Abstract
Depression has been associated with increased inflammation. However, only few large-scale, prospective studies have evaluated whether inflammation leads to new cases of depression and whether this association can be found in men and women. Longitudinal data of N = 10,357 adult participants with no evidence of depression at baseline (based on Patient Health Questionnaire (PHQ-9), lifetime diagnoses, and current antidepressant medication) were evaluated for depression 5 years later. Multivariate logistic regression models were used to predict the onset of depression based on C-reactive protein (CRP) and white blood cell count (WBC). We used interaction terms and separate analyses in men and women to investigate gender-dependent associations. Based on both markers, inflammation was predictive of new cases of depression 5 years later, even when adjusting for sociodemographic, physical health, health behavior variables, and baseline depression symptoms. As established by interaction terms and separate analyses, inflammatory markers were predictive of depression in men, but not in women. Additional predictors of new onset of depression were younger age, loneliness, smoking (only in men), cancer and less alcohol consumption (only in women). The study indicates gender differences in the etiology of depressive disorders within the community, with a greater role of physical factors in men.
Subject terms: Human behaviour, Medical research, Depression, Biomarkers, Inflammation, Risk factors, Public health, Psychiatric disorders
Introduction
Depression has been recognized as one of the most frequent and harmful mental disorders with an estimated life time risk of 15–25%; with prevalence rates twice as high among women compared to men1. Researchers have discussed a variety of factors underlying this observation, for instance genetic and biological vulnerabilities, environmental factors such as daily stressors, and differences in emotion regulation and stress responsiveness2.
In recent years, a growing body of research examining the temporal dynamics of depressive disorders has identified close associations of depression symptoms with chronic physical diseases3–5. The association with cardiovascular disease (CVD), which caused 37% of deaths in the German population in 20176, has been most extensively investigated. Studies showed (1) an increased risk of CVD in depressed individuals, (2) an even higher risk of depression following acute CVD, and (3) worse prognosis when CVD is complicated by depression7–10.
However, the mechanisms underlying this comorbidity remain an issue of investigation. Depression has been shown to aggravate health risks by poor health behaviors (smoking, sedentary behavior, poor diet) which can lead to central obesity, diabetes and CVD11. Studies have found that poor health behavior was more common among men than among women12. Chronic, low-grade inflammation plays a crucial role for atherosclerosis which underlies CVD13. Recently, inflammation has been studied as a potential link between depression and CVD14.
Already in the 1990s, meta-analyses15 have associated depression with alterations in cellular immunity. A recent meta-analysis16 found low-grade inflammation (C-reactive protein (CRP) > 3 mg/l) in 27% of depressed participants (Odds Ratio (OR) = 1.46; compared to healthy controls). Other cross-sectional studies indicated a link between depression symptoms and inflammation (e.g. CRP, interleukin-1 receptor antagonist (IL1-RA), fibrinogen)17,18. Lifestyle factors such as smoking and obesity, which are associated with inflammatory responses, accounted for the cross-sectional relationships. A recent meta-analysis, however, found that CRP and interleukin 12 (IL-12) not only had robust associations with depression (irrespective of cofounders such as smoking), but also showed a reduced variability in depressed participants vs. matched controls19.
The concept of an inflammatory phenotype of depression19 has attracted considerable attention. Chronic and persistent stress not only increases the risk for depression, but may also lead to a loss of the ability to regulate the inflammatory response. Chronically elevated cortisol levels contribute to a vicious cycle of immune dysregulation, hypertension, arteriosclerotic plaques and subsequent CVD. Systemic inflammation may drive depression via pro-inflammatory cytokines. As evidenced by immunotherapy (e. g. IFN–treatment in cancer patients), pro-inflammatory cytokines act on the brain to cause sickness behavior including early-onset somatic symptoms (such as flu-like complaints, pain, fatigue, loss of appetite, decreased motor activity and sleep disturbance), as well as late onset cognitive-emotional symptoms (such as mild cognitive dysfunction including impaired attention and memory, depressed mood, withdrawal, irritability and anxiety)20. Sickness behavior is an evolutionary adaptive constellation of changes, however, prolonged inflammatory activation is detrimental to physical and mental well-being21.
The cross-sectional findings raise important issues, (1) whether inflammatory mechanisms actually constitute risk factors for the onset of new depression, (2) which immunological markers are relevant, and (3) whether these findings are valid for men and for women.
The meta-analysis by Valkanova et al.22 identified a total of eight studies with 14,832 participants investigating the influence of baseline CRP on new onset of depression an average of 5 years later, and three studies (N = 3695) examining interleukin 6 (IL-6). Studies were predominantly conducted in the US, with few studies from Great Britain and the Netherlands. Elevated inflammatory markers had a small but statistically significant association with new onset of depression after adjustment for sociodemographic and other (unspecified) risk factors for depression, most importantly baseline depression. In a biobank investigation in a large cohort from Great Britain, Khandaker et al.8 established associations of depression with a family history of heart disease, as well as with IL-6, CRP and triglycerides.
Given the limited understanding of the mechanisms underlying the relationship between inflammation and depression, there is no consensus which inflammatory markers are particularly relevant. In their meta-analysis of longitudinal studies, Valkanova et al.22 demonstrated stronger effects for CRP vs. IL-6 on depression. By comparison, white blood cell count has been understudied as a marker. Yet, depression could lead to stimulation of hematopoietic cells and reduced vagal nerve activity accelerating the production of white blood cells (WBCs)23.
In recent years, awareness of the importance of sex- and gender-sensitive or -specific approaches in medical research has increased24–26. Given the substantial epidemiological gender differences in depressive disorders, it seems particularly promising to investigate potentially gender-dependent risk factors for depression. Knowledge about differential vulnerabilities could inform prevention and intervention efforts that are specially geared to the needs of men and women27,28.
The role of inflammation in the etiology of depression also needs to be investigated in a gender-specific manner as studies have found greater physiological dysregulation in women (including a host of systems and inflammatory markers), especially after menopause29. Experimental evidence supports a gender difference as well: a study found that women reported a stronger decline in their mood after being administered an endotoxin as inflammatory challenge30.
However, population-based findings regarding gender-dependent associations of depression and inflammation are scarce and mixed as participants’ outcomes were only seldom analyzed in gender- specific ways. In their large population-based cohort study, Khandaker et al.8 reported no gender differences regarding the association of CRP, IL-6 and depression. Surtees et al.31 found an association of both depression episode status and history of depression with higher leukocyte counts, but only in men. By contrast, Beydoun et al.14 reported that higher total white blood cell count was predictive of an increase of depression symptoms in women, but not in men. In a large Swedish twin study, Kendler et al.7 found that the manifestation of CVD was more predictive of the onset of major depression than reverse. However, the temporal pattern differed between men and women: The risk of depression following CVD was much greater for men than for women, particularly in the first year.
The present study
We used data of a prospective, representative cohort study to address these research gaps. The purpose of this longitudinal study was twofold:
to determine whether baseline indicators of inflammation (CRP and WBC) predict new onset of depression at follow-up 5 years later and;
to explore differences between men and women with respect to these associations.
In order to identify cases with new onset of depression, we excluded participants with acute depression, a lifetime diagnosis of depression and antidepressant medication at baseline. We controlled sociodemographic factors, socioeconomic status and lifestyle confounders such as smoking and body mass index (BMI) and, importantly, also for baseline depression symptoms.
Methods
Procedure and study sample
The Gutenberg Health Study (GHS) is a population-based, prospective, observational single-center cohort study in the Rhine-Main-Region, Germany32,33. Its primary aim is to analyze cardiovascular risk factors and their stratification and to foster health prevention in the community. The study protocol and documents were approved by the ethics committee of the Medical Chamber of Rhineland-Palatinate and the local data safety commissioner. All investigations were conducted in line with the Declaration of Helsinki and principles outlined in recommendations for Good Clinical Practice and Good Epidemiological Practice. Participants were included after informed consent. Insufficient knowledge of German and psychological or physical impairment hindering participation led to exclusion. The sample was drawn randomly from the local registry in the city of Mainz and the district of Mainz-Bingen, stratified 1:1 for gender and residence and in equal strata for decades of age. Inclusion criterion was age 35 to 74 years. The response rate (defined as the recruitment efficacy proportion, i.e. the number of persons with participation in or appointment for the baseline examination, divided by the total number of persons with participation in or appointment for the baseline examination, plus those with refusal and those who were not contactable) was 60.3%.
At baseline (assessments between 2007 and 2012), N = 15,010 participants were examined. A total of 12,423 of them took part in the follow-up examination (82.8%).
As the present study concerned new onset of depression, we excluded 2056 individuals with evidence for current depression or history of depression at baseline: 1424 had reported a diagnosis of depression; of the remaining sample, 213 had reported taking antidepressant medication. Lastly, there were 429 participants in the dataset who had surpassed the PHQ-9 cut-off score for relevant symptom burden (≥ 10) at baseline. They were also excluded. Thus, all analyses reported in the following were based on the analyses sample of N = 10,357 participants. Figure 1 shows the participant flow.
Figure 1.
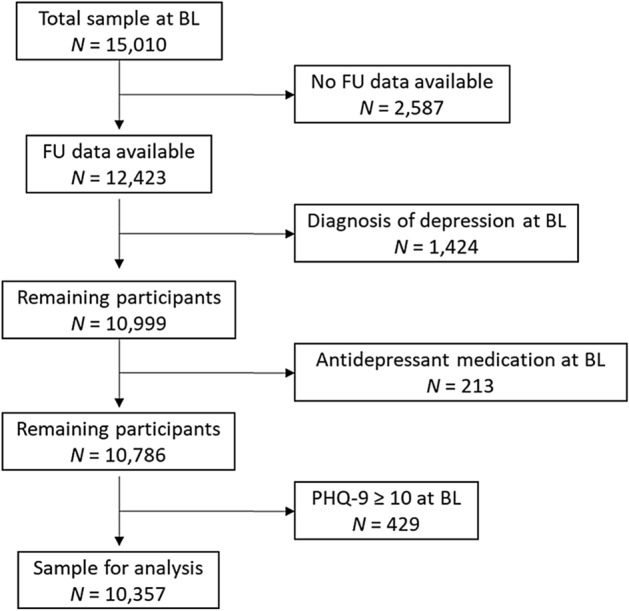
Participant flow. The present work is based on a community sample of N = 10,357 participants who took part in assessments at two measurement points and had no evidence for depression at baseline. BL baseline, FU follow-up.
Materials and assessment
The 5-h baseline-examination at the study center evaluated the classical cardiovascular risk factors and clinical variables. Participants also underwent a computer-assisted personal interview, and staff performed laboratory examinations from venous blood samples, blood pressure and anthropometric measurements. Examinations were performed according to standard operating procedures by certified medical technical assistants.
Sociodemographic information
Sociodemographic variables were assessed by self-report: gender, age in years, employment (no/yes), income, living with partner (no/yes), and socioeconomic status (SES). SES was defined according to Lampert et al.34. The index combines data based on education, profession and income and ranges from three (lowest socioeconomic status) to 27 (highest socioeconomic status).
Depression measures
Depression symptoms were assessed using the depression module of the Patient Health Questionnaire PHQ-9 (at baseline and follow-up). Participants rated the frequency of occurrence of the nine diagnostic criteria of major depression over the course of the last two weeks. (e.g. “Little interest or pleasure in doing things”) on a Likert scale (0 = not at all, 1 = several days, 2 = more than half the days, 3 = nearly every day). The sum scores ranges from 0 to 27. Presence of relevant symptom burden was defined as a sum score ≥ 10 which has previously shown a sensitivity of 81% and a specificity of 82% for detecting major depressive disorder35. Internal consistency was good (Cronbach’s α = 0.80).
Medication was registered on site by scanning the bar codes of original packages of drugs taken by participants. Active ingredients were recorded using ATC codes. Three classes of antidepressants were noted: nonselective monoamine reuptake inhibitors (ATC N06AA), selective serotonin reuptake inhibitors (ATC N06AB), and other antidepressants (ATC N06AX).
Loneliness was assessed using one item: ’I am frequently alone/have few contacts’ rated from 0 = no, does not apply, to 4 = yes, it applies, and I suffer strongly from it36.
Behavioral measures
Health behavior assessments included smoking. Participants’ reports were dichotomized into non-smokers (combining never smokers and ex-smokers) and smokers (combining occasional and frequent smokers).
Obesity was defined as a body mass index (BMI) ≥ 30 kg/m2.
Alcohol consumption was assessed via self-report. Participants reported how often, how many, and which kinds of beverages they consumed (e.g. beer, wine, spirits) in certain quantities. Following a standardized procedure, the number of grams/day was calculated from these responses. Alcohol consumption above the recommended limit was defined as daily consumption ≥ 10 g for women and ≥ 20 g for men (in line with the German threshold for alcohol consumption above tolerance).
Physical activity was inquired with the Short Questionnaire to Assess Health-Enhancing Physical Activity (SQUASH; Peters et al.37]). The SQUASH captures commuting, leisure time, household, work and school activities with reference to a typical week in recent months. Sleeping, lying, sitting and standing were classified as inactivity37. Active sports was presented in quartiles with Q1 denominating the lowest and Q4 the highest quartile of physical activity. Information on days per week, average time per day, and intensity of the activity is used for the calculation of an index score (total minutes of activity multiplied by intensity score, with higher scores reflecting more (intense) activity).
Interview assessments
During the computer-assisted personal interview, the presence of coronary heart disease was assessed by the question: ‘Were you diagnosed with a stenosis of your coronary vessels?’ Self-reported myocardial infarction (MI), heart failure (HF), stroke, deep vein thrombosis (DVT), pulmonary embolism (PE), and peripheral arterial disease (PAD) were summarized as cardiovascular disease (CVD). Participants were also asked whether they had ever received a definite cancer diagnosis from a physician, and whether they had ever received the definite diagnosis of any depressive disorder (i.e. lifetime diagnosis of depression).
Diabetes was defined in individuals with a definite diagnosis of diabetes by a physician or a blood glucose level of ≥ 126 mg/dl in the baseline examination after an overnight fast of at least 8 h or a blood glucose level of > 200 mg/dl after a fasting period of 8 h.
Laboratory analysis
Venous blood sampling was performed in supine position, and the prior fasting period was documented. C-reactive protein (CRP) concentration was measured in heparin-plasma by a high-sensitivity latex enhanced immunoturbidimetric assay (Abbott Laboratories, Abbott Park, IL). The limit of detection was 0.2 mg/l. Blood cell counts (including white blood cell count (WBC)) were performed on an ADVIA 2120 system (Siemens Healthcare Diagnostics, Eschborn, Germany). Measures of CRP and WBC were done immediately after sampling.
Statistical analysis
All data underwent quality control by a central data management unit. Data were reviewed for completeness by predefined algorithms and plausibility criteria. Descriptive analyses were performed as absolute and relative proportions for categorical data, means and standard deviations for continuous variables, and median with interquartile range if not fulfilling normal distribution. Inference tests between groups were calculated using t-tests or χ2 tests, where appropriate.
In order to identify predictors of incident depression in the analyses sample, we performed logistic regression analyses with depression (PHQ-9 sum score ≥ 10) at follow-up as the criterion variable. The models controlled for depression symptoms at baseline (PHQ-9 sum score), comprised inflammatory markers (CRP, WBC) and their interactions with gender, demographic variables (gender, age, living with a partner, SES) and physical health and health behavior variables (diabetes, cardiovascular disease, cancer, obesity, smoking, physical activity, and excessive consumption of alcohol) as predictors. For analyses, CRP was dichotomized into normal and elevated levels (≥ 3 mg/l, this cut-off value is based on the American Heart Association’s/Centers for Disease Control and Prevention’s guidelines38 and previous study procedures39). Raw WBCs were substantially left skewed, thus the natural log of WBC was used instead.
In order to determine gender-dependent effects (i.e. following observations of statistically significant interaction terms with gender), we also analyzed women and men separately.
All p-values should be regarded as continuous parameters that reflect the level of statistical evidence, and they are therefore reported exactly. Statistical analysis was carried out using R version 3.6.1.
Results
In the following, we first report univariate differences between individuals with and without new onset of depression (PHQ-9 sum score ≥ 10) at follow-up (which took place 5 years after the baseline assessment). We then present several logistic regression models predicting depression at follow-up: We tested elevated levels of CRP as a binary predictor (first within the whole sample using an interaction term with gender, secondly in separate analyses in men and women) as well as WBC as a continuous predictor (again both within the whole sample using an interaction term, and separately in men and women).
Sample characteristics
Table 1 yields an overview of baseline data of the study participants stratified by the presence of clinically relevant depression symptoms (according to the PHQ-9) at follow-up.
Table 1.
Baseline participant characteristics (stratified by PHQ-9 ≥ 10 at follow-up).
| Variable | All (N = 10,120) | PHQ-9 ≥ 10 at FU (N = 448) | PHQ-9 ≤ 9 at FU (N = 9672) | p |
|---|---|---|---|---|
| Sociodemographic | ||||
| Women (%) | 46.1% (4670) | 53.1% (238) | 45.8% (4432) | 0.003 |
| Age | 54.3 (10.9) | 51.2 (10.3) | 54.5 (10.9) | < 0.001 |
| Living with partner (%) | 84.5% (8547) | 79.7% (357) | 84.7% (8190) | 0.006 |
| Socioeconomic status | 13.45 (4.39) | 12.94 (4.26) | 13.48 (4.39) | 0.010 |
| Inflammation | ||||
| CRP ≥ 3 mg/l (%) | 23.9% (2419) | 28.0% (125) | 23.7% (2292) | 0.041 |
| WBC (leucocytes/nl) | 6.80 (5.75/8.10) | 7.10 (5.80/8.66) | 6.80 (5.75/8.10) | 0.002 |
| Physical health and health behavior | ||||
| Diabetes (%) | 7.3% (739) | 8.3% (37) | 7.3% (702) | 0.40 |
| CVD (%) | 8.3% (836) | 7.9% (35) | 8.3% (801) | 0.86 |
| Cancer (%) | 7.9% (800) | 8.9% (40) | 7.9% (760) | 0.42 |
| Obesity (%) | 22.7% (2297) | 24.8% (111) | 22.6% (2186) | 0.30 |
| Smoker (%) | 17.2% (1738) | 24.3% (108) | 16.9% (1630) | < 0.001 |
| Physical activity | 7260 (5174/9390) | 7590 (5500/10,140) | 7230 (5156/9360) | 0.019 |
| Alcohol (% > recom. limit) | 28.1% (2836) | 22.7% (101) | 28.3% (2735) | 0.01 |
| Mental distress | ||||
| Loneliness (%) | 6.6% (668) | 19.0% (85) | 6.1% (590) | < 0.001 |
| PHQ-9 sum at baseline | 3.13 (2.31) | 5.48 (2.37) | 3.02 (2.25) | < 0.001 |
Bold values indicate statistically significant differences.
Participant characteristics are shown as mean values and standard deviations, medians with interquartile ranges (if not fulfilling normal distribution) or as percentages and absolute numbers.
CRP C-reactive protein, CVD cardiovascular disease, FU follow-up, PHQ-9 patient health questionnaire-9, WBC white blood cell count.
At follow-up, 448 participants (210 men and 238 women) without evidence for depression at baseline reported relevant depression symptoms (4.4% of the overall sample). Group comparisons showed that those participants were more likely to be women, comparatively younger, more likely to live without a partner, and to have a lower socioeconomic status. Participants with new onset of depression were also more likely to smoke, to be physically inactive, and to consume less alcohol at baseline.
More women (5.1%) than men (3.9%) had new onset depression at follow-up. Supplementary Table S1 depicts differences between those with depression at follow-up and those without stratified by gender: Among women, new onset of depression was more common in those who were younger, smoked, and consumed less alcohol. The latter difference was not present among men. By contrast, new onset of depression among men was related to living without a partner, lower socioeconomic status, higher inflammation markers (both CRP and WBC), and being physically inactive.
Main analyses
We tested associations of CRP and WBC on new onset of depression in separate multivariate logistic regression models.
C-reactive protein
Table 2 shows the association of baseline CRP with depression at follow-up.
Table 2.
Results of the logistic regression analysis: Prediction of new onset of depression at follow-up (based on predictors measured at baseline 5 years earlier, including CRP ≥ 3 mg/l).
| Predictors | OR | 95% CI | p |
|---|---|---|---|
| Inflammation | |||
| CRP ≥ 3 mg/l | 1.58 | 1.10; 2.26 | 0.013 |
| Interaction: CRP by gender (women)* | 0.55 | 0.033; 0.91 | 0.021 |
| Sociodemographic information | |||
| Gender (women) | 1.34 | 1.02; 1.74 | 0.03 |
| Age | 0.98 | 0.97; 0.99 | < 0.001 |
| Living with partner | 1.14 | 0.84; 1.55 | 0.40 |
| Socioeconomic status | 0.99 | 0.96; 1.02 | 0.06 |
| Physical health and health behavior | |||
| Diabetes | 1.34 | 0.84; 2.12 | 0.22 |
| CVD | 1.15 | 0.75; 1.77 | 0.53 |
| Cancer | 1.38 | 0.92; 2.07 | 0.12 |
| Obesity | 0.88 | 0.66; 1.17 | 0.37 |
| Smoker | 1.40 | 1.07; 1.83 | 0.013 |
| Physical activity | 1.00 | 1.00; 1.00 | 0.81 |
| Alcohol > recom. limit | 0.83 | 0.64; 1.09 | 0.18 |
| Loneliness | 1.83 | 1.33; 2.52 | < 0.001 |
| PHQ-9 sum (baseline) | 1.47 | 1.40; 1.54 | < 0.001 |
Bold values indicate statistically significant predictors.
Total model: Nagelkerke R2 = 0.37.
CI confidence interval; CRP C-reactive protein; CVD cardiovascular disease, OR odds ratio, PHQ-9 patient health questionnaire-9.
*The odds ratio of the interaction term is a ratio of odds ratios (ROR). The OR for CRP > 3 mg/l of 1.58 is to be interpreted as the OR for men with elevated CRP. The OR for women with elevated CRP can be calculated from the ROR and the above OR for men as 1.58 × 0.55 = 0.87.
Elevated levels of CRP at baseline were predictive of depression at follow-up in the total sample (OR 1.58, 95% CI 1.10–2.26). The interaction term, however, showed that this effect was modified by gender. The interaction of elevated levels of CRP with gender was statistically significant (OR 0.55, 95% CI 0.33–0.91). As illustrated by the interaction plot (Fig. 2), elevated values of CRP predicted new onset depression only in men and not in women. Additional predictors of depression were being a woman, younger age, loneliness, and baseline depression score; there was a trend for lower SES. No significant effects were found for diabetes, cardiovascular disease, cancer, obesity, smoking, physical activity and alcohol consumption.
Figure 2.
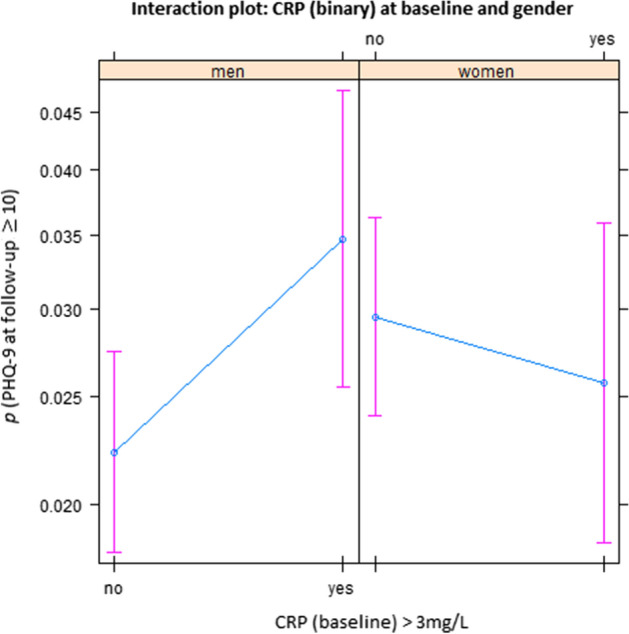
Association with baseline CRP levels and depression symptoms at follow-up in men and women. We observed gender differences regarding the association of elevated levels of CRP at baseline with new onset of depression at follow-up: in men, elevated CRP at baseline was associated with higher PHQ-9 scores at follow-up; whereas in women, no clear effect of baseline CRP on PHQ-9 at follow-up was apparent. The plot results show the probability (p) and 95% confidence intervals of depression (PHQ-9 ≥ 10) estimated based on the regression model of the total sample (reported in Table 2), depending on baseline CRP (normal versus elevated) and other predictors held fixed. (Figures were created using R version 3.6.1 https://www.R-project.org/).
Table 3 shows separate analyses for men and for women: CRP was predictive of depression (OR 1.4), 95% CI 1.03–2.16) in men only, along with younger age, smoking, loneliness and baseline depression symptoms. In women, lower age, a history of cancer, lower alcohol consumption, loneliness and baseline depression symptoms were predictive of depression, but not CRP.
Table 3.
Results of the logistic regression analysis: Prediction of new onset of depression at follow-up (based on predictors measured at baseline 5 years earlier, including CRP ≥ 3 mg/l), stratified by gender.
| Predictors | Men Nagelkerke R2 = 0.38 |
Women Nagelkerke R2 = 0.37 |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Inflammation | ||||||
| CRP ≥ 3 mg/l | 1.49 | 1.03; 2.16 | 0.033 | 0.92 | 0.63; 1.36 | 0.68 |
| Sociodemographic | ||||||
| Age | 0.99 | 0.97; 1.00 | 0.023 | 0.97 | 0.95; 0.99 | < 0.001 |
| Living with partner | 1.29 | 0.80; 2.10 | 0.30 | 1.06 | 0.71; 1.58 | 0.79 |
| Socioeconomic status | 0.99 | 0.95; 1.03 | 0.60 | 1.00 | 0.95; 1.04 | 0.87 |
| Physical health and health behavior | ||||||
| Diabetes | 1.54 | 0.89; 2.67 | .12 | 0.87 | 0.34; 2.23 | 0.76 |
| CVD | 1.01 | 0.58; 1.78 | 0.97 | 1.21 | 0.61; 2.42 | 0.59 |
| Cancer | 0.85 | 0.41; 1.75 | 0.65 | 1.82 | 1.11; 2.99 | 0.018 |
| Obesity | 0.98 | 0.67; 1.44 | 0.92 | 0.76 | 0.48; 1.18 | 0.22 |
| Smoker | 1.53 | 1.05; 2.22 | 0.027 | 1.31 | 0.89; 1.92 | 0.17 |
| Physical activity | 1.00 | 1.00; 1.00 | 0.54 | 1.00 | 1.00; 1.00 | 0.85 |
| Alcohol > recom. limit | 0.97 | 0.68; 1.40 | 0.88 | 0.67 | 0.44; 1.00 | 0.049 |
| Mental distress | ||||||
| Loneliness | 1.92 | 1.18; 3.10 | 0.008 | 1.75 | 1.14; 2.69 | 0.011 |
| PHQ-9 sum (baseline) | 1.56 | 1.46; 1.67 | < 0.001 | 1.38 | 1.30; 1.48 | < 0.001 |
Bold values indicate statistically significant predictors.
CI confidence interval, CRP C-reactive protein, CVD cardiovascular disease, OR odds ratio, PHQ-9 patient health questionnaire-9.
White blood cell count
Table 4 presents the association of baseline ln(WBC) with depression at follow-up.
Table 4.
Results of the logistic regression analysis: Prediction of new onset of depression at follow-up (based on predictors measured at baseline 5 years earlier, including the natural logarithm of WBC).
| Predictors | OR | 95% CI | p |
|---|---|---|---|
| Inflammation | |||
| Ln(WBC) | 1.88 | 1.04; 3.42 | 0.04 |
| Interaction: Ln(WBC)* Gender (women) | 0.37 | 0.16; 0.87 | 0.023 |
| Sociodemographic | |||
| Gender (women) | 7.91 | 1.48; 42.33 | 0.02 |
| Age | 0.98 | 0.97; 0.99 | < 0.001 |
| Living with partner | 1.13 | 0.83; 1.54 | 0.43 |
| Socioeconomic status | 0.99 | 0.97; 1.02 | 0.65 |
| Physical health and health behavior | |||
| Diabetes | 1.38 | 0.87; 2.19 | 0.17 |
| CVD | 1.14 | 0.74; 1.76 | 0.55 |
| Cancer | 1.39 | 0.93; 2.09 | 0.11 |
| Obesity | 0.89 | 0.67; 1.18 | 0.41 |
| Smoker | 1.37 | 1.04; 1.80 | 0.02 |
| Physical activity | 1.00 | 1.00; 1.00 | 0.81 |
| Alcohol > recom. limit | 0.86 | 0.66; 1.12 | 0.25 |
| Mental distress | |||
| Loneliness | 1.82 | 1.32; 2.50 | < 0.001 |
| PHQ-9 sum (baseline) | 1.47 | 1.4; 1.54 | < 0.001 |
Bold values indicate statistically significant predictors.
Total model: Nagelkerke R2 = 0.37.
CI confidence interval, CVD cardiovascular disease, OR odds ratio, PHQ-9 patient health questionnaire-9, WBC white blood cell count.
As Table 4 shows, ln(WBC) was predictive of depression (OR 1.88, 95% CI 1.04–3.42) in the total sample. Again, the interaction of the inflammation parameter with gender (OR 0.37, 95% CI 0.16–0.87) indicated a stronger increase of depression symptoms based on WBC in men (Fig. 3). Other statistically significant predictors were being a woman, younger age, smoking, loneliness, and baseline depression symptoms. In separate analyses for men and for women (Table 5), these analyses, the association of inflammation and depression in men showed a wide confidence interval, just beyond the conventional level of statistical significance (OR 1.80, 95% CI 0.96–3.37, p = 0.07). Risk factors were younger age, loneliness, and baseline depression symptoms. In women, younger age also played a role, and a history of cancer, lower alcohol consumption, loneliness, and baseline depression symptoms.
Figure 3.
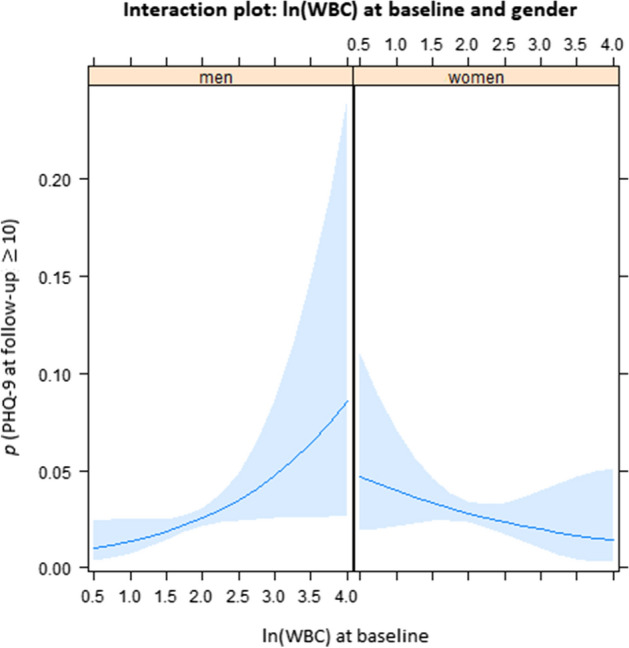
Interaction plot between gender and natural log of white blood cell count as predictors of new onset of depression 5 years later. There were differences between men and women with respect to the association of elevated levels of WBC at baseline with new onset of depression at follow-up: in men, elevated WBC at baseline was more closely associated with higher PHQ-9 sum scores at follow-up. The plot results show the probability (p) and 95%-confidence intervals (shaded areas) of depression (PHQ-9), estimated based on the regression model of the total sample (reported in Table 4), depending on baseline natural log of WBC and other predictors held fixed. (Figures were created using R version 3.6.1 https://www.R-project.org/).
Table 5.
Results of the logistic regression analysis: prediction of new onset of depression at follow-up (based on predictors measured at baseline 5 years earlier, including the natural logarithm of WBC), stratified by gender.
| Predictors | Men Nagelkerke R2 = 0.25 |
Women Nagelkerke R2 = 0.29 |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Inflammation | ||||||
| Ln(WBC) | 1.80 | 0.96; 3.37 | 0.07 | 0.72 | 0.38; 1.36 | 0.31 |
| Sociodemographic | ||||||
| Age | 0.99 | 0.97; 1.01 | 0.025 | 0.97 | 0.95; 0.99 | < 0.001 |
| Living with partner | 1.27 | 0.79; 2.07 | 0.33 | 1.05 | 0.70; 1.56 | 0.82 |
| Socioeconomic status | 0.99 | 0.95; 1.03 | 0.66 | 1.00 | 0.95; 1.04 | 0.83 |
| Physical health and health behavior | ||||||
| Diabetes | 1.58 | 0.91; 2.72 | 0.10 | 0.89 | 0.34; 2.29 | 0.80 |
| CVD | 1.00 | 0.57; 1.76 | 0.99 | 1.33 | 0.68; 2.62 | 0.41 |
| Cancer | 0.88 | 0.43; 1.82 | 0.73 | 1.79 | 1.09; 2.95 | 0.021 |
| Obesity | 1.02 | 0.70; 1.49 | 0.90 | 0.75 | 0.49; 1.15 | 0.19 |
| Smoker | 1.42 | 0.96; 2.10 | 0.08 | 1.36 | 0.92; 2.01 | 0.12 |
| Physical activity | 1.00 | 1.00; 1.00 | 0.51 | 1.00 | 1.00; 1.00 | 0.83 |
| Alcohol > recom. limit | 1.02 | 0.71; 1.46 | 0.93 | 0.66 | 0.44; 1.00 | 0.047 |
| Mental distress | ||||||
| Loneliness | 1.89 | 1.17; 3.05 | 0.01 | 1.75 | 1.14; 2.69 | 0.011 |
| PHQ-9 sum (baseline) | 1.56 | 1.45; 1.67 | < 0.001 | 1.38 | 1.30; 1.48 | < 0.001 |
Bold values indicate statistically significant predictors.
CVD cardiovascular disease, PHQ-9 patient health questionnaire-9, WBC white blood cell count.
Discussion
The present study investigated the longitudinal associations of inflammatory markers and new onset of depression symptoms in a large and representative German community sample. We had previously excluded all cases of acute depression, previous diagnosis of depression, and those on antidepressants at baseline in order to identify cases with new onset of depression at follow-up.
Based on two markers, CRP and WBC, we found inflammation at baseline predictive of new cases of depression at follow-up 5 years later. The effects of both markers were independent of depression symptoms at baseline. Increased CRP levels raised the risk of subsequent depression by 1.58 (95% CI 1.010 to 2.16) and ln WBC by 1.88 (95% CI 1.04 to 3.42), even when adjusting for sociodemographic, physical health, health behavior variables, and baseline depression symptoms.
Unlike previous studies, we used interaction terms with gender in our models and also conducted separate analyses for men and women. We found significant interactions of CRP and WBC with gender. In separate analyses, CRP was only predictive of new onset of depression in men, but not in women. These findings compliment previous research showing a stronger increase of CRP in depressed men vs. women40,41. Additional factors were lower age, smoking, loneliness and baseline depression symptoms. In women, lower age, a history of cancer, lower alcohol consumption, loneliness and baseline depression symptoms were predictive of depression, but not CRP. As consumption of alcohol is quite widespread in Germany, especially in social contexts, and as the recommended limit (for women) is reached with few drinks, the negative predictive valence of alcohol consumption might have been related to little social contact (and related social drinking) which aggravates the risk for depression42.
Regarding WBC, we found a trend for men (OR 1.80; 95% CI 0.96 to 3.37 per natural log-unit increase), along with loneliness, and baseline depression symptoms. These patterns of predictors corroborate previous findings of large cohort studies43.
In our study, inflammation increased the risk of incident depression in men. Especially with respect to CRP, it was suggested that the relationship with depression symptoms only applies to men44,45. A possible explanation is provided by the hypothesis that inflammation arises from the dysregulation of hormonal systems. In this context, estrogen might have beneficial effects (which are also indicated by the positive influence of hormone replacement therapy on women’s mood46), for instance due to its anti-inflammatory properties47,48.
What is more, there are differences in the basic immune response of men and women49 that could shape the association between inflammation markers and depression symptoms. Women’s response to infection is more pronounced; this pertained to the overwhelming majority of characteristics of immune components including both the innate and the adaptive immune system50. Although this response pattern has been deemed adaptive as women have a better prognosis after experiencing e. g. sepsis51, it might have downsides as they are more likely to suffer from autoimmune diseases than men52.
The exact mechanisms underlying the present findings, however, remain to be elucidated, as both inflammation and depression could be consequences of an underlying factor like allostatic load. Therefore, further characterization of an inflamed depression subtype could be a worthwhile pursuit. However, the gender difference and the high variability of the effect of inflammation on depression in men suggest that inflammation is not a necessary cause of depression. Instead, the results indicate gender-specific pathways in the development of depressive disorders. Regarding the generally more pronounced vulnerability for internalizing mental disorders, studies have shown that these disparities co-occur with the onset of puberty2. It has been discussed that differential risk depends on hormonal changes and societal gender role concepts alike (which become more salient around this time)2.
Within the present study, we observed that older age protected against new onset of depression. At baseline participants were between 35 and 74 years old. Correspondingly, the prevalence of major depression decreased with age in previous community studies53. Along these lines, it is important to note that hormonal changes over the course of a persons’ lifespan shape physiological regulation including inflammatory processes29. Moreover, studies have found that the gender gap in depression narrows after the fertile periods of women’s lives whereas women’s susceptibility to chronic inflammatory diseases approaches men’s as they age54. In a previous meta-analysis, depression in older adults was more likely to co-occur with physical complaints (including e.g. gastrointestinal symptoms) than in the young55.
As inflammation is a core mechanism of cardiovascular, metabolic and other chronic somatic diseases, the present results help to understand the high prevalence of somatic comorbidity in depression. In our investigation, presence of CVD was not associated with depression symptoms at follow-up (in models that controlled for inflammatory markers and lifestyle factors such as including bodyweight, physical activity, smoking, and alcohol consumption). Previous studies have found that depression was a risk factor for CVD56,57. Additionally, an earlier prospective cohort study showed that depression symptoms predicted health risk behaviors five years later, indicating a potential mediator11 of the relationship between depression and CVD. It is important to note that an individual’s lifestyle constitutes a modifiable risk factor that can be addressed by population-based intervention and prevention programs (e. g. smoking cessation; physical exercise). Beneficial effects of lifestyle changes on inflammation were recently reported58 and could contribute to recovery from depression symptoms, too. Other examples of interventions to reduce depression and CVD address nutrition (e.g. by incorporating anti-inflammatory foods and supplements23) and the reduction of chronic stress as a major driver of inflammation (e. g. by psychotherapy, online interventions, relaxation exercises).
Strengths and limitations
We report findings from a sizeable community cohort with equal proportions of men and women. As previously recommended19, we excluded participants with any indication of previous depression (based on baseline assessment, lifetime diagnosis, and intake of antidepressants). We also controlled for lifestyle variables associated with depression and with inflammation (smoking, obesity) and, importantly, for baseline depression symptoms.
In addition to CRP, one of the most frequently studied and robust indicators of inflammation, we included WBC to expand on previous research. Yet, we are aware that we used only two inflammatory markers, and other acute-phase markers (e. g. fibrinogen) or other soluble immune markers (e. g. cytokines such as IL-6) were not included. Given the fact that they were the focus of a large body of previous research concerning the etiology and/or maintenance of depression22, this limits the comparability of our investigation with previous studies. In addition, the combined study of a wider range of inflammatory markers could elicit different (gender-specific) pathogenic pathways.
Our primary outcome measure was the PHQ-9. It is one of the most-widely used self-report questionnaires to assess depression symptoms. While we inquired previous diagnoses of depression, we did not ascertain medical diagnoses of depression from medical files. Given the limited period of two weeks captured by the PHQ-9, we cannot preclude that participants were depressed at other times before the follow-up assessment took place. While we used all information available to exclude individuals who had previously been depressed, an unknown proportion of included participants might have fulfilled the criteria of depression in the past without proper diagnosis. Also, they might not have disclosed previous diagnoses. Other potential predictors such as family history of depression were not assessed. Further, alcohol consumption exceeding the recommended limit was defined based on self-reports that could not be objectively validated. We also assessed previous diagnosis of CVD and cancer via self-reported diagnoses. We did not differentiate for time since diagnosis or (in the case of cancer) stage and treatment status, which presents a limitation.
Supplementary Information
Acknowledgements
The authors thank all of the study participants for their willingness to provide data for this research project, and the authors are also indebted to all coworkers for their enthusiastic commitment. The Gutenberg Health Study is funded by the government of Rhineland-Palatinate (Stiftung Rheinland-Pfalz für Innovation, contract AZ 961-386261/733); by the research programs Wissen schafft Zukunft and Center for Translational Vascular Biology of the Johannes Gutenberg University of Mainz; and by a contract with Boehringer Ingelheim and Philips Medical Systems, including an unrestricted grant for the Gutenberg Health Study. Boehringer Ingelheim, Philips Medical Systems, and Novartis Pharma provided funding toward this study.
Author contributions
M.E., P.S.W., M.E.B. and K.J.L., conceived of and designed the study; M.N. analyzed the data; M.E. and M.E.B. wrote the paper; E.B., D.O., A.N.T., A.M.W., I.R., F.W., J.W., M.M., J.K., N.P., T.M., and A.B. critically revised the manuscript; and all authors read and approved the final manuscript.
Data availability
The written informed consent of the study participants is not suitable for public access to the data and this concept was not approved by the local data protection officer and ethics committee. Access to data at the local database in accordance with the ethics vote is offered upon request at any time. Interested researchers make their requests to the Principal Investigator of the GHS (Philipp.Wild@unimedizin-mainz.de).
Competing interests
Philipp S. Wild is funded by the Federal Ministry of Education and Research (BMBF 01EO1503). Philipp S. Wild and Thomas Münzel are PI of the German Center for Vascular Research (DZHK). There are no patents, products in development, or marketed products to declare. This does not alter the authors’ adherence to all of the journal policies on sharing data and materials. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The other authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-81927-9.
References
- 1.Malhi GS, Mann JJ. Depression. The Lancet. 2018;392:2299–2312. doi: 10.1016/s0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- 2.Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry. 2017;4:146–158. doi: 10.1016/s2215-0366(16)30263-2. [DOI] [PubMed] [Google Scholar]
- 3.Tibubos AN, et al. Course of depressive symptoms in men and women: Differential effects of social, psychological, behavioral and somatic predictors. Sci. Rep. 2019;9:18929. doi: 10.1038/s41598-019-55342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poole L, Steptoe A. Depressive symptoms predict incident chronic disease burden 10years later: Findings from the english longitudinal study of ageing (ELSA) J. Psychosom. Res. 2018;113:30–36. doi: 10.1016/j.jpsychores.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Dijkstra-Kersten SMA, et al. Longitudinal associations of multiple physical symptoms with recurrence of depressive and anxiety disorders. J. Psychosom. Res. 2017;97:96–101. doi: 10.1016/j.jpsychores.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 6.Statistisches Bundesamt. Verteilung der Häufigsten Todesursachen in Deutschland im Jahr 2017. (2020). https://de.statista.com/statistik/daten/studie/240/umfrage/verteilung-der-sterbefaelle-nach-todesursachen/. Accessed 12 Oct 2020.
- 7.Kendler KS, Gardner CO, Fiske A, Gatz M. Major depression and coronary artery disease in the Swedish twin registry: Phenotypic, genetic, and environmental sources of comorbidity. Arch. Gen. Psychiatry. 2009;66:857–863. doi: 10.1001/archgenpsychiatry.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandaker GM, et al. Shared mechanisms between coronary heart disease and depression: Findings from a large UK general population-based cohort. Mol. Psychiatry. 2020;25:1477–1486. doi: 10.1038/s41380-019-0395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penninx BW. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci. Biobehav. Rev. 2017;74:277–286. doi: 10.1016/j.neubiorev.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Pinquart M, Duberstein PR. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whooley MA, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinkhasov RM, et al. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int. J. Clin. Pract. 2010;64:475–487. doi: 10.1111/j.1742-1241.2009.02290.x. [DOI] [PubMed] [Google Scholar]
- 13.Raggi P, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108. doi: 10.1016/j.atherosclerosis.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Beydoun MA, et al. White blood cell inflammatory markers are associated with depressive symptoms in a longitudinal study of urban adults. Transl. Psychiatry. 2016;6:e895. doi: 10.1038/tp.2016.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbert TB, Cohen S. Stress and immunity in humans: A meta-analytic review. Psychosom. Med. 1993;55:364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol. Med. 2019;49:1958–1970. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duivis HE, Vogelzangs N, Kupper N, de Jonge P, Penninx BW. Differential association of somatic and cognitive symptoms of depression and anxiety with inflammation: Findings from the Netherlands study of depression and anxiety (NESDA) Psychoneuroendocrinology. 2013;38:1573–1585. doi: 10.1016/j.psyneuen.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Michal M, et al. Differential associations of depressive symptom dimensions with cardio-vascular disease in the community: Results from the Gutenberg health study. PLoS ONE. 2013;8:e72014. doi: 10.1371/journal.pone.0072014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osimo EF, et al. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav. Immunol. 2020;87:901–909. doi: 10.1016/j.bbi.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen S, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. U.S.A. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150:736–744. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Shao M, et al. Depression and cardiovascular disease: Shared molecular mechanisms and clinical implications. Psychiatry Res. 2020;285:112802. doi: 10.1016/j.psychres.2020.112802. [DOI] [PubMed] [Google Scholar]
- 24.Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and gender equity in research: Rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 2016;1:2. doi: 10.1186/s41073-016-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauvais-Jarvis F, et al. Sex and gender: Modifiers of health, disease, and medicine. The Lancet. 2020;396:565–582. doi: 10.1016/s0140-6736(20)31561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oquendo MA, et al. Sex differences in clinical predictors of depression: A prospective study. J. Affect. Disord. 2013;150:1179–1183. doi: 10.1016/j.jad.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendler KS, Gardner CO. Sex differences in the pathways to major depression: A study of opposite-sex twin pairs. Am. J. Psychiatry. 2014;171:426–435. doi: 10.1176/appi.ajp.2013.13101375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Y, Kozloski M. Sex differences in age trajectories of physiological dysregulation: Inflammation, metabolic syndrome, and allostatic load. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:493–500. doi: 10.1093/gerona/glr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moieni M, et al. Sex differences in depressive and socioemotional responses to an inflammatory challenge: Implications for sex differences in depression. Neuropsychopharmacology. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surtees P, et al. Association of depression with peripheral leukocyte counts in EPIC-Norfolk—Role of sex and cigarette smoking. J. Psychosom. Res. 2003;54:303–306. doi: 10.1016/s0022-3999(02)00456-7. [DOI] [PubMed] [Google Scholar]
- 32.Hohn R, et al. The ophthalmic branch of the Gutenberg health study: Study design, cohort profile and self-reported diseases. PLoS ONE. 2015;10:e0120476. doi: 10.1371/journal.pone.0120476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wild PS, et al. The Gutenberg health study. Bundesgesundheitsblatt Gesundh. Gesundh. 2012;55:824–829. doi: 10.1007/s00103-012-1502-7. [DOI] [PubMed] [Google Scholar]
- 34.Lampert T, Kroll L, Müters S, Stolzenberg H. Measurement of the socioeconomic status within the German health update 2009 (GEDA) Bundesgesundheitsblatt Gesundh. Gesundh. 2009;56:131–143. doi: 10.1007/s00103-012-1583-3. [DOI] [PubMed] [Google Scholar]
- 35.Lowe B, et al. Diagnosing ICD-10 depressive episodes: Superior criterion validity of the Patient Health Questionnaire. Psychother. Psychosom. 2004;73:386–390. doi: 10.1159/000080393. [DOI] [PubMed] [Google Scholar]
- 36.Beutel ME, et al. Loneliness in the general population: Prevalence, determinants and relations to mental health. BMC Psychiatry. 2017;17:97. doi: 10.1186/s12888-017-1262-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peters T, et al. Validity of a short questionnaire to assess physical activity in 10 European countries. Eur. J. Epidemiol. 2012;27:15–25. doi: 10.1007/s10654-011-9625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson TA, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for disease control and prevention and the American Heart association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 39.Michal M, et al. Complaints of sleep disturbances are associated with cardiovascular disease: Results from the Gutenberg health study. PLoS ONE. 2014;9:e104324. doi: 10.1371/journal.pone.0104324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danner M, Kasl SV, Abramson JL, Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom. Med. 2003;65:347–356. doi: 10.1097/01.PSY.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- 41.Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: Data from the Third National health and nutrition examination survey. Arch. Intern. Med. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 42.Goodman RJ, Samek DR, Wilson S, Iacono WG, McGue M. Close relationships and depression: A developmental cascade approach. Dev. Psychopathol. 2018 doi: 10.1017/S0954579418001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beutel ME, et al. New onset of depression in aging women and men—Contributions of social, psychological, behavioral, and somatic predictors in the community. Psychol. Med. 2018 doi: 10.1017/S0033291718001848. [DOI] [PubMed] [Google Scholar]
- 44.Elovainio M, et al. Depression and C-reactive protein: Population-based Health 2000 Study. Psychosom. Med. 2009;71:423–430. doi: 10.1097/PSY.0b013e31819e333a. [DOI] [PubMed] [Google Scholar]
- 45.Toker S, Shirom A, Shapira I, Berliner S, Melamed S. The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. J. Occup. Health Psychol. 2005;10:344–362. doi: 10.1037/1076-8998.10.4.344. [DOI] [PubMed] [Google Scholar]
- 46.Toffol E, Heikinheimo O, Partonen T. Hormone therapy and mood in perimenopausal and postmenopausal women: A narrative review. Menopause (New York) 2015;22:564–578. doi: 10.1097/GME.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 47.Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci. Rep. 2015;5:15224. doi: 10.1038/srep15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma L, Xu Y, Wang G, Li R. What do we know about sex differences in depression: A review of animal models and potential mechanisms. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019;89:48–56. doi: 10.1016/j.pnpbp.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 49.Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmunol. Rev. 2012;11:A479–A485. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 50.Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 51.Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis: Cardiovascular and immunological aspects. Virulence. 2014;5:12–19. doi: 10.4161/viru.26982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014;35:347–369. doi: 10.1016/j.yfrne.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Weinberger AH, et al. Trends in depression prevalence in the USA from 2005 to 2015: Widening disparities in vulnerable groups. Psychol. Med. 2018;48:1308–1315. doi: 10.1017/S0033291717002781. [DOI] [PubMed] [Google Scholar]
- 54.Gubbels Bupp MR. Sex, the aging immune system, and chronic disease. Cell Immunol. 2015;294:102–110. doi: 10.1016/j.cellimm.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Hegeman JM, Kok RM, van der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: Meta-analysis. Br. J. Psychiatry. 2012;200:275–281. doi: 10.1192/bjp.bp.111.095950. [DOI] [PubMed] [Google Scholar]
- 56.Frasure-Smith N, Lesperance F. Depression—A cardiac risk factor in search of a treatment. JAMA. 2003;289:3171–3173. doi: 10.1001/jama.289.23.3171. [DOI] [PubMed] [Google Scholar]
- 57.Goldstein BI, et al. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2015;132:965–986. doi: 10.1161/CIR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 58.van ‘t Klooster CC, et al. The relation between healthy lifestyle changes and decrease in systemic inflammation in patients with stable cardiovascular disease. Atherosclerosis. 2020;301:37–43. doi: 10.1016/j.atherosclerosis.2020.03.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The written informed consent of the study participants is not suitable for public access to the data and this concept was not approved by the local data protection officer and ethics committee. Access to data at the local database in accordance with the ethics vote is offered upon request at any time. Interested researchers make their requests to the Principal Investigator of the GHS (Philipp.Wild@unimedizin-mainz.de).


