Abstract
Air pollution is being shown to play an increasing causation role in our most common skin diseases. Acne, hyperpigmentation, atopic dermatitis, and psoriasis have been shown to be influenced by air pollution. It is important for pollution to be added as a risk factor for these skin disorders, and thus we must discuss mitigating its negative affects with patients. Air pollution is the contamination of outdoor (ambient) and indoor (household) environments by any chemical, physical, or biological agent that modifies the natural characteristics of the atmosphere. Nearly all (90%) of the world’s population experience daily pollution. In 2019, air pollution was considered by the World Health Organization to be the biggest environmental health risk to humans, responsible for killing more than 7 million people prematurely every year. Preliminary studies link air pollution to COVID-19 deaths, as there were high death tolls in some of the most globally polluted areas. Air pollution affects many organ systems such as cardiovascular, pulmonary, central nervous, reproductive, and integumentary systems. In this study, we detail the current evidence linking specific skin and health disorders to air pollution.
Keywords: Skin, Pollution, Air pollution, Skin disease
Air pollution and its effects on human health
Air pollution is this generation’s silent killer. In 2019, the World Health Organization (WHO) considered both ambient (outdoor) and household (indoor) air pollution as the biggest environmental health risk for humans, responsible for killing more than 7 million people prematurely every year. New estimates in 2018 revealed that 9 of 10 people breathe air containing high levels of pollutants (WHO, 2017). In the United States, nearly 134 million people—more than 40% of the population—are at risk for disease and premature death because of air pollution, according to the American Lung Association.
The WHO defines air pollution as the contamination of outdoor (ambient) and indoor (household) environments by any chemical, physical, or biological agent that modifies the natural characteristics of the atmosphere (National Geographic, 2016, World Health Organization, 2017) Air pollution constitutes a mixture of particles and gases that can reach harmful concentrations both outside and indoors. Soot, smoke, mold, pollen, methane, and carbon monoxide are a just few examples of common pollutants. Fine particles in polluted air can penetrate deeply into the lungs and gain access to the cardiovascular system. Air pollution affects many organ systems, including the cardiovascular system, causing heart disease and pulmonary causing lung cancer, chronic obstructive pulmonary disease, asthma, and respiratory infections. Higher stroke rates have been attributed to central nervous system involvement (Babdjouni et al., 2017; Brunekreef and Holgate, 2002, Hoek et al., 2013). There is also evidence that air pollution affects the reproductive (Carré et al., 2017) and integumentary systems (Krutmann, 2019, Mancebo and Wang, 2015).
Relationship between COVID-19 and pollution
Several studies report associations between susceptibility to and the severity of COVID-19 and air pollution. Recently, preliminary studies have linked exposure to air pollution with greater severity of COVID-19 infections, reporting higher death tolls in some of the most globally polluted areas (Wu et al., 2020) These authors suggest that hazardous air pollutant exposure is a contributing factor to COVID-19 mortality in the United States.
It has been observed that COVID-19 can be made more serious and, in some cases, more deadly by a specific type of industrial emission called hazardous air pollutants (Petrone et al., 2020). In a study published in 2020, scientific investigators examined air pollution and coronavirus deaths in roughly 3,100 US counties—both rural counties in Louisiana and highly populated communities in New York. In both of these settings they found a close correlation between levels of hazardous pollutants and the per-capita death rate from COVID-19.
Pollution is also being shown to play an increasing causation role in our most common skin diseases. Acne, hyperpigmentation, atopic dermatitis, and psoriasis have been shown to be influenced by air pollution (Araviiskaia et al., 2019, World Health Organization, 2006). It is important for pollution to be added as a risk factor for these skin disorders and for a discussion to be had on mitigating its negative effects on patients (WHO, 2017).
Sources and composition of air pollution
Table 1 summarizes the classes and sources of air pollutants. Most commonly, pollutants are generated through the burning of fossil fuels by vehicles and industries giving rise to components such as particulate matter (PM) and polycyclic aromatic hydrocarbons (PAHs). Additional pollutants are derived from a variety of unrelated sources, such as cigarette smoke, ultraviolet (UV) light, volatile organic compounds (VOCs), and tropospheric (ground-level) ozone. Particulate pollutants with a diameter of 2.5 µm or less (PM2.5) and 10 µm or less (PM10) and nanoparticles are the main constituents of PM. PAHs and VOCs are mainly generated by power plants, industries, vehicles, and domestic agricultural sources. PAHs and VOCs are often coated on PM and can easily get through the cutaneous lipid barrier due to their lipophilic properties (Millis et al., 1992, Piao et al., 2018) A pollutant can be of natural origin or manmade. Pollutants are classified as primary or secondary.
Table 1.
Classes and sources of pollutants.
| Air pollutant class | Name/Examples | Potential sources of pollutants |
|---|---|---|
| Gaseous | Carbon monoxide | Fossil‐fuel combustion, vehicle emission |
| Nitrogen dioxide | Fuel combustion, wood burning, vehicle emissions, waste incineration | |
| Ozone | Formed by interaction of VOCs and nitrogen oxide compounds upon UV photoactivation | |
| Sulphur dioxide | Fuel combustion, vehicle emissions, maritime transport, electric utilities, industrial facilities, volcanoes | |
| Heavy metals | Arsenic | Battery manufacture, minerals |
| Cadmium | Battery manufacturing, aircraft industry, television manufacturing | |
| Lead | facilities, leaded fuel, lead‐based paint, plumbing material | |
| Nickel | Casting, welding, battery manufacture | |
| Particulate matter (PM) | Coarse PM10 (2.5–10 μm) | Road dust, unpaved roads, forest fires, waste degradation including electronic waste, cooking processes |
| Fine PM2.5 (<2.5 μm) | Fossil‐fuel combustion, industrial facilities, maritime transport, biomass burning, waste incineration, cooking | |
| Ultrafine PM0.1 (<0.1 μm) | Vehicle emission, industrial facilities | |
| Persistent organic compounds | Dioxins, dioxin‐like polychlorinated biphenyls | Herbicides, pesticides, industrial processes, forest fires, volcanic eruptions |
| Semi-volatile organic compounds | Butylated hydroxytoluene, diethyl phthalate, geranyl acetone, nicotine (in free‐base form), parabens | Solvents, fragrances, bactericides, antimicrobial agents, flooring, furniture |
| VOCs | Acetaldehyde, dimethylformamide, formaldehyde, hexane, styrene, toluene, xylene | Fuel combustion, aircraft emission, household products, chemical solvents, paints, varnishes, cigarette smoke |
UV, ultraviolet; VOC, volatile organic compound.
Created with reference to Araviiskaia et al. (2019).
Primary pollutants are substances directly produced from a process. An example would be sulfur oxides released from factories. Other examples include ash from a volcanic eruption, black carbon, nitrogen oxides (NOx), carbon monoxide gas from motor vehicle exhaust, and heavy metals such as mercury and lead. Secondary pollutants are not emitted directly but instead form in the air when primary pollutants react or interact. Tropospheric ozone (O3) is an example of a secondary pollutant. The experimental evidence suggests a positive correlation of O3 exposure with oxidative damage, impaired antioxidant defense, and proinflammatory response in the skin (Fuks et al., 2019).
In time-series studies, it was observed that acute rises in O3 levels correlated with seeking medical help for skin conditions (Salvador et al., 2019). Other types of secondary pollutants include secondary PM (aka secondary inorganic aerosols) and oxidized VOCs (Centers for Disease Control and Prevention, 2020; WHO). Some pollutants can be both primary and secondary: They are both emitted directly and formed from other primary pollutants (WHO, 2017).
Mechanisms of injury from air pollution
Air pollution causes oxidative stress. Air pollutants enter the skin via nanoparticles and generate quinones, which are redox-cycling chemicals that produce reactive oxygen species (ROS). This increase in the amount of ROS and free radicals within the cell and its mitochondria overcomes the skin’s innate antioxidant defenses, including depletion of enzymatic (glutathione peroxidase, glutathione reductase, superoxide dismutase, and catalase) and nonenzymatic (vitamin E, vitamin C, and glutathione) antioxidant capacities (Thiele et al., 1997). The ROS and free radicals interact with the lipid-rich plasma membrane, initiating a lipid peroxidation reaction that unleashes proteolytic activity, causing further tissue injury. This cascade in turn triggers an increase of metalloproteinases (Farage et al., 2008, Millis et al., 1992). ROS also stimulate the release of proinflammatory mediators, which results in the accumulation of neutrophils and other phagocytic cells that further generate free radicals, thereby resulting in a vicious cycle of inflammation and metabolic impairments. Oxidative stress initiates complex biological processes resulting in genetic damage, activation of transcription factors such as activator protein 1 and nuclear factor-κB and signaling pathways such as extracellular signal-regulated kinases, c-Jun N-terminal kinases, and p38 mitogen-activated protein kinases, involved in cell growth and differentiation and in the degradation of the connective tissue of the dermis (Baek and Lee, 2016). These effects can be amplified by the deleterious synergy between pollution and sunlight (Burke and Wei, 2009). Repeated and frequent exposure to high levels of these pollutants may have deleterious effects on the skin, which include association with or causation of premature aging, photodamage, solar lentigines, melasma, and increased incidences of atopic dermatitis, psoriasis, skin cancers, and acne (Mancebo and Wang, 2015, Puri et al., 2017).
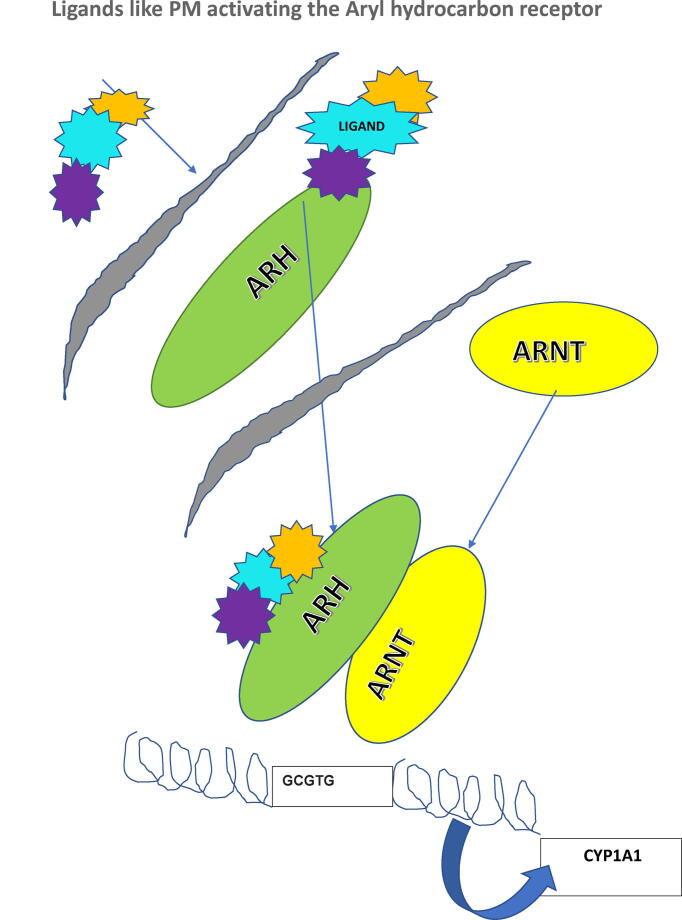
The role of the aryl hydrocarbon receptor (AHR) in causing the deleterious effects of pollution are being elucidated (Vogel et al., 2020). The AHR is a ligand-activated transcription factor that regulates the expression of genes, including those encoding the cytochrome P450 monooxygenases, CYP1A1, and CYP1A2, which are important enzymes in metabolism of xenobiotics. The AHR is activated upon binding of PAHs, persistent organic pollutants, and related ubiquitous environmental chemicals to mediate their biological and toxic effects. In addition, several endogenous and natural compounds can bind to AHR, thereby modulating a variety of physiologic processes. In recent years, ambient PM associated with traffic-related air pollution has been found to contain significant amounts of PAHs. PM containing PAHs are of increasing concern as a class of agonists, which can activate the AHR (Fig. 1).
Fig. 1.
The ARNT gene encodes the aryl hydrocarbon receptor nuclear translocator protein that forms a complex with ligand-bound aryl hydrocarbon receptor (AhR), P450A1 (CYP1A1) cytochrome system. The classical recognition motif of the AhR/ARNT ligand complex, referred to as the AhR-, dioxin-, or xenobiotic-responsive element (AHRE, DRE, or XRE), contains the core sequence 5′-GCGTG-3 within the consensus sequence 5′-T/GNGCGTGA/CG/CA-3 in the promoter of AhR responsive genes.
How pollution enters the skin
Skin interfaces with the atmosphere. Studies show that pollutants gain entrance to the skin by direct accumulation on the skin surface, absorption via the hair follicles, inhalation, ingestion, and circulation of pollutants in plasma that diffuse into deeper dermal tissues. PAHs can be detected not only in the air but also in all human bodily fluids and in hair (Krutmann et al., 2014).
There are two documented ways for pollution to permeate the skin: transepidermal and absorption through hair follicles and sweat ducts. The transepidermal route can be divided into the shorter, transcellular and the longer, intercellular routes for penetration of hydrophilic and lipophilic compounds, respectively. Absorption through hair follicles and sweat ducts is the shortest route. Although skin adnexal structures are 0.1% of the total skin surface of 18,000 cm2, they excel as a pathway for percutaneous penetration (Koohgoli et al., 2017).
Dermal uptake depends on the deposition of the hazardous substances on the skin surface, the composition and physical properties of epidermal lipids, and diffusion through the epidermis to the blood vessels (Rinnerthaler et al., 2015). Pesticides, solvents, mercury, isocyanates, polychlorinated biphenyls, acrylates, and PAHs are some of the main chemical groups that have been recognized as posing health problems via percutaneous absorption (Rinnerthaler et al., 2015; Soeur et al., 2020).
Inhalation uptake of pollution is dependent on two factors: alveolar concentration of inhalation agents and diffusion and solubility, which is also called the blood–gas partition coefficient (Weschler and Nazaroff, 2014). Pollutants can also be ingested, as in grilled meats. All these sources—percutaneous, inhaled, and ingested—can provide access to the bloodstream and systemic distribution.
Skin disorders associated with pollution
Photoaging
We have known for decades that smoking leads to premature aging (Morita, 2007, Morita et al., 2009). Epidemiological studies discovered a direct link between airborne PM exposure and the occurrence of prominent skin aging signs especially pigment spots, as well as wrinkles (Okada et al., 2013). Another study documented that an increase in soot and particles from traffic were associated with 20% more pigment spots on forehead and cheeks (Huls et al., 2016). Before industrialization, the skin of farmers had extensive photoaging, which may have been due to the perfect storm of agricultural pollutants such as VOCS and unprotected UV exposure. With urbanization, the cities became the new pollution pockets, with PAH and PM from industry and traffic as major culprits. Pollution with and without UV synergy has been proven to be causative in premature ephelides, lentigines, and rhytids (Huls et al., 2016, Yin et al., 2001).
Lentigines
A lentigo (plural lentigines) is a small brown macule located typically on the face, hands, and chest. Variously called liver spots, Hori’s macules, solar lentigines, and senile lentigines, they are typically associated with advancing age but may be seen in some unique dermatologic syndromes (Hamm et al., 2019). Solar lentigines can be considered a sign of premature aging. In a well-done study, Peng et al. (2017) demonstrated that exposure to PM2.5 was significantly associated with senile lentigines on the cheeks and on the back of hands; the higher the PM, the more lentigines. Other variables associated with skin aging were secondhand smoke and exposure to fossil fuels. Both were associated with a higher number of spots on the cheeks. In another study, investigators found that a distance of 100 m or less from a busy road was associated with 35% more senile lentigines on the forehead and 15% more on the cheeks (Huls et al., 2016). NO2 exposure has been linked to pigment spot formation on the cheeks in both Caucasian and Asian women (Schikowski and Krutmann, 2019).
In one proposed mechanism, organic compounds present on the surface of PM may penetrate the skin and directly affect viable skin cells, such as keratinocytes and melanocytes. In addition, it has been shown that both UV radiation and PAH‐loaded particles activate AHR in human skin and thereby contribute to lentigenes. Notably, recent studies demonstrate that AHR activation in skin cells induced skin pigmentation in a variety of pigmentary disorders (Napolitano and Patruno, 2018, Schikowski and Krutmann, 2019).
Melasma
Melasma is a benign, acquired, irregularly patterned, light- to dark-brown hyperpigmentation, with symmetric distribution mostly over the face but at times also involving the neck, chest, and shoulders. The etiology of melasma includes genetic predisposition, hormones, and most importantly UV radiation (Trivedi et al., 2017). Recent studies suggest pollution as a possible emerging risk factor for the development of melasma (Roberts, 2015). Airborne PM and PAHs present in the polluted air enter the skin via nanoparticles and generate quinones that lead to the generation of ROS. These ROS then trigger high metalloproteinase levels that lead to skin pigmentation. Interestingly, the incidence of melasma is higher in persons with skin type III-VI residing in India, China, Southeast Asia, and the United States, countries that are also the most polluted geographical regions in the world (Handel et al., 2014, Mannucci and Franchini, 2017, Roberts, 2015).
Atopic dermatitis/eczema
Atopic dermatitis (AD) is a common, heterogenous, chronic‐intermittent, eczematous skin disorder that starts at infancy or early childhood and persists for a large part of life. It is characterized by frequent relapse with intense pruritus that deteriorates the quality of life and decreases treatment satisfaction of afflicted patients (Kim et al., 2019).
The lifetime incidence of AD is as high as 20% in the general population (Kim et al., 2013, Mannucci and Franchini, 2017) The incidence in children may be higher (Schachtel et al., 2020) Despite the large number of clinical, laboratory, and experimental studies, the pathophysiology of AD remains to be clarified. We know that AD is characterized by T-helper cell (Th2)–deviated skin inflammation, barrier disruption, and chronic pruritus (Bonamonte et al., 2019, Rerknimitr et al., 2017). The increasing prevalence of AD parallels a global rise in industrialization, urban living, and increasing air pollution over recent decades (Bonamonte et al., 2019, Kim et al., 2013).
We have strong evidence-based medicine demonstrating that outdoor air pollution is related to exacerbation of preexisting asthma, development of atopic diseases, and allergic sensitization (Bonamonte et al., 2019). In contrast, there are few studies to our knowledge that show the association between outdoor air pollution and AD. In one study, investigators in Seoul collected symptom records of 1,880 person-days (Kim et al., 2019). They found that AD symptoms were associated with the levels of outdoor air pollutants such as PM, toluene, and VOC. Interestingly, this association varied according to season.
Skin barrier dysfunction
Skin barrier dysfunction is associated with the reduced production of terminal differentiation molecules such as filaggrin (Del Rosso et al., 2016). Abnormal skin barrier integrity also causes an increased colonization of microbes such as Staphylococcus aureus, which further exacerbate Th2-deviated skin inflammation (Del Rosso et al., 2016, Furue et al., 2019). There are new molecules and drugs in the therapeutic pipeline for AD (Schachtel et al., 2020). One such treatment under study includes the drug tapinarof (Psomadakis and Han, 2019), which has gained increased attention because its topical application is efficacious for patients with AD. In clinical trials, tapinarof activates the AHR/CYP1A1 axis and augments the expression of filaggrin and involucrin. Tapinarof is a high-affinity AHR ligand with antioxidative activity via NRF2 (nuclear factor-erythorid2-related factor-2) activation and a ROS-scavenging structure. Earlier in this article, we discussed AHR high-affinity ligands such as PM, which promoted oxidative stress by activating the cytokine class I cascade to increase ROS resulting in inflammation. Even in barrier-disrupted AD patients, systemic absorption of topical tapinarof is limited and likely decreases during the treatment course in parallel with treatment success that restores the barrier function (Cotter et al., 2018, Psomadakis and Han, 2019).
Psoriasis
We know that pollution negatively affects psoriasis by the same mechanism seen in AD (Puri et al., 2017). Cadmium, found in battery and television manufacturing and the aircraft industry, is one of the newly identified air pollutants that affect the pathogenesis of psoriasis (Kamiya et al., 2019). In this study, patients with severe psoriasis had higher blood cadmium compared with the general population. It is proposed that environmental exposure to cadmium may compromise immunity, and microenvironmental perturbations can predispose to worsening of psoriasis (Kamiya et al., 2019, Liaw et al., 2017).
Acne
A robust body of evidence indicates a link between pollution and inflammatory acne (Dréno et al., 2018, Krutmann et al., 2017, Liu et al., 2018). The prevalence of acne is similar between Asian and Caucasian women, but Asian women were reported to have a higher prevalence of inflammatory than comedonal acne (20 vs 10%), compared with Caucasian women, for whom comedonal acne was more prevalent (14%) than inflammatory acne (10%; Perkins et al., 2011).
In addition to a higher prevalence of inflammatory acne, Asian women also report that their symptoms are exacerbated during periods of high air pollution. Subsequent studies provided evidence for this impression (Krutmann et al., 2017). Lefebvre et al., 2015, Lefebvre et al., 2016) looked at skin quality changes, defined as reduced vitamin E and squalene levels, in acne patients residing in Shanghai and Mexico City, two cities with heavy pollution levels. They observed that these measures of skin quality declined with chronic exposures to ambient pollution. Although both markers are signs of sebum oxidation, clinical signs of acne severity were not assessed. Nevertheless, increased sebum is known to be an early pathophysiological pillar of acne (Eudier et al., 2019, Gollnick and Dreno, 2015).
Skin cancer
As discussed in detail earlier in this journal (Parker, 2020), pollution is associated with an increased risk for skin cancer (Baudouin et al., 2002). The “pollutants” that react most specifically with the skin are UV radiation, PAHs (eg, benzo[a]pyrene), VOCs (eg, benzene), heavy metals, and O3. UV radiation, a “physical” pollutant, is thought to be the factor responsible for most skin cancers in humans. The genotoxicity of UV light is well documented and its carcinogenic effect is clearly demonstrated in vivo in humans. A few epidemiological studies describe the carcinogenicity of certain pollutants such as arsenic or lead on the skin. However, most of the evidence for the role of pollutants in skin cancers comes from in vivo animal studies or from in vitro studies using PAHs.
Prevention strategies
In addition to advocating for clean air, non-fossil fuel energy sources, and environmental protections, we need to protect ourselves and our patients from the deleterious effects of pollution. Here we discuss some strategies for personal protection.
Diet
Water may dilute the toxin load within the bloodstream. The recommended daily water intake for an adult is 2 L or 0.5 gallons per day (Jéquier and Constant, 2010).
Antioxidants
Free radicals are unstable molecules that can cause inflammation and are postulated to underlie a number of age-related chronic diseases, as well as the aging response, itself (Rinnerthaler et al., 2015, Sies, 1997). Antioxidants neutralize the free radicals produced as a result of toxic air pollutants entering the body. Thus, a diet rich in antioxidants may help protect from the damage caused by air pollution. The most important antioxidant supplements are vitamins B3, C, and E; omega-3; β-carotene; selenium; coenzyme Q10; polypodium leucotomos; green tea; and lipoic acid (Prasad et al., 2017).
The Mediterranean diet involves the consumption of foods high in antioxidants, such as fruits, vegetables, whole grains, legumes, olive oil, fish, and poultry (Widmer et al., 2015). For more than17 years, researchers at New York University School of Medicine prospectively collected data from 550,000 individuals with an average age of 62 years from around the United States. This cohort was grouped on how closely their eating habits mirrored the Mediterranean diet and on estimates of their long-term exposures to air pollution, including PM2.5, nitric oxide, NOx, and O3 (Lim et al., 2019). Among the individuals who least adhered to the Mediterranean eating pattern, death from all causes increased by 5% for every 10 parts per billion increase in long-term average nitrous oxide exposure, compared with only 2% among the cohort who were best at following the diet. Deaths from cardiovascular disease increased 17% for every 10 μg/m3 increase in long-term average PM2.5 exposure among the individuals who did not closely follow the diet, compared with 5% among those who did. Similar patterns were seen for deaths from heart attacks (Lim et al., 2019). There is certainly a case to be made for eating a healthy, high-antioxidant diet. Although its benefits for skin health have not yet been studied, it seems plausible to predict that it would benefit.
Anti-pollution skin care regimen
Cleansing the skin is the backbone of an anti-pollution skin regimen because airborne pollutants can bind to the skin and weaken the skin barrier, rendering it more susceptible to UV damage, dryness, wrinkles, and hyperpigmentation. Evidence-based data have demonstrated improved cleansing with an ultrasonic vibrational handheld brush (Peterson et al., 2017). Although the cost of this device may put it out of reach for some, good facial cleansing can be accomplished manually. Protecting skin during the daytime with sun protective skin care products is another critical cornerstone of prevention. Emollients augmented with evidence-based topical antioxidants, such as vitamins A, B3 (niacinamide), C, and E; green tea; coenzyme Q10; and resveratrol is another important preventive strategy (Burke, 2019).
We advise a twice-daily skin care regimen, starting each time with a cleanse. The morning cleanse prepares the face for the anticipated environmental impacts of the day. After cleansing, one attempts to “build a shield” can protect facial skin from environmental pollutants. Acne-prone patients should be recommended products that will not induce disease flares. This regimen is also beneficial for patients with melasma because it creates additional barriers to decrease the skin’s UV absorption. The skin care program we recommend in our clinic includes the following sequence:
-
1)
Use of a gentle cleanser, washing the face with hands or a washcloth.
-
2)
Apply witch hazel or an alcohol-based toner/serum to help close pores.
-
3)
Use of a silicone-based primer and/or moisturizer (select a vehicle that is most compatible with dry, T-zone, or oily skin type) as an additional barrier to environmental pollutants.
-
4)
Use of a broad-spectrum sunscreen with protection against all UV wavelengths as well as infra-red radiation.
-
5)
Finally, use a foundation makeup if desired to complete the skin pollution barrier.
The evening cleanse is designed to remove airborne particles, soot, and other possible skin contaminants that may lie at the skin surface. Because PM is being removed, this cleanse should be longer and stronger than the morning cleanse. The evening regimen should follow this sequence:
-
1.
Cleanse with a handheld sonic/vibrational cleansing device (with or without grains for sensitive skin types) to stretch pores and help expel surface debris. If this device is not available, cleanse well using a washcloth in contact with all areas of the face.
-
2.
Use a makeup removal wipe to ensure all makeup was removed by cleansing.
-
3.
Finally, use an evening moisturizer containing repair ingredients, such as some DNA repair creams, or products containing one or more of the following: resveratrol, retinoids, or hyaluronic/chondroitin sulfate. Product rotation may offer patients the benefit of synergism.
Summary
Pollution has been shown to increasingly play a role in some of our most common diseases, and effects on the skin is no exception. It is important to consider pollution as a risk factor for causation of a variety of skin disorders and to include management strategies in patient discussions. To those practitioners in high-pollution areas, a generational approach of prevention is recommended. Introduce anti-pollution dietary and skin care routines at an early age to promote the healthiest, cleanest skin possible and to mitigate evolving pollution-related skin disorders (Roberts, 2013).
Conflicts of interest
None.
Funding
None.
Study approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
References
- Araviiskaia E., Berardesca E., Bieber T., Gontijo G., Sanchez Viera M., Marrot L. The impact of airborne pollution on skin. J Eur Acad Dermatol Venereol. 2019;33(8):1496–1505. doi: 10.1111/jdv.15583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J., Lee M.G. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016;21(4):164–169. doi: 10.1179/1351000215Y.0000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudouin C., Charveron M., Tarroux R., Gall Y. Environmental pollution and skin cancer. Cell Biol Toxicol. 2002;18(5):341–348. doi: 10.1023/a:1019540316060. [DOI] [PubMed] [Google Scholar]
- Bonamonte D., Filoni A., Vestita M., Romita P., Foti C., Angelini G. The role of the environmental risk factors in the pathogenesis and clinical outcome of atopic dermatitis. Biomed Res Int. 2019;2019:2450605. doi: 10.1155/2019/2450605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunekreef B., Holgate S.T. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Burke K.E. Protection from environmental skin damage with topical antioxidants. Clin Pharmacol Ther. 2019;105(1):36–38. doi: 10.1002/cpt.1235. [DOI] [PubMed] [Google Scholar]
- Burke K.E., Wei H. Synergistic damage by UVA radiation and pollutants. Toxicol Ind Health. 2009;25:219–224. doi: 10.1177/0748233709106067. [DOI] [PubMed] [Google Scholar]
- Carré J., Gatimel N., Moreau J., Parinaud J., Léandri R. Does air pollution play a role in infertility? A systematic review Environ Health. 2017;16:82. doi: 10.1186/s12940-017-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Air pollutants [Internet]. Available from: https://www.cdc.gov/air/pollutants.htm, 2020.
- Cotter D.G., Schairer D., Eichenfield L. Emerging therapies for atopic dermatitis: JAK inhibitors. J Am Acad Dermatol. 2018;78:S53–S62. doi: 10.1016/j.jaad.2017.12.019. [DOI] [PubMed] [Google Scholar]
- Del Rosso J, Zeichner J, Alexis A, Cohen D, Berson D, MD understanding the epidermal barrier in healthy and compromised skin: clinically relevant information for the dermatology practitioner: Proceedings of an Expert Panel Roundtable Meeting. J Clin Aesthet Dermatol 2016;9(4 suppl 1):S2–8. [PMC free article] [PubMed]
- Dréno B., Bettoli V., Araviiskaia E., Sanchez Viera M., Bouloc A. The influence of exposome on acne. J Eur Acad Dermatol Venereol. 2018;32(5):812–819. doi: 10.1111/jdv.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudier F., Hucher N., Picard C., Savary G., Grisel M. Squalene oxidation induced by urban pollutants: Impact on skin surface. Physico-Chemistry. Chem Res Toxicol. 2019;32(2):285–293. doi: 10.1021/acs.chemrestox.8b00311. [DOI] [PubMed] [Google Scholar]
- Farage M.A., Miller K.W., Elsner P., Maibach H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int J Cosmet Sci. 2008;30:87–95. doi: 10.1111/j.1468-2494.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- Furue M., Hashimoto-Hachiya A., Tsuji G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int J Mol Sci. 2019;20(21):5424. doi: 10.3390/ijms20215424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick H.P., Dreno B. Pathophysiology and management of acne. J Eur Acad Dermatol Venereol. 2015;29(suppl 4):1–2. doi: 10.1111/jdv.13182. [DOI] [PubMed] [Google Scholar]
- Handel A.C., Miot L.D.B., Helios A.M. Melasma: A clinical and epidemiological review. An Bras Dermatol. 2014;89(5):771–782. doi: 10.1590/abd1806-4841.20143063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm H., Emmerich K., Olk J. Pigmented macules as possible early signs of genetic syndromes. Hautarzt. 2019;70(7):506–513. doi: 10.1007/s00105-019-4416-6. [DOI] [PubMed] [Google Scholar]
- Hoek G., Krishnan R.M., Beelen R., Peters A., Ostro B., Bryunekreef B. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huls A., Vierkotter A., Gao W., Kramer U., Yang Y., Ding A. Traffic-related air pollution contributes to development of facial lentigines: further epidemiological evidence from Caucasians and Asians. J Invest Dermatol. 2016;136:1053–1056. doi: 10.1016/j.jid.2015.12.045. [DOI] [PubMed] [Google Scholar]
- Jéquier E., Constant F. Water as an essential nutrient: the physiological basis of hydration. Eur J Clin Nutr. 2010;64(2):115–123. doi: 10.1038/ejcn.2009.111. [DOI] [PubMed] [Google Scholar]
- Kamiya K., Kishimoto M., Sugai J., Komine M., Ohtsuki M. Risk factors for the development of psoriasis. Int J Mol Sci. 2019;20(18):4347. doi: 10.3390/ijms20184347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim B.E., Leung D.Y.M. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. 2019;40(2):84–92. doi: 10.2500/aap.2019.40.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim E.H., Oh I., Jung K., Han Y., Cheong H.K. Symptoms of atopic dermatitis are influenced by outdoor air pollution. J Allergy Clin Immunol. 2013;132:495–498. doi: 10.1016/j.jaci.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Koohgoli R., Hudson L., Naidoo K., Wilkinson S., Chavan B., Birch-Machin M.A. Bad air gets under your skin. Exp Dermatol. 2017;26:384–387. doi: 10.1111/exd.13257. [DOI] [PubMed] [Google Scholar]
- Krutmann J. Air pollution and the skin. Hautarzt. 2019;70(3):156–157. doi: 10.1007/s00105-018-4349-5. [DOI] [PubMed] [Google Scholar]
- Krutmann J., Liu W., Li L., Pan X., Crawford M., Sore G. Pollution and skin: from epidemiological and mechanistic studies to clinical implications. J Dermatol Sci. 2014;76:163–168. doi: 10.1016/j.jdermsci.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Krutmann J., Moyal D., Liu W., Kandahari S., Lee G.S., Nopadon N. Pollution and acne: Is there a link? Clin Cosmet Investig Dermatol. 2017;10:199–204. doi: 10.2147/CCID.S131323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre M.A., Pham D.M., Boussouira B., Bernard D., Camus C., Nguyen Q.L. Evaluation of the impact of urban pollution on the quality of skin: A multicentre study in Mexico. Int J Cosmet Sci. 2015;37(3):329–338. doi: 10.1111/ics.12203. [DOI] [PubMed] [Google Scholar]
- Lefebvre M.A., Pham D.M., Boussouira B., Qiu H., Ye C., Long X. Consequences of urban pollution upon skin status. A controlled study in Shanghai area. Int J Cosmet Sci. 2016;38(3):217–223. doi: 10.1111/ics.12270. [DOI] [PubMed] [Google Scholar]
- Liaw F.Y., Chen W.L., Kao T.W., Chang Y.W., Huang C.F. Exploring the link between cadmium and psoriasis in a nationally representative sample. Sci Rep. 2017;7(1):1723. doi: 10.1038/s41598-017-01827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.C., Hayes R.B., Ahn J., Shao Y., Silverman D.T., Jones R.R., Thurston G.D. Mediterranean diet and the association between air pollution and cardiovascular disease mortality risk. Circulation. 2019;139(15):1766–1775. doi: 10.1161/CIRCULATIONAHA.118.035742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Pan X., Vierkotter A., Guo Q., Wang X., Wang Q. A time-series study of the effect of air pollution on outpatient visits for acne vulgaris in Beijing. Skin Pharmacol Physiol. 2018;31:107–113. doi: 10.1159/000484482. [DOI] [PubMed] [Google Scholar]
- Mancebo S.E., Wang S.Q. Recognizing the impact of ambient air pollution on skin health. J Eur Acad Dermatol Venereol. 2015;29:2326–2332. doi: 10.1111/jdv.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannucci P.M., Franchini M. Health effects of ambient air pollution in developing countries. Int J Environ Res Public Health. 2017;14:1048–1055. doi: 10.3390/ijerph14091048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis A.J., Hoyle M., McCue H.M., Martini H. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp. Cell Res. 1992;201:373–379. doi: 10.1016/0014-4827(92)90286-h. [DOI] [PubMed] [Google Scholar]
- Morita A. Tobacco smoke causes premature skin aging. J Dermatol Sci. 2007;48(3):169–175. doi: 10.1016/j.jdermsci.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Morita A., Torii K., Maeda A., Yamaguchi Y. Molecular basis of tobacco smoke-induced premature skin aging. J Investig Dermatol Symp Proc. 2009;14(1):53–55. doi: 10.1038/jidsymp.2009.13. [DOI] [PubMed] [Google Scholar]
- Napolitano M., Patruno C. Aryl hydrocarbon receptor (AhR) a possible target for the treatment of skin disease. Med Hypotheses. 2018;116:96–100. doi: 10.1016/j.mehy.2018.05.001. [DOI] [PubMed] [Google Scholar]
- National Geographic. Air pollution causes, effects, and solutions. Available from: https://www.nationalgeographic.com/environment/global-warming/pollution/, 2016.
- Okada H.C., Alleyne B., Varghai K., Kinder K., Guyuron B. Facial changes caused by smoking: a comparison between smoking and nonsmoking identical twins. Plast Reconstr Surg. 2013;132(5):1085–1092. doi: 10.1097/PRS.0b013e3182a4c20a. [DOI] [PubMed] [Google Scholar]
- Parker E.R. The influence of climate change on skin cancer incidence – a review of the evidence. Int J Women’s Dermatol. 2021;7(1):17–27. doi: 10.1016/j.ijwd.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng F., Xue C.H., Hwang S.K., Li W.H., Chen Z., Zhang J.Z. Exposure to fine particulate matter associated with senile lentigo in Chinese women: a cross-sectional study. J Eur Acad Dermatol Venereol. 2017;31(2):355–360. doi: 10.1111/jdv.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A.C., Cheng C.E., Hillebrand G.G., Miyamoto K., Kimball A.B. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25(9):1054–1060. doi: 10.1111/j.1468-3083.2010.03919.x. [DOI] [PubMed] [Google Scholar]
- Peterson G., Rapaka, Koski N., Kearney M., Ortblad K., Tadlock L. A robust sebum, oil, and particulate pollution model for assessing cleansing efficacy of human skin. Int J Cosmet Sci. 2017;39(3):351–354. doi: 10.1111/ics.12378. [DOI] [PubMed] [Google Scholar]
- Petrone M., Hill D., Younes L., Barkman L., Howard S., Howell B. Hazardous air pollutant exposure as a contributing factor to COVID-19 mortality in the United States. Environ Res Lett. 2020;9:15. [Google Scholar]
- Piao M.J., Ahn M.J., Kang K.A., Ryu Y.S., Hyun Y.J., Shilnikova K. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch Toxicol. 2018;92(6):2077–2091. doi: 10.1007/s00204-018-2197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S., Gupta S.C., Tyagi A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017;387:95–105. doi: 10.1016/j.canlet.2016.03.042. [DOI] [PubMed] [Google Scholar]
- Psomadakis C.E., Han G. New and emerging topical therapies for psoriasis and atopic dermatitis. J Clin Aesthet Dermatol. 2019;12(12):28–34. [PMC free article] [PubMed] [Google Scholar]
- Puri P., Nandar S.K., Kathuria S., Ramesh V. Effects of air pollution on the skin: a review. Indian J Dermatol Venereol Leprol. 2017;83:415–423. doi: 10.4103/0378-6323.199579. [DOI] [PubMed] [Google Scholar]
- Rerknimitr P., Otsuka A., Nakashima C., Kabashima K. The etiopathogenesis of atopic dermatitis: Barrier disruption, immunological derangement, and pruritus. Inflamm Regen. 2017;37:14. doi: 10.1186/s41232-017-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinnerthaler M., Bischof J., Streubel M.K., Trost A., Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545–589. doi: 10.3390/biom5020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W.E. Generational dermatology: model for prevention and multi decade approach toward the evolving, aging patient. J Drugs Dermatol. 2013;12(12):1396–1399. [PubMed] [Google Scholar]
- Roberts W.E. Pollution as a risk factor for the development of melasma and other skin disorders of facial hyperpigmentation – is there a case to be made? J Drugs Dermatol. 2015;14(4):337–341. [PubMed] [Google Scholar]
- Salvador C.M., Beko G., Weschler C.J., Morrison G., LeBreton M., Hallquist M. Indoor ozone/human chemistry and ventilation strategies. Indoor Air. 2019;29(6):913–925. doi: 10.1111/ina.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikowski T., Krutmann J. Air pollution (particulate matter and nitrogen dioxide) and skin aging. Hautarzt. 2019;70(3):158–162. doi: 10.1007/s00105-018-4338-8. [DOI] [PubMed] [Google Scholar]
- Schachtel A, Dyer JA, Boos MD. Climate change and pediatric skin health. Int J Womens Dermatol 2020;In Press. [DOI] [PMC free article] [PubMed]
- Sies H. Oxidative stress: Oxidants and antioxidants. Exp Physiol. 1997;82(2):291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- Thiele J.J., Traber M.G., Tsang K., Cross C.E., Packer L. In vivo exposure to ozone depletes vitamins C and E and induces lipid peroxidation in epidermal layers of murine skin. Free Radic Biol Med. 1997;23:385–391. doi: 10.1016/s0891-5849(96)00617-x. [DOI] [PubMed] [Google Scholar]
- Trivedi M.K., Yang F.C., Cho B.K. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3(1):11–20. doi: 10.1016/j.ijwd.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C.F.A., VanWinkle L.S., Esser C., Stemmann T.H. The aryl hydrocarbon receptor as a target of environmental stressors – Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler C.J., Nazaroff W.W. Dermal uptake of organic vapors commonly found in indoor air. Environ Sci Technol. 2014;48:1230–1237. doi: 10.1021/es405490a. [DOI] [PubMed] [Google Scholar]
- Widmer R.J., Flammer A.J., Lerman L.O., Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128(3):229–238. doi: 10.1016/j.amjmed.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Nethery RC, Sabath BM, BraunD, Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: a nationwide cross-sectional study. Sci Adv 2020;6:eabd4049. [DOI] [PMC free article] [PubMed]
- Yin L., Morita A., Tsuji T. Skin aging induced by ultraviolet exposure and tobacco smoking: evidence from epidemiological and molecular studies. Photodermatol Photoimmunol Photomed. 2001;17:178–183. doi: 10.1034/j.1600-0781.2001.170407.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Air pollution [Internet]. Available from: https://www.who.int/health-topics/air-pollution#tab=tab_1, 2017.
- World Health Organization. Systematic review of air pollution, a global update. 2006 http://www9.who.int/airpollution/en/.