Abstract
Background
Sunscreens are topical preparations containing one or more compounds that filter, block, reflect, scatter, or absorb ultraviolet (UV) light. Part 2 of this review focuses on the environmental, ecological effects and human toxicities that have been attributed to UV filters.
Methods
Literature review using NIH databases (eg, PubMed and Medline), FDA and EPA databases, Google Scholar, the Federal Register, and the Code of Federal Regulations (CFR).
Limitations
This was a retrospective literature review that involved many different types of studies across a variety of species. Comparison between reports is limited by variations in methodology and criteria for toxicity.
Conclusions
In vivo and in vitro studies on the environmental and biological effects of UV filters show a wide array of unanticipated adverse effects on the environment and exposed organisms. Coral bleaching receives considerable attention from the lay press, but the scientific literature identifies potential toxicities of endocrine, neurologic, neoplastic and developmental pathways. These effects harm a vast array of aquatic and marine biota, while almost no data supports human toxicity at currently used quantities (with the exception of contact allergy). Much of these data are from experimental studies or field observations; more controlled environmental studies and long-term human use data are limited. Several jurisdictions have prohibited specific UV filters, but this does not adequately address the dichotomy of the benefits of photoprotection vs lack of eco-friendly, safe, and FDA-approved alternatives.
Abbreviations: 4-MBC, 4-methylbenzylidene camphor; AAD, American Academy of Dermatology; BP-3, Benzophenone-3 or Oxybenzone; CDER, Center for Drug Evaluation and Research (part of FDA); EPA, Environmental Protection Agency; Europa, European Union Commission for Public Health; FDA, Food and Drug Administration; GRASE, Generally Recognized As Safe and Effective; GBRMPA, Great Barrier Reef Marine Park Authority; NHANES, National Health and Nutrition Examination Survey; OC, Octocrylene; OMC, Octyl methoxycinnamate or octinoxate; OTC, Over-the-counter; PABA, Para-aminobenzoic acid; PPCP, Pharmaceuticals and personal care products; PCPC, Personal care products and cosmetics; UV, Ultraviolet; UVF, Ultraviolet filter; WWTP, Wastewater treatment plant; NDA, New drug application; TiO2, Titanium dioxide; NanoTiO2, Nanoparticle titanium dioxide
Keywords: UV filter, Coral bleaching, Aquatic organism toxicity of UV filters, Bioaccumulation, Nanoparticle toxicity, Sunscreen side effects, Human toxicity of UV filters
Key Points.
-
•
Man-made UV filters are ubiquitous in the environment with human and animal absorption being well documented, long term studies and bioaccumulation have not been well characterized.
-
•
There is little data to support direct toxicity of UV filters in humans to date beyond contact and photocontact allergy, while the mechanisms for coral bleaching and coral death are better understood and are areas of active research.
-
•
Animal, marine and aquatic organisms have evidence for in vitro and ex vivo toxicity, but in vivo toxicity is less well characterized as much of the work to date shows water levels below toxicity thresholds. These studies lack control for high fluxes of UVF release in waste water treatment plants or at popular beaches during peak tourism.
Introduction
In Part 1, we describe the regulatory recommendations that the U.S. Food and Drug Administration (FDA) issued in February 2019 for non-prescription, over-the-counter (OTC) sunscreens to ensure their safety, efficacy, and consistency in labeling. We reviewed practical uses of UVFs and the AAD’s recommendations for sun protection as well as the need for more options for safe use in children and adults. In part 2 we will review the ecologic and biologic potential toxicities of UVFs. This part of the review is a survey of data regarding UVF effects and is not meant to give guidance on choices of UVF or the appropriate use of sunscreen agents as these were reviewed in part 1 (Sabzevari N, Qiblawi S, Norton S, Fivenson D, 2020).
Definitions
When reviewing scientific data, it is essential that readers understand the terminology. For example, titanium dioxide (TiO2) is not a sunscreen. It is a UV filter (UVF) that is included in many commercial products known as sunscreens.
Sunscreen: a commercial product sold to consumers for protection of human skin from UV radiation. Sunscreens contain one or more UVFs that may be physical, chemical, or both. In addition, they contain many other substances, such as emollients, preservatives or stabilizers, emulsifiers, fragrances, and coloring compounds. Broad spectrum sunscreens are defined by the FDA as products that provide UVA protection that is proportional to its UVB protection (Food and Drug Administration (US), 2017, Food and Drug Administration (US), 2019a).
According to the FDA, “a product that includes the term “sunscreen” in its labeling or in any other way represents or suggests that it is intended to prevent, cure, treat, or mitigate disease or to affect a structure or function of the body comes within the definition of a drug in section 201(g)(1) of the act. Sunscreen active ingredients affect the structure or function of the body by absorbing, reflecting, or scattering the harmful, burning rays of the sun, thereby altering the normal physiological response to solar radiation. These ingredients also help to prevent diseases such as sunburn and may reduce the chance of premature skin aging, skin cancer, and other harmful effects due to the sun when used in conjunction with limiting sun exposure and wearing protective clothing.” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=700.35
UV filter: a specific compound that impedes the passage of UV light. These are typically divided these into chemical (absorbing UV rays and converting to thermal energy) vs. physical agents (reflecting UV rays). Environmental chemists categorize them in several ways, for example, organic vs. inorganic, lipophilic vs. hydrophilic. The National Library of Medicine databases sometimes refer to these compounds as sunscreening agents (confusing to all of us at times), and define them as chemical or physical agents that protect the skin from sunburn and erythema by absorbing or blocking ultraviolet radiation. UVFs are also used in consumer cosmetics (makeup, nail polish, shampoo, etc.) and industry (plastics, paints, sealants, etc.) to protect against photodegradation.
Environment: the surroundings or conditions in which a person, animal, or plant lives or operates.
Ecosystem: the interactions between the environment and the organisms that dwell within it. GRASE: defined by the FDA OTC Glossary (https://www.accessdata.fda.gov/scripts/cder/training/otc/topic3/images/Glossary.pdf)
“A drug is not considered a new drug only when it is generally recognized as safe and effective (GRASE). In order to conclude a GRASE determination, a drug must satisfy three criteria: 1. The particular drug product must have been subjected to adequate and well-controlled clinical investigations that establish the product as safe and effective. 2. Those investigations must have been published in the scientific literature available to qualified experts. 3. Experts must generally agree, based on those published studies, that the product is safe and effective for its intended uses. At a minimum, the general acceptance of a product as GRASE must be supported by the same quality and quantity of scientific and/or clinical data necessary to support the approval of a New Drug Application.”
Few UVFs used in FDA-approved sunscreen products are considered GRASE but are sold under the definition of a ‘Marketed Unapproved Drugs’ as they have been in use for a long time, but may be lacking the rigorous testing described in this OTC Glossary definition (see Table 1) (Food and Drug Administration (US), 1978, Food and Drug Administration (US), 2006).
Table 1.
UV filters in use worldwide.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Sources: BASF Sunscreen Simulator- https://www.sunscreensimulator.basf.com/Sunscreen_Simulator/login/register, The Skin Cancer Foundation https://www.skincancer.org/skin-cancer-prevention/sun-protection/sunscreen/, in part from the FDA Fact Sheet on sunscreen issued in February of 2019 and from Federal Register FDA Proposed Rule February 2019 https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective.

-
1.Janjua N, Mogensen B, Andersson AM, Petersen J, Henriksen M, Skakkebaek N, et al. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol 2004;123:57–61.
-
2.Janjua NR, Kongshoj B, Petersen JH, Wulf HC. Sunscreens and thyroid function in humans after short-term whole-body topical application: a single-blinded study, Brit J Dermatol 2007;156:1080–1082.
-
3.Sarveiya V, Risk S, Benson HA. Liquid chromatographic assay for common sunscreen agents: application to in vivo assessment of skin penetration and systemic absorption in human volunteers. J Chromatogr B Analyt Technol Biomed Life Sci 2004;803(2):225–31.
-
4.Gonzalez H, Farbrot A, Larkö O, Wennberg AM. Percutaneous absorption of the sunscreen benzophenone-3 after repeated whole-body applications, with and without ultraviolet irradiation. Br J Dermatol. 2006;154(2):337–40.
-
5.Rodríguez E, Valbuena MC, Rey M, Porras de Quintana L.Causal agents of photoallergic contact dermatitis diagnosed in the national institute of dermatology of Colombia. Photodermatol Photoimmunol Photomed. 2006;22(4):189–92.
-
6.Krause M, Klit A, Blomberg Jensen M, Søeborg T, Frederiksen H, Schlumpf M, Lichtensteiger W, Skakkebaek NE, Drzewiecki KT. Sunscreens: Are They Beneficial for Health? An Overview of Endocrine Disrupting Properties of UV-Filters. Int J Androl. 2012;35(3):424–36. doi: https://doi.org//10.1111/j.1365–2605.2012.01280.x.
-
7.Ghazipura M, McGowan R, Arslan A, Hossain T. Exposure to benzophenone-3 and reproductive toxicity: A systematic review of human and animal studies. Reprod Toxicol. 2017;73:175–183. doi: https://doi.org//10.1016/j.reprotox.2017.08.015. Epub 2017 Aug 24.
-
8.Klinubol P, Asawanonda P, Wanichwecharungruang SP.Transdermal penetration of UV filters. Skin Pharmacol Physiol. 2008;21(1):23–9. Epub 2007 Oct 2.
-
9.Europa, SCCNFP, Opinion on Homosalate. 2007 https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_097.pdf.
-
10.Walters KA, Brain KR, Howes D, James VJ, Kraus AL, Teetsel NM, Toulon M, Watkinson AC, Gettings SD. Percutaneous penetration of octyl salicylate from representative sunscreen formulations through human skin in vitro. Food Chem Toxicol. 1997;35(12):1219–25.
-
11.Shaw DW. Allergic Contact Dermatitis from Octisalate and cis-3-Hexenyl Salicylate. Dermatitis, 2006; 17(3):152–5.
-
12.Singh M, Beck MH, Octyl Salicylate: A New Contact Sensitivity. Contact Dermatitis, 2007; 56(1):48.
-
13.Bryden AM, Moseley H, Ibbotson SH, Chowdhury MM, Beck MH, Bourke J, English J, Farr P, Foulds IS, Gawkrodger DJ, George S, Orton DI, Shaw S, McFadden J, Norris P, Podmore P, Powell S, Rhodes LE, Sansom J, Wilkinson M, van Weelden H, Ferguson JA. Photopatch Testing of 1155 Patients: Results of the U.K. Multicentre Photopatch Study Group. Brit J Dermatol 2006;155(4):737–47.
-
14.C.G.J. Hayden et al., Sunscreen Penetration of Human Skin and Related Keratinocyte Toxicity After Topical Application. Skin Pharmacol Physiol, 2005, 18(4):170–4.
-
15.Montenegro L, Carbone C, Paolino D, Drago R, Stancampiano AH, Puglisi G.n Vitro Skin Permeation of Sunscreen Agents from O/W EmulsionsInt J Cosmet Sci. 2008 Feb;30(1):57–65. doi: https://doi.org//10.1111/j.1468-2494.2008.00417.x.
-
16.Nash JF, Tanner PR. Relevance of UV Filter/Sunscreen Product Photostability to Human Safety. Photodermatol Photoimmunol Photomed. 2014 Apr-Jun;30(2–3):88–95. doi: https://doi.org//10.1111/phpp.12113. Epub 2014 Feb 19.
-
17.Knezevic NZ, Ilic N, D̵okic V, Petrovic R, Janackovic D. Mesoporous silica and organosilica nanomaterials as UV-blocking agents. ACS App Mater Interfaces 2018;10(24):20231–6. doi: https://doi.org//10.1021/acsami.8b04635.
-
18.Schlumpf M, Durrer S, Faass O, Ehnes C, Fuetsch M, Gaille C, Henseler M, Hofkamp L, Maerkel K, Reolon S, Timms B, Tresguerres JA, Lichtensteiger W. Developmental toxicity of UV filters and environmental exposure: a review. Int J Androl. 2008;31(2):144–51. doi: https://doi.org//10.1111/j.1365-2605.2007.00856.x. Epub 2008 Jan 10.
-
19.White I.R. (1995) Phototoxic and Photoallergic Reactions. In: Rycroft R.J.G., Menné T., Frosch P.J. (eds) Textbook of Contact Dermatitis. Springer, Berlin, Heidelberg.
Marine: relating to bodies of saltwater such as oceans and seas.
Aquatic: relating to bodies of freshwater such as lakes, streams, rivers, ponds, etc.
Estuarine: relating to bodies of water formed where freshwater from rivers and streams flowinto the ocean, mixing with the seawater. Estuaries and the lands surrounding them are places of transition from land to sea, and from freshwater to saltwater.
Biota: living things in an ecosystem.
Legislative actions related to the environmental impacts of UV filters
In the FDA proposed rule of February 2019, under CFR 25.31 for Human Drugs and Biologics, Section XIV, (FDA in, US-FDA, 2019b,c), it is stated “this action is of a type that does not individually or cumulatively have a significant effect on the human environment. Therefore, neither an environmental assessment nor an environmental impact statement is required.”
Nevertheless, many potentially harmful environmental effects of UVFs have been identified (Blitz and Norton, 2008) and led to the restriction of specific ingredients believed responsible for these changes (see Table 1, Table 2)). Hawaii, Key West and the United States Virgin Islands (USVI) have recently passed ordinances and/or legislation that prohibits the use of chemical sunscreens BP-3 and octinoxate (OMC), as correlation was found between these substances and coral reef bleaching (Bever, 2018, Fleshler, 2018, Schneider and Lim, 2019a, Schneider and Lim, 2019b). There are similar bans passed or in discussion in Palau, Bonaire, Aruba, Mexico, Brazil and the EU. In June 2019, USVI joined Hawaii and Key West in banning specific sunscreen products that have been deemed harmful to coral reefs and marine life (Blum, 2019).
Table 2.
Broad-spectrum or UVA I filter.
|
UV filter |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BP1 | BP2 | BP3 | BP4 | BP8 | EHMC/OMC | OC | 4-MBC | OD-PABA | B-MDM | 3-BC | PBSA | HMS | ||
| Organism | Class Citation # | |||||||||||||
| Arthrobacter globiformis | Bacteria 27,28 | *** | NE | NE | ||||||||||
| Isochrysis glabana | Algae 3,32 rank order |
*3 | *4 | *1 | *2 | |||||||||
| Desmodesmus subspicatus | Algae 12 | ** | ** | ** | ** | |||||||||
| Tetrahymena thermophila | Protozoan 6 | *** | NE | NE | *** | |||||||||
| Chironomus riparius | Insect-midge 26 | NE | ||||||||||||
| Pocillopora damicornis | Coral 29-33,35,37 rank order |
1 or 2 | 2 | 3 | 1 | ** | ||||||||
| Seriatopora caliendrum | Coral 33,35,37 | * | * | ** | ** | |||||||||
| Mytilus galloprovincialis | Mollusk-mussel 31,32 rank order |
*2 | *3 | *1 | ** | *1 | * | |||||||
| Melanoides tuberculata | Mollusk 28 | ** | ||||||||||||
| Potamopyrgus antipodarum | Mollusk-mud snail 27,28 | *** | NE | NE | ||||||||||
| Lumbriculus variegatus | Annelid- freshwater worm 27,28 | NE | NE | NE | ||||||||||
| Daphnia magna | Crustacean 12,13 | ** | *** | ** | ** | *** | ||||||||
| Siriella armata | Crustacean-carnivorous worm 32 | * | * | * | * | |||||||||
| Gammarus fossarum | Crustacean 11 | ** | ||||||||||||
| Tigriopus japonicus | Crustacean 30 | * | ||||||||||||
| Acartis tonsa | Crustacean 33 | *** | ||||||||||||
| Paracentrotus lividus | Echinoderm-sea urchin 31,32 rank order |
*2 | *3 | *** | *1 | ** | *1 | * | ||||||
| Danio rerio | Vertebrate/fish Zebrafish 14–16,27,2834,36,37 | *** | ** | ** | *** | ** | NE | |||||||
| Pimephales promelas | Vertebrate/fishFathead minnow 13, 23, 25 |
** | ** | |||||||||||
| Oncorrhynchus mykiss | vertebrate/fish trout 8,23,24 | x | *** | * | ||||||||||
| Wistar rat | Vertebrate/mammal 9,10,16–22 |
^T3, ^T4, lowTSH | *** | ** | *** | |||||||||
| Human leiomyoma, | Human cell line 7 | X | X | X | X | X | ||||||||
| Breast cancer cells | Human cell line 1, 2 | X | X | *** | X | |||||||||
| FLG loss of function | Human cell line 4 | X,XXX | X,XXX | |||||||||||
| Hirschsprung’s | 3 | XX | ||||||||||||
Legend: 2,4-dihydroxybenzophenone (BP1), Benzophenone- 2 (BP2), Oxybenzone, Benzophenone- 3 (BP3), Sulisobenzone, Benzophenone- 4 (BP4), Dioxybenzone (BP8), 4-methylbenzylidene-camphor (4-MBC), Ethylhexyl dimethyl para-aminobenzoic acid (OD-PABA), Ethylhexylmethoxycinnamate (EHMC, also known as oxymethyl cinnamate [OMC] or octinoxate), homosalate (HMS), Octocrylene (OC), Butyl-methoxydibenzoylmethane (B-MDM, avobenzone), 3-benzylidene camphor (3-BC), 2-phenylbenzimidazole-5-sulfonic acid (PBSA), ^= increased, NE= no effect, *= toxicity <100ug/L, **=toxicity 100ug-1mg/L, ***=toxicity 1-100mg/L X= toxicity in vitro, not quantified, XX=clinical association, XXX=increased absorption in vivo.
-
1.Alamer M, Darbre PD.Effects of exposure to six chemical ultraviolet filters commonly used in personal care products on motility of MCF-7 and MDA-MB-231 human breast cancer cells in vitro. J Appl Toxicol 2018;38(2):148-59. doi:10.1002/jat.3525. Epub 2017 Oct 9.
-
2.Mueller SO, Kling M, Arifin Firzani P, Mecky A, Duranti E, Shields-Botella J, Delansorne R, Broschard T, Kramer PJ. Activation of estrogen receptor alpha and beta by 4-methylbenzylidene camphor in human and rat cells: comparison with phyto- and xenoestrogens. Toxicol Lett 2003;142(1-2):89-101.
-
3.Huo W, Cai P, Chen M, Li H, Tang J, Xu C, Zhu D, Tang W, Xia Y.The relationship between prenatal exposure to BP-3 and Hirschsprung's disease. Chemosphere 2016;144:1091-7. doi:10.1016/j.chemosphere.2015.09.019. Epub 2015 Oct 23.
-
4.Joensen UN, Jørgensen N, Thyssen JP, Petersen JH, Szecsi PB, Stender S, Andersson AM, Skakkebæk NE, Frederiksen H. Exposure to phenols, parabens and UV filters: Associations with loss-of-function mutations in the filaggrin gene in men from the general population. Environ Int 2017;105:105-11. doi:10.1016/j.envint.2017.05.013. Epub 2017 May 17.
-
5.Fong HC, Ho JC, Cheung AH, Lai KP, Tse WK. Developmental toxicity of the common UV filter, benzophenone-2, in zebrafish embryos. Chemosphere. 2016;164:413-20. doi:10.1016/j.chemosphere.2016.08.073. Epub 2016 Sep 3.
-
6.Gao L, Yuan T, Zhou C, Cheng P, Bai Q, Ao J, Wang W, Zhang H.Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere 2013;93(10):2507-13. doi: 10.1016/j.chemosphere.2013.09.041. Epub 2013 Oct 13.
-
7.Pollack AZ, Buck Louis GM, Chen Z, Sun L, Trabert B, Guo Y, Kannan K. Bisphenol A, benzophenone-type ultraviolet filters, and phthalates in relation to uterine leiomyoma. Environ Res. 2015 Feb;137:101-7. doi:10.1016/j.envres.2014.06.028. Epub 2014 Dec 19.
-
8.Grabicova K, Fedorova G, Burkina V, Steinbach C, Schmidt-Posthaus H, Zlabek V, Kocour Kroupova H, Grabic R, Randak T. Presence of UV filters in surface water and the effects of phenylbenzimidazole sulfonic acid on rainbow trout (Oncorhynchus mykiss) following a chronic toxicity test. Ecotoxicol Environ Saf 2013;96:41-7. doi:10.1016/j.ecoenv.2013.06.022. Epub 2013 Jul 29.
-
9.Broniowska Ż, Ślusarczyk J, Starek-Świechowicz B, Trojan E, Pomierny B, Krzyżanowska W, Basta-Kaim A, Budziszewska B.The effect of dermal benzophenone-2 administration on immune system activity, hypothalamic-pituitary-thyroid axis activity and hematological parameters in male Wistar rats. Toxicology. 2018;402-403:1-8. doi:10.1016/j.tox.2018.04.002. Epub 2018 Apr 13.
-
10.Krzyżanowska W, Pomierny B, Starek-Świechowicz B, Broniowska Ż, Strach B, Budziszewska B.The effects of benzophenone-3 on apoptosis and the expression of sex hormone receptors in the frontal cortex and hippocampus of rats. Toxicol Lett 2018 Oct 15;296:63-72. doi:10.1016/j.toxlet.2018.08.006. Epub 2018 Aug 9.
-
11.Scheil V, Triebskorn R, Köhler HR. Cellular and stress protein responses to the UV filter 3-benzylidene camphor in the amphipod crustacean Gammarus fossarum (Koch 1835). Arch Environ Contam Toxicol 2008;54(4):684-9. Epub 2007 Nov 6.
-
12.Sieratowicz A, Kaiser D, Behr M, Oetken M, Oehlmann J. Acute and chronic toxicity of four frequently used UV filter substances for Desmodesmus subspicatus and Daphnia magna. J Environ Sci Health A Tox Hazard Subst Environ Eng 2011;46(12):1311-9. doi:10.1080/10934529.2011.602936.
-
13.Christen V, Zucchi S, Fent K. Effects of the UV-filter 2-ethyl-hexyl-4-trimethoxycinnamate (EHMC) on expression of genes involved in hormonal pathways in fathead minnows (Pimephales promelas) and link to vitellogenin induction and histology. Aquat Toxicol 2011;102(3-4):167-76. doi:10.1016/j.aquatox.2011.01.013. Epub 2011 Feb 2
-
14.Bluthgen N, Zucchi S, Fent K. Effects of the UV filter benzophenone-3 (oxybenzone) at low concentrations in zebrafish (Danio rerio). Toxicol Appl Pharmacol 2012;263:184-194
-
15.Zucchi, N. Blüthgen, A. Ieronimo, K. Fent. The UV-absorber benzophenone-4 alters transcripts of genes involved in hormonal pathways in zebrafish (Danio rerio) eleuthero-embryos and adult males. Toxicol Appl Pharmacol 2011;250:137-46
-
16.Faass O, Schlumpf M, Reolon S, Henseler M, Maerkel K, Durrer S, Lichtensteiger W. Female sexual behavior: estrous cycle and gene expression in sexually dimorphic brain regions after pre- and postnatal exposure to endocrine active UV filters. Neurotoxicol 2009; 30:249-260..
-
17.Carou E, Szwarcfarb B, Deguiz ML, Reynoso R, Carbone S, Moguilevsky JA, Scacchi P, Ponzo OJ. Impact of 4-methylbenzylidene-camphor (4-MBC) during embryonic and fetal development in the neuroendocrine regulation of testicular axis in prepubertal and peripubertal male rats. Exp. Clin. Endocrinol. Diabetes, 2009;117:449-454
-
18.Axelstad M, Boberg J, Hougaard KS, Christiansen S, Jacobsen PR, Mandrup KR, Nellemann C, Lund SP, Hass U. Effects of pre- and postnatal exposure to the UV-filter octyl methoxycinnamate (OMC) on the reproductive: auditory and neurological development of rat offspring. Toxicol. Appl. Pharmacol., 2011;250:278-290.
-
19.Klammer H, Schlecht C, Wuttke W, Schmutzler C, Gotthardt I, Köhrle J, Jarry H. Effects of a 5-day treatment with the UV-filter octyl-methoxycinnamate (OMC) on the function of the hypothalamo-pituitary-thyroid function in rats. Toxicol 2007; 238:192-199.
-
20.Carbone S, Szwarcfarb B, Reynoso R, Ponzo OJ, Cardoso N, Ale E, Moguilevsky JA, Scacchi P. In vitro effect of octyl − methoxycinnamate (OMC) on the release of Gn-RH and amino acid neurotransmitters by hypothalamus of adult rats. Exp. Clin. Endocrinol. Diabetes 2010;118: 298-303.
-
21.Szwarcfarb B, Carbone S, Reynoso R, Bollero G, Ponzo O, Moguilevsky J, Scacchi P. Octyl-methoxycinnamate (OMC), an ultraviolet (UV) filter, alters LHRH and amino acid neurotransmitters release from hypothalamus of immature rats. Exp. Clin. Endocrinol. Diabetes, 2008;116: 94-98.
-
22.Weisbrod C, Kunz P, Zenker A, Fent K. Effects of the UV filter benzophenone-2 on reproduction in fish. Toxicol Appl Pharm 2017;225:255-66.
-
23.Holbech H, Norum U, Korsgaard B, Bjerregaard P. The chemical UV filter 3-benzylidene camphor causes an oestrogenic effect in an in vivo fish assay. Pharmacol Toxicol 2002;91:204-8.
-
24.Kunz PY, Gries T, Fent K. The ultraviolet filter 3-benzylidene camphor adversely affects reproduction in fathead minnow (Pimephales promelas). Toxicol Sci, 2006;93:311-21.
-
25.Muniz-Gonzalez AB, Martínez-Guitarte JL. Effects of single exposure and binary mixtures of ultraviolet filters octocrylene and 2-ethylhexyl 4-(dimethylamino) benzoate on gene expression in the freshwater insect Chironomus riparius. Environ Sci Pollut Res Int. 2018;25(35):35501-14. doi:10.1007/s11356-018-3516-7. Epub 2018 Oct 22
-
26.Fent K, Kunz PY, Zenker A, Rapp M. A tentative environmental risk assessment of the UV-filters 3-(4-methylbenzylidene-camphor), 2-ethyl-hexyl-4-trimethoxycinnamate, benzophenone-3, benzophenone-4 and 3-benzylidene camphor. Mar Environ Res. 2010;69Suppl:S4-6. doi:10.1016/j.marenvres.2009.10.010. Epub 2009 Nov 11.
-
27.Kaiser D, Sieratowicz A, Zielke H, Oetken M, Hollert H, Oehlmann J. Ecotoxicological effect characterisation of widely used organic UV filters. Environ Pollut 2012;163:84-90. doi: 10.1016/j.envpol.2011.12.014. Epub 2012 Jan 11
-
28.He T, Tsui MMP, Tan CJ, Ng KY, Guo FW, Wang LH, Chen TH, Fan TY, Lam PKS, Murphy MB. Comparative toxicities of four benzophenone ultraviolet filters to two life stages of two coral species. Sci Total Environ. 2019;651(Pt 2):2391-9. doi: 10.1016/j.scitotenv.2018.10.148. Epub 2018 Oct 11.
-
29.Chen L, Li X, Hong H, Shi D. Multigenerational effects of 4-methylbenzylidene camphor (4-MBC) on the survival, development and reproduction of the marine copepod Tigriopus japonicus. Aquat Toxicol 2018;194:94-102. doi:10.1016/j.aquatox.2017.11.008. Epub 2017 Nov 16.
-
30.Giraldo A, Montes R, Rodil R, Quintana JB, Vidal-Linan L, Beiras R. Ecotoxicological evaluation of the UV filters ethylhexyl dimethyl p-aminobenzoic acid and octocrylene using marine organisms Isochrysis galbana, Mytilus galloprovincialis and Paracentrotus lividus. Arch Environ Contam Toxicol 2017;72(4):606-11. doi:10.1007/s00244-017-0399-4. Epub 2017 Apr 8.
-
31.Paredes E, Perez S, Rodil R, Quintana JB, Beiras R. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere 2014;104:44-50. doi: 10.1016/j.chemosphere.2013.10.053. Epub 2013 Dec 19
-
32.He T, Tsui MMP, Tan CJ, Ma CY, Yiu SKF, Wang LH, Chen TH, Fan TY, Lam PKS, Murphy MB. Toxicological effects of two organic ultraviolet filters and a related commercial sunscreen product in adult corals. Environ Pollut. 2019;245:462-71. doi: 10.1016/j.envpol.2018.11.029. Epub 2018 Nov 13.
-
33.Kusk KO, Avdolli M, Wollenberger L. Effect of 2,4-dihydroxybenzophenone (BP1) on early life-stage development of the marine copepod Acartia tonsa at different temperatures and salinities. Environ Toxicol Chem. 2011;30(4):959-66. doi: 10.1002/etc.458. Epub 2011 Feb 19.
-
34.Kunz PY, Galicia HF, Fent K. Comparison of in vitro and in vivo estrogenic activity of UV filters in fish. Toxicol Sci 2006;90(2): 349-61.
-
35.Corinaldesi C, Marcellini F, Nepote E, Damiani E, Danovaro R. Impact of inorganic UV filters contained in sunscreen products on tropical tony corals (Acropora spp.). Sci Total Environ. 2018;637-8:1279-85. doi:10.1016/j.scitotenv.2018.05.108. Epub 2018 May 22.
-
36.Schreurs R, Lanser P, Seinen W, van der Burg B. Estrogenic activity of UV filters determined by an in vitro reporter gene assay and an in vivo transgenic zebrafish assay. Arch Toxicol 2002;76:257-61.
-
37.Wood E. Impacts of sunscreens on corals - International Coral Reef Initiative (ICRI) briefing 2018. (Cited 4-11-2020) https://www.icriforum.org/sites/default/files/ICRI_Sunscreen_0.pdf.
The Hawaii and Key West bans are set to start to take effect in January 2021 and prohibit the sale of sunscreens containing the UVFs BP-3 or OMC without a physician’s prescription. The USVI began banning importation of sunscreens on December 31, 2019 with importing of sunscreens. On March 30, 2020, the sale or distribution of sunscreen products containing these UVFs was added to the ban. After January 1, 2021, transporting them into the USVI or possessing them will be completely banned, with first time violators facing potential fines of up to $1,000. The Virgin Islands National Park has stated that mineral sunscreen products with zinc oxide and titanium dioxide are the only sunscreens permitted for use by visitors and residents (Fajardo, 2019). The Hawaii ban was challenged by the AAD and the Hawaii Dermatological Society, citing that removing accessibility to broad spectrum sunscreen ingredients could create a public health concern.
These bans will lead to fewer products that can prevent skin cancers like melanoma, but may contribute to a public perception of sunscreens being unsafe products in general. Furthermore, these bans legislation does not emphasize that we are in need of newer, safer, and highly effective sunscreen ingredients as we reviewed in Part 1 of this review. (1 (Sabzevari et al., 2020, American Academy of Dermatology, 2019a, American Academy of Dermatology, 2019b).
UV filter effects on coral reefs
BP-3, OMC, OC and sulisobenzone have been considered as threats to coral reefs around the world and an estimated 14,000 tons of sunscreen, some containing as much as 10% BP-3, are washed off swimmers into coral reef areas annually (Schneider and Lim, 2019a, Schneider and Lim, 2019b, Mitchelmore et al., 2019, Du et al., 2017). The impact of sunscreen pollution is possibly being magnified by public health messaging on skin health and skin cancer prevention. However, it is important to note the magnitude of UVF effects is far below other factors endanging coral reefs, (e.g. rising ocean temperatures, acidification and loss of CO2 metabolism from plankton) which is expanded below in section 4 (Schneider and Lim, 2019a, Schneider and Lim, 2019b; 2018).
Coral bleaching refers to the loss of the essential symbiotic unicellular algae called zooxanthellae (Symbiodinium spp), that live within the newly developing tips of living coral called coral polyps. This results in a loss of color on the outer margins and a whitening or bleaching effect. Coral reef ecosystems support many marine biota, so many other species can be affected by repeated bleaching events that lead to coral death.
Numerous studies have shown that some UVFs may contribute to and exacerbate widespread coral bleaching in marine ecosystems especially in coastal areas popular with recreational swimmers (Mitchelmore et al., 2019, Environmental Working Group, 2019b, Environmental Working Group, 2019a, Corinaldesi et al., 2018, Wood, 2018, Danovaro et al., 2008). These studies have included UVF concentration data from many beaches and urban ports as well as remote and unpopulated marine environments. Most studies suggest that UVFs are present in beach water and sand in steady state concentrations ranging from 10 ng/L to 1ug/L but changes occur in relation to degrees of human activity (Scheil et al., 2008, Downs et al., 2016, Mao et al., 2018, Mitchelmore et al., 2019). There is little data on the high flux of UVF washing off swimmers or divers at peak recreational times or sites. Recent studies along beaches of the French Riviera, Hawaii, as well as rivers and lakes near these tourist populations do support this as a toxicity risk (Kung et al., 2018, Mitchelmore et al., 2019, Tovar-Sanchez et al., 2013, Tovar-Sanchez et al., 2019, Labille et al., 2020, Guo et al., 2020, Tang et al., 2018)
Several species of hard coral have been studied in situ using living corals in laboratories that keep cultures bathed in seawater circulated from adjacent beachfronts. Other studies use in vitro cultures of algae to test toxicity of UVF exposure directly (Sieratowicz et al., 2011, He et al., 2019a, He et al., 2019b). The studies have shown that toxicity is found in the ranges of 10-300ug/L depending on UVF and species (10-100x the reported concentrations from various locales worldwide (Labille et al., 2020, Mitchelmore et al., 2019, Downs et al., 2016, Du et al., 2017, Narla and Lim, 2020). Gross effects were noticed within 18–48 hours, followed by complete bleaching within 96 hours. Untreated controls showed no change.
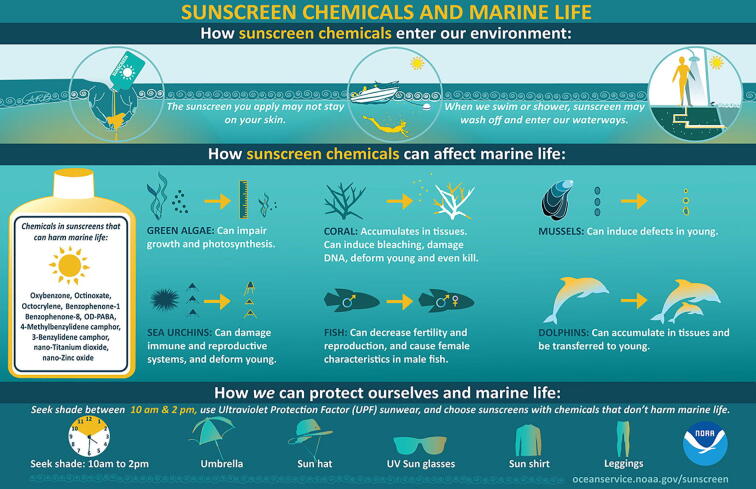
There is also a suggestion that UVF promote the propagation of latent viral infections in the zooxanthellae which force them to enter a lytic cycle and then be expelled from the coral polyp (Danovaro and Corinaldesi, 2003, Downs et al., 2014; Paredes et al., 2013; Giraldo et al., 2017, Corinaldesi et al., 2018). The subsequent die-off of zooxanthellae creates stressful survival conditions for the coral. Corals can survive the stress of a transient bleaching event, but when corals are stressed they are subject to mortality. Recovery can begin once the stress is removed and algae repopulate the tender coral polyps, however, continued exposure can kill corals. Other studies have shown UVFs to have direct effects on ossification and DNA structure of larval coral (Fig. 1 NOAA Infographic- https://oceanservice.noaa.gov/news/sunscreen-corals.html,) (Ruszkiewicz et al., 2017, Downs et al., 2016, see references with Table 2). Approximately 60% of the world’s coral ecosystems are currently threatened due to various causes, many of which are anthropogenic (i.e. related to human activity), including UVF contamination (Danorvaro et al., 2008). Thus coral bleaching may be a consequence of UVF pollution but the magnitude of their effects is not clear as many other factors can affect corals (see below). Caution with use of organic/chemical UVF containing sunscreens with preferences for inorganic/physical UVF products containing ZnO and/or TiO2 is still the best advice for patients, along with UV-protective clothing and avoidance of peak hours of sun exposure and follows the guidelines of the AAD.
Fig. 1.
NOAA's National Ocean Service Sunscreen Infographic. Published with permission of National Oceanic and Atmospheric Administration, National Ocean Service. New NOAA infographic V2 here Infographic: Sunscreen Chemicals and Marine Life.
Other causes of coral bleaching
Warming of ocean water temperatures (as well as sudden cooling) can also lead to coral bleaching, with numerous cycles of this phenomenon reported in the Pacific over the last century (Narla and Lim, 2020, Cheng et al., 2019, Slattery et al., 2019, Hughes et al., 2019, Authority GBRMP, 2016, Barkley et al., 2018). Thus global warming and changes in warmer ocean currents (el niño) can impact coral health (Eakin, 2016). Inorganic UVF (eg. ZnO, TiO2) and organic UVF (eg. BP-3, octinoxate and OCTO) may also promote this effect in ocean water (Corinaldesi et al., 2018, Jovanovic and Guzman, 2014, Schneider and Lim, 2019a, Schneider and Lim, 2019b). By absorbing or refracting UV rays, UVFs transfer thermal energy which creates localized increases in water temperatures, much the same as when applied to human skin (Lim, Thomas, Rigel Photoprotection in Photoaging, Marcel Dekker 2008). Blocking UV transmission through water can also indirectly damage coral by inhibiting photosynthesis within zooxanthellae (Danovaro et al., 2008).
While studies quantifying the magnitude of these UVF effects, it is generally accepted that they are smaller than other factors which are toxic to corals. Rising temperatures also due to higher CO2 in the atmosphere, acidification due to CO2 dissolving in oceans, toxic chemicals and microplastic pollution with resulting die-off of plankton are all major factors. According to Dryden, if our oceans were clean and had healthy plankton (which are one of most efficient metabolizers of CO2), they could absorb twice the CO2 they do today – (12 to 24 billion (giga) tonnes/year (current human-related CO2 emissions are estimated 16–17 billion (giga) tonnes per year (Dryden, 2020). Thus UVF pollution is only one of many factors that lead to coral bleaching and premature death.
UVF pollution is ubiquitous
Human water sources are also affected by UV filters in the environment. Studies have shown that man-made organic UVFs, such as BP-3, OCTO, octinoxate, and ethylhexyl salicylate have been found in almost all water sources worldwide. Reviews by DiNardo and Downs (2016, 2017), Schneider and Lim (2018) and Narla and Lim (2020), note that wastewater treatment plants (WWTP) are not effective at removing these compounds due to their innate chemical properties (low water solubility, high lipophilicity, and high organic carbon–water coefficient). Ozonation is a common method of disinfection in WWTPs and has been shown to not reduce toxicity of BP-3, OMC and OC (Hopkins et al., 2017). WWTP influents have been shown to have BP-3 concentrations > 10 ug/L in some locales (Kim and Choi, 2014, Wu et al., 2018). The organic filters are found in higher concentrations in urban areas, and tend to fluctuate based on the season, density of near shore beach activity and with currents (Balmer et al., 2005, Ekpeghere et al., 2016, Tovar-Sanchez et al., 2019).
Studies in marine and aquatic locations with higher density of human activity (see section 3) have also drawn attention to the possibility that UVFs can persist for a long time in water and sediments, and that tides and currents might carry them great distances to previously pristine areas (Balmer et al., 2005, Emnet et al., 2014, Tang et al., 2018). UVFs were identified in the sewage of two large Antarctic research stations, McMurdo Station and Scott Base, and the same compounds were also identified in the surrounding seawater up to 25 km away (Emnet et al., 2014). The presence of these UVFs is particularly concerning in the Antarctic because the environment factors (long periods of darkness, presence of sea ice, and cold temperatures) slow down microbial and photo-degradation of these compounds as well as increasing ocean temperatures that speed ice melting (Downs et al., 2016, Blitz and Norton, 2008, Emnet et al., 2014).
In addition to natural water sources, organic UVFs have also been found in chlorinated water sources like swimming pools and WWTP discharges. In vitro studies with human diploid fibroblast cultures have shown that chlorinated BP-3, OMC, BP-3 and avobenzone lead to a higher rate of cell death compared to non-chlorinated controls in vitro (Manasfi et al., 2017, Sherwood et al., 2012). It is unknown what impact these chlorinated byproducts have on human health and further studies are necessary (see Table 2) (Schneider and Lim, 2019a, Schneider and Lim, 2019b).
UV filters from industrial use as protectants against photodegradation and from other PCPCuses (makeup, nailpolish, shampoo, conditioners, etc.) also make their way through WWTP and rainwater runoff into our waterways and add to the burden of UVF pollution as well (Hahladakisa et al., 2018).
UVF effects on aquatic and marine organisms
In late 2019 and early 2020 we performed a series of literature searches using NIH databases (eg, PubMed and Medline), EPA databases and Google Scholar using the terms UV filter, sunscreen, toxicity and aquatic life. These resulted in studies on 20 different species including corals (He et al., 2019a, He et al., 2019b), planktonic crustaceans (e.g. Sieratowicz et al., 2011), amphipod crustaceans (e.g. Scheil et al., 2007), mollusks (e.g. Kaiser et al., 2012), algae (e.g. Paredes et al., 2014), bacteria (e.g. Gao et al., 2013), sea urchins (e.g. Giraldo et al., 2017), zebrafish (e.g Fong et al., 2016), fathead minnows (e.g. Christen et al., 2011), and rainbow trout (e.g. Grabicova et al., 2013). Most toxicity studies reported UVF effects in the range of 100 ug/l to5 mg/l concentrations, with most of the published UVF concentrations in high density beach or metropolitan areas being in the 10–1000 ng/l range. Some locales have reports of 10–100 ug/l concentrations of some UVFs (Balmer et al., 2005, Ekpeghere et al., 2016, Langford et al., 2015, Guo et al., 2020, Tang et al., 2018, Kung et al., 2018, Kusk et al., 2011). The organisms and relative toxicities of UV filters are summarized in Table 2 (along with more extensive references), highlighting where specific UVFs and aquatic or marine biota overlap on this threshold for environmentally relevant toxicity (10–100 ug/l). This table of specific organisms and the reported UVF effects highlights the diversity of environmental, metabolic and toxic effects reported across human cell lines, other mammals, fish, coral, mollusks, algae and bacteria.
Laboratory studies have also shown that there are some pronounced effects of UV filters in fish (Kunz et al., 2006b, Fent et al., 2008). In zebra fish, octocrylene alters the development of the brain and liver (Fong et al., 2016). In Japanese rice fish, high levels of BP-3 in a laboratory setting led to decreased egg production, significantly fewer hatchings, as well as the induction of vitellogenin protein, a precursor of the egg yolk only found in females, in male fish (Schneider and Lim, 2019a, Schneider and Lim, 2019b, Wang et al., 2016). Species vary considerably as Chen et al. (2018) have shown no effects of BP-3 in the false clown anemonefish which inhabits many coral reefs, as well as in Siamese fighting fish (Chen et al., 2016).
As mentioned earlier, these are steady state findings and do not take into account the potentially higher levels locally seen near wastewater discharge or when a group of divers all jump into a prime reef sightseeing location and all that freshly applied sunscreen begins to wash off (Blitz and Norton, 2008, Mitchelmore et al., 2019, Matta et al., 2019, Bhatia and Friedman, 2020, Downs et al., 2016, Akhiyat and Olasz-Harken, 2019, Tingley, 2020, Wang and Lim, 2019).
Taken together, UVF pollution appears to have relevant in vitro and in vivo effects on marine biota but the long term implications of these effects are still unknown. Concentrations of these agents range from 10-1000x fold lower in local waters compared to that of the amount associated with biologic effects (Table 2). The reader is advised to follow local regulations when going to bodies of water for recreation and preferentially use TiO2 or ZnO sunscreen products, wear UV-protective clothing, and/or avoid peak hours of exposure whenever possible to mitigate the potential effects of organic/chemical UVFs on local biota.
Human health impact of UV filter exposure
Historically, studies on the environmental effects of man-made chemicals have attempted to assess the disruption of normal endocrine pathways in a variety of species (National Institute of Health, 2020, Environmental Protection Agency (US), 2010, Environmental Protection Agency (US), 2010). In 2001, the first articles to suggest that UVFs can disrupt endocrine pathways created an immediate concern among European environmental scientists and the European Union’s Commission for Public Health (Europa) asked its Scientific Committee on Cosmetic Products and Non-Food Products for further evaluation (Europa, 2001; Schlumpf et al., 2001, Schlumpf and Lichtensteiger, 2001, Nash, 2006). In vivo studies in humans, rats, frogs, fish and worms, as well as in vitro studies suggest that many commonly used organic UVFs have endocrine-disrupting properties, however these studies vary widely in dosage and exposure to specific UVFs (Janjua et al., 2004, Schneider et al., 2005, Schlumpf et al., 2001, Schlumpf et al., 2004, Morohoshi et al., 2005, Carou et al., 2008, Carou et al., 2009, Fent et al., 2008, Kunz et al., 2006a, Kunz et al., 2006b, Weisbrod et al., 2017, Klammer et al., 2007, Carbone et al., 2010, Szwarcfarb et al., 2008, Holbech et al., 2002, Wang et al., 2011).
Endocrine disruption has been associated with several organic UVFs (Heneweer et al., 2005, Schlumpf et al., 2001, Coronado et al., 2008, Krause et al., 2012, Broniowska et al., 2018, Krzyzanowska et al., 2018) (see Table 2). BP-3 has also been reported to have systemic effects on sex and thyroid hormone pathways in animal models (Schreurs et al., 2002, Krause et al., 2012, Broniowska et al., 2018, Akhiyat and Olasz-Harken, 2019). OMC has been associated with lower levels of thyroid hormone (T4) due to its ability to inhibit 5′-deiodinase (Ma et al., 2003, Janjua et al., 2007, Krause et al., 2012, Broniowska et al., 2018). This enzyme is responsible for converting the inactive form of thyroid hormone (T4) to the active triiodothyronine (T3). BP-3, 4-MBC, and OMC have also been associated with minor changes in testosterone, estradiol, and inhibin B in male patients, decreased sperm counts, and delayed puberty (Joensen et al., 2017, Mueller et al., 2003, Schlumpf et al., 2008). None of these human studies have yet to show any real world human biologic consequences.
BP-3 can be absorbed at a rate of 1% to 9% with topical application in some models (Klimova et al., 2015, Environmental Working Group, 2019b, Environmental Working Group, 2019a). Recent single application (2 mg/m2 to 75% of body surface area) and maximal use application studies (TEA testing in 2011 Final Rule) (75% of body surface area, four times daily) result in plasma and stratum corneum levels 10–2000 times the FDA guideline of 0.5 ng/ml for plasma levels of organic UV filters. Tissue levels were 10–1000 fold higher than plasma levels (Klimova et al., 2015, Janjua et al., 2004, Janjua et al., 2007, Matta et al., 2019, Matta et al., 2020). The Matta et al., studies showed detectable plasma and skin levels of all UV filters beyond the 21d study duration. As with the endocrine studies in humans, no acute or chronic toxicity data has been reported from these absorption studies (Klimova et al., 2015, Matta et al., 2019, Matta et al., 2020). Earlier work by Walters et al., has suggested that some of the salicylate UVFs can increase the risk for salicylism through percutaneous absorption (Walters et al., 1978).
Individuals with compromised skin barrier function such as the filaggrin loss-of-function mutations (FLG null- see in 40+% of atopic dermatitis patients) may absorb UVFs more rapidly (Joensen et al., 2017). UVFs have been found in breast milk (Schlumpf et al., 2008, Schlumpf et al., 2001), placental tissues (Kim and Choi, 2014) and is detected in nearly every American’s urine (Olson, 2006, DiNardo and Downs, 2018, Environmental Working Group, 2019b, Environmental Working Group, 2019a). Exposure to BP-3 during pregnancy has been reported to be associated with an increased incidence of Hirschprung’s disease, a neonatal intestinal dysfunction (Huo et al., 2016, DiNardo and Downs, 2019). The pathogenesis is likely related to the failure of neural crest cells to migrate to the distal hindgut during fetal organogenesis, specifically during weeks 5 to 12. Other studies suggest possible correlations with uterine leiomyoma formation and increased mobility of breast and lung cancer cells (Alamer and Darbre, 2018, Pollack et al., 2015, Phiboonchaiyanan et al., 2017, Wang et al., 2018) (see Table 2).
The UVFs (especially BP-3, OC, amiloxate, avobenzone and PABA) have been reported to cause various forms of irritant dermatitis as well as allergic contact and/or photo-allergens. According to a study by the European Scientific Committee on Consumer Safety, out of 6378 patients, 159 tested positive on photo patch tests for BP-3 between 1981 and 2003 (Lim et al., 2004, DiNardo and Downs, 2019). The spectrum of allergic reactions to UVF has been extensively reviewed elsewhere and will not be reviewed here (Schauder and Ippen, 1997, Heurung et al., 2014).
Similar to effects on aquatic and marine biota, humans can be exposed to UVF from WWTP and other industrial and cosmetic sources as well as from sunscreens (Schneider and Lim, 2019b, Matta et al., 2019, Matta et al., 2020, da Silva et al., 2015, Balmer et al., 2005, Brausch and Rand, 2011). As mentioned earlier, in vivo studies in which subjects ingest or undergo subcutaneous injection with UVFs found evidence of broad endocrine disruption biochemically but without any lasting effects (Schlumpf et al., 2004, Schlumpf et al., 2001, Bolt et al., 2001).
Public health agencies including the EU’s Commission for Public Health (Europa - Hansen and Baun 2012), the National Institute of Health, 2020, Environmental Protection Agency (US), 2010, National Institute of Health, 2020, Environmental Protection Agency (US), 2010 and FDA (US - Matta et al., 2020) have all concluded that current organic UVFs do not pose short or long-term endocrinologic risks to human health. These regulatory bodies have not been able to effectively address long-term effects on humans or the environment from sustained systemic exposure to UVFs and with their prolonged existence in the environment (see below bioaccumulation and biomagnification), low level exposures may continue for much of a human’s lifetime.
Narla and Lim (2020) nicely summarize these potential human biological effects, pointing out that UVF-induced disruptions in thyroid and sex hormones in experimental animals were reversible. In humans, similar dose-dependent endocrinopathies would require 30–250 years of daily use under real world use conditions (Ma et al., 2003, Heneweer et al., 2005, Schlumpf et al., 2001, Coronado et al., 2008, Janjua et al., 2007).
Thus we agree with the AAD still strongly supporting the use of both organic and inorganic UVF as part of their ‘Practice Safe Sun’ initiatives, as reviewed in part 1 of this review.
UV filter effects on the marine food chain and bioaccumulation
Organic/lipophilic substances cross cell membranes easily and are therefore more likely to be biologically active and capable of altering physiologic processes (Emnet et al., 2014). Many organic UVFs are also lipophilic and have been found to accumulate in the fat of many freshwater and marine species, making them theoretically capable ofbioaccumulation up the food chain. Bioaccumulation in human adipose tissue has been well documented with freshwater fish consumption in areas including the Great Lakes with mercury, DDT, polychlorinated biphenyls (PCBs) (EPA-US) 2017). Organic UVFs have been shown to follow similar metabolic pathways, thus when people eat those fish, the lipophilic compounds are further concentrated in human adipose tissue (Balmer et al., 2005, Langford et al., 2015, Saunders et al., 2020).
Trace amounts of UV filters, mostly 4-MBC, were found in fish species including: perch, white fish, and roach in lakes in Switzerland (Balmer et al., 2005, Buser et al., 2006). Surveys of Swiss rivers detected hormonally active UVFs in all fauna samples (mussels, several fish species, and cormorants). The concentrations of UVFs in the biota’s tissues increased as one ascended trophic levels of the aquatic food web, suggesting biomagnification of these compounds (Fent et al., 2010a, Fent et al., 2010b). In Norway, cod liver specimens contained organic UV filters, most notably octocrylene (found in 80% of specimens) and BP-3 (found in 50% of specimens). In Spain, similar UV filters were found in fish species including: white fish, rainbow trout, barb, perch, chub, and mussels (Blitz and Norton, 2008, Schneider and Lim, 2019a, Schneider and Lim, 2019b, Narla and Lim, 2020, Saunders et al., 2020). Similar findings have also been seen in aquatic biota in the Pearl River Estuarine of the South China Sea (Peng et al., 2017).
Laboratory studies have also shown that there to be variability between species of UVF absorptioned in (Kunz et al., 2006b, Fent et al., 2008). In zebra fish, OC alters the development of the brain and liver (Fong et al., 2016). In Japanese rice fish, high levels of BP-3 in a laboratory setting led to decreased egg production, significantly fewer hatchings, as well as the induction of vitellogenin protein, a precursor of the egg yolk only found in females, in male fish (Schneider and Lim, 2019a, Schneider and Lim, 2019b, Wang et al., 2016). Many of these toxicology studies are summarized in Table 2.
Bioaccumulation of UVF in marine mammals was first reported by Gago-Ferrero et al. (2013) in a Brazilian coastal study. These authors screened liver tissue samples, from dead LaPlata dolphins (Pontoporia blainvillei) that had been beached or accidentally caught, for UV filters. OC was found in 21 of 56 specimens at concentrations between 89 and 782 ng/g lipid and mirrored the local levels found in biota consumed by these dolphins (Gago-Ferrero et al., 2013). Marine UVF bioaccummulation has also been shown in vivo over a 10 year span in mollusks from the Chinese Bohai Sea (Liao and Kannan, 2019), in Japan’s Ariake Sea of invertebrates, hammerhead sharks and coastal birds (Nakata et al., 2009).
Thus long term studies of these marine biosystems should provide more meaningful data to guide future human use recommendations as the bioaccumulation of UVF up the food chain is now well established.
Human bioaccumulation
These findings imply that humans with a mainly seafood-based diet may be at risk for bioaccumulating UVFs, but there limited long term studies compared to those for PCBs or mercury as mentioned above (Gago-Ferrero et al., 2012). During a 2003–4 NHANES survey, Calafat et al. (2008) detected BP-3 in 96.8% of urine samples from 2517 US adults. The mean level was 22.9 µg/L, varying from 0.4 µg/L to 21,700 µg/L and a subset of 30 volunteers with no documented exposure to BP-3 had it detected in 90% of urine samples. Schlumpf et al. (2008) reported the results of a 2004–2006 Swiss study on BP-3, 4-MBC, OMC, OC, and other common UVFs in the breastmilk of 34 women. 27 women reported current use of some type of UVF-containing cosmetic product. UVFs were detected in 26 breast milk samples, with a strong correlation found between exposure to a specific UVF and its presence in the individual’s milk sample. These findings reflect the widespread presence of BP-3 in PPCPs (various cosmetics and sunscreens) as well as possible consequences of indirect exposure to BP-3 through the environment (as mentioned above) (EPA (US), 2005). As mentioned above, there are some correlations also reported for UVFs in relationship to uterine leiomyoma (Pollack et al., 2015) and on the motility of breast and lung cancer cell lines (Alamer et al., 2018), making the potential for bioaccumulation effects more poignant for the average woman’s diet.
Thus human bioaccumulation remains unproven and an area that the FDA could encourage further research, especially long term studies. Sunscreen recommendations should not be altered at this time, but these findings should give us pause and require further study. Women in particular should carefully weigh the risks and benefits of these agents in light of these data, and consider use of physical blockers, UV protective clothing, and sun avoidance when possible, especially during pregnancy.
Nanoparticle UV filters
The use of nanotechnology has become commonplace in a wide array of chemical and biological products and processes. Nanoparticles, named for sizes in the nanometer range (one-billionth of a meter), are chemically identical to the conventional forms. However, the small size of nanoparticles confers increased photoelectric reactivity due to the relatively greater surface area per unit of mass (Environmental Protection Agency (US), 2010, Environmental Protection Agency (US), 2010). This technology employs the use of particles on the microscopic or atomic scale to improve the performance of hundreds of consumer products, ranging from energy drinks, protective clothing, sports equipment, cosmetics, storage containers, pharmaceuticals, and sunscreens.
Although TiO2 and ZnO have long been used as physical blockers in sunscreens, nanoparticulate versions are relatively new and have become popular as they appear ‘relatively’ transparent on the skin compared to older formulations with their telltale thick, pasty white appearance (Environmental Protection Agency (US), 2010, Environmental Protection Agency (US), 2010, Schlossman et al., 2015). Nanoparticles (especially nano-TiO2) are often coated with compounds to prevent or reduce photoelectric reactions. Although the ecotoxicological effects of nanoparticles on marine and aquatic organisms have not been studied extensively, scientists caution that these particles may have adverse biological and environmental effects at concentrations as low as ug/L, the equivalent of a few drops of liquid in an Olympic-sized swimming pool (Gruden and Mileyeva-Biebesheimer, 2009, Schlossman et al., 2006).
We mentioned earlier that inorganic UVFs can block UV rays from coral algae and inhibit photosynthesis and may add local increases in water temperatures. Nanoparticle ZnO and TiO2 should be assumed to do the same but data is less robust. Nano-TiO2 was shown to affect algae by Jovanovic and Guzman (2014), and nano-ZnO was more toxic to algae than ZnO (Narla and Lim, 2020, Miller et al., 2012). Both nano-TiO2 and nano-ZnO can aggregate on organism’s surfaces, where they can be toxic even without entering the cells (Corinaldesi et al., 2018).
Federici et al. (2007) observed severe damage to gills of trout from environmental exposure to TiO2, and, dietary contamination with nano-TiO2 is toxic in some species of fish (Ramsden et al., 2009, Ramsden et al., 2013, Chen et al., 2011, Fouqueray et al., 2013). While some aspects of nanoparticle ecotoxicity are beginning to be understood, the degradable nanomaterial coating these particles has been studied very little, both release of these agents in vivo and unmasking of the free radical oxygen on the surface of nanoparticles are potentially causes of damage to biota (Fouqueray et al., 2013, Handy et al., 2008).
Human use of nano-ZnO and nano-TiO2 make the application and appearance of these sunscreen products more cosmetically appealing (see part 1 of these reviews, Narla and Lim, 2020). Some studies indicate that large doses of these nanoparticles can harm human cells and organs (mainly when inhaled), but no evidence has been published that enough nano-ZnO or nano-TiO2 can be absorbed percutaneously and cause systemic effects. Variations in particle size and whether there is a surface coating of the nanoparticles (mainly TiO2 using silica, magnesium or aluminum (Lewicka et al., 2013, Grande and Tucci, 2016) in sunscreen products to neutralize free radical oxygen moieties) remain variables in need of further toxicology research (Schneider and Lim, 2018; Schilling et al., 2010). Inhaled nanoparticles are difficult for the lungs to clear, and can be transferred to the bloodstream and may be pulmonary carcinogens. Nanoparticles in the bloodstream can cause organ damage through oxidative stress and/or activation of proinflammatory pathways (Grande and Tucci, 2016, Nohynek and Dufour, 2012, Hansen and Baun, 2012, Europa, 2007, Ze et al., 2014). Based on these findings, the International Agency for Research on Carcinogens has classified nano-TiO2 as a possible carcinogen when inhaled in large doses.
There is also some evidence that nanoparticles have environmental effects, including coral bleaching (inhibition of photosynthesis) and adding to ocean temperatures by transmission of heat energy when blocking UV (similar to other UVFs}. Marine and or aquatic biota that ingest nanoparticles may be at increased risks for carcinogenesis and genotoxicity over time (bioaccumulation) In support of this are reports showing both nano-ZnO and nano-TiO2can have cumulative neurotoxicity to microglia (Kwon et al., 2014, Rihane et al., 2016; Schneider and Lim, 2018; Corinaldesi et al., 2018). Bioaccummulation studies with nano-TiO2 have shown that algae bathed in nano-TiO2-laden growth medium, then fed to freshwater fleas (Daphnia magna), and finally feeding the fleas to zebrafish resulted in no nanoTiO2 accumulation (Chen et al., 2011, Fouqueray et al., 2013, Zucchi et al., 2011).
Thus, nanoparticles may have far more complex biologic effects than the older forms of ZnO and TiO2, and caution is advisable when counselling patients, especially with spray sunscreen products which have higher risk for inhalation.
Expanding options for UV filters in the US market and beyond
The global sunscreen industry is estimated to be worth in excess of $24B USD by 2024 with approximately one third of that being in the North American market. (https://www.transparencymarketresearch.com/sun-care-market.html). As part of the 2019 Final Rule, the FDA is encouraging manufacturers to accelerate testing and applications for approval to GRASE status or through the NDA process (FDA-US 2019). High throughput testing has been proposed to help with some of the toxicity studies needed for this process (Erickson, 2018, Matta et al., 2020, Wang and Lim, 2011).
In such a competitive market, the testing and approval processes may seem a deterrent to new product development. Gradual decreases in successive batches of the concentrations of UVF that have the most evidence of toxic effects, might lead to competitive edge for environmentally conscious manufacturers. Partnering with EU and Australian manufacturers may also help bring more eco-friendly products to the US market. We encourage the FDA to do whatever it can to help make it financially viable for manufacturers to perform the necessary testing, as well as to bring other agents (as in Europe) into the US market are part of the NDA process (a well-traveled path for pharmaceuticals entering the US).
Conclusion – call to action (opinions of the authors)
The use of sunscreen has been shown to reduce the incidence of squamous cell carcinoma by 40% and melanoma by 50% (American Academy of Dermatology, 2019a, American Academy of Dermatology, 2019b, Green et al., 2011). New legislation in Hawaii, the USVI, and other locations have begun to ban the use of certain organic UVFs in PPCPs. Currently the evolution of regulatory guidelines about sunscreen products is not keeping pace with the growing bodies of research on toxicities we have reviewed. Consequently, there is concern amongst dermatologists that a growing skepticism about certain sunscreens may lead to an overall decrease in their use (Schwen, 2005). To prevent this, and provide safe eco-friendly product options, it is imperative that more research on both the long term human effects and the cumulative effects on our environment, be done before deeming certain organic and/or inorganic UVFs as safe (GRASE) or unsafe for use. The AAD and FDA still recommend using sunscreen to protect the skin from UV to prevent skin cancer and photoaging. We hope that there can be better collaboration between regulatory, industry and advocacy groups to move the process forward to best provide a portfolio of safe, effective options to help protect our patients from UV damage and skin cancer, as well as protect our environment.
Conflict of Interest
None.
Funding
None.
Study Approval
NA.
Contributor Information
David Fivenson, Email: dfivenson@fivensondermatology.com.
Sultan Qiblawi, Email: qiblawis@msu.edu.
Jason Blitz, Email: Jason.b.blitz.mil@mail.mil.
References
- Akhiyat S., Olasz-Harken E.B. Update on human safety and the environmental impact of physical and chemical sunscreen filters: What do we know about the effects of these commonly used and important molecules? Pract Dermatol. 2019:48–51. https://practicaldermatology.com/articles/2019-feb (cited 4-11-2020) [Google Scholar]
- Alamer M., Darbre P.D. Effects of exposure to six chemical ultraviolet filters commonly used in personal care products on motility of MCF-7 and MDA-MB-231 human breast cancer cells in vitro. J Appl Toxicol. 2018;38(2):148–159. doi: 10.1002/jat.3525. Epub 2017 Oct 9. [DOI] [PubMed] [Google Scholar]
- American Academy of Dermatology, How to apply sunscreen. https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent/how-to-apply-sunscreen, 2019 (cited 4-11-2020).
- American Academy of Dermatology. Sunscreen FAQs. https://www.aad.org/public/everyday-care/sun-protection/sunscreen-patients/sunscreen-faqs, 2019 (cited 4-11-2020).
- Authority GBRMP, Final report: 2016 coral bleaching event on the Great Barrier Reef, GBRMPA, Townsville, 2017 Search PubMed.
- Bhatia N, Friedman A. Sunscreen chemicals found in the bloodstream: Expert reaction. https://practicaldermatology.com/articles/2019-june/recentdevelopments (cited 4-11-2020).
- Balmer M., Buser H.R., Muller M., Poiger T. Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from Swiss lakes. Environ Sci Technol. 2005;39:953–962. doi: 10.1021/es040055r. CrossRef CAS PubMed. [DOI] [PubMed] [Google Scholar]
- Barkley H.C., Cohen A.L., Mollica N.R., Brainard R.E., Rivera H.E., DeCarlo T.M., Lohmann G.P., Drenkard E.J., Alpert A.E., Young C.W., Vargas-Angel B., Lino K.C., Oliver T.A., Pietro K.R., Luu V.H. Repeat bleaching of a central Pacific coral reef over the past six decades (1960–2016) Commun Biol. 2018;1:177. doi: 10.1038/s42003-018-0183-7. eCollection 2018. CrossRef PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bever L. Hawaii just banned your favorite sunscreen to protect its coral reefs. The Washington Post 2018. https://www.washingtonpost.com/news/energy-environment/wp/2018/07/02/hawaii-is-about-to-ban-your-favorite-sunscreen-to-protect-its-coral-reefs/?utm_term=.883c2c288c08. (cited 4-11-2020).
- Blitz J., Norton S.A. Possible environmental effects of sunscreen run-off. J Am Acad Dermatol. 2008;59:898. doi: 10.1016/j.jaad.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Blum A. 2019 Stream2Sea. Sunscreen Bans - Travel EcoConsciously! https://stream2sea.com/sunscreen-ban/ (cited 4-11-2020).
- Bolt H., Guhe C., Degeti G. Comments on “In vitro and in vivo estrogenicity of UV screens”. Environ Health Perspect. 2001;109:A358–A359. doi: 10.1289/ehp.109-a358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brausch J.M., Rand G.M. A review of personal care products in the aquatic environment: environmental concentrations and toxicity. Chemosphere. 2011;8:1518–1532. doi: 10.1016/j.chemosphere.2010.11.018. CrossRef CAS PubMed. [DOI] [PubMed] [Google Scholar]
- Broniowska Z., Slusarczyk J., Starek-Swiechowicz B., Trojan E., Pomierny B., Krzyzanowska W., Basta-Kaim A., Budziszewska B. The effect of dermal benzophenone-2 administration on immune system activity, hypothalamic-pituitary-thyroid axis activity and hematological parameters in male Wistar rats. Toxicology. 2018;1(402–403):1–8. doi: 10.1016/j.tox.2018.04.002. Epub 2018 Apr 13. [DOI] [PubMed] [Google Scholar]
- Buser H.R., Balmer M., Schmid P., Kohler M. Occurrence of UV filters 4-methylbenzylidene camphor and octocrylene in fish from various Swiss rivers with inputs from wastewater treatment plants. Environ Sci Technol. 2006;40:1427–1431. doi: 10.1021/es052088s. [DOI] [PubMed] [Google Scholar]
- Calafat A.M., Wong L.N., Ye X., Reidy J., Needham L. Concentrations of the sunscreen agent benzophenone-3 in residents of the United States: National Health and Nutrition Examination Survey 2003–2004. Environ Health Perspect. 2008;116:893–989. doi: 10.1289/ehp.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carou E., Szwarcfarb B., Deguiz M.L., Reynoso R., Carbone S., Moguilevsky J.A., Scacchi P., Ponzo O.J. Impact of 4-methylbenzylidene-camphor (4-MBC) during embryonic and fetal development in the neuroendocrine regulation of testicular axis in prepubertal and peripubertal male rats. Exp Clin Endocrinol Diabetes. 2009;117:449–454. doi: 10.1055/s-0028-1112153. [DOI] [PubMed] [Google Scholar]
- Carou M., Ponzo O., Gutierrez R., Szwarcfarb B., Deguiz M., Reynoso R. Low dose 4-MBC effect on neuroendocrine regulation of reproductive axis in adult male rat. J Environ Toxicol Pharmacol. 2008;26:222–224. doi: 10.1016/j.etap.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Carbone S., Szwarcfarb B., Reynoso R., Ponzo O.J., Cardoso N., Ale E., Moguilevsky J.A., Scacchi P. In vitro effect of octyl - methoxycinnamate (OMC) on the release of Gn-RH and amino acid neurotransmitters by hypothalamus of adult rats. Exp Clin Endocrinol Diabetes. 2010;118:298–303. doi: 10.1055/s-0029-1224153. [DOI] [PubMed] [Google Scholar]
- Chen J., Dong X., Xin Y., Zhao M. Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (Danio rerio) after a long-term exposure. Aquat Toxicol. 2011;101:493–499. doi: 10.1016/j.aquatox.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Chen T.H., Hsieh C.Y., Ko F.C., Cheng J.O. Effect of the UV-filter benzophenone-3 on intra-colonial social behaviors of the false clown anemonefish (Amphiprion ocellaris) Sci Total Environ. 2018;644:1625–1629. doi: 10.1016/j.scitotenv.2018.07.203. Epub 2018 Jul 23. [DOI] [PubMed] [Google Scholar]
- Chen T.H., Wu Y.T., Ding W.H. UV-filter benzophenone-3 inhibits agonistic behavior in male Siamese fighting fish (Betta splendens) Ecotoxicology. 2016;25(2):302–309. doi: 10.1007/s10646-015-1588-4. Epub 2015 Nov 20. [DOI] [PubMed] [Google Scholar]
- Cheng L., Abraham J., Hausfather Z., Trenberth K.E. How fast are the oceans warming? Science. 2019;363:128–129. doi: 10.1126/science.aav7619. CrossRef CAS PubMed. [DOI] [PubMed] [Google Scholar]
- Christen V., Zucchi S., Fent K. Effects of the UV-filter 2-ethyl-hexyl-4-trimethoxycinnamate (EHMC) on expression of genes involved in hormonal pathways in fathead minnows (Pimephales promelas) and link to vitellogenin induction and histology. Aquat Toxicol. 2011;102:167–176. doi: 10.1016/j.aquatox.2011.01.013. Epub 2011 Feb 2. [DOI] [PubMed] [Google Scholar]
- Corinaldesi C., Marcellini F., Nepote E., Damiani E., Danovaro R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.) Sci Total Environ. 2018;637–638:1279–1285. doi: 10.1016/j.scitotenv.2018.05.108. Epub 2018 May 22. [DOI] [PubMed] [Google Scholar]
- Coronado M., De Haro H., Deng X., Rempel M.A., Lavado R. Estrogenic activity and reproductive effects of the UV-filter oxybenzone (2-hydroxy-4-methoxyphenyl-methanone) in fish. Aquat Toxicol. 2008;90:182–187. doi: 10.1016/j.aquatox.2008.08.018. [DOI] [PubMed] [Google Scholar]
- da Silva C.P., Emidio E.S., de Marchi M.R. The occurrence of UV filters in natural and drinking water in Sao Paulo State (Brazil) Environ Sci Pollut Res Int. 2015;22:19706–19715. doi: 10.1007/s11356-015-5174-3. [DOI] [PubMed] [Google Scholar]
- Danovaro R., Bongiorni L., Corinaldesi C., Giovannelli D., Damiani E., Astolfi P. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116:441–447. doi: 10.1289/ehp.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danovaro R., Corinaldesi C. Sunscreen products increase virus production through prophage induction in marine bacterioplankton. Microb Ecol. 2003;45(2):109–118. doi: 10.1007/s00248-002-1033-0. [DOI] [PubMed] [Google Scholar]
- DiNardo J.C., Downs C.A. Can oxybenzone cause Hirschsprung’s disease? Reprod Toxicol. 2019;86:98–100. doi: 10.1016/j.reprotox.2019.02.014. [DOI] [PubMed] [Google Scholar]
- DiNardo J.C., Downs C.A. Dermatological and environmental toxicological impact of the sunscreen ingredient oxybenzone/benzophenone-3. J Cosmet Dermatol. 2018;17:15–19. doi: 10.1111/jocd.12449. [DOI] [PubMed] [Google Scholar]
- Downs C.A., Kramarsky-Winter E., Segal R., Fauth J., Knutson S., Bronstein O., Ciner F.R., Jeger R., Lichtenfeld Y., Woodley C.M., Pennington P., Cadenas K., Kushmaro A., Loya Y. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265–288. doi: 10.1007/s00244-015-0227-7. [DOI] [PubMed] [Google Scholar]
- Downs C.A., Kramarsky-Winter E., Segal R., Fauth J., Knutson S., Bronstein O., Ciner F.R., Jeger R., Lichtenfeld Y., Woodley C.M., Pennington P., Cadenas K., Kushmaro A., Loya Y. Toxicopathological effects of the sunscreen UV filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Arch Environ Contam Toxicol. 2016;70:265–288. doi: 10.1007/s00244-015-0227-7. Epub 2013 Dec 19. [DOI] [PubMed] [Google Scholar]
- Dryden H. 2020 Corals are dying. https://www.goesfoundation.com/media/coral-by-goes-foundation/ (cited 4-11-2020).
- Du Y, Wang WQ, Pei ZT, Ahmad F, Xu RR, Zhang YM, Sun LW. Acute toxicity and ecological risk assessment of benzophenone-3 (BP-3) and benzophenone-4 (BP-4) in ultraviolet (UV)-filters. Int J Environ Res Public Health. 2017;19:14(11). pii: E1414. doi: 10.3390/ijerph14111414.CrossRef PubMed. [DOI] [PMC free article] [PubMed]
- Eakin M. El Niño prolongs longest global coral bleaching event. NOAA Coral Reef Watch. 2016 https://www.noaa.gov/media-release/el-ni-o-prolongs-longest-global-coral-bleaching-event (cited 4-11-2020) [Google Scholar]
- Ekpeghere K.I., Kim U.J., Sung-Hee O, Kim H.Y., Oh J.E. Distribution and seasonal occurrence of UV filters in rivers and wastewater treatment plants in Korea. Sci Total Environ. 2016;542(121–128) doi: 10.1016/j.scitotenv.2015.10.033. Epub 2015 Oct 28. CrossRef CAS PubMed. [DOI] [PubMed] [Google Scholar]
- Emnet P., Gaw S., Northcott G., Storey B., Graham L. Personal care products and steroid hormones in the Antarctic coastal environment associated with two Antarctic research stations, McMurdo Station and Scott Base. Environ Res. 2014;136:331–342. doi: 10.1016/j.envres.2014.10.019. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (US). Nanomaterial case studies: Nanoscale titanium dioxide in water treatment and in topical sunscreen (Final). EPA/600/R-09/057F, 2010. https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=230972 (cited 4-11-2020).
- Environmental Protection Agency (US) “Endocrine Disruptor Screening Program.” 2010. https://www.epa.gov/endocrine-disruption (cited 4-11-2020).
- Environmental Protection Agency (US). Daughton CG. Introduction to pharmaceuticals and personal care products. At ‘Non-Regulated Pollutants Workshop: Brominated Flame Retardants (BFRs) and Pharmaceuticals & Personal Care Products (PPCPs)’, New York, Oct 2005. https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&TIMSType=&count=10000&dirEntryId=141544&searchAll=&showCriteria=2&simpleSearch=0&startIndex=20001 (cited 4-11-2020) (cited 4-11-2020).
- Environmental Protection Agency (US). Murohy E. 2017 https://www.epa.gov/great-lakes-monitoring/great-lakes-fish-monitoring-and-surveillance (cited 4-11-2020).
- Environmental Working Group. The Trouble With Ingredients in Sunscreens. 2019. https://www.ewg.org/sunscreen/report/the-trouble-with-sunscreen-chemicals/ (cited 4-11-2020).
- Environmental Working Group, Sunscreen Guide 2019. https://www.ewg.org/sunscreen/report/the-trouble-with-sunscreen-chemicals/ (cited 4-11-2020).
- Erickson B. Sunscreen approval delays prompt push for high-throughput tests. Chem Eng News. 2018;96(5):17. (cited 4-11-2020) [Google Scholar]
- Europa, European Commission on Public Health. 2007. What are potential harmful effects of nanoparticles? https://ec.europa.eu/health/scientific_committees/opinions_layman/en/nanotechnologies/index.htm#6 (cited 4-11-2020).
- Fajardo R. USVI bans sunscreen products that are harmful to coral reefs. The Weekly Journal. Jul 2019. https://www.theweeklyjournal.com/lifestyle/usvi-bans-sunscreen-products-that-are-harmful-to-coral-reefs/article_23421b3c-ad6e-11e9-a1b4-2fb2ae56b6e4.html (cited 4-11-2020).
- Federici G., Shaw B.J., Handy R.D. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquatic Toxicol. 2007;84:415–430. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Fent K., Kunz P.Y., Zenker A., Rapp M. A tentative environmental risk assessment of the UV-filters 3-(4-methylbenzylidene-camphor), 2-ethyl-hexyl-4-trimethoxycinnamate, benzophenone-3, benzophenone-4 and 3-benzylidene camphor. Mar Environ Res. 2010;(69 Suppl):S4–S6. doi: 10.1016/j.marenvres.2009.10.010. Epub 2009 Nov 11. [DOI] [PubMed] [Google Scholar]
- Fent K., Kunz P.Y., Gomez E. UV filters in the aquatic environment induce hormonal effects and affect fertility and reproduction in fish. Chimia. 2008;62:368–375. [Google Scholar]
- Fent K., Zenker A., Rapp M. Widespread occurrence of estrogenic UV-filters in aquatic ecosystems in Switzerland. Environ Pollut. 2010;158:1817–1824. doi: 10.1016/j.envpol.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (US). Guidance on marketed, unapproved drugs. 2006. https://www.federalregister.gov/documents/2006/06/09/E6-9032/guidance-on-marketed-unapproved-drugs-compliance-policy-guide-availability (cited 4-11-20).
- Food and Drug Administration (US). FDA advances new proposed regulation to make sure that sunscreens are safe and effective. Federal Register 84FR6204, 2019-03019. 2019. (cited 4-11-2020) https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective.
- Food and Drug Administration (US), Sunscreen Drug Products for Over-the-Counter Human Use. Federal Register. 2019 https://www.federalregister.gov/documents/2019/02/26/2019-03019/sunscreen-drug-products-for-over-the-counter-human-use (cited 4-11-2020).
- Food and Drug Administration (US). Sunscreen Drug Products for Over-The-Counter Human Use; Proposal to Amend and Lift Stay on Monograph, Federal. Register 1978. https://www.fda.gov/media/122882/ (cited 4-11-2020).
- Food and Drug Administration (US), CFR - Code of Federal Regulations Title 21; 2017, FDA approved UV filters for sunscreens. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr. (cited 4-11-2020).
- Fleshler D. Ban on sunscreen chemicals proposed to protect coral reefs. Sun Sentinel; 2018, http://www.sun-sentinel.com/news/florida/fl-reg-sunscreen-coral-20180523-story.html. (cited 4-11-2020).
- Fong H.C., Ho J.C., Cheung A.H., Lai K.P., Tse W.K. Developmental toxicity of the common UV filter, benzophenone-2, in zebrafish embryos. Chemosphere. 2016;164:413–420. doi: 10.1016/j.chemosphere.2016.08.073. Epub 2016 Sep 3. [DOI] [PubMed] [Google Scholar]
- Fouqueray M., Noury P., Dherret L., Chaurand P., Abbaci K., Labille J., Rose J., Garric J. Exposure of juvenile Danio rerio to aged TiO₂ nanomaterial from sunscreen. Environ Sci Pollut Res Int. 2013;20(5):3340–3350. doi: 10.1007/s11356-012-1256-7. Epub 2012 Oct 25. [DOI] [PubMed] [Google Scholar]
- Gago-Ferrero P., Alonso M.B., Bertozzi C.P., Marigo J., Barbosa L., Cremer M., Secchi E.R., Domit C., Azevedo A., Lailson-Brito J., Torres J.P., Malm O., Eljarrat E., Díaz-Cruz M.S., Barcelo D. First determination of UV filters in marine mammals: octocrylene levels in Franciscana dolphins. Environ Sci Technol. 2013;47:5619–5625. doi: 10.1021/es400675y. Epub 2013 May 14. [DOI] [PubMed] [Google Scholar]
- Gago-Ferrero P., Díaz-Cruz M.S., Barcelo D. An overview of UV-absorbing compounds (organic UV filters) in aquatic biota. Anal Bioanal Chem. 2012;404:2597–2610. doi: 10.1007/s00216-012-6067-7. CrossRef CAS PubMed. [DOI] [PubMed] [Google Scholar]
- Gao L., Yuan T., Zhou C., Cheng P., Bai Q., Ao J., Wang W., Zhang H. Effects of four commonly used UV filters on the growth, cell viability and oxidative stress responses of the Tetrahymena thermophila. Chemosphere. 2013;93(10):2507–2513. doi: 10.1016/j.chemosphere.2013.09.041. Epub 2013 Oct 13. [DOI] [PubMed] [Google Scholar]
- Giraldo A., Montes R., Rodil R., Quintana J.B., Vidal-Linan L., Beiras R. Ecotoxicological evaluation of the UV filters ethylhexyl dimethyl p-aminobenzoic acid and octocrylene using marine organisms isochrysis galbana, mytilus galloprovincialis and paracentrotus lividus. Arch Environ Contam Toxicol. 2017;72(4):606–611. doi: 10.1007/s00244-017-0399-4. Epub 2017 Apr 8. [DOI] [PubMed] [Google Scholar]
- Grabicova K., Fedorova G., Burkina V., Steinbach C., Schmidt-Posthaus H., Zlabek V., Kocour Kroupova H., Grabic R., Randak T. Presence of UV filters in surface water and the effects of phenylbenzimidazole sulfonic acid on rainbow trout (Oncorhynchus mykiss) following a chronic toxicity test. Ecotoxicol Environ Saf. 2013;96:41–47. doi: 10.1016/j.ecoenv.2013.06.022. Epub 2013 Jul 29. [DOI] [PubMed] [Google Scholar]
- Grande F., Tucci P. Titanium dioxide nanoparticles: a risk for human health? Mini-Rev Med Chem. 2016;16:762–769. doi: 10.2174/1389557516666160321114341. [DOI] [PubMed] [Google Scholar]
- Green A.C., Williams G.M., Logan V., Strutton G.M. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257–263. doi: 10.1200/JCO.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- Gruden C, Mileyeva-Biebesheimer O. American Chemical Society. (2009, March 27). Nanoparticles In Cosmetics, Personal Care Products May Have Adverse Environmental Effects. ScienceDaily. www.sciencedaily.com/releases/2009/03/090326162747.htm. (cited 4-11-2020).
- Guo Q., Wei D., Zhao H., Du Y. Predicted no-effect concentrations determination and ecological risk assessment for benzophenone-type UV filters in aquatic environments. Environ Pollut. 2020;256 doi: 10.1016/j.envpol.2019.113460. Epub 2019 Oct 25. [DOI] [PubMed] [Google Scholar]
- Hahladakisa J.N., Costas A., Velisa C.A., Weber R., Iacovidoua E., Purnella P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Haz Mater. 2018;344(15):179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Handy R., Henry T., Scown T., Johnston B., Tyler C. Manufactured nanoparticles: their uptake and effects on fish: a mechanistic analysis. Ecotoxicol. 2008;17:396–409. doi: 10.1007/s10646-008-0205-1. [DOI] [PubMed] [Google Scholar]
- Hansen S.F., Baun A. European Regulation affecting nanomaterials - review of limitations and future recommendations. Dose Response. 2012;10(3):364–383. doi: 10.2203/dose-response.10-029.Hansen. Epub 2011 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T., Tsui M.M.P., Tan C.J., Ng K.Y., Guo F.W., Wang L.H., Chen T.H., Fan T.Y., Lam P.K.S., Murphy M.B. Comparative toxicities of four benzophenone ultraviolet filters to two life stages of two coral species. Sci Total Environ. 2019;651(Pt 2):2391–2399. doi: 10.1016/j.scitotenv.2018.10.148. Epub 2018 Oct 11. [DOI] [PubMed] [Google Scholar]
- He T., Tsui M.M.P., Tan C.J., Ma C.Y., Yiu S.K.F., Wang L.H., Chen T.H., Fan T.Y., Lam P.K.S., Murphy M.B. Toxicological effects of two organic ultraviolet filters and a related commercial sunscreen product in adult corals. Environ Pollut. 2019;245:462–471. doi: 10.1016/j.envpol.2018.11.029. Epub 2018 Nov 13. [DOI] [PubMed] [Google Scholar]
- Heneweer M., Muusse M., van den Berg M., Sanderson J.T. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170–177. doi: 10.1016/j.taap.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Heurung A.R., Raju S.I., Warshaw E.M. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25(6):289–326. doi: 10.1097/DER.0000000000000079. [DOI] [PubMed] [Google Scholar]
- Holbech H., Norum U., Korsgaard B., Bjerregaard P. The chemical UVfilter 3-benzylidene camphor causes an oestrogenic effect in an in vivo fish assay. Pharmacol Toxicol. 2002;91:204–208. doi: 10.1034/j.1600-0773.2002.t01-3-910403.x. [DOI] [PubMed] [Google Scholar]
- Hopkins Z.R., Snowberger S., Blaney L. Ozonation of the oxybenzone, octinoxate, and octocrylene UV-filters: Reaction kinetics, absorbance characteristics, and transformation products. J Hazard Mater. 2017;338:23–32. doi: 10.1016/j.jhazmat.2017.05.016. Epub 2017 May 10. [DOI] [PubMed] [Google Scholar]
- Hughes T.P., Kerry J.T., Baird A.H., Connolly S.R., Chase T.J., Dietzel A., Hill T., Hoey A.S., Hoogenboom M.O., Jacobson M., Kerswell A., Madin J.S., Mieog A., Paley A.S., Pratchett M.S., Torda G., Woods R.M. Global warming impairs stock-recruitment dynamics of corals. Nature. 2019;568(7752):387–390. doi: 10.1038/s41586-019-1081-y. Epub 2019 Apr 3. [DOI] [PubMed] [Google Scholar]
- Huo W., Cai P., Chen M., Li H., Tang J., Xu C., Zhu D., Tang W., Xia Y. The relationship between prenatal exposure to BP-3 and Hirschsprung's disease. Chemosphere. 2016;144:1091–1097. doi: 10.1016/j.chemosphere.2015.09.019. Epub 2015 Oct 23. [DOI] [PubMed] [Google Scholar]
- Janjua N., Mogensen B., Andersson A.M., Petersen J., Henriksen M., Skakkebaek N. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol. 2004;123:57–61. doi: 10.1111/j.0022-202X.2004.22725.x. [DOI] [PubMed] [Google Scholar]
- Janjua N.R., Kongshoj B., Petersen J.H., Wulf H.C. Sunscreens and thyroid function in humans after short-term whole-body topical application: a single-blinded study. Br J Dermatol. 2007;156:1080–1082. doi: 10.1111/j.1365-2133.2007.07803.x. [DOI] [PubMed] [Google Scholar]
- Joensen U.N., Jorgensen N., Thyssen J.P., Petersen J.H., Szecsi P.B., Stender S., Andersson A.M., Skakkebaek N.E., Frederiksen H. Exposure to phenols, parabens and UV filters: associations with loss-of-function mutations in the filaggrin gene in men from the general population. Environ Int. 2017;105:105–111. doi: 10.1016/j.envint.2017.05.013. Epub 2017 May 17. [DOI] [PubMed] [Google Scholar]
- Jovanovic B., Guzman H.M. Effects of titanium dioxide (TiO2) nanoparticles on caribbean reef-building coral (Montastraea faveolata) Environ Toxicol Chem. 2014;33(6):1346–1353. doi: 10.1002/etc.2560. Epub 2014 Apr 22. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Sieratowicz A., Zielke H., Oetken M., Hollert H., Oehlmann J. Ecotoxicological effect characterisation of widely used organic UV filters. Environ Pollut. 2012;163:84–90. doi: 10.1016/j.envpol.2011.12.014. Epub 2012 Jan 11. [DOI] [PubMed] [Google Scholar]
- Kim S., Choi K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: a mini-review. Environ Int. 2014;70:143–157. doi: 10.1016/j.envint.2014.05.015. Epub 2014 Jun 14. [DOI] [PubMed] [Google Scholar]
- Klammer H., Schlecht C., Wuttke W., Schmutzler C., Gotthardt I., Köhrle J., Jarry H. Effects of a 5-day treatment with the UV-filter octyl-methoxycinnamate (OMC) on the function of the hypothalamo-pituitary-thyroid function in rats. Toxicology. 2007;238:192–199. doi: 10.1016/j.tox.2007.06.088. [DOI] [PubMed] [Google Scholar]
- Klimova Z., Hojerova J., Beránkova M. Skin absorption and human exposure estimation of three widely discussed UV filters in sunscreens–In vitro study mimicking real-life consumer habits. Food Chem Toxicol. 2015;83:237–250. doi: 10.1016/j.fct.2015.06.025. Epub 2015 Jul 4. [DOI] [PubMed] [Google Scholar]
- Krause M., Klit A., Blomberg Jensen M., Søeborg T., Frederiksen H., Schlumpf M., Lichtensteiger W., Skakkebaek N.E., Drzewiecki K.T. Sunscreens: are they beneficial for health? An overview of endocrine disrupting properties of UV-Filters. Int J Androl. 2012;35(3):424–436. doi: 10.1111/j.1365-2605.2012.01280.x. [DOI] [PubMed] [Google Scholar]
- Krzyzanowska W., Pomierny B., Starek-Swiechowicz B., Broniowska Ż., Strach B., Budziszewska B. The effects of benzophenone-3 on apoptosis and the expression of sex hormone receptors in the frontal cortex and hippocampus of rats. Toxicol Lett. 2018;296:63–72. doi: 10.1016/j.toxlet.2018.08.006. Epub 2018 Aug 9. [DOI] [PubMed] [Google Scholar]
- Kung T.A., Lee S.H., Yang T.C., Wang W.H. Survey of selected personal care products in surface water of coral reefs in Kenting National Park, Taiwan. Sci Total Environ. 2018;635:1302–1307. doi: 10.1016/j.scitotenv.2018.04.115. Epub 2018 Apr 24. [DOI] [PubMed] [Google Scholar]
- Kunz P.Y., Galicia H.F., Fent K. Comparison of in vitro and in vivo estrogenic activity of UV filters in fish. Toxicol Sci. 2006;90(2):349–361. doi: 10.1093/toxsci/kfj082. Epub 2006 Jan 10. [DOI] [PubMed] [Google Scholar]
- Kunz P.Y., Gries T., Fent K. The ultraviolet filter 3-benzylidene camphor adversely affects reproduction in fathead minnow (Pimephales promelas) Toxicol Sci. 2006;93:311–321. doi: 10.1093/toxsci/kfl070. [DOI] [PubMed] [Google Scholar]
- Kusk K.O., Avdolli M., Wollenberger L. Effect of 2,4-dihydroxybenzophenone (BP1) on early life-stage development of the marine copepod Acartia tonsa at different temperatures and salinities. Environ Toxicol Chem. 2011;30(4):959–966. doi: 10.1002/etc.458. Epub 2011 Feb 19. [DOI] [PubMed] [Google Scholar]
- Kwon JY, Koedrith P, Seo RK. Current investigations into the genotoxicity of zinc oxide and silica nanoparticles in mammalian models in vitro and in vivo: carcinogenic/genotoxic potential, relevant mechanisms and biomarkers, artifacts, and limitations. Int J Nanomed 2014; 9(Suppl 2):271–286. 5. doi:10.2147/IJN.S57918. Epub 2014 Dec 1. [DOI] [PMC free article] [PubMed]
- Labille J., Slomberg D., Catalano R., Robert S., Apers-Tremelo M.L., Boudenne J.L., Manasfi T., Radakovitch O. Assessing UVfilter inputs into beach waters during recreational activity: afield study of three French Mediterranean beaches from consumer survey to water analysis. Sci Total Environ. 2020;706 doi: 10.1016/j.scitotenv.2019.136010. Epub 2019 Dec 9. [DOI] [PubMed] [Google Scholar]
- Langford K.H., Reid M.J., Fjeld E., Oxnevad S., Thomas K.V. Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway. Environ Int. 2015;80:1–7. doi: 10.1016/j.envint.2015.03.012. Epub 2015 Mar 30. CrossRef CAS PubMed. [DOI] [PubMed] [Google Scholar]
- Lewicka Z.A., Li Y., Bohloul A., Yu W.W., Colvin V.L. Nanorings and nanocrescents formed via shaped nanosphere lithography: a route toward large areas of infrared metamaterials. Nanotechnology. 2013;24(11) doi: 10.1088/0957-4484/24/11/115303. Epub 2013 Feb 28. [DOI] [PubMed] [Google Scholar]
- Lim H.W., Thomas L., Rigel D.S. Photoprotection. In: Rigel D.S., Weiss R.A., Lim H.W., editors. Photoaging. Marcel Dekker; New York: 2004. pp. 73–88. [Google Scholar]
- Liao C., Kannan K. Species-specific accumulation and temporal trends of bisphenols and benzophenones in mollusks from the Chinese Bohai Sea during 2006–2015. Sci Total Environ. 2019;653:168–175. doi: 10.1016/j.scitotenv.2018.10.271. Epub 2018 Oct 21. [DOI] [PubMed] [Google Scholar]
- Ma R., Cotton B., Lichtensteiger W., Schlumpf M. UV filters with antagonistic action at androgen receptors in the MDA-kb2 cell transcriptional-activation assay. Toxicol Sci. 2003;74:43–50. doi: 10.1093/toxsci/kfg102. [DOI] [PubMed] [Google Scholar]
- Manasfi T., Coulomb B., Ravier S., Boudenne J.L. Degradation of organic UV filters in chlorinated seawater swimming pools: transformation pathways and bromoform formation. Environ Sci Technol. 2017;51(23):13580–13591. doi: 10.1021/acs.est.7b02624. Epub 2017 Nov 16. [DOI] [PubMed] [Google Scholar]
- Mao F., You L., Reinhard M., He Y., Gin K.Y. Occurrence and fate of benzophenone-type UV filters in a tropical urban watershed. Environ Sci Technol. 2018;52(7):3960–3967. doi: 10.1021/acs.est.7b05634. Epub 2018 Mar 13. [DOI] [PubMed] [Google Scholar]
- Matta M.K., Florian J., Zusterzeel R., Pilli N.R., Patel V., Volpe D.A., Yang Y., Oh L., Bashaw E., Zineh I., Sanabria C., Kemp S., Godfrey A., Adah S., Coelho S., Wang J., Furlong L.A., Ganley C., Michele T., Strauss D.G. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. J Am Med Assoc. 2020;323(3):256–267. doi: 10.1001/jama.2019.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta M.K., Florian J., Zusterzeel R., Pilli N.R., Patel V., Volpe D.A., Yang Y., Oh L., Bashaw E., Zineh I., Sanabria C., Kemp S., Godfrey A., Adah S., Coelho S., Wang J., Furlong L.A., Ganley C., Michele T., Strauss D.G. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. J Am Med Assoc. 2020;323(3):256–267. doi: 10.1001/jama.2019.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.J., Bennett S., Keller A.A., Pease S., Lenihan H.S. TiO2 nanoparticles are phototoxic to marine phytoplankton. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchelmore C.L., He K., Gonsior M., Hain E., Heyes A., Clark C., Younger R., Schmitt-Kopplin P., Feerick A., Conway A., Blaney L. Occurrence and distribution of UV-filters and other anthropogenic contaminants in coastal surface water, sediment, and coral tissue from Hawaii. Sci Total Environ. 2019;670:398–410. doi: 10.1016/j.scitotenv.2019.03.034. CrossRef CAS PubMed. [DOI] [PubMed] [Google Scholar]
- Morohoshi K., Yamamoto H., Kamata R., Shiraishi F., Koda T., Morita M. Estrogenic activity of 37 components of commercial sunscreen lotions evaluated by in vitro assays. Toxicol in Vitro. 2005;19:457–469. doi: 10.1016/j.tiv.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Mueller S.O., Kling M., Arifin Firzani P., Mecky A., Duranti E., Shields-Botella J., Delansorne R., Broschard T., Kramer P.J. Activation of estrogen receptor alpha and beta by 4-methylbenzylidene camphor in human and rat cells: comparison with phyto- and xenoestrogens. Toxicol Lett. 2003;142(1–2):89–101. doi: 10.1016/s0378-4274(03)00016-x. [DOI] [PubMed] [Google Scholar]
- Nakata H., Murata S., Filatreau J. Occurrence and concentrations of benzotriazole UV stabilizers in marine organisms and sediments from the Ariake Sea, Japan. Environ Sci Technol. 2009;43:6920–6926. doi: 10.1021/es900939j. [DOI] [PubMed] [Google Scholar]
- Narla S., Lim H.W. Sunscreen: FDA regulation, and environmental and health impact. Photochem Photobiol Sci. 2020;19(1):66–70. doi: 10.1039/c9pp00366e. [DOI] [PubMed] [Google Scholar]
- Nash J. Human safety and efficacy of ultraviolet filters and sunscreen products. Dermatol Clin. 2006;24:35–51. doi: 10.1016/j.det.2005.09.006. [DOI] [PubMed] [Google Scholar]
- National Institute of Health 2020. “Endocrine Disruptors.” https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm (cited 4-11-2020).
- Nohynek G.J., Dufour E.K. Nano-sized cosmetic formulations or solid nanoparticles in sunscreens: a risk to human health? Arch Toxicol. 2012;86:1063–1075. doi: 10.1007/s00204-012-0831-5. [DOI] [PubMed] [Google Scholar]
- Olson E. The rub on sunscreen. New York Times. June 2006;19 [Google Scholar]
- Paredes E., Perez S., Rodil R., Quintana J.B., Beiras R. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere. 2014;104:44–50. doi: 10.1016/j.chemosphere.2013.10.053. Epub 2013 Dec 19. [DOI] [PubMed] [Google Scholar]
- Peng X., Fan Y., Jin J., Xiong S., Liu J., Tang C. Bioaccumulation and biomagnification of ultraviolet absorbents in marine wildlife of the Pearl River Estuarine, South China Sea. Environ Pollut. 2017;225:55–65. doi: 10.1016/j.envpol.2017.03.035. Epub 2017 Mar 26. [DOI] [PubMed] [Google Scholar]
- Phiboonchaiyanan P.P., Busaranon K., Ninsontia C., Chanvorachote P. Benzophenone-3 increases metastasis potential in lung cancer cells via epithelial to mesenchymal transition. Cell Biol Toxicol. 2017;33(3):251–261. doi: 10.1007/s10565-016-9368-3. Epub 2016 Oct 28. [DOI] [PubMed] [Google Scholar]
- Pollack A.Z., Buck Louis G.M., Chen Z., Sun L., Trabert B., Guo Y., Kannan K. Bisphenol A, benzophenone-type ultraviolet filters, and phthalates in relation to uterine leiomyoma. Environ Res. 2015;137:101–107. doi: 10.1016/j.envres.2014.06.028. Epub 2014 Dec 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden C.S., Henry T.B., Handy R.D. Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat Toxicol. 2013;126:404–413. doi: 10.1016/j.aquatox.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Ramsden C.S., Smith T.J., Shaw B.J., Handy R.D. Dietary exposure to titanium dioxide nanoparticles in rainbow trout, (Oncorhynchus mykiss): no effect on growth, but subtle biochemical disturbances in the brain. Ecotoxicology. 2009;18:939–951. doi: 10.1007/s10646-009-0357-7. Epub 2009 Jul 10. [DOI] [PubMed] [Google Scholar]
- Rihane N., Nury T., M'Rad I., El Mir L., Sakly M., Amara S., Lizard G. Microglial cells (BV-2) internalize titanium dioxide (TiO2) nanoparticles: toxicity and cellular responses. Environ Sci Pollut Res Int. 2016;23:9690–9699. doi: 10.1007/s11356-016-6190-7. Epub 2016 Feb 5. [DOI] [PubMed] [Google Scholar]
- Ruszkiewicz J.A., Pinkas A., Ferrer B., Peres T.V., Tsatsakis A., Aschner M.L. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol Rep. 2017;2017(4):245–259. doi: 10.1016/j.toxrep.2017.05.006. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabzevari N, Qiblawi S, Norton S, Fivenson D Sunscreens: UV filters that protect us. Part 1-Changing regulations and choices for optimal sun protection. Int. J Womens Dermatology 2020, in press. [DOI] [PMC free article] [PubMed]
- Saunders L.J., Hoffman A.D., Nichols J.W., Gobas F.A.P.C. Dietary bioaccumulation and biotransformation of hydrophobic organic sunscreen agents in rainbow trout. Environ Toxicol Chem. 2020;39(3):574–586. doi: 10.1002/etc.4638. Epub 2020 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder S., Ippen H. Contact and photocontact sensitivity to sunscreens. Review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37(5):221–232. doi: 10.1111/j.1600-0536.1997.tb02439.x. [DOI] [PubMed] [Google Scholar]
- Scheil V., Triebskorn R., Köhler H.R. Cellular and stress protein responses to the UV filter 3-benzylidene camphor in the amphipod crustacean Gammarus fossarum (Koch 1835) Arch Environ Contam Toxicol. 2008;54(4):684–689. doi: 10.1007/s00244-007-9072-7. Epub 2007 Nov 6. [DOI] [PubMed] [Google Scholar]
- Schlossman D, Shao Y, Detrieuv P. Perspectives on supplying attenuation grades of titanium dioxide and zinc oxide for sunscreen applications. 2006. https://www.fda.gov/science-research/science-and-research-special-topics/nanotechnology-programs-fda. (cited 4-11-2020).
- Schlumpf M., Cotton B., Conscience M., Hailer V., Steinmann B., Lichtensteiger W. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109:239–244. doi: 10.1289/ehp.01109239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumpf M., Lichtensteiger W. In vitro and in vivo estrogenicity of UV screens: Response. Environ Health Perspect. 2001;109:A359–A361. doi: 10.1289/ehp.01109239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumpf M., Schmid P., Durrer S., Conscience M., Maerkel K., Henseler M. Endocrine activity and developmental toxicity of cosmetic UV filters: (an) update. Toxicology. 2004;205:113–122. doi: 10.1016/j.tox.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Schlumpf M., Durrer S., Faass O., Ehnes C., Fuetsch M., Gaille C., Henseler M., Hofkamp L., Maerkel K., Reolon S., Timms B., Tresguerres J.A., Lichtensteiger W. Developmental toxicity of UV filters and environmental exposure: a review. Int J Androl. 2008;31(2):144–151. doi: 10.1111/j.1365-2605.2007.00856.x. Epub 2008 Jan 10. [DOI] [PubMed] [Google Scholar]
- Schneider S.L., Lim H.W. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J Am Acad Dermatol. 2019;80(1):266–271. doi: 10.1016/j.jaad.2018.06.033. [DOI] [PubMed] [Google Scholar]
- Schneider S.L., Lim H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol Photoimmunol Photomed. 2019;35(6):442–446. doi: 10.1111/phpp.12439. Epub 2018 Dec 10 Search PubMed. [DOI] [PubMed] [Google Scholar]
- Schneider S., Deckardt K., Hellwig J., Kuttler K., Mellert W., Schulte S., van Ravenzwaay B. Octyl methoxycinnamate: two generation reproduction toxicity in Wistar rats by dietary administration. Food Chem Toxicol. 2005;43:1083–1092. doi: 10.1016/j.fct.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Schilling K., Bradford B., Castelli D., Dufour E., Nash J.F., Pape W., Schulte S., Tooley I., van den Bosch J., Schellauf F. Human safety review of “nano” titanium dioxide and zinc oxide. Photochem Photobiol Sci. 2010;9(4):495–509. doi: 10.1039/b9pp00180h. [DOI] [PubMed] [Google Scholar]
- Schreurs R., Lanser P., Seinen W., van der Burg B. Estrogenic activity of UV filters determined by an in vitro reporter gene assay and an in vivo transgenic zebrafish assay. Arch Toxicol. 2002;76:257–261. doi: 10.1007/s00204-002-0348-4. [DOI] [PubMed] [Google Scholar]
- Schwen RJ. Safety consideration for sunscreen in the USA. In: Sunscreens: regulations and commercial development (Shaath NA, ed) CRC Press 2005, 57–69.
- Sherwood V.F., Kennedy S., Zhang H., Purser G.H., Sheaff R.J. Altered UV absorbance and cytotoxicity of chlorinated sunscreen agents. Cutan Ocul Toxicol. 2012;31:273–279. doi: 10.3109/15569527.2011.647181. [DOI] [PubMed] [Google Scholar]
- Sieratowicz A., Kaiser D., Behr M., Oetken M., Oehlmann J. Acute and chronic toxicity of four frequently used UV filter substances for Desmodesmus subspicatus and Daphnia magna. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2011;46(12):1311–1319. doi: 10.1080/10934529.2011.602936. [DOI] [PubMed] [Google Scholar]
- Slattery M., Pankey M.S., Lesser M.P. Annual thermal stress increases a soft coral's susceptibility to bleaching. Sci Rep. 2019;9:8064. doi: 10.1038/s41598-019-44566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwarcfarb B., Carbone S., Reynoso R., Bollero G., Ponzo O., Moguilevsky J., Scacchi P. Octyl-methoxycinnamate (OMC), an ultraviolet (UV) filter, alters LHRH and amino acid neurotransmitters release from hypothalamus of immature rats. Exp Clin Endocrinol Diabetes. 2008;116:94–98. doi: 10.1055/s-2007-1004589. [DOI] [PubMed] [Google Scholar]
- Tang Z., Han X., Li G., Tian S., Yang Y., Zhong F., Han Y., Yang J. Occurrence, mdistribution and ecological risk of ultraviolet absorbents in water and sediment from Lake Chaohu and its inflowing rivers, China. Ecotoxicol Environ Saf. 2018;164:540–547. doi: 10.1016/j.ecoenv.2018.08.045. Epub 2018 Aug 24. [DOI] [PubMed] [Google Scholar]
- Tingley, K Studies Show:When You Wear Sunscreen, You’re Taking Part in a Safety Study. https://www.nytimes.com/2019/07/23/magazine/when-you-wear-sunscreen-youre-taking-part-in-a-safety-study.html (cited 4-11-2020).
- Tovar-Sanchez A., Sanchez-Quiles D., Rodríguez-Romero A. Massive coastal tourism influx to the Mediterranean Sea: the environmental risk of sunscreens. Sci Total Environ. 2019;656:316–321. doi: 10.1016/j.scitotenv.2018.11.399. Epub 2018 Nov 27. [DOI] [PubMed] [Google Scholar]
- Tovar-Sanchez A., Sanchez-Quiles D., Basterretxea G., Benede J.L., Chisvert A., Salvador A., Moreno-Garrido I., Blasco J. Sunscreen products as emerging pollutants to coastal waters. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0065451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.Q., Burnett M.E., Lim H.W. Safety of oxybenzone: putting numbers into perspective. Arch Dermatol Res. 2011;147(7):865–866. doi: 10.1001/archdermatol.2011.173. [DOI] [PubMed] [Google Scholar]
- Wang S.Q., Lim H.W. Highlights and implications of the 2019 proposed rule on sunscreens by the US Food and Drug Administration. J Am Acad Dermatol. 2019;81:650–651. doi: 10.1016/j.jaad.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Wang S.Q., Lim H.W. Current status of the sunscreen regulation in the United States: 2011 Food and Drug Administration's final rule on labeling and effectiveness testing. J Am Acad Dermatol. 2011;65:863–869. doi: 10.1016/j.jaad.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Wang J., Pan L., Wu S., Lu L., Xu Y., Zhu Y., Guo M., Zhuang S., Wang J. Recent advances on endocrine disrupting effects of UV filters. Int J Environ Res Public Health. 2016;13(8) doi: 10.3390/ijerph13080782. pii: E782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.Q., Duan H.X., Pei Z.T., Xu R.R., Qin Z.T., Zhu G.C., Sun L.W. Evaluation by the Ames assay of the mutagenicity of UV filters using benzophenone and benzophenone-1. Int J Environ Res Public Health. 2018;15(9) doi: 10.3390/ijerph15091907. pii: E1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod C., Kunz P., Zenker A., Fent K. Effects of the UV filter benzophenone-2 on reproduction in fish. Toxicol App Pharm. 2017;225:255–266. doi: 10.1016/j.taap.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Wood E. Impacts of sunscreens on corals https://www.oceangrants.org/ocean-news/2018/5/29/impacts-of-sunscreens-on-coral-reefs. 2018. (cited 4-11-2020).
- Wu M.H., Li J., Xu G., Ma L.D., Li J.J., Li J.S., Tang L. Pollution patterns and underlying relationships of benzophenone-type UV-filters in wastewater treatment plants and their receiving surface water. Ecotoxicol Environ Saf. 2018;152:98–103. doi: 10.1016/j.ecoenv.2018.01.036. Epub 2018 Feb 4. [DOI] [PubMed] [Google Scholar]
- Ze Y., Hu R., Wang X., Sang X., Ze X., Li B., Su J., Wang Y., Guan N., Zhao X., Gui S., Zhu L., Cheng Z., Cheng J., Sheng L., Sun Q., Wang L., Hong F. Neurotoxicity and gene-expressed profile in brain-injured mice caused by exposure to titanium dioxide nanoparticles. J Biomed Mater Res A. 2014;102(2):470–478. doi: 10.1002/jbm.a.34705. Epub 2013 Jun 1 CrossRef PubMed. [DOI] [PubMed] [Google Scholar]
- Zucchi S., Bluthgen N., Ieronimo A., Fent K. The UV-absorber benzophenone-4 alters transcripts of genes involved in hormonal pathways in zebrafish (Danio rerio) eleuthero-embryos and adult males. Toxicol Appl Pharmacol. 2011;250:137–146. doi: 10.1016/j.taap.2010.10.001. [DOI] [PubMed] [Google Scholar]