Abstract
Sunscreens are topical preparations containing any number of ultraviolet filters (UVFs). The first part of the review will focus on the recent Food and Drug Administration (FDA) regulations of 2019 and general use of these agents. While sunscreen products are becoming more regulated in the United States, we still lag behind other countries in our options for UVFs. Sun protection to prevent skin cancer and aging changes should be a combination of sun avoidance, protective structures, and clothing as well as use of sunscreen products. Newer and safer products are needed to help supplement and replace older agents as well as improve their cosmetic acceptability. This will be a review of ingredients, local toxicities (i.e. contact dermatitis, photocontact dermatitis), special considerations for children, and cosmesis of sunscreen preparations. Part 2 will focus on the environmental, ecological and human toxicities that have been increasingly related to UVFs.
Abbreviations: 4-MBC, 4-methylbenzylidene camphor; AAD, American Academy of Dermatology; BP-3, Benzophenone-3; CDER, Center for Drug Evaluation and Research (part of FDA); EPA, Environmental Protection Agency; FDA, Food and Drug Administration; GRASE, Generally Recognized As Safe and Effective; NHANES, National Health and Nutrition Examination Survey; OCTO, Octocrylene; OMC, Octyl methoxycinnamate; OTC, Over-the-counter; PABA, Para-aminobenzoic acid; PPCP, Pharmaceuticals and personal care products; PCPC, Personal care products and cosmetics; UV, Ultraviolet; UVF, Ultraviolet filter; WWTP, Wastewater treatment plant; NDA, New drug application; NLM, National Library of Medicine; TiO2, Titanium dioxide; NanoTiO2, Nanoparticle titanium dioxide
Keywords: Sunscreen, Ultraviolet filter (UVF), Generally Recognized As Safe And Effective (GRASE), Skin cancer, Sun protection factor (SPF), Ultraviolet protection
Key Points.
-
•
Sunscreen regulation is evolving since the 2019 FDA Proposed Rule but many UV filters are not allowed in the US.
-
•
UV filters are treated as drugs in the US but as cosmetics in other parts of the world, resulting in longer pathways to approval for use.
-
•
Practicing ‘Safe Sun’ is an important platform that the AAD promotes and there are many options available that can be cosmetically as well as clinically acceptable for women and children. Special care in small children and infants is important.
Introduction
In February 2019, the U.S. Food and Drug Administration (FDA) issued a proposed rule (Federal Register 84FR6204, 2019-03019) to update the regulatory requirements for non-prescription, over-the-counter (OTC) sunscreens to ensure their safety, efficacy, and consistency in labeling. While there has long been a need for this action (Food and Drug Administration, 1978, Wang et al., 2011) and the intent is to educate/benefit consumers, enactment of the rule may have unintentionally added to the confusion and skepticism of ultraviolet filters (UVFs) that has also been triggered by concerns/local legislative bans of some UVFs.
This has become a hot topic in dermatology practices across the nation with numerous commentaries published in the last 10 years (Wang et al., 2011, Erickson, 2018, see also AAD.ORG Sunscreen FAQ 2019 and Talking points: JAMA study on absorption of sunscreen ingredients 2020). As dermatologists, we are responsible for educating our patients and their families, our colleagues, public health officials, community leaders, and legislators regarding the health benefits and risks of sunlight exposure, as well as the health benefits and risks of sunscreens.
In this review we cover this recent FDA announcement, the wide range of UVF in sunscreen products and cosmetics, and variations on different continents, how to safely and properly use/choose a sunscreen product in the overall scheme of UV protection, and sun safety practices in children and adults. In part 2, we will address concerns of products that we are recommending to protect our patients and their families from skin cancer by reviewing reported types of human and ecological toxicities of UVFs once they are applied and subsequently enter the environment.
We hope this overview will be a useful reference and help to clarify many of the issues we face as dermatologists trying to do the best job of advising and protecting our patients under the Hippocratic Mantra of ‘Do No Harm’.
Definitions
It is essential that readers use the terms correctly, rather than colloquially. For example, titanium dioxide (TiO2) is not a sunscreen. It is a UV filter that is included in many commercial products known as sunscreens.
Sunscreen: the commercial product sold to consumers for protection of human skin from ultraviolet radiation. Sunscreens contain one or more UVFs that may be physical, chemical, or both. In addition, they contain many other substances, such as emollients, preservatives or stabilizers, emulsifiers, fragrances, and coloring compounds. Broad spectrum sunscreens are defined by the FDA as products that provide UVA protection that is proportional to its UVB protection (FDA-US, 2017).
According to the FDA, “a product that includes the term “sunscreen” in its labeling or in any other way represents or suggests that it is intended to prevent, cure, treat, or mitigate disease or to affect a structure or function of the body comes within the definition of a drug in section 201(g)(1) of the act. Sunscreen active ingredients affect the structure or function of the body by absorbing, reflecting, or scattering the harmful, burning rays of the sun, thereby altering the normal physiological response to solar radiation. These ingredients also help to prevent diseases such as sunburn and may reduce the chance of premature skin aging, skin cancer, and other harmful effects due to the sun when used in conjunction with limiting sun exposure and wearing protective clothing” (https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=700.35).
UV filter: a specific compound that impedes the passage of ultraviolet light. Dermatologists typically divide these into chemical (absorbing UV rays and converting to thermal energy) vs. physical agents (reflecting UV rays). Environmental chemists categorize them in several ways, for example, organic vs. inorganic, lipophilic vs. hydrophilic. The National Library of Medicine databases call these compounds, sunscreening agents, and define them as chemical or physical agents that protect the skin from sunburn and erythema by absorbing or blocking ultraviolet radiation. UVFs are also used in consumer cosmetics (makeup, nail polish, shampoo, etc.) and industry (plastics, paints, sealants, etc.) to protect against photodegradation.
Environment: the surroundings or conditions in which a person, animal, or plant lives or operates.
Ecosystem: the interactions between the environment and the organisms that dwell within it. It is likened to a community that functions as one unit.
GRASE: defined by the FDA OTC Glossary (https://www.accessdata.fda.gov/scripts/cder/training/otc/topic3/images/Glossary.pdf)
“A drug is not considered a new drug only when it is generally recognized as safe and effective (GRASE). In order to conclude a GRASE determination, a drug must satisfy three criteria: 1. The particular drug product must have been subjected to adequate and well-controlled clinical investigations that establish the product as safe and effective. 2. Those investigations must have been published in the scientific literature available to qualified experts. 3. Experts must generally agree, based on those published studies, that the product is safe and effective for its intended uses. At a minimum, the general acceptance of a product as GRASE must be supported by the same quality and quantity of scientific and/or clinical data necessary to support the approval of a New Drug Application.”
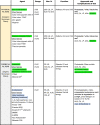
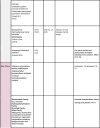
Few UVFs used in FDA-approved sunscreen products are considered GRASE but are sold under the definition of a ‘Marketed Unapproved Drugs’ as they have been in use for a long time, but may be lacking the rigorous testing described in the OTC Glossary definition (Table 1) (FDA-US, 2006).
Table 1.
UV filters in use worldwide.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Sources: BASF Sunscreen Simulator- https://www.sunscreensimulator.basf.com/Sunscreen_Simulator/login/register, The Skin Cancer Foundation https://www.skincancer.org/skin-cancer-prevention/sun-protection/sunscreen/, in part from the FDA Fact Sheet on sunscreen issued in February of 2019 and from Federal Register FDA Proposed Rule February 2019 https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective.

-
1.Janjua N, Mogensen B, Andersson AM, Petersen J, Henriksen M, Skakkebaek N, et al. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol 2004;123:57–61.
-
2.Janjua NR, Kongshoj B, Petersen JH, Wulf HC. Sunscreens and thyroid function in humans after short-term whole-body topical application: a single-blinded study, Brit J Dermatol 2007;156:1080–1082.
-
3.Sarveiya V, Risk S, Benson HA. Liquid chromatographic assay for common sunscreen agents: application to in vivo assessment of skin penetration and systemic absorption in human volunteers. J Chromatogr B Analyt Technol Biomed Life Sci 2004;803(2):225–31.
-
4.Gonzalez H, Farbrot A, Larkö O, Wennberg AM. Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation. Br J Dermatol. 2006;154(2):337–40.
-
5.Rodríguez E, Valbuena MC, Rey M, Porras de Quintana L.Causal agents of photoallergic contact dermatitis diagnosed in the national institute of dermatology of Colombia. Photodermatol Photoimmunol Photomed. 2006;22(4):189–92.
-
6.Krause M, Klit A, Blomberg Jensen M, Søeborg T, Frederiksen H, Schlumpf M, Lichtensteiger W, Skakkebaek NE, Drzewiecki KT. Sunscreens: Are They Beneficial for Health? An Overview of Endocrine Disrupting Properties of UV-Filters. Int J Androl. 2012;35(3):424–36. doi: https://doi.org//10.1111/j.1365–2605.2012.01280.x.
-
7.Ghazipura M, McGowan R, Arslan A, Hossain T. Exposure to benzophenone-3 and reproductive toxicity: A systematic review of human and animal studies. Reprod Toxicol. 2017;73:175–183. doi: https://doi.org//10.1016/j.reprotox.2017.08.015. Epub 2017 Aug 24.
-
8.Klinubol P, Asawanonda P, Wanichwecharungruang SP.Transdermal penetration of UV filters. Skin Pharmacol Physiol. 2008;21(1):23–9. Epub 2007 Oct 2.
-
9.Europa, SCCNFP, Opinion on Homosalate. 2007 https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_097.pdf.
-
10.Walters KA, Brain KR, Howes D, James VJ, Kraus AL, Teetsel NM, Toulon M, Watkinson AC, Gettings SD. Percutaneous penetration of octyl salicylate from representative sunscreen formulations through human skin in vitro. Food Chem Toxicol. 1997;35(12):1219–25.
-
11.Shaw DW. Allergic Contact Dermatitis from Octisalate and cis-3-Hexenyl Salicylate. Dermatitis, 2006; 17(3):152–5.
-
12.Singh M, Beck MH, Octyl Salicylate: A New Contact Sensitivity. Contact Dermatitis, 2007; 56(1):48.
-
13.Bryden AM, Moseley H, Ibbotson SH, Chowdhury MM, Beck MH, Bourke J, English J, Farr P, Foulds IS, Gawkrodger DJ, George S, Orton DI, Shaw S, McFadden J, Norris P, Podmore P, Powell S, Rhodes LE, Sansom J, Wilkinson M, van Weelden H, Ferguson JA. Photopatch Testing of 1155 Patients: Results of the U.K. Multicentre Photopatch Study Group. Brit J Dermatol 2006;155(4):737–47.
-
14.C.G.J. Hayden et al., Sunscreen Penetration of Human Skin and Related Keratinocyte Toxicity After Topical Application. Skin Pharmacol Physiol, 2005, 18(4):170–4.
-
15.Montenegro L, Carbone C, Paolino D, Drago R, Stancampiano AH, Puglisi G.n Vitro Skin Permeation of Sunscreen Agents from O/W EmulsionsInt J Cosmet Sci. 2008 Feb;30(1):57–65. doi: https://doi.org//10.1111/j.1468-2494.2008.00417.x.
-
16.Nash JF, Tanner PR. Relevance of UV Filter/Sunscreen Product Photostability to Human Safety. Photodermatol Photoimmunol Photomed. 2014 Apr-Jun;30(2–3):88–95. doi: https://doi.org//10.1111/phpp.12113. Epub 2014 Feb 19.
-
17.Knezevic NZ, Ilic N, D̵okic V, Petrovic R, Janackovic D. Mesoporous silica and organosilica nanomaterials as UV-blocking agents. ACS App Mater Interfaces 2018;10(24):20231–6. doi: https://doi.org//10.1021/acsami.8b04635.
-
18.Schlumpf M, Durrer S, Faass O, Ehnes C, Fuetsch M, Gaille C, Henseler M, Hofkamp L, Maerkel K, Reolon S, Timms B, Tresguerres JA, Lichtensteiger W. Developmental toxicity of UV filters and environmental exposure: a review. Int J Androl. 2008;31(2):144–51. doi: https://doi.org//10.1111/j.1365-2605.2007.00856.x. Epub 2008 Jan 10.
-
19.White I.R. (1995) Phototoxic and Photoallergic Reactions. In: Rycroft R.J.G., Menné T., Frosch P.J. (eds) Textbook of Contact Dermatitis. Springer, Berlin, Heidelberg.
Sunscreen ingredients and safety
In its February 2019 document, the FDA recognized 22 UVF compounds in use in sunscreen products and classified them as Generally Recognized As Safe and Effective (GRASE) (Category I), those that are Non GRASE (Category II), and those that require further evaluation (Category III). In addition, several compounds remained unclassified because they require further evaluation or ‘reserved’. Two UVF agents, titanium dioxide (TiO2) and zinc oxide (ZnO), known to consumers (and dermatologists) as mineral sunscreens or physical blockers, were designated as GRASE-Category I (Federal Register 84FR6204-6275, 2019-03019).
An additional 20 UVFs, some/many of which are approved in Europe, are listed in the proposed rule as Non GRASE and must undergo the FDA’s New Drug Application (NDA) approval process prior to marketing. Two of these 20 UVFs, aminobenzoic acid (including available variants of para-aminobenzoic acid (PABA), glyceryl-PABA, ethyl dihydroxypropyl-PABA) and trolamine salicylate were classified as Non-GRASE-Category II. Consequently, they are no longer permitted in sunscreen products sold in the U.S. PABA was found to cause endocrine abnormalities in some individuals, specifically altering thyroid activity, in addition to allergic contact dermatitis (cross-reacting with sulfonamides) and a tendency to stain clothing (Lim et al., 2004, Schauder and Ippen, 1997). Trolamine salicylate, an ingredient found in some OTC topical analgesics for relief of arthritis and muscle pain, is also a UVB UVF. However, trolamine salicylate can be absorbed across the skin into underlying tissues and systemically (Morra et al., 1996), potentially leading to salicylism (elevated serum concentrations of salicylic acid following topically applied salicylate products).
Among the “reserved” products, which remain unclassified, are lawsone + dihydroxyacetone (Henna dye skin surface oxidation product, Munt et al., 2015), DEA methoxycinnamate (a UVF used mainly in hair care products, such as shampoos and conditioners), digalloyl triolate (unapproved in the US and banned in EU) and red petrolatum (a technical grade form of petrolatum that contains intrinsic red pigments from crude oil, now used mainly in veterinary medicine). Table 1 summarizes all these UVFs along with many other compounds not in regular use for sunscreen products and highlights some associated human and environmental toxicities of these agents, which are detailed in Part 2 of this review.
In the proposed rule, the FDA has designated the remaining 12 currently marketed UVFs (avobenzone, cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octocrylene, octinoxate, octisalate, oxybenzone, padimate O, and sulisobenzone) to require further study (GRASE Category III) on their safety as topical agents (Table 1). The FDA also raised concerns over the lack of toxicity data for all UVFs in spray and powder formulations due to potential risks of inhalation and/or flammability. Another area that requires more study is the group of sunscreens that also contain insect repellents.
Another 22 agents await further FDA-regulated testing (FDA-US CDER 2016 including topical safety studies (irritation, sensitization, and photo safety), bioavailability (absorption), and evaluation of adverse events observed in clinical studies as well as reproductive and carcinogenic animal studies (Kunz et al., 2006, Weisbrod et al., 2017) to approval for use in the United States. Ecamsule (Mexoryl SX) was approved through the FDA NDA process for sale in the US as an active ingredient in La Roche-Posay products in 2016 and is not listed in the FDA monograph, therefore, no GRASE categorization was made in the proposed rule (FDA, 2008).
The availability of newer, and more effective sunscreen ingredients in the US lags behind European countries (Reisch, 2015). The FDA’s stringent approval process is slower than the European process [EU Cosmetic Regulation (EC No.1223/2009) by the Scientific Committee on Consumers’ Safety (SCCS). Per federal law, the FDA classifies new UVFs intended for use in sunscreens (i.e., not already deemed GRASE) as OTC drugs rather than cosmetics as they are treated in Europe and other parts of the world (FDA-US, 1978). Accordingly, these agents require extensive clinical data and NDA approval to be deemed safe for use in patients.
The Federal Sunscreen Innovation Act (SIA) of 2014 was created as an alternative process to review the safety and effectiveness of active ingredients used in nonprescription sunscreen products. The SIA provides strict deadlines for the FDA to take certain actions on UVFs contained in sunscreens, but it does not relax the FDA’s requirement for adequate data to determine the safety and efficacy. The SIA gave the FDA 12 months to respond to the existing backlog of UVF approval requests and then an additional 18 months to reply to any future applications. The passing of the SIA was a major step for the US to expedite the review process of many UVFs that are already in wide use in European and Asian countries, but no new filters have been added since 2014. It also requires the Secretary of Health and Human Services (HHS) to make determinations on the testing and labeling of aerosol sunscreens, as well as the question whether any sunscreens can be labeled with a sun protection factor (SPF) greater than 50 and any sunscreen products combined with insect repellants.
How to practice safe sun
According to the American Academy of Dermatology (AAD), sunscreen is a part of a safe and effective comprehensive sun protection program (AAD.ORG, 2019). The AAD recommends that individuals seek shade from 10:00am to 2:00 pm; dress to protect skin from the sun by wearing lightweight long-sleeve shirts, pants, wide brimmed hats, and sunglasses; and apply a broad spectrum sunscreen of SPF 30 or higher on areas not covered by clothing (AAD.ORG, 2019). Another component is UV protective clothing, which in recent years has improved in availability, style, comfort, and cost. Some clothing lines (eg. Solumbra, Coolibar) produce only UV protective garments, but many/most manufacturers of general clothing lines include some garments made with UV protective fabric. Fashionable options are available from such manufacturers as Patagonia, J Crew, Uniqlo, Mott 50, Solumbra, and Coolibar.
Using a water-resistant, broad-spectrum sunscreen, that are protective against both UVA and UVB rays with SPF 30 or higher, has been shown to reduce the risk of developing skin cancer, decrease the incidence (and severity) of sunburn, and prevent signs of aging (van der Pols et al., 2006; Green et al., 2011). Sun protection factor, also known as SPF is a measure of sunscreen efficacy and is primarily related to the amount of UVB protection it provides. The SPF is the multiple of times that a standardized amount of UV filter application (2 mg/cm2) increases the UV threshold to attain minimal erythema compared to unprotected skin. Therefore when using a properly applied product with SPF 30, it will take 30 times more UV exposure to burn than without sunscreen (FDA-US CDER 2017). Note it is only 30 times more time if exposure is under the exact same time and UV intensity as without the product.
Sunscreen should be applied at least 15 minutes before going outside. Most adults require one ounce (about 2 tablespoonfuls) of sunscreen to adequately cover the entire body. It is important to use a sun-protective lip balm as well. For sustained protection, sunscreen should be applied every two hours, immediately after swimming, and after excessive sweating. Few people apply sufficient sunscreen in the first place and even fewer re-apply it as often as it is recommended. Sunburns are typically a result of insufficient volume of sunscreen, infrequent reapplication, or using expired or denatured products (such as those stored in a car glovebox all summer). It is important to remind patients that UV rays are present from sunrise to sunset, even on cloudy days, and in the winter. Furthermore, UVA can penetrate window-glass in buildings or cars making sun protection a daily necessity.
When visiting their dermatologists, middle-aged and older adults commonly remark that sun protection was not mentioned when we were growing up. Nowadays, UVFs are found in many cosmetic products including facial moisturizers, eye creams, foundations, “beauty balm/blemish balm” (BB) and “color correcting/complexion corrector” (CC) creams, primers, lip products, and even hair styling products. Adding UVFs to a cosmetic product changes its status from a cosmetic alone to subject to FDA regulations for both cosmetics and drugs. While adding UVFs to cosmetic products is beneficial, it is rare for a UVF-containing cosmetic product to provide adequate protection against both UVA and UVB radiation. As a result, individuals who rely solely on their make-up foundations to provide facial sun protection are likely to be underprotected (Wadyka, 2019).
Practical issues with sunscreen use
Studies published in the Journal of the American Medical Association by Matta et al., 2019, Matta et al., 2020 measured plasma concentrations of the UVF agents applied under under maximal use conditions in an effort to meet the Time and Extent Application (TEA) process mandated in the FDA Final Rule of 2011 (Wang et al., 2011, Narla and Lim, 2020). These studies showed systemic absorption of seven commercially available UVF ingredients tested (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, octinoxate, and ecamsule), making it into headline news and social media. The authors clearly stated that their findings provide no evidence of systemic toxicity (more on this below) and should not deter the use of sunscreens. Nevertheless, patients might question ‘whether these products might cause some harm’ … perhaps even ‘more harm than good?’ Could a protective product they had applied diligently to themselves and their family members have unexpectedly detrimental effects on their health? In response, the AAD re-emphasized that sunscreens are merely one part of the overall ‘Safe Sun Program’ and that these products have been used for many decades to reduce the risk of skin cancer without any reported internal side effects (Lim et al., 2004, Schneider and Lim, 2019).
Cosmetic acceptability of sunscreen products
Some people find that facial sunscreens do not fully vanish, despite being rubbed in well, or that they leave a subtle Kabuki-like white hue to the skin. This residual pallor is often more conspicuous/apparent/noticeable in photographs than in person (Bond, 2019). Another concern is that applying make-up on top of a sunscreen leads to a “pilling” quality/phenomenon. When products with different consistencies are applied to one’s skin, it is often difficult to create a uniform, smooth surface. This can cause flaking or pilling of the more superficial products. A Google Search of terms including ‘best selling facial sunscreens on Amazon, most popular facial sunscreens and most highly recommended cosmetics containing sunscreens’ revealed popular brands Olay, EltaMD, Cerave, Neutrogena, LaRoche Posay, Purito, Sun Bum with prices ranging from $2.66 to $22.20 per ounce, (Amazon.com, 2020, Quinn, 2019, Corsillo and Morrill, 2019). For reference, a similar search of lowest cost sunscreens for children (containing only ZnO or TiO2) resulted in prices ranging from $0.61 to $2.16 per ounce.
Many facial sunscreen products are now available in tinted varieties, however not all shades and skin tones are available, which makes color matching difficult. Tinted products can adhere to fabrics and may stain clothing. But tinted sunscreens aren’t the only ones that can leave a residue behind on clothing. While both chemical and physical sunscreens can leave a residue behind, those with higher amounts of physical blockers, such as titanium dioxide and zinc oxide, are more likely to rub off on clothing and leave behind a chalk-dust-like appearance that can be removed with a variety of stain removal tricks (Kerr, 2016). An innovative product that may appeal to women who will be attending public or highly visible functions but still provide sun protection are powder brush-on sunscreen products. These are probably best used for reapplication (i.e., touch up) rather than primary UV protection, which would be provided for in the foundation. They apply easily and blend in well on top of make-up. Consumers should note that while cosmetically acceptable products may feel smoother and provide a more even skin tone, they may also have many additive ingredients that can cause irritant or allergic contact dermatitis.
Chemical sunscreens may cause both allergic and irritant contact dermatitis. Oxybenzone, a UVA chemical filter, is the most common sunscreen to cause irritant or allergic contact or photocontact dermatitis. PABA was a common allergen but is no longer found in sunscreens. Other sunscreen agents that can cause photo-allergic reactions include cinnamates, dibenzoylmethanes, and benzophenones (Lim, Thomas, Rigel Photoprotection in Photoaging, Marcel Dekker 2004), but these potential adverse effects were not included in the FDA Proposed Rule of February 2019.
Sunscreen use for infants and children
There are sunscreen products that are specifically marketed for babies but the FDA recommends not using these on infants under six months of age (FDA-US, 2019). These products tend to contain only titanium dioxide and zinc oxide as their active ingredients that work by sitting on the stratum corneum in order to scatter, reflect, and absorb UVA and UVB. As a result, it may take parents a longer period of time to evenly apply these physical blockers that can leave behind a white, pasty appearance. There are other products with some chemical components (Table 1) that help provide for a more even application but these are not approved for use in children (FDA-US, 2019). Some delivery vehicles, such as sprays and rub-on sticks, are regarded as less messy, but they are difficult to apply evenly. This leads to missed spots and an unfortunate pattern of sunburn. Sprays may also result in inhalation and eye exposure in a fidgeting child.
While in general, sunscreen is recommended for infants six months of age and older, the best practices for sun safety for infants less than six months old is to keep them in the shade and in lightweight, sun protective clothing. Their skin is immature and they have a higher surface-area to body-weight ratio, putting them at risk for greater absorption of the chemicals in sunscreens (West et al., 1981). In regards to pregnancy and breastfeeding, there are very limited data on this topic. However, chemical sunscreens can be absorbed systemically and studies have shown effects on the fetus of animal models-- covered in greater detail in Part 2 of this review. Overall, physical blockers are preferred, however in the case of breastfeeding, they should not be applied too close to the areola where they may interfere with suckling.
Summary
This review of the practical implications of recent sunscreen regulations has highlighted that, despite more regulation in the United States, there is still a lag behind other countries in our options for UVFs. Irritants, allergens, photoallergens as well as cosmesis all are points to consider when advising patients about proper sun protection. The AAD’s Practice Safe Sun Program emphasizes protection to prevent skin cancer and aging with a combination of sun avoidance, protective structures, clothing as well as regular use of sunscreen products, and newer and safer products along with special considerations for children.
Conflicts of interest
None.
Funding
None.
Study approval
N/A.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2019.104640. For patient information on skin cancer in women, please click on Supplemental Material to bring you to the Patient Page.
Contributor Information
Sultan Qiblawi, Email: qiblawis@msu.edu.
David Fivenson, Email: dfivenson@fivensondermatology.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Amazon.com 2020 Best-selling facial sunscreens. (https://www.amazon.com/Best-Sellers-Beauty-Facial-Sunscreens/zgbs/beauty/7792567011).
- American Academy of Dermatology 2019. How to apply sunscreen. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent/how-to-apply-sunscreen.
- American Academy of Dermatology 2019 Sunscreen FAQs. https://www.aad.org/media/stats-sunscreen.
- American Academy of Dermatology 2020.Talking points: JAMA study on absorption of sunscreen ingredients. https://www.aad.org/member/advocacy/promote/practice/social-news-jama-sunscreen.
- Bond P. Why do Some Sunblocks Give off a White Cast, and How Can I Avoid it? https://www.adorebeauty.com.au/skin-care/sunscreen/guide/avoid-white-cast. 2019.
- Corsillo L, Morrill H. What Are the Best Sunscreens? https://nymag.com/strategist/article/best-sunscreens-review-face-spray.html 2019.
- Erickson B. Sunscreen approval delays prompt push for high-throughput tests. Chem Eng News. 2018;96(5):17. https://cen.acs.org/articles/96/i5/Sunscreen-approval-delays-prompt-push.html. [Google Scholar]
- Food and Drug Administration (US). Sunscreen Drug Products for Over-The-Counter Human Use; Proposal to Amend and Lift Stay on Monograph, Federal. Register 1978. https://www.fda.gov/media/122882/download Search PubMed.
- Food and Drug Administration (US)-CDER Sun Protection Factor, 2017. https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/sun-protection-factor-spf.
- Food and Drug Administration (US), Over-the-Counter Drug Products; Safety and Efficacy Review; Additional Sunscreen Ingredients. Federal Register 2006, FDCA § 201(p)(1) “Guidance for FDA Staff and Industry: Marketed Unapproved Drugs — Compliance Policy Guide” (PDF). https://www.govinfo.gov/app/details/FR-2006-07-26.
- Food and Drug Administration (US), Sunscreen Drug Products for Over-the-Counter Human Use. Federal Register, 2019. https://www.federalregister.gov/documents/2019/02/26/2019-03019/sunscreen-drug-products-for-over-the-counter-human-use. [PubMed]
- Food and Drug Administration (US) Ecamsule Eligibility for Inclusion in Monograph; Over-the-Counter Sunscreen Drug Products for Human Use; Request for Safety and Effectiveness Data-Federal Register 2008. https://www.govinfo.gov/content/pkg/FR-2008-09-12.
- Kunz P.Y., Galicia H.F., Fent K. Comparison of in vitro and in vivo estrogenic activity of UV filters in fish. Toxicol Sci. 2006;90(2):349–361. doi: 10.1093/toxsci/kfj082. Epub 2006Jan 10. [DOI] [PubMed] [Google Scholar]
- Lim H.W., Thomas L., Rigel D.S. Photoprotection. In: Rigel D.S., Weiss R.A., Lim H.W., editors. Photoaging. Marcel Dekker; New York: 2004. pp. 73–88. [Google Scholar]
- Munt D.J., Grana A., Hulce M., Fusaro R.M., Dash A.K. Effect of simultaneous administration of dihydroxyacetone on the diffusion of Lawsone through various in vitro skin models. AAPS Pharm Sci Tech. 2015;16(6):1425–1433. doi: 10.1208/s12249-015-0335-8. ePub 2015 May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S.L., Lim H.W. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J Am Acad Dermatol. 2019;80(1):266–271. doi: 10.1016/j.jaad.2018.06.033. [DOI] [PubMed] [Google Scholar]
- Matta MK, Florian J, Zusterzeel R, Pilli NR, Patel V, Volpe DA, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: A randomized clinical trial. J Am Med Assoc 2020;21:323(3):256-267. doi: 10.1001/jama.2019.20747. [DOI] [PMC free article] [PubMed]
- Matta MK, Zusterzeel R, Pilli NR, Patel V, Volpe DA, Florian JA, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: A randomized clinical trial, J Am Med Assoc 2019;321:2082–2091. ePub 2019 May 6. doi: 10.1001/jama.2019.5586. [DOI] [PMC free article] [PubMed]
- Morra P., Bartle W.R., Walker S.E., Lee S.N., Bowles S.K., Reeves R.A. Serum concentrations of salicylic acid following topically applied salicylate derivatives. Ann Pharmacother. 1996;30(9):935–940. doi: 10.1177/106002809603000903. [DOI] [PubMed] [Google Scholar]
- Narla S., Lim H.W. Sunscreen: FDA regulation, and environmental and health impact. Photochem Photobiol Sci. 2020;19(1):66–70. doi: 10.1039/c9pp00366e. [DOI] [PubMed] [Google Scholar]
- Quinn J 10 best sunscreens for year-round protection. Dermstore 2019 https://www.dermstore.com/blog/top_ten/best-spf-sunscreens/.
- Reisch M. After more than a decade, FDA still won’t allow new sunscreens. Chem Eng News. 2015;93(20):10–15. [Google Scholar]
- Schauder S., Ippen H. Contact and photocontact sensitivity to sunscreens: review of a 15-year experience and the literature. Contact Derm. 1997;37:221–232. doi: 10.1111/j.1600-0536.1997.tb02439.x. [DOI] [PubMed] [Google Scholar]
- van der Pols J.C., Williams G.M., Pandeya N., Logan V., Green A.C. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15:2546–2548. doi: 10.1158/1055-9965.EPI-06-0352. [DOI] [PubMed] [Google Scholar]
- Wadyka S. Sunscreens in makeup and moisturizers. Consumer Reports. 2019 https://www.consumerreports.org/sunscreens/will-moisturizers-with-spf-protect-your-skin/ [Google Scholar]
- Wang S.Q., Burnett M.E., Lim H.W. Safety of oxybenzone: putting numbers into perspective. Arch Dermatol Res. 2011;147(7):865–866. doi: 10.1001/archdermatol.2011.173. [DOI] [PubMed] [Google Scholar]
- Weisbrod C., Kunz P., Zenker A., Fent K. Effects of the UV filter benzophenone-2 on reproduction in fish. Toxicol Appl Pharm. 2017;225:255–266. doi: 10.1016/j.taap.2007.08.004. [DOI] [PubMed] [Google Scholar]
- West D.P., Worobec S., Solomon L.M. Pharmacology and toxicology of infant skin. J, Invest Dermatol. 1981;76(3):147–150. doi: 10.1111/1523-1747.ep12525553. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


