Abstract
Background
The percentage of patients in intensive care who are 80 years old or older is continually increasing. Such patients already made up more than 20% of all patients in intensive care in Germany in the years 2007–2011. Meanwhile, effective treatments that support the organs of the body and keep severely ill patients alive are also being continually developed and refined. Frailty is a key prognostic parameter. The scientifically based assessment of frailty can be highly useful in intensive care medicine with regard to consented decision-making, individualized prognostication, treatment planning, and aftercare.
Methods
Pertinent publications were retrieved by a selective search in the PubMed database. On the basis of the literature assessment, a variety of screening instruments were used to assess frailty and its significance for very old, critically ill patients in German intensive care units.
Results
Only a small number of screening instruments are suitable for routine use in German intensive care units. The scores vary in diagnostic precision. The Clinical Frailty Scale (CFS) enables highly accurate prognostication; it considers the patient in relation to his or her social environment, and to the reference population. Categorization is achieved by means of pictograms that are supplemented with brief written descriptions. The CFS can be used prospectively and is easy to learn. Its interrater reliability is high (weighted Cohen’s κ: 0.85 [0.84; 0.87]), and it has been validated for routine use in intensive care units in Germany.
Conclusion
None of the available scores enable perfect prognostication. In Germany, frailty in intensive-care patients is currently best assessed on a simple visual scale (CFS).
The very old (aged 80 years and over) and the old (65 and over) constitute a growing subgroup of intensive care patients. It is estimated that by the year 2030, more than 25% of the population of western Europe will be in the 65+ age group (1). In Austria, the proportion of very old intensive care patients rose from 11.5% in 1998 to 15.3% in 2008 (2). Another study found that more than 20% of German intensive care patients were aged 80 or over in the period 2007 to 2011 (3). Comparable increases have been observed in Denmark, the Netherlands, and Australia (e1– e3). In Scandinavia, the proportion of very old patients in intensive care is expected to rise to 36% by 2025 (e4).
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. The questions on this article can be found at http://daebl.de/RY95. The deadline for submission is 1 October 2021
Participation is possible only via: cme.aerzteblatt.de
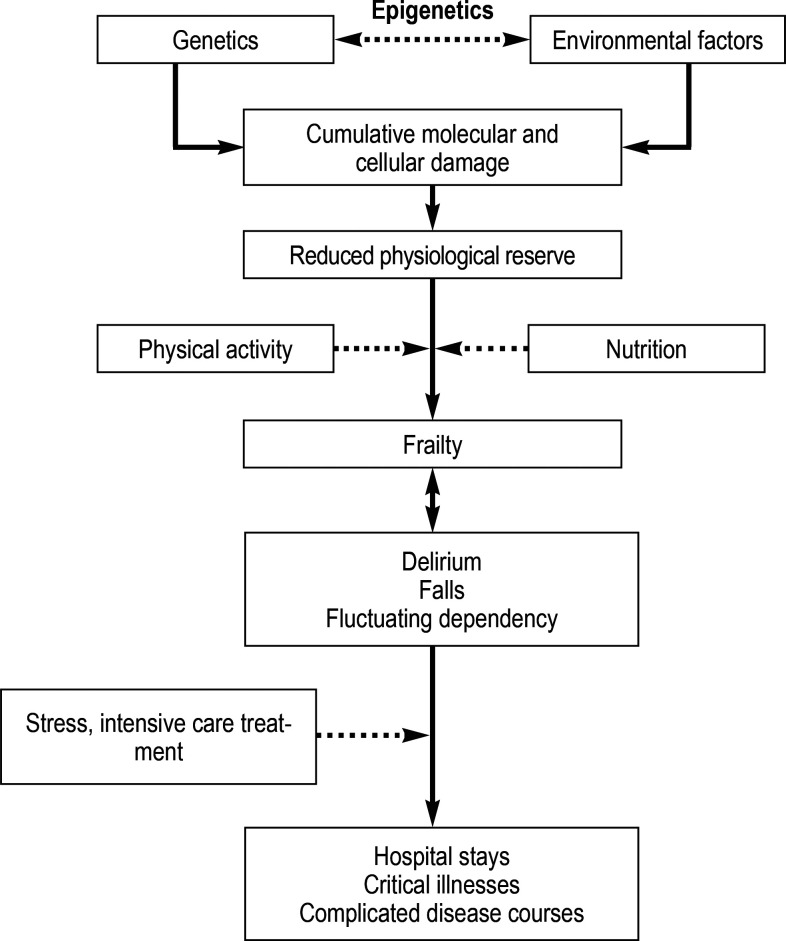
An estimated 25 to 50% of patients aged ≥ 80 years are classified as frail (e5). The WHO defines frailty as a clinically discernible state in which the ability of older persons to cope with both routine and acute stressors is reduced. Advanced age is not, however, synonymous with frailty; rather, many different factors are involved (e6). The cumulative molecular and cellular damage caused by genetic, epigenetic, and environmental factors results in a reduced physiological reserve, potentially affecting all organ systems (figure). These aging processes vary in pace of progress and take place over a period of decades. This leads to considerable biological and functional heterogeneity among very old patients (e7). It is not always easy to distinguish frailty from multimorbidity. Large clinical studies have shown that multimorbidity—measured using a comorbidity and polypharmacy score (CPS) (e8)—alone has no predictive value for 30-day mortality in acutely critically ill elderly patients (4).
Figure.
Pathophysiological processes in connection with the development and consequences of frailty. (modified from Clegg et al. [e5])
Frail patients have a much greater degree of functional impairment than non-frail patients following critical illness and are more likely to have to be admitted to a care home (5, e9). Overall, frail patients run a much higher risk of morbidity and mortality than non-frail patients of the same age (5). Intensive care personnel have a particular responsibility to assess the prognosis of elderly critically ill patients. A subgroup analysis of the ELDICUS study showed that the relative benefit—that is, the difference in mortality between patients with and without intensive care treatment—is greatest in the very old (2.3% in the age group 18 to 44 years versus 17% in those over 84 years) (1). This relative advantage existed despite initially comparable results on the intensive care scores used to measure severity of illness (SOFA, APACHE, SAPS II). This finding underlines the fact that these established instruments do not capture all relevant dimensions, in that they do not take sufficient account of the functional heterogeneity of this age group (6).
Alongside documentation of the acute illness that has led to intensive care admission and its specific prognosis, assessment of frailty can help to evaluate the overall prognosis and assist in planning individual patient-specific treatment measures at various times: it can make a contribution to the assessment of the patient’s status prior to the acute illness that resulted in hospital admission. Thus, from the time of admission to intensive care onward, the evaluation of frailty can help to estimate the long-term prognosis, assess the patient’s capacity for rehabilitation after intensive care, and (re)evaluate the utility of continued intensive care treatment (e10). The focal point should be agreement with the patient and their family members about the course of action to be pursued. In common with every other medical decision, this evaluation should follow the principles of doing good (“bonum facere”), doing no harm (“non nocere”), and acting according to the patient’s wishes (“voluntas aegroti suprema lex” [“the will of the patient is paramount”]). Numerous instruments have been designed to this end, differing with regard to the underlying concepts, the information needed for scoring purposes, and clinical validation. No randomized controlled trial has yet demonstrated that development of a score is associated with a survival benefit. Nevertheless, frailty is, independently of the specific acute illness, a predictor for survival, complication rate, and quality of life after intensive care treatment (8).
This review follows on from the articles on frailty published in Deutsches Ärzteblatt International by Mende et al. and Olotu et al. (9, 10). While Mende et al. (9) provided an excellent overview of the many different screening instruments available, Olotu et al. (10) elucidated the role of frailty in perioperative medicine. In this review article we explore the significance of frailty for the prognosis and for treatment planning in critically ill patients of advanced age and discuss the suitability of various established screening methods for use in German intensive care units. Since documentation and administrative tasks already take up much of the working time of intensivists, one important aspect is whether swift and reliable assessment of frailty can feasibly be integrated into the daily intensive care routine in Germany.
Method
For the purposes of this review we conducted a selective search of the PubMed database, including original articles, national and international guidelines, meta-analyses, and selective reviews. Analysis was restricted to records published between January 2000 and 28 March 2020. The search criteria and search terms are presented in eBox 1.
eBOX 1. Literature search.
The literature search for this review article was conducted using the PubMed database.
The following terms were used for the primary search: frailty*[tiab]) AND (intensive care*[tiab]) OR frailty*[tiab]) AND (Critical Care*[tiab]) OR frailty*[tiab]) AND (assessment*[tiab]) OR (frailty*[tiab]) AND (emergency department*[tiab]) OR (frailty*[tiab]) AND (Risk stratification*[tiab]).
These terms were extended for the corresponding subtopics: (CFS/Clinical frailty scale / FI / MFI / EFI / Fried’s phenotype / HFRS / FRAIL-Scale)
The search was restricted to articles published between 1 January 2001 and 28 March 2020.
Only articles in English were considered.
Results
Screening methods
The concepts behind the various operationalized measurements for quantification of frailty differ from one another, in some cases widely (e11, 11). For this reason, a large number of different instruments have been developed for qualitative and quantitative classification of individual frailty. A overview of the instruments discussed herein is provided in Table 1. A more detailed synopsis can be found in the article by Mende et al. (9). The scores differ—from study to study—in their diagnostic accuracy. None of the scores enables prediction of the prognosis with absolute certainty. The diagnostic characteristics of the screening methods discussed herein, as depicted in selected studies with the emphasis on intensive care, are presented in Table 2 and the eTable.
Table 1. Techniques for screening of frailty.
| Information required | Definition of frailty | |
| Fried’s phenotype (12) | Five questions to the patient: 1. Unintentional weight loss > 4.5 kg in previous year? 2. Subjective exhaustion? 3. Muscular weakness(grip strength measurement)? 4. Slow walking speed? 5. Low physical activity? |
≥ 3 positive answers(1–2 = prefrail) |
| FRAIL scale (e12) | Five questions to the patient: – Fatigue? – Resistance (muscle strength, ability to walk up one flight of stairs)? – Ambulation (ability to walk 100 m)? – Illnesses (more than five)? – Loss of weight (> 5 % or 5 kg in previous 6 months)? |
≥ 3 positive answers(1–2 = prefrail) |
| Clinical Frailty Scale (CFS) (4, 28, 34) | Nine pictograms | Pictogram ≥ 5 (4 = prefrail) |
| Frailty index (FI) (17) | Cumulative deficit model with 11–92 variables (self-reported) | Depends on the version |
| Electronic frailty index (eFI) (18) | As for FI, except that routinely acquired electronic data are used (see eBox 2 for examples) | Depends on the version |
| Hospital Frailty Risk Score (HFRS) (20) | Routinely acquired electronic data for 109 ICD-10 codes | <5 = low risk 5–15 = intermediate 15 = high risk |
Table 2. Selected studies on instruments for frailty screening that are relevant for German intensive care units*.
| Instrument(s) | Study | Number/age of patients | Design |
Diagnostic accuracy [95% CI] |
Conclusion |
| CFS FP |
Le Maguet et al. (25) |
196/≥ 65 | Multicenter prospective observational study(intensive care patients) | Significant correlation between CFS and FP (R2 = 0.66, p <0.001) Multivariate analysis ICU survival: FP score ≥ 3 (HR: 3.3; [1.6; 6.6]; p <0.001) Six-month mortality: CFS ≥ 5 (HR: 2.4; [1.49; 3.87]; p <0.001) |
The CFS is not inferior to FP, but is much easier to ascertain. |
| CFS | De Geer et al. (27) |
872/≥ 18 | Single-center prospective observational study(intensive care patients) | Thirty-day survival: CFS AUC: 0.74; [0.69; 0.79] versus SAPS 3 (0.79; [0.75; 0.83]; p = 0.53) Thirty-day mortality: CFS adjusted for SAPS 3 (HR: 2,12; [1.44; 3.14]; p <0.001) |
The CFS offers additional prognostic benefit in intensive care patients. |
| CFS | Flaatten et al. (VIP-1) (34) |
5 021/≥ 80 | Multicenter prospective observational study(intensive care patients) | Dichotomized CFS ≥ 5 = independently elevated risk of 30-day mortality (HR: 1.54; [1.38; 1.73] |
Frailty according to the CFS is an independent risk factor in intensive care patients. |
| CFS | Guidet et al. (VIP-2) (4) | 3 920/≥ 80 | Multicenter prospective observational study(intensive care patients) | Multivariable analysis showed the ordinally scaled CFS to be a risk factor for 1-month mortality (HR: per point CFS 1.1; [1.05; 1.15]; p <0.001) | Frailty according to the CFS is an independent risk factor in intensive care patients. |
| HFRS | Bruno et al. (21) | 1 498/≥ 75 | Single-center retrospective observational study(intensive care patients) | No independent association of HFRS with ICU mortality after adjustment of multivariable model for APACHE II (HR: 1.03; [0.98; 1.09]; p = 0.27) SAPS II (HR 1.05; [0.99; 1.11]; p = 0.14) |
The HFRS yields no additional benefit in German intensive care patients. |
| FP | Ferrante et al. (5) | 391/≥ 70 | Single-center prospective observational study(intensive care patients) | Frailty according to FP: 41% higher rate of disability at 6 months after ICU (adjusted risk ratio: 1.41; [1.12; 1.78]) (OR: 3.52; [1.23; 10.08]) and higher risk of admission to a care home Six-month mortality: doubling with each FP point (HR: 2.00; [1.33; 3.00] |
FP helps to assess the long-term care needs. |
*To determine whether the instruments examined in the studies are suitable for use in German intensive care units, the studies were analyzed with regard to the following questions: Were intensive care patients investigated? Were a sufficient number of patients analyzed? Did the validation take place in German/European intensive care units? Was the intended age group investigated? Do the results justify the conclusion that the instrument is suitable?
APACHE II, Acute Physiology and Chronic Health Score II; AUC, area under the curve; aOR, adjusted odds ratio; CFS, Clinical Frailty Scale; CHS, Cardiovascular Health Study Scale; eFI, electronic frailty index; FRAIL, FRAIL scale; FP, Fried’s phenotype; FI, frailty index; HR, hazard ratio; HFRS, Hospital Frailty Risk Score; mFI, modified frailty index; OR, odds ratio; SAPS II/3, Simplified Acute Physiology Score II/3; SOFA, Sequential Organ Failure Assessment Score; SOF, Study of Osteoporotic Fractures scale; ROC, receiver operating characteristic; 95% CI, 95% confidence interval
eTable. Diagnostic characteristics of the individual instrument in further selected studies with no immediate relevance for German intensive care units*.
| Instrument(s) | Study | Number/age of patients | Design | Diagnostic accuracy[95% CI] | Conclusion | Utility for German intensive care |
| HFRS | Redfern et al. (22) | 31 812/≥ 75 | Two-center retrospective observational study (including intensive care patients) | No association between HFRS and hospital mortality in intensive care patients (p = 0.178). | The HFRS has no additional mortality benefit in intensive care patients. | None |
| FRAIL | Lopez et al. (15) | 132/≥ 65 | Single-center prospective observational study(intensive care patients) | Frailty associated with 1-month mortality (OR: 3.5; [1.22; 10.03]; p <0.05) 6-month mortality (OR: 2.62; [1.04; 6.56]; p <0.05) |
FRAIL is a predictor of mortality in intensive care patients. | Limited, because no very old patients were investigated |
| mFI with 52 criteria | Zeng et al. (e13) | 155/≥ 65 | Single-center prospective observational study(intensive care patients) | Thirty-day mortality with mFI cut-off of > 0.46 and AUC = 0.89 ± 0.03 with FI <0.22 = 0% mortality | The mFI is very complex to measure, but can predict mortality. | Limited |
| mFI | Kizilarslanoglu et al. (e14) | 122/mean age 71 | Single-center prospective observational study(intensive care patients) | In the multivariate analysis the FI—as used by these authors—was an independent predictor of mortality in the intensive care unit (HR: 39.1; [1.2; 1232.5]). | The mFI is very complex to measure, but can predict mortality. | Limited |
| CFS FP |
Ritt et al. (32) | 307/≥ 65 | Single-center prospective observational study(no intensive care patients) | Six-month survival:CFS: AUC 0.867; [0.807; 0.926]; p <0.001 FP: AUC: 0.754; [0.688; 0.821]; p <0.001) Secondary endpoints, such as unplanned hospital admission or falls: CFS superior to FP |
The CFS is superior to FP in some cases. | Limited (no intensive care patients) |
| CFS PRISMA-7 ISAR |
O’Caoihm et al. (35) | 280/≥ 70 | Single-center prospective observational study(no intensive care patients) | CFS had good diagnostic accuracy (AUC 0.83; 95% CI 0.77–0.88). This was lower than PRISMA-7 (AUC: 0.88; [0.83; 0.93], but the difference between the two was not significant (p = 0.15). Youden’s index for the CFS with c ut-off of ≥ 4 at 0.5 (sensitivity 78%, specificity 72%, positive predictive value 80%, negative predictive value 69%, false positive 20%, false negative 31%) Cut-off of ≥ 5 at 0.45 (sensitivity 51%, specificity 94%, positive predictive value 57%, negative predictive value 7%, false positive 7%, false negative 43%) „Gold standard = geriatric consultant’s assessment |
The CFS has good diagnostic accuracy. | Limited (no intensive care patients) |
| FRAIL FI CHS SOF |
Malstrom et al. (e9) | 998 | Single-center prospective observational study(exclusively Afro-American cohort) | Longitudinal cohort study over 9 years On ROC analyses, the FI and the FRAIL scale were superior to the other scores for prediction of disability and mortality |
FRAIL is a valid parameter. | Limited (no intensive care patients, only Afro-Americans) |
| CFS FRAIL |
Chong et al. (36, 37) | 210/ mean age 89.4) | Single-center prospective observational study | Hospital mortality:FRAIL (aOR: 3.31; [1.43; 7.67]; p = 0.005) CFS (aOR: 2.57; [1.14; 5.83]; p = 0.023) Twelve-month mortality: CFS (aOR: 5.78; [3.19; 10.48]; p <.001) |
The CFS is superior for 12-month mortality, FRAIL for hospital mortality. | Limited (no intensive care patients) |
| CFS | Shears et al. (30) | 150/≥ 18 | Single-center prospective observational study(intensive care patients) | Assessments of various operators (researcher, intensivist, geriatric consultant) not significantly different (p > 0.05) | The CFS possesses high inter-rater reliability. | Limited (young patients also included) |
| CFS | Kaeppeli et al. (31) | 2393/≥ 65 | Single-center prospective observational study(emergency patients) | Thirty-day mortality and hospitalization (AUC 0.81; [0.77; 0.85] Good inter-rater reliability (weighted Cohen‘s κ: 0.74; [0.64; 0.85] |
The CFS possesses high inter-rater reliability and is validated for emergency patients. | Limited, because emergency patients were investigated |
| FP | Baldwin et al. (38) | 22/65 | Single-center prospective observational study(intensive care patients) | For every FP point the rate of disability after 1 month rose by 90% (rate ratio: 1.9; [0.7; 4.9]) after 3 months, threefold (rate ratio: 3.0; [1.4; 6.3]) |
FP helps to assess the long-term care need. | Limited owing to the very low number of patients |
*To determine whether the instruments examined in the studies are suitable for use in German intensive care units, the studies were analyzed with regard to the following questions: Were intensive care patients investigated? Were a sufficient number of patients analyzed? Did the validation take place in German/European intensive care units? Was the intended age group investigated? Do the results justify the conclusion that the instrument is suitable?
APACHE II, Acute Physiology and Chronic Health Score II; AUC, area under the curve; aOR, adjusted odds ratio; CFS, Clinical Frailty Scale; CHS, Cardiovascular Health Study Scale;
eFI, electronic frailty index; FRAIL, FRAIL scale; FP, Fried’s phenotype; FI, frailty index; HR, hazard ratio; HFRS, Hospital Frailty Risk Score; mFI, modified frailty index; OR, odds ratio; SAPS II/3, Simplified Acute Physiology Score II/3; SOFA, Sequential Organ Failure Assessment Score; SOF, Study of Osteoporotic Fractures scale; ROC, receiver operating characteristic; 95% CI, 95% confidence interval
Fried’s phenotype
Fried’s phenotype of frailty (FP) is based on five criteria of individual functional capacity (12):
Unintentional loss of weight
General exhaustion
Lack of muscle strength
Slow walking speed
Reduced physical activity
One point is assigned for each criterion fulfilled. A patient who scores 0 points is classified as robust, 1–2 points as prefrail, and ≥ 3 points as frail. The FP was recently validated prospectively in old (≥ 70 years) intensive care patients (5): long-term follow-up showed that among all patients who left the intensive care unit alive, those who were frail had a significantly higher incidence of both lasting dependence of care and mortality. A limitation of the FP is the necessity for active participation by the patient, which is not possible for the critically ill.
FRAIL scale
The FRAIL scale is based on Fried’s phenotype and, like the FP, asks about the following:
Fatigue
Resistance (muscle strength, ability to walk up one flight of stairs)
Ambulation (ability to walk 100 m)
Illnesses (more than five)
Loss of weight (> 5 % or 5 kg in previous 6 months)
The score is evaluated as for the FP. The difference from the FP is that the items are verifiable (13). This score has been validated in various studies (all in the USA) (e12, 14). A study on intensive care patients ≥ 65 years of age revealed that—with comparable SOFA scores—mortality at 1 month and at 6 months was higher in patients classified as frail using the FRAIL scale (15). The FRAIL scale has not yet been validated for German intensive care units.
Frailty index, modified frailty index, and electronic frailty index
Frailty indexes (FI) are models for measuring cumulative deficits. They are based on symptoms, clinical signs, illnesses, and laboratory test results that have been identified as deficits for the frail patient. Originally, 70 to 92 variables were defined (16). Each variable must be biologically plausible, must increase with age, and must not be fulfilled by all patients at too young an age (17). The difference between FI and the derivative electronic frailty index (eFI) is that FI require the patients themselves to complete an exhaustive questionnaire, while the eFI evaluates routinely available digital data (18). Subsequent studies succeeded in reducing the variables to 11 items (modified frailty index, mFI) (table 2) with no loss of accuracy (19). A modified FI with 52 criteria was used in patients ≥ 65 years in Chinese intensive care units. Depending on the cut-off, this FI predicted 30-day mortality with high accuracy (e13). These findings were replicated in another study (e14). However, the results are very difficult to transfer to the situation in Germany. One problem is that many studies use different FI. Both FI and the eFI are time-consuming and require data that are generally not available at the time of admission to the intensive care unit. There is currently no validation for German intensive care units.
Hospital Frailty Risk Score
The Hospital Frailty Risk Score (HFRS), derived from the eFI, uses ICD-10 codes to estimate frailty (20). By means of a cluster analysis in older patients who displayed higher mortality and morbidity than others of the same age, Gilbert et al. identified 109 ICD-10 codes that were overrepresented in this cohort. Every individual ICD code was weighted differently. A score of >5 equated to intermediate risk of frailty, and >15 to high risk. The studies that investigated the HFRS were predominantly retrospective analyses (20). In most cases the admitting physician in Germany has no access to a list of the patient’s ICD-10 codes. Studies have shown that the HFRS yields no additional benefit for the estimation of mortality (21, 22). Overall, the data indicate that the HFRS is neither practicable nor of prognostic utility in Germany.
Clinical Frailty Scale
The Clinical Frailty Scale (CFS) is based on the FI, correlates well with it, and is a greatly simplified instrument (23). Moreover, the CFS has been modified specifically for use in intensive care units (24). The CFS looks at the patient’s social environment (“needs help with washing”) and compares them to the reference population (“much fitter than others in their age group”). This distinguishes the CFS from the FP, which, for its part, looks more closely at sarcopenia—a syndrome complementary to frailty with many features in common (25). Different levels of frailty are represented in pictograms (1 = very fit, ≥ 5 = frail, 9 = terminally ill). The pictograms have been translated into German with the involvement of the German Society of Geriatrics (Deutsche Gesellschaft für Geriatrie; DGG) and are available free of charge (www.uniklinik-duesseldorf.de/cfs).
The CFS integrates both physical and cognitive elements and also delivers brief written descriptions. A statement (secondary medical history) from a family member or care provider is generally necessary for classification, although assessment also functions reliably without the involvement of relatives (26). The CFS has been successfully validated for patients above 50 years of age (8). In intensive care patients, the CFS with a cut-off of ≥ 5 is equivalent to the SAPS 3 intensive care score for prediction of 30-day survival (27). Even after adjustment for SAPS 3, CFS remained a strong independent predictor of 30-day mortality. In intensive care patients over 65 with initially identical SOFA and SAPS II scores, CFS (cut-off ≥ 5) is superior to both FP and conventional intensive care scores (SOFA, SAPS II) in prediction of 6-month mortality (25). In a subgroup analysis from an international multicenter prospective study, the CFS in German intensive care units was also independently associated with the prognosis (28). An exhaustive meta-analysis confirmed both the practicability and the informative value of the CFS in the field of intensive care (29). The CFS possesses “almost perfect” inter-rater reliability when evaluated by several different persons (weighted Cohen’s κ 0.85; 95% confidence interval [0.84; 0.87]) (4). The assessment of the intensive care team does not differ significantly from that of the geriatric consultant (30). In emergency patients, too, the CFS has high predictive power for 30-day mortality and hospitalization as well as good inter-rater reliability (weighted Cohen’s κ 0.74; [0.64; 0.85]) (31). The CFS can also be used in patients with dementia syndrome. It has not been validated, however, in patients with stable permanent disabilities, such as infantile brain damage. This must be taken into account when assigning patients with dementia syndrome to levels 1 to 9. The extent to which the patient needs assistance with activities of daily life is crucial. Assessment by means of FP is more complex than with CFS, but the predictive power of the latter is equivalent or inferior (25, 32). The two scores show high correlation.
In summary, the CFS—used prospectively or retrospectively—has been validated for the intensive care setting, is simple to use, and assesses a patient’s frailty with high reliability despite the low consumption of resources (classification by means of pictograms). Use of the CFS has been recommended by the National Institute for Health and Care Excellence (e15). In Germany, the DGG and the German Interdisciplinary Association for Intensive Care and Emergency Medicine (Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin; DIVI) have translated and annotated the CFS. The suitability of the CFS for prediction of the prognosis in COVID-19 in the intensive care setting is currently being assessed in a multicenter prospective study (NCT04321265).
Other scores
Large prospective multicenter studies have shown that the use of other scores ascertaining cognitive performance (IQCODE) and the extent of independence in performing daily tasks (Katz Index of Independence for Activities of Daily Living) in more detail yield no additional benefit relative to the CFS (4). Attempts have been made to identify suitable biomarkers for frailty (“frailty troponin”) (33). The decisive processes that have to be elucidated for this purpose in laboratory tests are inflammation, mitochondrial function, calcium homeostasis, markers of fibrosis, neuronal damage, and markers of the cytoskeleton. To date, no single marker for the degree of frailty has been pinpointed (e16). With the exception of clinical studies, these approaches are not currently being pursued in intensive care.
Summary
Frailty is a powerful independent predictor of the short-term and long-term prognosis of very old patients in the intensive care setting. A number of instruments are available for assessment of frailty, but the bulk of them are of limited applicability, if at all, in German intensive care units (etable) (15, 22, 30– 32, 35– 38, e9, e13, e14). The CFS, with its superior practicability and good prediction of the prognosis, has proved useful for routine application in intensive care units.
Key Messages.
The proportion of intensive care beds occupied by very old patients is increasing.
Frailty is a multimodal and age-associated model, but very old patients are not necessarily frail.
Frailty is an independent parameter, that can be used to predict the prognosis of critically ill patients.
A number of diagnostic methods with varying quality of validation are available for the assessment of frailty.
The Clinical Frailty Scale is simple to use, possesses high inter-rater reliability, and has been successfully validated for use in German intensive care units.
eBOX 2. Modified frailty index (mFI).
Diabetes mellitus
Cardiac insufficiency
Arterial hypertension
Status post cerebral ischemia
Myocardial infarction
Peripheral arterial occlusive disease
Neurological deficit after stroke
Status post chronic obstructive pulmonary disease or pneumonia
Status post coronary intervention/operation or angina pectoris
Functional status: not independent
Modified and adapted from (19)
Questions on the article in issue 40/2020: Frailty as a Prognostic Indicator in Intensive Care.
The closing date for answers is 1 October 2020. Only one answer per question is possible. Please select the answer that is most appropriate.
Question 1
What is the primary purpose of frailty assessment?
It helps to ensure correct coding in the DRG system.
It serves to predict the long-term prognosis and the prospects of rehabilitation.
Performance of the assessment is associated with a survival advantage for the patient.
It serves to motivate patients to improve their score.
It helps to evaluate the severity of an acute illness.
Question 2
According to the WHO, which of the following defines frailty?
Primarily the patient’s age.
The number of previous hospital stays.
The number of medications taken on a regular basis.
Reduced walking capacity in old age.
Reduced ability to cope with routine and acute stressors.
Question 3
Which of the following is one of the five criteria for evaluating frailty according to Fried?
Unintentional weight loss, general exhaustion, lack of muscle strength
General exhaustion, loss of speech, lack of muscle strength
Slow walking speed, loss of reading ability, lack of muscle strength
Unintentional weight loss, depression, confusion
Slow walking speed, loss of speech, general dissatisfaction
Question 4
Which of the following instruments has been validated for the assessment of frailty in intensive care and is recommended for use in German intensive care units?
The visual analog scale (VAS)
The modified frailty index (mFI)
The Hospital Frailty Risk Score (HFRS)
The Clinical Frailty Scale (CFS)
The FRAIL scale
Question 5
The FRAIL scale is based on Fried’s phenotype; what advantage does it offer?
The score is simpler to evaluate.
The items are more verifiable.
It considers a higher number of items.
It takes no account of weight loss.
The FRAIL scale has been validated for German intensive care units.
Question 6
On what information is the electronic frailty index (eFI) based?
On details of medical history provided by the patient in an exhaustive questionnaire
On details of the patient’s medical history provided in a questionnaire for relatives
On routinely available digital data
On details of medical history provided by the patient and by relatives in two separate questionnaires
On laboratory test results from the preceding 6 months
Question 7
What forms the basis of the Clinical Frailty Scale?
-
Five pictograms
(1 = very fit; 5 = terminally ill)
-
Three pictograms
(1= very fit; 2 = frail; 3 = very frail)
-
Nine pictograms
(1= terminally ill; 9 = very fit)
-
Five pictograms
(1 = very frail; 5 = very fit)
-
Nine pictograms
(1 = very fit; ≥ 5 = frail; 9 = terminally ill)
Question 8
Which of the following is one of the limitations of assessment by means of Fried’s phenotype (FP)?
The FP has not been prospectively validated.
The FP can only be used in patients < 70 years.
The patient’s active participation is required.
Only three criteria are assessed.
The FP has not been validated for use in intensive care patients.
Question 9
According to a study, what proportion of German intensive care patients in the period 2007 to 2011 were very old?
Over 40%
Around 10%
Over 60%
Over 20%
Under 15%
Question 10
Which of the following restrictions applies to the use of the CFS in intensive care units?
It has not been validated for patients with stable, permanent disability.
Its inter-rater reliability is relatively low.
It is not suitable for patients with dementia syndrome.
The patients should not be more than 75 years old.
Its high consumption of resources means it is difficult to use in German intensive care units.
Acknowledgments
Translated from the original German by David Roseveare
Footnotes
Conflict of interest statement The authors declare that no conflict of interest exists.
References
- 1.Sprung CL, Artigas A, Kesecioglu J, et al. The Eldicus prospective, observational study of triage decision making in European intensive care units Part II: intensive care benefit for the elderly. Crit Care Med. 2012;40:132–138. doi: 10.1097/CCM.0b013e318232d6b0. [DOI] [PubMed] [Google Scholar]
- 2.Ihra GC, Lehberger J, Hochrieser H, et al. Development of demographics and outcome of very old critically ill patients admitted to intensive care units. Intensive Care Med. 2012;38:620–626. doi: 10.1007/s00134-012-2474-7. [DOI] [PubMed] [Google Scholar]
- 3.Yayan J. Trends in intensive care in patients over 90 years of age. Clin Interv Aging. 2012;7:339–347. doi: 10.2147/CIA.S31780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidet B, de Lange DW, Boumendil A, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2020;46:57–69. doi: 10.1007/s00134-019-05853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. The association of frailty with post-ICU disability, nursing home admission, and mortality: a longitudinal study. Chest. 2018;153:1378–1386. doi: 10.1016/j.chest.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaatten H, de Lange DW, Artigas A, et al. The status of intensive care medicine research and a future agenda for very old patients in the ICU. Intensive Care Med. 2017;43:1319–1328. doi: 10.1007/s00134-017-4718-z. [DOI] [PubMed] [Google Scholar]
- 7.Beauchamp T, Childress J. Oxford UniversityPress. New York: 1994. Respect for autonomy, nonmaleficence, beneficence, justice Principles of biomedical ethics 4th ed; pp. 120–394. [Google Scholar]
- 8.Bagshaw SM, Stelfox HT, McDermid RC, et al. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186:E95–E102. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mende A, Riegel AK, Plümer L, Olotu C, Goetz AE, Kiefmann R. The determinants of perioperative outcome in frail older patients. Dtsch Arztebl Int. 2019;116:73–82. doi: 10.3238/arztebl.2019.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olotu C, Weimann A, Bahrs C, Schwenk W, Scherer M, Kiefmann R. The perioperative care of older patients—time for a new, interdisciplinary approach. Dtsch Arztebl Int. 2019;116:63–69. doi: 10.3238/arztebl.2019.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidet B, Vallet H, Boddaert J, et al. Caring for the critically ill patients over 80: a narrative review. Ann Intensive Care. 2018;8 doi: 10.1186/s13613-018-0458-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 13.Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008;9:71–72. doi: 10.1016/j.jamda.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Maxwell CA, Dietrich MS, Miller RS. The FRAIL Questionnaire: a useful tool for bedside screening of geriatric trauma patients. J Trauma Nurs. 2018;25:242–247. doi: 10.1097/JTN.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 15.Lopez Cuenca S, Oteiza Lopez L, Lazaro Martin N, et al. Frailty in patients over 65 years of age admitted to intensive care units (FRAIL-ICU) Med Intensiva. 2019;43:395–401. doi: 10.1016/j.medin.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. Bmc Geriatr. 2008;8 doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45:353–360. doi: 10.1093/ageing/afw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farhat JS, Velanovich V, Falvo AJ, et al. Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 2012;72:1526–1530. doi: 10.1097/TA.0b013e3182542fab. discussion 30-1. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391:1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruno RR, Wernly B, Flaatten H, Scholzel F, Kelm M, Jung C. The hospital frailty risk score is of limited value in intensive care unit patients. Crit Care. 2019;23 doi: 10.1186/s13054-019-2520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redfern OC, Harford M, Gerry S, Prytherch D, Watkinson PJ. Frailty and unplanned admissions to the intensive care unit: a retrospective cohort study in the UK. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06020-7. 10.1007 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 23.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermid RC, Stelfox HT, Bagshaw SM. Frailty in the critically ill: a novel concept. Crit Care. 2011;15 doi: 10.1186/cc9297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Maguet P, Roquilly A, Lasocki S, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40:674–682. doi: 10.1007/s00134-014-3253-4. [DOI] [PubMed] [Google Scholar]
- 26.Fisher C, Karalapillai DK, Bailey M, Glassford NG, Bellomo R, Jones D. Predicting intensive care and hospital outcome with the Dalhousie Clinical Frailty Scale: a pilot assessment. Anaesth Intensive Care. 2015;43:361–368. doi: 10.1177/0310057X1504300313. [DOI] [PubMed] [Google Scholar]
- 27.De Geer L, Fredrikson M, Tibblin AO. Frailty predicts 30-day mortality in intensive care patients: A prospective prediction study. Eur J Anaesthesiol. 2020 doi: 10.1097/EJA.0000000000001156. 10.1097 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 28.Muessig JM, Nia AM, Masyuk M, et al. Clinical Frailty Scale (CFS) reliably stratifies octogenarians in German ICUs: a multicentre prospective cohort study. Bmc Geriatr. 2018;18 doi: 10.1186/s12877-018-0847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pugh RJ, Ellison A, Pye K, et al. Feasibility and reliability of frailty assessment in the critically ill: a systematic review. Crit Care. 2018;22 doi: 10.1186/s13054-018-1953-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shears M, Takaoka A, Rochwerg B, et al. Assessing frailty in the intensive care unit: a reliability and validity study. J Crit Care. 2018;45:197–203. doi: 10.1016/j.jcrc.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Kaeppeli T, Rueegg M, Dreher-Hummel T, et al. Validation of the clinical frailty scale for prediction of thirty-day mortality in the emergency department. Ann Emerg Med. 2020 doi: 10.1016/j.annemergmed.2020.03.028. S0196 0644(20)30218-3 (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 32.Ritt M, Schwarz C, Kronawitter V, et al. Analysis of Rockwood et al‘s clinical frailty scale and Fried et al‘s frailty phenotype as predictors of mortality and other clinical outcomes in older patients who were admitted to a geriatric ward. J Nutr Health Aging. 2015;19:1043–1048. doi: 10.1007/s12603-015-0667-9. [DOI] [PubMed] [Google Scholar]
- 33.Kane AE, Sinclair DA. Frailty biomarkers in humans and rodents: Current approaches and future advances. Mech Ageing Dev. 2019;180:117–128. doi: 10.1016/j.mad.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flaatten H, De Lange DW, Morandi A, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years) Intens Care Med. 2017;43:1820–1828. doi: 10.1007/s00134-017-4940-8. [DOI] [PubMed] [Google Scholar]
- 35.O‘Caoimh R, Costello M, Small C, et al. Comparison of frailty screening instruments in the emergency department. Int J Environ Res Public Health. 2019;16 doi: 10.3390/ijerph16193626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chong E, Ho E, Baldevarona-Llego J, Chan M, Wu L, Tay L. Frailty and risk of adverse outcomes in hospitalized older adults: A comparison of different frailty measures. J Am Med Dir Assoc. 2017;18:638 e7–638 e11. doi: 10.1016/j.jamda.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Chong E, Ho E, Baldevarona-Llego J, et al. Frailty in hospitalized older adults: Comparing different frailty measures in predicting short- and long-term patient outcomes. J Am Med Dir Assoc. 2018;19 doi: 10.1016/j.jamda.2017.10.006. 450 7 e3. [DOI] [PubMed] [Google Scholar]
- 38.Baldwin MR, Reid MC, Westlake AA, et al. The feasibility of measuring frailty to predict disability and mortality in older medical intensive care unit survivors. J Crit Care. 2014;29:401–408. doi: 10.1016/j.jcrc.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Bagshaw SM, Webb SA, Delaney A, et al. Very old patients admitted to intensive care in Australia and New Zealand: a multi-centre cohort analysis. Crit Care. 2009;13 doi: 10.1186/cc7768. R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Haas LE, Karakus A, Holman R, Cihangir S, Reidinga AC, de Keizer NF. Trends in hospital and intensive care admissions in the Netherlands attributable to the very elderly in an ageing population. Crit Care. 2015;19 doi: 10.1186/s13054-015-1061-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3.Nielsson MS, Christiansen CF, Johansen MB, Rasmussen BS, Tonnesen E, Norgaard M. Mortality in elderly ICU patients: a cohort study. Acta Anaesthesiol Scand. 2014;58:19–26. doi: 10.1111/aas.12211. [DOI] [PubMed] [Google Scholar]
- E4.Laake JH, Dybwik K, Flaatten HK, Fonneland IL, Kvale R, Strand K. Impact of the post-World War II generation on intensive care needs in Norway. Acta Anaesthesiol Scand. 2010;54:479–484. doi: 10.1111/j.1399-6576.2009.02170.x. [DOI] [PubMed] [Google Scholar]
- E5.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.World Health O. World Health Organization. Geneva: 2018. WHO clinical consortium on healthy ageing 2017: focus: development of comprehensive assessments and care plans. Report of consortium meeting, 21-22 November 2017 in Geneva Switzerland. [Google Scholar]
- E7.Howlett SE, Rockwood K. New horizons in frailty: ageing and the deficit-scaling problem. Age Ageing. 2013;42:416–423. doi: 10.1093/ageing/aft059. [DOI] [PubMed] [Google Scholar]
- E8.Evans DC, Cook CH, Christy JM, et al. Comorbidity-polypharmacy scoring facilitates outcome prediction in older trauma patients. J Am Geriatr Soc. 2012;60:1465–1470. doi: 10.1111/j.1532-5415.2012.04075.x. [DOI] [PubMed] [Google Scholar]
- E9.Malmstrom TK, Miller DK, Morley JE. A comparison of four frailty models. J Am Geriatr Soc. 2014;62:721–726. doi: 10.1111/jgs.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E10.Hogan DB, Maxwell CJ, Afilalo J, et al. A scoping review of frailty and acute care in middle-aged and older individuals with recommendations for future research. Can Geriatr J. 2017;20:22–37. doi: 10.5770/cgj.20.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Flaatten H, Clegg A. Frailty: we need valid and reliable tools in critical care. Intensive Care Med. 2018;44:1973–1975. doi: 10.1007/s00134-018-5404-5. [DOI] [PubMed] [Google Scholar]
- E12.Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16:601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E13.Zeng A, Song X, Dong J, et al. Mortality in relation to frailty in patients admitted to a specialized geriatric intensive care unit. J Gerontol A Biol Sci Med Sci. 2015;70:1586–1594. doi: 10.1093/gerona/glv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E14.Kizilarslanoglu MC, Civelek R, Kilic MK, et al. Is frailty a prognostic factor for critically ill elderly patients? Aging Clin Exp Res. 2017;29:247–255. doi: 10.1007/s40520-016-0557-y. [DOI] [PubMed] [Google Scholar]
- E15.NICE. COVID-19 rapid guideline: critical care in adults. www.nice.org.uk/guidance/ng159/resources/critical-care-admission-algorithm-pdf-8708948893 (last accessed on 14 July 2020) [Google Scholar]
- E16.Cardoso AL, Fernandes A, Aguilar-Pimentel JA, et al. Towards frailty biomarkers: Candidates from genes and pathways regulated in aging and age-related diseases. Ageing Res Rev. 2018;47:214–277. doi: 10.1016/j.arr.2018.07.004. [DOI] [PubMed] [Google Scholar]