aspects, cutaneous malignancy imposes a profound economic burden on health care systems. In the United States alone, an estimated $8.1 billion was spent on skin cancer in 2011 (Guy et al., 2015). Given the increasing skin cancer incidence and the substantial psychosocial and economic implications, it should come as no surprise that the U.S. Surgeon General issued a Call to Action in 2014 (Watson et al., 2014).
UVR is implicated as a primary driver in the pathogenesis of skin cancer, but the etiology is multifactorial. The contributions of key risk factors, such as genetic mutations, skin type, age, and viral infections, will not be further addressed here (Apalla et al., 2017, Chen et al., 2013, Gandhi and Kampp, 2015, Gilchrest et al., 1999, Xiang et al., 2014). Rather, this review aims to explore the overall impact of climate change and global warming in further forcing the increasing global incidence of cutaneous malignancies.
Photocarcinogenesis
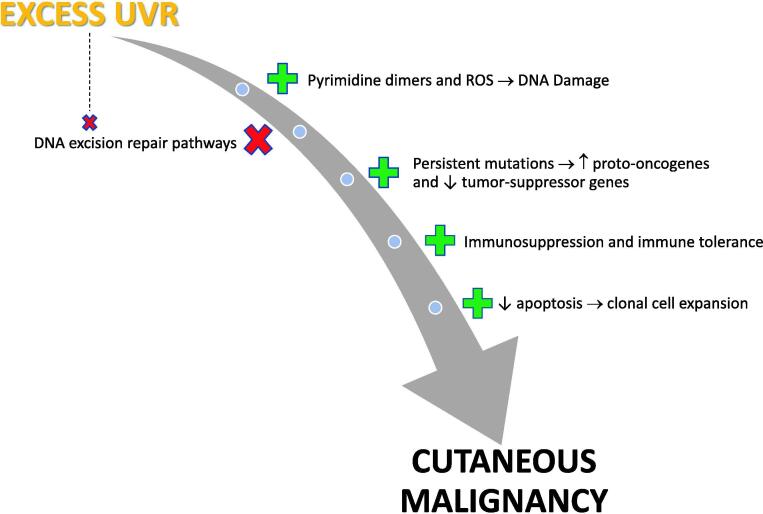
It is well acknowledged that UVR exposure is carcinogenic and implicated as the primary cause of skin cancer (El Ghissassi et al., 2009, Forbes, 1981, Moan et al., 2015). In fact, UVR is considered a complete carcinogen due to its ability to act as mutagen and promote and initiate tumor formation without inducement by another agent (D’Orazio et al., 2013). Although it is beyond the scope of this review to detail these complex molecular pathways, it is worthwhile to provide a simplistic overview of the cascade of events in photocarcinogenesis that ultimately end with the development of cutaneous malignancies (Fig. 2).
Fig. 2.
Schematic illustration of the multi-step process of photocarcinogenesis whereby exposure of the skin to ultraviolet radiation results in DNA damage via the formation of pyrimidine dimers and reactive oxygen species. Excisional repair of DNA may reverse some damage, but the reparative mechanisms are overwhelmed when ultraviolet radiation exposure is excessive. This allows the progression of mutagenesis, immune suppression, and clonal cell expansion, thus promoting tumor formation.
In summary, ultraviolet A stimulates the production of reactive oxygen species, which in turn leads to DNA damage (single-strand DNA breaks, crosslinks, altered bases); however, DNA acts as an actual chromophore for ultraviolet B such that direct absorption results in the formation of pyrimidine dimers (Martens et al., 2018, Pfeifer and Besaratinia, 2012). DNA excision repair mechanisms become overwhelmed by excessive UVR exposure and eclipsed by mutagenesis, including upregulation of proto-oncogene expression and downregulation of tumor suppressor genes, allowing dysregulation of apoptosis with reduced cell death and subsequent proliferation of clonal cell populations (Melnikova and Ananthaswamy, 2005).
Photocarcinogenesis is further promoted by UVR-induced immune suppression (Hart and Norval, 2018). Although the pattern and intensity of UVR exposure and number of sunburns may differentially affect the risk of developing various forms of cutaneous malignancy, the World Health Organization (WHO) deems that strong evidence of causality exists for the ultraviolet-induction of NMSC and CMM, so it stands without argument among the scientific and medical community that UVR is the most important causative environmental factor in skin cancer development and, as such, is designated as a carcinogen by the IARC (Lucas, 2010, Moan et al., 2015).
Stratospheric ozone depletion and ultraviolet radiation
The stratospheric ozone layer serves as a critical UVR filter, effectively shielding the earth from ultraviolet C and a large portion of ultraviolet B. This function is essential for the survival of plant and animal life on earth, yet human activity has caused substantial SOD over the last century. As a less toxic and nonflammable alternative refrigerant, the first chlorofluorocarbon (CFC) was developed in 1928. Mass production under the trade name Freon began in 1930 as a joint venture between General Motors and DuPont, and CFCs grew to include almost 100 chemicals that became widely used as refrigerants, aerosol propellants, and components in the manufacture of insulation (Andersen et al., 2013).
These so-called “wonder gases” were lauded until the 1970s, when CFCs ability to catalytically degrade ozone in the presence of UVR was identified (Molina and Rowland, 1974). Following this seminal work, British scientists published the first documentation of stratospheric ozone loss over Antarctica in 1985, and subsequent aircraft data confirmed the inverse relationship between concentrations of chlorine monoxide and ozone in the stratosphere (Farman et al., 1985, National Aeronautics and Space Administration, 2020a). CFCs and their related chemicals were determined to be very long-lived, potent greenhouse gases (GHGs) that cleave ozone molecules, leading to SOD and a resultant increase in UVR at the earth’s surface. Consequently, they are justly designated as ozone-depleting substances (Andersen et al., 2013).
Accordingly, these substances were deemed a great threat to human health and earth’s ecological systems (Dugo et al., 2012, U.S. Environmental Protection Agency, 2020). Based on these discoveries and the urgent need to preserve the stratospheric ozone layer, the 1987 Montreal Protocol on Substances That Deplete the Ozone Layer was promulgated. This sweeping international environmental policy effectively mandated the incremental phasing out of ozone-depleting substances and was universally ratified by every nation. The United Nations Environment Programme (UNEP) and World Meteorological Organization (WMO) formally reassess the state of the ozone quadrennially. Since its inception, the treaty has been amended and strengthened multiple times based on these assessments, with drastic reductions in ozone-depleting substances now observed (WMO et al., 2019).
Increased ultraviolet radiation secondary to stratospheric ozone depletion
Because of severe and persistent seasonal SOD over Antarctica, average ultraviolet B was 55% to 85% greater between 1991 to 2017 than between 1963 to 1980. Additionally, surface erythemal radiation increased on average 3% globally between 1979 and 2008, with long-term changes being the most significant at mid-latitudes (UNEP, 2018). The specific case of Australia is illustrative. Clear-sky UVR levels have increased overall since 1970, with an annual increase in UVR of 2% to 6% since 1990. These increases in UVR coincide with SOD and increasing skin cancer incidences over the same period (Lemus-Deschamps and Makin, 2012).
Stratospheric ozone depletion and skin cancer incidence
As a basic corollary, continued SOD will result in increased UVR at the earth’s surface and an increased incidence of skin cancer. For every 1% decrease in ozone layer thickness, the incidence of melanoma is projected to increase 1% to 2%; the SCC incidence would increase 3% to 4.6% and BCC by up to 2.7% (López Figueroa, 2011). Based on compliance with the 1992 amendments to the Montreal Protocol, a 10% increase in skin cancer incidence by mid-century due to ozone depletion is predicted (Slaper et al., 1996). However, as discussed later in this review, SOD and climate change influences will not be uniformly observed across the globe. Given that these threats will have regional variability and skin type also greatly influences malignancy risk, geographic variations in excess skin cancer cases will likely be evident. Based on full compliance with the Montreal Protocol and accounting for skin color variances, van Dijk et al. (2013) simulated that, by the mid to latter 21st century, the greatest increase in additional cases of cutaneous malignancy (NMSC and CMM combined) is estimated for Australia, with up to 200 extra cases per million people per year, but no increase is expected for the Congo. The simulated results for the remaining regions further demonstrate this geographic variation: 20 to 50/million in Patagonia, 30 to 40/million in Western Europe, 80 to 110/million in the Southwest United States, 90 to 100/million in the Mediterranean, 90 to 120/million in China, and 100 to 150/million in New Zealand.
The future: Climate change, ozone recovery, and impact on skin cancer incidence
The Montreal Protocol is deemed a success due to the drastic reduction in atmospheric concentrations of ozone-depleting substances resulting from its enforcement. Based on models of excess UVR avoided by implementation of this treaty, millions of cases of skin cancer have been prevented worldwide, yet the threat is far from resolved (van Dijk et al., 2013, World Meteorological Organization, 2019). As of 2014 to 2017, the total global ozone remained 2.2% below the 1964 to 1980 averages, with notable regional differences. Specifically, the current stratospheric ozone concentrations in the northern mid-latitudes (35°N-60°N) are 3.0% lower than historical averages, 5.0% lower in the southern mid-latitudes (35°S-60°S), and <1% lower in the tropics (20°S-20°N; WMO et al., 2019). Because ozone-depleting substances are long-lived in the stratosphere, ozone depletion over Antarctica is not expected to fully recover until at least 2060, perpetuating the hazard posed by increased UVR for many decades to come.
Additionally, as of 2011, a large hole in the stratospheric ozone layer has developed in the Northern Hemisphere over the Arctic (Bais et al., 2018, World Meteorological Organization, 2019). This new Arctic ozone hole resulted in a 60% increase in ultraviolet irradiance at the earth’s surface over long-term averages for the involved area. In mid latitudes, mean ultraviolet irradiance has increased on average 2% to 6% per decade in the last 20 years (Andrady et al., 2017). Due to the latency between UVR exposure and cutaneous cancer development, decades of loss combined with slow ozone recovery translate into persistent increased levels of UVR and continued peril in global rates of cutaneous malignancy.
Future amelioration of SOD is dependent on multiple factors but, in part, remains tenuous because the continued release of ozone-depleting substances jeopardizes ongoing ozone recovery. Hydrofluorocarbons are widely used replacements for ozone-depleting substances, and emissions have rapidly increased, presenting a large impact to climate change because hydrofluorocarbons possess high global warming potential. Accumulation of halogenated very short-lived substances, such as dichloromethane, is also an emerging hazard because they act as ozone-depleting substances but are unregulated by the Montreal Protocol (Hossaini et al., 2017, World Meteorological Organization, 2019). Additionally, an unexpected increase in banned CFC-11 emissions has resulted from illegal production in Asia. This long-lived ozone-depleting substance acts as a potent GHG such that continued release enhances global warming and SOD (Montzka et al., 2018). These setbacks in the release of ozone-depleting substances further augment climate forcing, with recent modeling suggesting a potential delay in stratospheric ozone recovery of 30 years (Hossaini et al., 2017).
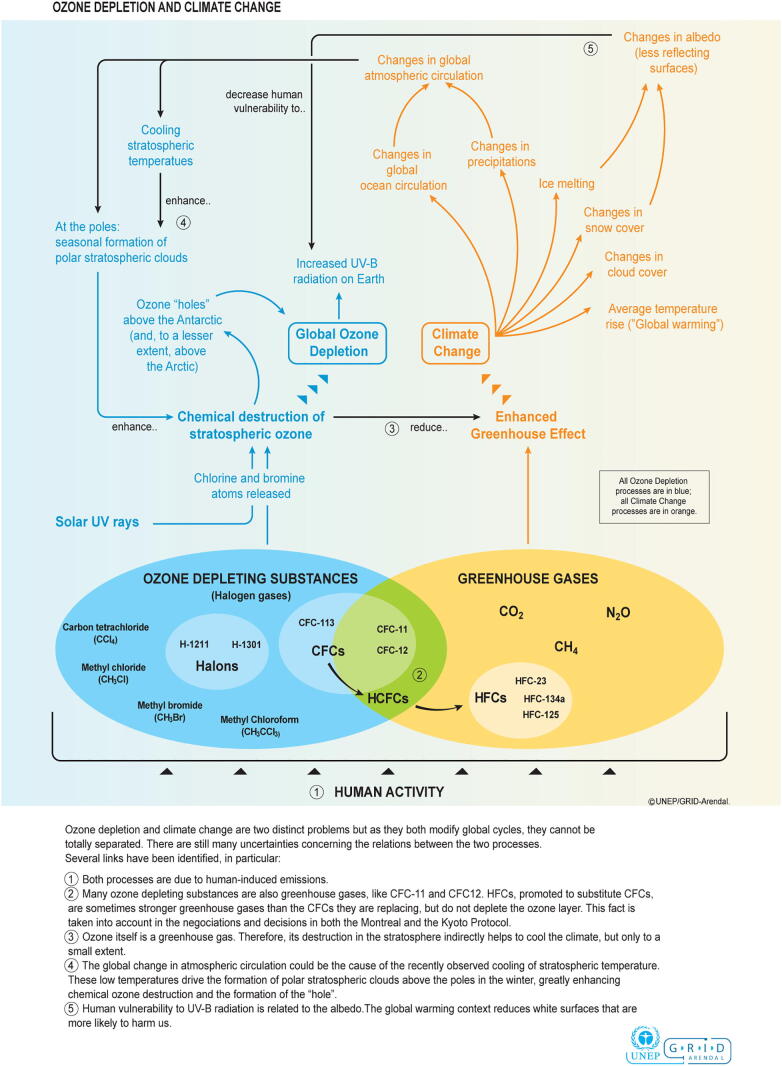
Although the relationship was not previously well accounted for in older simulations, climate change forcing is now considered the largest threat to stratospheric ozone recovery. Future projections of GHG concentrations and climate change impact will be highly dependent on current international mitigation efforts. When this is coupled with the complexity of interactions between SOD and climate change, which are yet to be fully elucidated, modeling accurate scenarios for ozone recovery has inherent limitations, contradictions, and uncertainty (Bais et al., 2018, World Meteorological Organization, 2019). What we do understand is that a multiplex of interrelated feedback mechanisms exists between ozone depletion and climate change, illustrating that these two processes are bidirectional and, arguably, mutually destructive forces (Fig. 3). To distill this complexity, GHG emissions and climate forcing affect both chemical cycles and overturning circulation in the stratosphere and will serve as the major determinant of stratospheric ozone concentrations in the second half of this century, especially if levels of ozone-depleting substances continue to decline. Furthermore, stratospheric ozone levels similarly affect climate systems and alter atmospheric circulation in both the troposphere and stratosphere (Environmental Effects Assessment Panel UNEP, 2019). In fact, Antarctic SOD is already a dominant driver of climate change in the Southern Hemisphere and the tropics, resulting in altered patterns in tropospheric circulation, temperature, and precipitation (WMO et al., 2019). Furthermore, Arctic SOD has demonstrably shifted the North Atlantic jet stream, resulting in temperature and precipitation changes in the Northern Hemisphere and influenced El Nino southern oscillation events (Bais et al., 2018).
Fig. 3.
Schematic illustration of the complex interactions between stratospheric ozone depletion and climate change (Bournay, 2007).
To better understand the interplay between GHG emissions and SOD and how this relationship in turn affects climate in the aforementioned manner, it is first helpful to explain the dynamic process of seasonal ozone losses over the polar regions. Brewer-Dobson atmospheric circulation patterns push equatorial air upward from the troposphere to the stratosphere and transport it poleward, widely distributing ozone-depleting substances throughout the stratosphere, yet unique geographical, meteorological, and chemical factors preferentially combine at the poles to trigger seasonal SOD. As winter temperatures plummet below −78 °C (−108.4°F) in the atmosphere over Antarctica and the Arctic, polar stratospheric clouds form, lasting 1 to 2 months over the Arctic and up to 5 months over Antarctica. Varying topography and land mass sizes of the two regions account for these differences.
In addition to polar stratospheric clouds, strong polar vortexes also form, preventing transport and mixing of stratospheric air and serving to isolate these portions of the stratosphere overlying the poles. Consequently, chemical reactions commence on the surfaces of polar stratospheric clouds, significantly increasing the abundance of reactive halogen gases that cleave ozone molecules in the presence of UVR (Environmental Effects Assessment Panel UNEP, 2019). A second foundational, but perhaps counterintuitive, point necessary in conceptualizing the bidirectional relationship between the forces of climate change and SOD is that of stratospheric cooling. An intact ozone layer traps UVR, heating the stratosphere. Thus, persistent ozone depletion serves to cool the stratosphere, creating positive feedback mechanisms that prolong polar vortexes and lead to further ozone loss with subsequent influences on tropospheric climate patterns. Moreover, evidence suggests that GHGs may increase the tropospheric-stratospheric temperature differential and accelerate Brewer-Dobson circulation patterns (Dugo et al., 2012).
Although particular simulations of unabated GHG concentrations predict stratospheric warming and thus super recovery of the global stratospheric ozone column by the end of the century (Andrady et al., 2017, Lucas et al., 2019), others have offered an alternative and grimmer perspective that instead suggest GHG emissions actually exacerbate ozone column degradation (Anderson and Clapp, 2018, Lucas et al., 2019). This latter notion is supported by currently observed climate-influenced losses occurring in the lower portion of the stratospheric ozone layer since 1998 in regions outside the polar latitudes, coinciding with the areas where the vast majority of the human population resides (Ball et al., 2018). Specifically, as GHGs force climate change, a reduction in temperature gradient from the poles to the tropics will develop. When this is coupled with other effects of climate change, such as the intensification of storm systems, loss of cryosystems, rising sea levels, stratospheric cooling, and increased water vapor, favorable conditions ensue for enhanced catalytic SOD.
The effects of climate change during the summer months in the central United States present ideal conditions for this very scenario of increased regional SOD. The concomitant increase in UVR that would occur with these losses is such that a 3% increase in skin cancer incidence would occur as a result of a 1% fractional decrease in the ozone layer (Anderson and Clapp, 2018). Co-dependent dynamics between climate change and SOD will culminate in differential patterns of climate and UVR intensity, with lower latitudes experiencing decreased cloud cover and higher ultraviolet irradiance, translating into future adverse effects on skin cancer incidence at mid and low latitudes (Newman and McKenzie, 2011). Enhanced understanding of the interactions between SOD, climate change, and UVR, with specific emphasis on the impact to human health, will be critically important moving forward (Andrady et al., 2017).
The UNEP concluded that “when considering the effects of climate change, it has become clear that processes resulting in changes in stratospheric ozone are more complex than previously believed. As a result of this, human health and environmental issues will be longer-lasting and more regionally variable” (Andrady et al., 2017). The effects from continued ozone depletion risk further harm to human health, specifically persistent elevations in UVR and continued increases in rates of cutaneous malignancy. If one broadens the focus beyond merely skin cancer risk, the implications of stratospheric ozone loss are wide-reaching, extending directly to deleterious effects on ocular health, immune system function, ecological impacts on terrestrial and aquatic systems that imperil our food supply, and overall amplification of the health effects attributable to climate change (Andrady et al., 2017, Bais et al., 2018, Lucas et al., 2019). SOD is alarmingly detrimental to humans, and restitution is paramount to preserving more than just future skin health.
Global warming
Due to continued anthropogenic emissions of GHG, the earth’s temperature has increased by more than 1 °C (1.8°F) since 1880, with the majority of warming occurring in the last 45 years (National Aeronautics and Space Administration, 2020b). The Intergovernmental Panel on Climate Change has urged limiting the global temperature increase to 1.5 °C (2.7°F) of warming by 2100 to mitigate human health effects (WMO and UNEP, 2018), but warming beyond this may be inevitable. Increasing temperatures due to global warming have been implicated as a contributory factor in increasing skin cancer incidence worldwide. Because UVR and temperature are geographically and climatologically related and co-exposure commonly occurs when outdoors, clearly establishing the degree to which temperature influences skin cancer risk is challenging, but data clearly support this as a secondary influence (Fabbrocini et al., 2010). In fact, the notion that increased temperature may amplify cutaneous photocarcinogenesis has been suggested since the 1940s (Bain et al., 1943, Freeman and Knox, 1964).
Early mouse models demonstrated an increased rate of UVR-induced tumor formation at higher temperatures (Bain et al., 1943, Freeman and Knox, 1964). Later work reinforced the synergistic effect of UVR and heat on cutaneous carcinogenesis by demonstrating that the production of heat shock proteins in response to heat stress inhibits cell-death signaling pathways, resulting in greater survival of DNA-damaged cells (Calapre et al., 2013). The specific finding that heat exposure leads to a reduction in p53-mediated cell cycle arrest and apoptosis in ultraviolet B-exposed cells augments this theory (Calapre et al 2016). Not surprisingly, a statistically significant increase in skin temperature has been documented as a result of sun bathing, providing a realistic conceptualization for how co-exposure to heat and temperature can commonly occur over large surface areas of the skin (Petersen et al., 2014). A 2 °C (3.6°F) increase in ambient temperature is estimated to increase skin cancer incidence 11% globally by 2050 (Van Der Leun and de Gruijl, 2002).
Heat also has an indirect effect on skin cancer incidence by altering human behavior. Predictably, as temperatures increase, people spend greater time outdoors and do so with less clothing, thus increasing UVR exposure (Bélanger et al., 2009, Dobbinson et al., 2008, Makin, 2011). This is of particular concern in children, for whom significantly more UVR exposure is documented during the summer months (Diffey et al., 1996). Moreover, sunburn risk is correlated with increased temperatures. Above 22 °C (72°F), adolescents and adults were two to three times more likely to develop sunburn, and above 28 °C (82°F), the likelihood of sunburn increased more than 3-fold (Dobbinson et al., 2008). Although extremely high temperatures may exceed an individual’s thermal comfort threshold, triggering individuals to remain indoors, especially when air conditioning is accessible, the aforementioned increase in time spent outdoors during warmer weather is likely to be most prominent in mid to upper latitudes (Elnabawi and Hamza, 2019, Makin, 2011). Since the 1970s, UVR has doubled during the winter months (Lemus-Deschamps and Makin, 2012). As global temperatures rise, a shorter winter duration in more temperate regions combined with greater UVR intensity during these months will likely contribute to increased year-round sun exposure and exacerbate skin cancer incidence (Makin, 2011, Lemus-Deschamps and Makin, 2012).
The increasing threat of extreme heat events due to climate change is causative in wider-reaching adverse health effects. Specifically, cyclical episodes of dehydration as a result of increased temperatures and decreased access to clean water during extreme weather events are implicated in an increased incidence of acute and chronic kidney disease (Borg et al., 2017, Harari Arjona et al., 2011, Tomson and Connor, 2015). Similarly, extreme heat increases the risk of ischemic heart disease and myocardial infarction and exacerbates heart failure and pulmonary disease (Bernstein and Rice, 2013, Chen et al., 2019, De Blois et al., 2015). A markedly increased risk of skin cancer has been demonstrated in recipients of organ transplants and those on hemodialysis for end-stage renal disease (España, 2004, Wang et al., 2016). This has prompted the author to ponder whether the overall adverse health effects of increasing ambient temperature due to global warming will result in a greater need for renal dialysis and solid-organ transplantation in the future and thus further contribute to increasing skin cancer incidence.
Ambient air pollution
Combustion of fossils fuels not only contributes to increasing concentrations of GHGs that drive global warming and climate change, it is also the primary source of ambient AP. Much like the complex feedback systems between climate change and SOD, a similar intertwined relationship exists with AP. Short-lived climate pollutants, such as methane, particulate matter (PM), and tropospheric ozone, are major components of AP, act as important climate forcers, and are harmful to human health (Climate and Clean Air Coalition [CCAC], 2014). In addition to these compounds, AP contains a complex mix of many chemicals, including polycyclic aromatic hydrocarbons, volatile organic compounds, nitrogen oxide species, carbon monoxide, sulfur dioxide, and heavy metals (Balakrishnan et al., 2015).
Health effects due to air pollution
In 2013, the IARC deemed AP to be a human carcinogen, and as of 2016, >90% of the world’s population reside in areas with AP levels above the healthy limits set by the WHO’s Air Quality Guidelines (WHO, 2020a). Although the deleterious impacts on lung, neurologic, and ischemic cardiovascular disease, as well as the association with premature death, have been well established (Schraufnagel et al., 2019a, Schraufnagel et al., 2019b), we are just beginning to understand how these pollutants also influence cutaneous carcinogenesis. Both UVR and the prominent components of AP share a similar pathogenetic mechanism by acting as exogenous ligands for the aryl hydrocarbon receptor (AHR), a key regulator of keratinocyte proliferation and differentiation, melanogenesis, skin barrier function, and immune function (Vogeley et al., 2019). Binding of AHR by AP results in chronic activation, triggering the formation of reactive oxygen species, an increase in extracellular matrix metalloproteinases, cytokine production, and DNA damage, subsequently leading to tumorigenesis (Kim et al., 2016, Parrado et al., 2019, Vogeley et al., 2019). In general, higher concentrations of ambient AP have been linked with an increased risk of NMSC, and specific components of pollution are also linked with CMM (Datzmann et al., 2018, Kim et al., 2016).
Air pollution and skin cancer risk
As the most important constituent of ambient AP, PM is generally subclassified based on size: course PM < 10 μm, fine PM < 2.5 μm (PM2.5), and ultrafine particles <0.1 μm. PM2.5 is considered one of the most important indicators of air quality, with increasing exposure to PM closely linked with mortality risk (Climate and Clean Air Coalition, 2014, Di et al., 2017, World Health Organization, 2020b). Global increases in the population-weighted mean concentration of PM2.5 were observed from 1990 to 2013, with a 20% increase noted. Importantly, due to their nanoparticle size, when inhaled, PM2.5 and ultrafine particles embed deeply into the lungs and from there readily enter the systemic circulation. These particles also penetrate the epidermis and may pass transdermally via follicular and eccrine structures (Parrado et al., 2019). Studies on exposure to PM from traffic emissions demonstrated a 20% increase in pigmented facial lesions, suggesting its proliferative effect on the skin and enhancement of melanogenesis (Vierko et al., 2010). Black carbon comprises a large fraction of PM2.5 and is a known carcinogen shown to increase CMM risk (Kim et al., 2016, Puntoni et al., 2004). Furthermore, the carcinogenicity of PM2.5 is enhanced when it forms aerosols with adsorbed toxic metals and polycyclic aromatic hydrocarbons, which have been linked with the induction of SCC and CMM (Grant, 2009, Liu et al., 2019).
As a key component of AP, the Janus-faced nature of ozone warrants a brief explanation to dispel any confusion because stratospheric and tropospheric ozone concentrations are unrelated entities. Ozone in the upper atmosphere comprises a critical ultraviolet-filtering layer of the stratosphere that is essential for the viability of all life on earth, whereas tropospheric or ground-level ozone is a major component of smog and a highly toxic air pollutant. Although not emitted directly from the combustion of fossil fuels, ground-level ozone forms when combustion products in ambient AP, such as nitrogen oxide species, volatile organic compounds, and carbon monoxide, react with oxygen in the presence of UVR to create ozone molecules (Balakrishnan et al., 2015, Climate and Clean Air Coalition, 2014, World Health Organization, 2020b). Population-weighted mean tropospheric ozone concentrations were observed to have increased by almost 9% globally between 1990 and 2013 (Brauer et al., 2016), and this pollutant is well established as a cause of chronic lung disease and premature mortality (Climate and Clean Air Coalition, 2014, Lelieveld et al., 2015). In addition to binding AHR (Parrado et al., 2019), ozone reacts with unsaturated lipids on exposed skin, forming lipid peroxidation products that result in oxidative stress and inflammatory cytokine production (Burke, 2018).
Polycyclic aromatic hydrocarbons (PAHs) are classified as persistent organic pollutants that remain in a gaseous state or bind PM to form complex mixtures in ambient air, where they may be inhaled or cutaneously absorbed. PAHs represent a ubiquitous component of AP and are known carcinogens. Because of their lipophilic properties, direct exposure of PAHs to the skin results in transepidermal absorption (Parrado et al., 2019). PAHs are implicated in the etiology of NMSC and CMM (Grant, 2009, Mehlman, 2006). Topical application of PAHs in animal studies induces the formation of SCC (Siddens et al., 2012). Moreover, PAHs demonstrate a synergistic effect on cutaneous carcinogenesis via enhancement of oxidative DNA damage when combined with simultaneous ultraviolet A exposure (Burke, 2018). A number of occupational studies have further demonstrated a link between PAH exposure and skin cancer (Boffetta et al., 1997, Espina et al., 2015, Stenehjem et al., 2017).
Volatile organic compounds (VOCs) comprise a large group of gaseous chemicals with varied natural and anthropogenic sources, including combustion of fossil fuels. Those arising from industrial manufacturing as well as motor vehicle and aviation emissions are hazardous air pollutants implicated in multiple adverse health effects, including neurologic disorders, hematologic and visceral malignancies, respiratory disease, and hepato- and nephrotoxicity (Montero-Montoya et al., 2018). VOCs act as both primary and secondary air pollutants, and their lipophilic nature allows for ready tissue penetration via multiple routes, including pulmonary, gastrointestinal, and dermal absorption (Balakrishnan et al., 2015, International Agency for Research on Cancer, 2018).
Moreover, VOCs are important precursor pollutants, undergoing photochemical oxidation to produce ground-level ozone and the formation of aerosols that become components of PM2.5 (Okada et al., 2012, World Meteorological Organization, 2017). Benzene is one of the most important VOCs in ambient air pollution. Exposure routes include inhalation, allowing entry into the systemic circulation, and cutaneous absorption. Classified as a Group 1 carcinogen (sufficient evidence of carcinogenicity in humans) by the IARC, benzene exposure results in oxidative stress and induction of inflammatory cytokines with associated DNA damage, genotoxicity, and immune suppression (International Agency for Research on Cancer, 2018, Wilmer et al., 1997). Although the majority of benzene exposure is occupational, exposure to AP serves as an important source for the general population, especially in densely populated geographic regions such as China, where ambient levels routinely exceed European limits of 5 μg/m3 by an order of magnitude (IARC, 2018). Exposure to VOCs in AP are linked to an increased risk of CMM (Boeglin et al., 2006), and benzene specifically is associated with the induction of cutaneous squamous cell papillomas and carcinomas in animal models (Huff et al., 1989), as well as an increased incidence of CMM in humans (Mehlman, 2006, Stenehjem et al., 2017).
Exposure to AP is associated with significant disease burden in humans and is implicated in eight million deaths annually worldwide (Brauer et al., 2016, Schraufnagel et al., 2019a, World Health Organization, 2020b). As with increased heat due to global warming, the known deleterious pulmonary and cardiovascular impacts again raise the question of whether increasing exposure to AP in the future will translate to a greater need for solid organ transplantation to manage heart and lung disease. Dually, AP is well documented to exacerbate inflammatory skin conditions, especially atopic dermatitis (Koohgoli et al., 2017). Similarly, worsening of inflammatory skin diseases due to AP may mandate greater use of systemic immunosuppressant medications in the future. Both scenarios would be expected to exacerbate the risk of skin cancer. Much remains to be studied, but exposure to AP clearly contributes to the increasing the incidence of cutaneous malignancy both via direct and indirect mechanisms.
Vulnerable populations
Requisite to a discussion on the effects of climate change on skin cancer incidence is recognition of those who will disproportionally bear that burden, along with the ethnic, comorbid, geographic, occupational, and socioeconomic determinants of this burden. Large disparities are expected globally in fair-skinned populations, as evidenced by the aforementioned regional variability in simulations of extra skin cancer cases expected in the future due to stratospheric ozone depletion (van Dijk et al., 2013). Moreover, patients with vitiligo, oculocutaneous albinism, and genetic cutaneous cancer syndromes such as xeroderma pigmentosa, basal cell nevus syndrome, and familial atypical multiple mole melanoma syndrome will be differentially affected by the excess skin cancer risk posed by climate change.
Perhaps at greatest risk are those on immunosuppressant medications, especially organ transplant recipients. The incidence of cutaneous malignancy in this population is markedly increased, with an incidence rate of SCC up to 250-fold greater than that in the general population. Additionally, incidence rates in transplant patients are increased for other forms of skin cancer: BCC is up to 16 times higher and CMM is up to 8 times higher (Bais et al., 2018, Mittal and Colegio, 2017). Moreover, skin cancers in transplant patients are more aggressive and carry a 10-fold greater mortality than in the general population (Bais et al., 2018, Garrett et al., 2016). Transplant recipients carry substantially more skin cancer risk due to climate change effects, and as previously discussed, an increased need for transplantation may theoretically arise in the future due to adverse impacts from heat and AP on other organ systems.
Geographically, those living at higher elevations receive greater UVR. For every 1000 m increase in altitude, UVR intensity increases by 10% to 12% (Narayanan et al., 2010). Consequently, residents of alpine regions have a higher incidence of skin cancer, including CMM (Haluza et al., 2014). In Ecuador especially, outdoor workers shoulder particular risk due to the extreme elevations of the Andes, their inherent occupational risk, and lower socioeconomic status. Great concern for the health effects resulting from climate change extends to outdoor workers worldwide because they have markedly increased exposure to UVR, rising temperatures, extreme heat events, and ambient AP (Espina et al., 2015, Harari Arjona et al., 2011, Schulte et al., 2016).
Currently, 10% of the world lives in extreme poverty (The World Bank, 2020) and will generally experience enormous health impacts as a result of climate change (Friel et al., 2011). With respect to skin cancer prevention, access to sunscreen and sun protective clothing, air conditioning, and air filtration systems are luxuries not available to many, especially the impoverished. This will be severely compounded by health care inequities among the socially disadvantaged and those residing in low-income nations (Friel et al., 2011). Conceptualizing that planetary health is emblematic of human health and embracing the moral imperative of environmental ethics should be as much as part of our daily discussions and vernacular as any other medically relevant topic because, as dermatologists, we shoulder the important responsibility to advocate even more loudly for vulnerable populations as climate impacts continue to adversely affect human health in the future (Dunk et al., 2019).
Conclusions
The contributions of many factors are frequently offered to explain increasing global skin cancer rates over the last 50 years, yet climate change is frequently omitted from this conversation. Although much of our current knowledge and future extrapolations are based on computer modeling and mouse studies, this review documents well the likely contribution of climate change to increased skin cancer incidence and makes a compelling argument that climate change will likely play a larger role in cutaneous carcinogenesis in the future. More epidemiologic studies examining climate change impacts on cutaneous carcinogenesis are needed to better establish a direct and definitive causality. Adoption of sun avoidance and sun-protective behaviors and remaining in temperature-controlled indoor environments with air filtration systems, both ideally powered by alternative energy sources, would mitigate individual risk for those privileged to access these measures, but the larger impact of climate change on skin cancer incidence demands wide-scale, global efforts to halt the progression of climate change.
We have entered a climate crisis, and the time to act to level set prior anthropogenic harm to our planet is now (Haines and Ebi, 2019, Solomon et al., 2019). At a time when our nation has exited the Paris Climate Accord and the current administration has already repealed 100 environmental laws and standards (Mccarthy and Bernstein, 2019, Popovich et al., 2019), ignoring the contribution of climate change to the most common malignancy affecting humans is unconscionable, and this author urges that proper consideration be given to the effects of climate change on human health in general and skin cancer specifically.
Consequently, this topic deserves greater acknowledgement and legitimacy with respect to research focus and funding, public health awareness, environmental advocacy, rapid and widespread implementation of fossil-fuel alternatives, more stringent industrial emission standards, meaningful federal and international policy directives, and engagement of key stakeholders in nongovernmental agencies, health care, industry, technology, and government to identify sustainable and cost-effective co-benefits. The evidence for overall harm to human health as a direct result of climate change is overwhelming, and global action to mitigate the negative impacts to humans and the environment is imperative.
Financial disclosures
None.
Funding
None.
Study approval
N/A.
References
- Andersen S.O., Halberstadt M.L., Borgford-Parnell N. Stratospheric ozone, global warming, and the principle of unintended consequences—An ongoing science and policy success story. J Air Waste Manage Assoc. 2013;63(6):607–647. doi: 10.1080/10962247.2013.791349. [DOI] [PubMed] [Google Scholar]
- Anderson J.G., Clapp C.E. Coupling free radical catalysis, climate change, and human health. Phys Chem Chem Phys. 2018;20(16):10569–10587. doi: 10.1039/c7cp08331a. [DOI] [PubMed] [Google Scholar]
- Andrady A., Aucamp P.J., Austin A.T., Bais A.F., Ballaré C.L., Barnes P.W. Environmental effects of ozone depletion and its interactions with climate change: progress report, 2016. Photochem Photobiol Sci. 2017;16(2):107–145. doi: 10.1039/c7pp90001e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apalla Z., Lallas A., Sotiriou E., Lazaridou E., Ioannides D. Epidemiological trends in skin cancer What does the future hold. Dermatol Pract Concept. 2017;7(2):1–6. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Institute of Health and Welfare. Skin cancer in Australia [Internet]. 2016 [cited 2020 August 30]. Available from: https://www.aihw.gov.au/reports/cancer/skin-cancer-in-australia/contents/table-of-contents.
- Bain J.A., Rusch H.P., Kline M.S. The effect of temperature upon ultraviolet carcinogenesis with wave lengths 2800–3400 Å. Cancer Res. 1943;3(9):610–612. [Google Scholar]
- Bais F., Lucas R.M., Bornman J.F., Williamson C.E., Sulzberger B., Austin A.T. Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017. Photochem Photobiol Sci. 2018;17(2):127–179. doi: 10.1039/c7pp90043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan K., Brauer M., Chen G., Chow J. International Agency for Research on Cancer Monographs; Lyon, France: 2015. Outdoor air pollution; Vol. 109. [Google Scholar]
- Ball W.T., Alsing J., Mortlock D.J., Staehelin J., Haigh J.D., Peter T. Evidence for a continuous decline in lower stratospheric ozone offsetting ozone layer recovery. Atmos Chem Phys. 2018;18(2):1379–1394. [Google Scholar]
- Bélanger M., Gray-Donald K., O’Loughlin J., Paradis G., Hanley J. Influence of weather conditions and season on physical activity in adolescents. Ann Epidemiol. 2009;19(3):180–186. doi: 10.1016/j.annepidem.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Bernstein A.S., Rice M.B. Lungs in warming world: climate change and respiratory health. Chest. 2013;143(5):1455–1459. doi: 10.1378/chest.12-2384. [DOI] [PubMed] [Google Scholar]
- De Blois J., Kjellstrom T., Agewall S., Ezekowitz J.A., Armstrong P.W., Atar D. The effects of climate change on cardiac health. Cardiology. 2015;131(4):209–217. doi: 10.1159/000398787. [DOI] [PubMed] [Google Scholar]
- Boeglin M.L., Wessels D., Henshel D. An investigation of the relationship between air emissions of volatile organic compounds and the incidence of cancer in Indiana counties. Environ Res. 2006;100(2):242–254. doi: 10.1016/j.envres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Boffetta P., Jourenkova N., Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8(3):444–472. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- Borg M., Bi P., Nitschke M., Williams S., McDonald S. The impact of daily temperature on renal disease incidence: an ecological study. Environ Heal A Glob Access Sci Source. 2017;16(1):1–30. doi: 10.1186/s12940-017-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bournay E. GRID-A: ozone depletion and climate change [Internet]. 2007 [cited 2020 August 30]. Available from: https://grid-arendal.herokuapp.com/resources/7510.
- Brauer M., Freedman G., Frostad J., Donkelaar A Van, Martin R.V., Dentener F. Ambient air pollution exposure estimation for the global burden of disease 2013. Environ Sci Technol. 2016;50:79–88. doi: 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- Burke K.E. Mechanisms of aging and development—A new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech Ageing Dev. 2018;172:123–130. doi: 10.1016/j.mad.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Calapre L., Gray E.S., Kurdykowski S., David A., Hart P., Descargues P. Heat-mediated reduction of apoptosis in UVB-damaged keratinocytes in vitro and in human skin ex vivo. BMC Dermatol. 2016;16(1):1–11. doi: 10.1186/s12895-016-0043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calapre L., Gray E.S., Ziman M. Heat stress: a risk factor for skin carcinogenesis. Cancer Lett. 2013;337(1):35–40. doi: 10.1016/j.canlet.2013.05.039. [DOI] [PubMed] [Google Scholar]
- Climate and Clean Air Coalition. Time to act to reduce short-lived climate pollutants [Internet]. 2014 [cited 2020 August 30]. Available from: https://www.ccacoalition.org/es/resources/time-act-reduce-short-lived-climate-pollutants#:∼:text=Time%20to%20Act%2C%20released%20in,tons%20of %20crops%20every%20year.
- Chen K., Breitner S., Wolf K., Hampel R., Meisinger C., Heier M. Temporal variations in the triggering of myocardial infarction by air temperature in Augsburg, Germany, 1987–2014. Eur Heart J. 2019;40:1600–1608. doi: 10.1093/eurheartj/ehz116. [DOI] [PubMed] [Google Scholar]
- Chen S.T., Geller A.C., Tsao H. Update on the epidemiology of melanoma. Curr Dermatol Rep. 2013;2(1):24–34. doi: 10.1007/s13671-012-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Orazio J., Jarrett S., Amaro-Ortiz A., Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datzmann T., Markevych I., Trautmann F., Heinrich J., Schmitt J., Tesch F. Outdoor air pollution, green space, and cancer incidence in Saxony: a semi-individual cohort study. BMC Public Health. 2018;18(1):1–10. doi: 10.1186/s12889-018-5615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Q., Dai L., Wang Y., Zanobetti A., Choirat C., Schwartz J.D. Association of short-term exposure to air pollution with mortality in older adults. JAMA. 2017;318(24):2446–2456. doi: 10.1001/jama.2017.17923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffey B.L., Gibson C.J., Haylock R., Mckinlay A.F. Outdoor ultraviolet exposure of children and adolescents. Br J Dermatol. 1996;134(6):1030–1034. [PubMed] [Google Scholar]
- Dobbinson S., Wakefield M., Hill D., Girgis A., Aitken J., Beckmann K. Prevalence and determinants of Australian adolescents’ and adults’ weekend sun protection and sunburn, summer 2003–2004. J Am Acad Dermatol. 2008;59(4):602–614. doi: 10.1016/j.jaad.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Dugo M.A., Han F., Tchounwou P.B. Persistent polar depletion of stratospheric ozone and emergent mechanisms of ultraviolet radiation-mediated health dysregulation. Rev Environ Health. 2012;27(2–3):103–116. doi: 10.1515/reveh-2012-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunk J.H., Jones D.S., Capon A., Anderson W.H. Human health on an ailing planet—historical perspectives on our future. NEJM. 2019;381(8):778–782. doi: 10.1056/NEJMms1907455. [DOI] [PubMed] [Google Scholar]
- Elnabawi M.H., Hamza N. Behavioural perspectives of outdoor thermal comfort in urban areas: a critical review. Atmosphere (Basel) 2019;11(1):51. [Google Scholar]
- Environmental Effects Assessment Panel UNEP . United Nations Environment Programme; Nairobi, Kenya: 2019. Environmental effects and interactions of stratospheric ozone depletion, UV radiation, and climate change; 2018 assessment report. [Google Scholar]
- Erdmann F., Lortet-Tieulent J., Schüz J., Zeeb H., Greinert R., Breitbart E.W. International trends in the incidence of malignant melanoma 1953–2008-are recent generations at higher or lower risk? Int J Cancer. 2013;132(2):385–400. doi: 10.1002/ijc.27616. [DOI] [PubMed] [Google Scholar]
- España A. Skin cancer in organ transplant recipients. Skin Cancer. 2004;19(3):115–136. [Google Scholar]
- Espina C., Straif K., Friis S., Kogevinas M., Saracci R., Vainio H. European Code against cancer 4th edition: environment, occupation and cancer. Cancer Epidemiol. 2015;39:S84–S92. doi: 10.1016/j.canep.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Fabbrocini G., Triassi M., Mauriello M.C., Torre G., Annunziata M.C., de Vita V. Epidemiology of skin cancer: role of some environmental factors. Cancers (Basel) 2010;2(4):1980–1989. doi: 10.3390/cancers2041980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farman J.C., Gardiner B.G., Shanklin J.D. Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature. 1985;315(6016):207–210. [Google Scholar]
- Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- Forbes P.D. Photocarcinogenesis: an overview. J Invest Dermatol. 1981;77(1):139–143. doi: 10.1111/1523-1747.ep12479351. [DOI] [PubMed] [Google Scholar]
- Freeman R.G., Knox J.M. Influence of temperature on ultraviolet injury. Arch Dermatol. 1964;89(6):858. doi: 10.1001/archderm.1964.01590300086023. [DOI] [PubMed] [Google Scholar]
- Friel S., Hancock T., Kjellstrom T., Mcgranahan G., Monge P., Roy J. Urban health inequities and the added pressure of climate change: an action-oriented research agenda. J Urban Health. 2011;88(5):886–895. doi: 10.1007/s11524-011-9607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S.A., Kampp J. Skin cancer epidemiology, detection, and management. Med Clin North Am. 2015;99(6):1323–1335. doi: 10.1016/j.mcna.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Garbe C., Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27(1):3–9. doi: 10.1016/j.clindermatol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Garrett G., Lowenstein S., Singer J., He S., Arron S. Trends of skin cancer mortality after transplantation in the United States: 1987 to 2013. J Am Acad Dermatol. 2016;75(1):106–112. doi: 10.1016/j.jaad.2016.02.1155. [DOI] [PubMed] [Google Scholar]
- El Ghissassi F., Baan R., Straif K., Grosse Y., Secretan B., Bouvard V. A review of human carcinogens–part D: radiation. Lancet Oncol. 2009;10(8):751–752. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- Gilchrest B.A., Eller M.S., Geller A.C., Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- Godar D.E. Worldwide increasing incidences of cutaneous malignant melanoma. J Skin Cancer. 2011;2011:1–6. doi: 10.1155/2011/858425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W.B. Air pollution in relation to U.S. cancer mortality rates: an ecological study; likely role of carbonaceous aerosols and polycyclic aromatic hydrocarbons. Anticancer Res. 2009;29(9):3537–3545. [PubMed] [Google Scholar]
- Haines A., Ebi K. The imperative for climate action to protect health. NEJM. 2019;380(3):263–273. doi: 10.1056/NEJMra1807873. [DOI] [PubMed] [Google Scholar]
- Haluza D., Simic S., Moshammer H. Temporal and spatial melanoma trends in Austria: an ecological study. Int J Environ Res Public Health. 2014;11(1):734–748. doi: 10.3390/ijerph110100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harari Arjona R., Piñeiros J., Ayabaca M., Harari Freire F. Climate change and agricultural workers’ health in Ecuador: occupational exposure to UV radiation and hot environments. Ann Ist Super Sanita. 2011;52(3):368–373. doi: 10.4415/ANN_16_03_08. [DOI] [PubMed] [Google Scholar]
- Hart P.H., Norval M. Ultraviolet radiation-induced immunosuppression and its relevance for skin carcinogenesis. Photochem Photobiol Sci. 2018;17(12):1872–1884. doi: 10.1039/c7pp00312a. [DOI] [PubMed] [Google Scholar]
- Hossaini R., Chipperfield M.P., Montzka S.A., Leeson A.A., Dhomse S.S., Pyle J.A. The increasing threat to stratospheric ozone from dichloromethane. Nat Commun. 2017;8:15962. doi: 10.1038/ncomms15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff J.E., Haseman J.K., DeMarini D.M., Eustis S., Maronpot R.R., Peters A.C. Multiple-site carcinogenicity of benzene in fischer 344 rats and B6C3F 1 mice. Environ Health Perspect. 1989;82:125. doi: 10.1289/ehp.8982125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Association of Nordic Cancer Registries [Internet]. 2020 [cited 2020 August 30]. Available from: https://nordcan.iarc.fr/en.
- International Agency for Research on Cancer. Benzene, Vol. 120: IARC monographs on the evaluation of carcinogenic risks to humans. Geneva, Switzerland: World Health Organization Press; 2018.
- Guy G.P., Jr, Machlin S.R., Ekwueme D.U., Yabroff K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med. 2015;48(2):183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimkhani C., Green A.C., Nijsten T., Weinstock M.A., Dellavalle R.P., Naghavi M. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol. 2017;177(1):134–140. doi: 10.1111/bjd.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.E., Cho D., Park H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016;152:126–134. doi: 10.1016/j.lfs.2016.03.039. [DOI] [PubMed] [Google Scholar]
- Koohgoli R., Hudson L., Naidoo K., Wilkinson S., Chavan B., Birch-Machin M.A. Bad air gets under your skin. Exp Dermatol. 2017;26(5):384–387. doi: 10.1111/exd.13257. [DOI] [PubMed] [Google Scholar]
- Lelieveld J., Evans J.S., Fnais M., Giannadaki D., Pozzer A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–371. doi: 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Lemus-Deschamps L., Makin J.K. Fifty years of changes in UV index and implications for skin cancer in Australia. Int J Biometeorol. 2012;56(4):727–735. doi: 10.1007/s00484-011-0474-x. [DOI] [PubMed] [Google Scholar]
- Van Der Leun J.C., de Gruijl F.R. Climate change and skin cancer. Photochem Photobiol Sci. 2002;1(5):324–326. doi: 10.1039/b201025a. [DOI] [PubMed] [Google Scholar]
- Liu J., Chen Y., Cao H., Zhang A. Burden of typical diseases attributed to the sources of PM2.5-bound toxic metals in Beijing: an integrated approach to source apportionment and QALYs. Environ Int. 2019;131:105041. doi: 10.1016/j.envint.2019.105041. [DOI] [PubMed] [Google Scholar]
- Lomas A., Leonardi-Bee J., Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- López Figueroa F. Climate change and the thinning of the ozone layer: implications for dermatology. Actas Dermosifiliogr. 2011;102(5):311–315. doi: 10.1016/j.ad.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Lucas R. Solar ultraviolet radiation: Assessing the environmental burden of disease at national and local levels. Prüss-Ustün A and Perkins van Deventer E, eds. Geneva, World Health Organization, 2010 (Environmental Burden of Disease Series, No. 17). [Internet]. [cited 2020 August 30]. Available from: https://www.who.int/quantifying_ehimpacts/publications/UV.pdf.
- Lucas R.M., Yazar S., Young A.R., Norval M., De Gruijl F.R., Takizawa Y. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem Photobiol Sci. 2019;18(3):641–680. doi: 10.1039/c8pp90060d. [DOI] [PubMed] [Google Scholar]
- Makin J. Implications of climate change for skin cancer prevention in Australia. Heal Promot J Aust. 2011;22(4):39–41. doi: 10.1071/he11439. [DOI] [PubMed] [Google Scholar]
- Martens M.C., Seebode C., Lehmann J., Emmert S. Photocarcinogenesis and skin cancer prevention strategies: an update. Anticancer Res. 2018;38(2):1153–1158. doi: 10.21873/anticanres.12334. [DOI] [PubMed] [Google Scholar]
- Matthews N.H., Li W.Q., Qureshi A.A., Weinstock M.A., Cho E. Codon Publications; Brisbane, Australia: 2017. Cutaneous melanoma: etiology and therapy. [Google Scholar]
- Mccarthy G., Bernstein A. Combating EPA rollbacks—health care’s response to a retreat on climate. NEJM. 2019;81(8):696–698. doi: 10.1056/NEJMp1909643. [DOI] [PubMed] [Google Scholar]
- Mehlman M.A. Causal relationship from exposure to chemicals in oil refining and chemical industries and malignant melanoma. Ann N Y Acad Sci. 2006;1076:822–828. doi: 10.1196/annals.1371.005. [DOI] [PubMed] [Google Scholar]
- Melnikova VO, Ananthaswamy HN. Cellular and molecular events leading to the development of skin cancer. Mutat Res 2005;571(1-2 SPEC. ISS.):91–106. [DOI] [PubMed]
- Miller D.L., Weinstock M.A. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30(5):774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- Mittal A., Colegio O. Skin cancers in organ transplant recipients. Am J Transplant. 2017;17(10):2509–2530. doi: 10.1111/ajt.14382. [DOI] [PubMed] [Google Scholar]
- Moan J., Grigalavicius M., Baturaite Z., Dahlback A., Juzeniene A. The relationship between UV exposure and incidence of skin cancer. Photodermatol Photoimmunol Photomed. 2015;31(1):26–35. doi: 10.1111/phpp.12139. [DOI] [PubMed] [Google Scholar]
- Molina M.J., Rowland F.S. Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone. Nature. 1974;249(5460):810–812. [Google Scholar]
- Montero-Montoya R., López-Vargas R., Arellano-Aguilar O. Volatile organic compounds in air: sources, distribution, exposure and associated illnesses in children. Ann Glob Heal. 2018;84(2):225–238. doi: 10.29024/aogh.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montzka S.A., Dutton G.S., Yu P., Ray E., Robert W., Daniel J.S. An unexpected and persistent increase in global emissions of ozone-depleting CFC-11. Nature. 2018;557:413–417. doi: 10.1038/s41586-018-0106-2. [DOI] [PubMed] [Google Scholar]
- Narayanan D.L., Saladi R.N., Fox J.L. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49(9):978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- National Aeronautics and Space Administration. Earth observatory [Internet]. 2020a [cited 2020 August 4]. Available from: https://earthobservatory.nasa.gov/world-of-change/global-temperatures.
- National Aeronautics and Space Administration. NASA ozone watch [Internet]. 2020b [cited 2020 April 4]. Available from: https://ozonewatch.gsfc.nasa.gov/facts/history_SH.html.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program [Internet]. 2020 [cited 2020 August 30]. Available from: https://seer.cancer.gov/statfacts/html/melan.html.
- Newman P.A., McKenzie R. UV impacts avoided by the Montreal Protocol. Photochem Photobiol Sci. 2011;10(7):1152–1160. doi: 10.1039/c0pp00387e. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nakagoshi A., Tsurukawa M., Matsumura C., Eiho J., Nakano T. Environmental risk assessment and concentration trend of atmospheric volatile organic compounds in Hyogo Prefecture, Japan. Environ Sci Pollut Res. 2012;19(1):201–213. doi: 10.1007/s11356-011-0550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrado C., Mercado-Saenz S., Perez-Davo A., Gilaberte Y., Gonzalez S., Juarranz A. Environmental stressors on skin aging. Mechanistic insights. Front Pharmacol. 2019;10:1–17. doi: 10.3389/fphar.2019.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera E., Gnaneswaran N., Staines C., Win A.K., Sinclair R. Incidence and prevalence of non-melanoma skin cancer in Australia: a systematic review. Australas J Dermatol. 2015;56(4):258–267. doi: 10.1111/ajd.12282. [DOI] [PubMed] [Google Scholar]
- Petersen B., Philipsen P.A., Wulf H.C. Skin temperature during sunbathing-relevance for skin cancer. Photochem Photobiol Sci. 2014;13(8):1123–1125. doi: 10.1039/c4pp00066h. [DOI] [PubMed] [Google Scholar]
- Pfeifer G.P., Besaratinia A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem Photobiol Sci. 2012;11(1):90–97. doi: 10.1039/c1pp05144j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich N, Albeck-Ripka L, Pierre-Louis K. 100 environmental rules being rolled back under Trump. The New York Times [Internet]. 2020 [cited 2020 August 30]. Available from: https://nyti.ms/2uY0GQa.
- Puntoni R., Ceppi M., Gennaro V., Ugolini D., Puntoni M., Manna G. La. Occupational exposure to carbon black and risk of cancer. Cancer Causes Control. 2004;15:511–516. doi: 10.1023/B:CACO.0000036446.29787.94. [DOI] [PubMed] [Google Scholar]
- Răducu L., Avino A., Purtan R.P., Balcangiu-Stroescu A.E., Balăn D.G., Timofte D. Quality of life in patients with surgically removed skin tumors. Medicina (Kaunas) 2020;56(2):3–9. doi: 10.3390/medicina56020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H.W., Weinstock M.A., Feldman S.R., Coldiron B.M. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- Rogers H.W., Weinstock M.A., Harris A.R., Hinckley M.R., Feldman S.R., Fleischer A.B. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- Sampogna F., Paradisi A., Iemboli M.L., Ricci F., Sonego G., Abeni D. Comparison of quality of life between melanoma and non-melanoma skin cancer patients. Eur J Dermatol. 2019;29(2):185–191. doi: 10.1684/ejd.2019.3523. [DOI] [PubMed] [Google Scholar]
- Schraufnagel D.E., Balmes J.R., Cowl C.T., De Matteis S., Jung S.H., Mortimer K. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, Part 1: The damaging effects of air pollution. Chest. 2019;155(2):409–416. doi: 10.1016/j.chest.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufnagel D.E., Balmes J.R., Cowl C.T., De Matteis S., Jung S.H., Mortimer K. Air pollution and noncommunicable diseases: a review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air pollution and organ systems. Chest. 2019;155(2):417–426. doi: 10.1016/j.chest.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte P.A., Bhattacharya A., Butler C.R., Chun H.K., Jacklitsch B., Jacobs T. Advancing the framework for considering the effects of climate change on worker safety and health. J Occup Environ Hyg. 2016;13(11):847–865. doi: 10.1080/15459624.2016.1179388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotto J, Fears TR, Fraumeni JF. Incidence of nonmelanoma skin cancer in the United States. 1983 [cited 2020 August 30]. Available from: http://www.ciesin.org/docs/001-526/001-526.html.
- Siddens L.K., Larkin A., Krueger S.K., Bradfield C.A., Waters K.M., Tilton S.C. Polycyclic aromatic hydrocarbons as skin carcinogens: comparison of benzo[a]pyrene, dibenzo[def, p] chrysene and three environmental mixtures in the FVB/N mouse. Toxicol Appl Pharmacol. 2012;264(3):377–386. doi: 10.1016/j.taap.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaper H., Velders G.J.M., Daniel J.S., De Gruijl F.R., Van der Leun J.C. Estimates of ozone depletion and skin cancer incidence to examine the Vienna Convention achievements. Nature. 1996;384(6606):256–258. doi: 10.1038/384256a0. [DOI] [PubMed] [Google Scholar]
- Solomon C.G., Larocque R.C., Division B. Climate change—A health emergency. NELM. 2019;380(3):209–211. doi: 10.1056/NEJMp1817067. [DOI] [PubMed] [Google Scholar]
- Stenehjem J.S., Robsahm T.E., Bråtveit M., Samuelsen S.O., Kirkeleit J., Grimsrud T.K. Aromatic hydrocarbons and risk of skin cancer by anatomical site in 25 000 male offshore petroleum workers. Am J Ind Med. 2017;60:679–688. doi: 10.1002/ajim.22741. [DOI] [PubMed] [Google Scholar]
- The World Bank. Poverty [Internet]. 2020 [cited 2020 August 30]. Available from: https://www.worldbank.org/en/topic/poverty/overview.
- Tomson C., Connor A. Outlook: implications of climate change for nephrology. Nat Rev Nephrol. 2015;11(1):8–9. doi: 10.1038/nrneph.2014.199. [DOI] [PubMed] [Google Scholar]
- United Nations Environment Programme. Ozone: 20 questions and answers [Internet]. 2018 [cited 2020 May 4]. Available from: https://ozone.unep.org/20-questions-and-answers.
- U.S. Environmental Protection Agency. Health and environmental effects of ozone layer depletion [Internet]. 2020 [cited 2020 August 30]. Available from: https://www.epa.gov/ozone-layer-protection/health-and-environmental-effects-ozone-layer-depletion.
- van Dijk A., Slaper H., Den Outer P.N., Morgenstern O., Braesicke P., Pyle J.A. Skin cancer risks avoided by the Montreal protocol—Worldwide modeling integrating coupled climate-chemistry models with a risk model for UV. Photochem Photobiol. 2013;89(1):234–246. doi: 10.1111/j.1751-1097.2012.01223.x. [DOI] [PubMed] [Google Scholar]
- Vierko A., Schikowski T., Ranft U., Sugiri D., Matsui M., Kra U. Airborne particle exposure and extrinsic skin aging. J Invest Dermatol. 2010;130:2719–2726. doi: 10.1038/jid.2010.204. [DOI] [PubMed] [Google Scholar]
- Vogeley C., Esser C., Tüting T., Krutmann J., Haarmann-Stemmann T. Role of the aryl hydrocarbon receptor in environmentally induced skin aging and skin carcinogenesis. Int J Mol Sci. 2019;20(23):6005. doi: 10.3390/ijms20236005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Tang C., Wang C., Huang S., Sue Y. Risk of skin cancer in patients on chronic haemodialysis: a nationwide, population-based study in Taiwan. Br J Dermatol. 2016;175:1136–1137. doi: 10.1111/bjd.14789. [DOI] [PubMed] [Google Scholar]
- Watson M., Garnett E., Guy G.P., Holman D.M. The Surgeon General’s call to action to prevent skin cancer. Skin Cancer Prev. 2014:7–152. [Google Scholar]
- World Health Organization. Air pollution [Internet]. 2020a [cited 2020 May 17]. Available from: http://www.who.int/airpollution/en/.
- World Health Organization. Ambient (outdoor) air pollution [Internet]. 2020b [cited 2020 August 30]. Available from: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health.
- Wilmer J.L., Simeonova P.P., Germolec D.R., Luster M.I. Benzene and its principal metabolites modulate proinflammatory cytokines and growth factors in human epidermal keratinocyte cultures. Vitr Toxicol. 1997;10(4):429–436. [PMC free article] [PubMed] [Google Scholar]
- World Meteorological Organization . WMO; Geneva, Switzerland: 2017. WMO reactive gases bulletin: highlights from the Global Atmospheric Watch Programme. [Google Scholar]
- World Meteorological Organization, United Nations Environment Programme. Global warming of 1.5°C. An IPCC special report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change [Internet]. 2018 [cited 2020 August 30]. Available from: https://www.ipcc.ch/sr15/.
- World Meteorological Organization, United Nations Environment Programme, National Oceanic and Atmospheric Administration, National Aeronautics and Space Administration, European Commission. Scientific assessment of ozone depletion: 2018, World Meteorological Organisation global ozone research and monitoring project-Report No. 58 [Internet]. 2019 [cited 2020 August 30]. Available from: http://ozone.unep.org/science/assessment/sap.
- Xiang F., Lucas R., Hales S., Neale R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978–2012 empirical relationships. JAMA Dermatol. 2014;150(10):1063–1071. doi: 10.1001/jamadermatol.2014.762. [DOI] [PubMed] [Google Scholar]