Abstract
Acute myeloid leukemia (AML) represents a major therapeutic challenge in the elderly. Because of the high treatment-related mortality and poor overall outcomes of remission induction therapy, many older patients are not considered candidates for intensive chemotherapy. The current study evaluated prognostic factors for achievement of complete remission (CR) in newly diagnosed elderly AML patients who were treated with initial intensive chemotherapy. The study included 62 newly diagnosed AML patients ≥70 years who were treated with intensive chemotherapy. The overall response rate (CR and CRp) was 56%. Patients with favorable or intermediate cytogenetics (p = 0.0036) as well as those with primary AML (p = 0.0212) had a higher response rate. The median overall survival for all patients was 6.85 months (95% CI 3.7–13.5 months). The median overall survival for patients achieving remission after intensive induction chemotherapy was significantly higher than those who did not respond to therapy (20.4 months vs. 3.5 months, p < 0.001). The all-cause 4-week mortality rate was 11%, and the all-cause 8-week mortality rate was 17.7%. A subgroup of elderly patients may benefit more from initial intensive induction chemotherapy, specifically those patients with performance status able to tolerate induction chemotherapy and favorable cytogenetic status. However, despite high rates of initial CR, relapse rates are still high, suggesting that alternative strategies of postremission therapy are warranted.
Key words: Acute myeloid leukemia (AML), Induction chemotherapy, Elderly patients, Prognostic factors, Survival
INTRODUCTION
Acute myeloid leukemia (AML) is the most common type of acute leukemia in adults with a median age at diagnosis of 67 years (1). The biological and clinical features of leukemia change gradually with increasing age, contributing to worse outcomes for elderly patients with AML. Older AML patients are more likely to have adverse cytogenetics, antecedent hematologic disorders or secondary AML, and a higher expression of multidrug resistance genes (2). Given their higher incidence of comorbidities and decreased performance status at the time of diagnosis, older patients tend to have poorer tolerance to therapy compared to younger patients (3).
The goal of induction chemotherapy in AML is to achieve complete remission (CR) with restoration of normal bone marrow (4,5). Attainment of CR is an important first goal in the treatment of AML, as this is associated with improved survival. The first course of intensive chemotherapy for newly diagnosed AML has not changed appreciably over the past few decades; it consists of an anthracycline combined with cytarabine (6). CR can be expected in 60–70% of newly diagnosed AML patients with the current intensive chemotherapy regimens, but this depends on multiple factors including age, cytogenetics and molecular abnormalities, performance status, and organ function at diagnosis.
Treatment options for elderly AML patients range from intensive chemotherapy (e.g., combination of cytarabine and idarubicin) to less-intensive therapies (e.g., hypomethylating agents) to supportive care only. Because of the perceived risks for poor outcomes and the increased toxicity of intensive chemotherapy, elderly AML patients may not be considered candidates for intensive chemotherapy. To characterize the outcomes of intensive chemotherapy in patients age 70 or older with newly diagnosed AML, we performed a retrospective study of the efficacy and toxicity associated with intensive chemotherapy. We also assessed factors that are potentially predictive of response to induction chemotherapy.
MATERIALS AND METHODS
Study Group
Between January 2000 and January 2013, 62 newly diagnosed AML patients ≥70 years were treated with intensive chemotherapy at the University of Pittsburgh Cancer Institute. Patients with acute promyelocytic leukemia were not included in this analysis. Cases of AML were classified as secondary on the basis of having a history of previous treatment with chemotherapy or radiotherapy or antecedent hematologic conditions including myelodysplasia and myeloproliferative neoplasms. The study was approved by the University of Pittsburgh Institutional Review Board according to institutional guidelines.
Therapy
Fifty-four elderly patients (87%) with AML received induction intensive chemotherapy that consisted of cytarabine (100 mg/m2 per day, days 1–7) and idarubicin (12 mg/m2 per day, days 1–3). The remaining eight patients (13%) received mitoxantrone (10 mg/m2 per day, days 1–5) and etoposide (100 mg/m2 per day, days 1–5) for induction intensive chemotherapy. For patients who had residual leukemia and received additional intensive chemotherapy, the combination of mitoxantrone and etoposide was used in the majority of patients (19/24 patients) as second course therapy. Topotecan and cytarabine, mitoxantrone and cytarabine, and idarubicin and cytarabine were also used as second course therapy. Fludarabine (30 mg/m2 per day, days 1–5) and cytarabine (2 gm/m2 per day, days 1–5) were administered as third course therapy in two patients, and gemtuzumab ozogamicin (GO) and cytarabine were given to one patient.
Cytogenetics
Cytogenetic abnormalities were defined based on published criteria (7,8). The favorable-risk category included patients with abnormalities (abn) of inv(16)/t(16;16)/del(16q) or t(8;21) without del(9q) or as part of a complex karyotype. The intermediate-risk category included patients characterized by +8, −Y, +6, del (12p), or normal karyotype. The unfavorable-risk category was defined by the presence of one or more of −5/del(5q), −7/del(7q), inv(3q), abn 11q, 20q, or 21q, del(9q), t(6;9), t(9;22), abn 17p, or complex karyotype defined as three or more abnormalities.
Criteria for Response
Patients underwent bone marrow biopsy and aspiration 14 days after a course of induction intensive chemotherapy and a bone marrow biopsy/aspiration after peripheral blood count recovery. Using established criteria (9), CR was defined by the presence of less than 5% blasts in the bone marrow, absence of extramedullary leukemia, and peripheral blood count recovery with a neutrophil count of at least 1 × 109/L and a platelet count of at least 100 × 109/L. CRp was defined by the presence of less than 5% blasts in the bone marrow, absence of extramedullary leukemia, and incomplete platelet regeneration (<100 × 109/L).
Statistical Methods
Logistic regression was used to evaluate the association of different factors and the occurrence of CR or CRp. Overall survival (OS) and relapse-free survival (RFS) were estimated by the Kaplan–Meier method from our study database, which was locked on April 29, 2013. The Cox proportional hazard model (CoxPH) was employed to examine the relationship between survival experience and demographic and baseline characteristics. The log-rank test was used to compare the OS between the responders and nonresponders. OS was defined as the date of starting induction chemotherapy until the date of recorded death. RFS was defined as the date on which complete response was achieved to the date of documented relapse or death. All tests were two-sided. Statistical analysis was performed using SAS 12.1, and the figures were produced by the R package (version 2.14.0).
RESULTS
Baseline Characteristics
The study cohort consisted of 62 elderly patients with newly diagnosed AML; the median age was 73 years (range, 70–87 years). Patient demographics and baseline characteristics are presented in Table 1. Twenty patients had secondary AML. Cytogenetic data were available for 56 patients; 16 (26%) had an unfavorable-risk karyotype, 37 (60%) had an intermediate-risk karyotype, and 3 (5%) had a favorable-risk karyotype.
Table 1.
Patient Characteristics
| Characteristic | No. |
|---|---|
| Total number | 62 |
| Median age (years, range) | 73 (70–87) |
| Sex | |
| Male | 37 (60%) |
| Female | 25 (40%) |
| AML at diagnosis | |
| De novo | 42 (68%) |
| Secondary | 20 (32%) |
| Cytogenetic risk category at AML diagnosis | |
| Unfavorable | 16 (26%) |
| Intermediate | 37 (60%) |
| Favorable | 3 (5%) |
| Not reported | 6 (9%) |
| WBC count at AML diagnosis (range) × 109/L | 5.8 (0.8–293) |
| % blasts in bone marrow at AML diagnosis (range) | 50 (17–96) |
| Hemoglobin at AML diagnosis (g/dl, range) | 9.1 (5.8–13.9) |
| Platelet count at AML diagnosis (× 109/L, range) | 54 (3–263) |
| LDH count at AML diagnosis (range) | 557 (103–4734) |
AML, acute myeloid leukemia; WBC, white blood cell.
Responses
The overall response rate (CR and CRp) was 56%; 28 patients (45%) achieved CR and 7 patients (11%) achieved CRp. Twenty-four patients achieved remission after a single course of intensive chemotherapy, 10 patients after two courses, and 1 patient after three courses. Twenty-one patients (34%) had persistent leukemia after one to three courses of intensive chemotherapy (10 patients received one course of therapy, 9 patients received two courses, and 2 patients received three courses). Five patients had a significant reduction of blasts (<5%) in their bone marrow after therapy but died prior to count recovery and reevaluation of the bone marrow. One patient died prior to performing the bone marrow biopsy to evaluate response.
Four patients who were treated with cytarabine and idarubicin had the cytarabine discontinued on the fourth day due to the development of cardiac arrhythmias and elevated liver enzymes. Two of these patients achieved CR, and two had persistent leukemia.
Favorable or intermediate cytogenetics (p = 0.0036) and primary AML (p = 0.0212) were associated with a higher response rate (including CR and CRp) (Tables 2 and 3). In the multivariable model, after controlling for each other, cytogenetic status (p = 0.0038) and AML status (0.0186) were still significantly associated with response.
Table 2.
CR Rates by Patient Characteristics
| CR Rates | p Value | |
|---|---|---|
| Sex | 0.21 | |
| Female | 16/22 (73%) | |
| Male | 19/34 (56%) | |
| AML etiology | 0.0212 | |
| De novo | 28/39 (72%) | |
| Secondary | 6/16 (37%) | |
| Cytogenetic risk category at diagnosis | 0.0036 | |
| Unfavorable | 4/13 (31%) | |
| Intermediate/favorable | 29/37 (78%) |
Table 3.
Comparisons of Patient Characteristics Between CR and NR
| CR Pts (n = 35) | NR Pts (n = 21) | p Value | |
|---|---|---|---|
| Median age (years, range) at diagnosis | 73 (70–83) | 73 (70–87) | 0.50 |
| Median WBC count (× 109/L, range) at AML diagnosis | 8.0 (1.2–126.8) | 9.5 (0.8–293.3) | 0.66 |
| Median (%, range) blasts at AML diagnosis | 51 (21–96) | 30.5 (20–67) | 0.76 |
| Median hemoglobin (g/dl, range) at AML diagnosis | 9.1 (7.8–13.9) | 9.45 (7.5–12.0) | 0.42 |
| Median platelet count (× 109/L, range) at AML diagnosis | 64 (16–263) | 51.5 (3–163) | 0.09 |
| Median LDH (range) at AML diagnosis | 539 (113–4734) | 631 (119–3,008) | 0.90 |
| Median total bilirubin (range) at AML diagnosis | 0.7 (0.2–1.4) | 0.4 (0.1–1.8) | 0.67 |
| Median creatinine (range) at AML diagnosis | 0.95 (0.6–2.1) | 1.1 (0.6–1.9) | 0.11 |
CR, complete remission; NR, patients not responsive to induction chemotherapy.
Among the patients who achieved remission, the majority of patients (71%) received additional postremission therapy with cytarabine. Other postremission therapies included decitabine and thalidomide, and two patients underwent reduced intensity allogeneic hematopoietic cell transplantation.
Survival
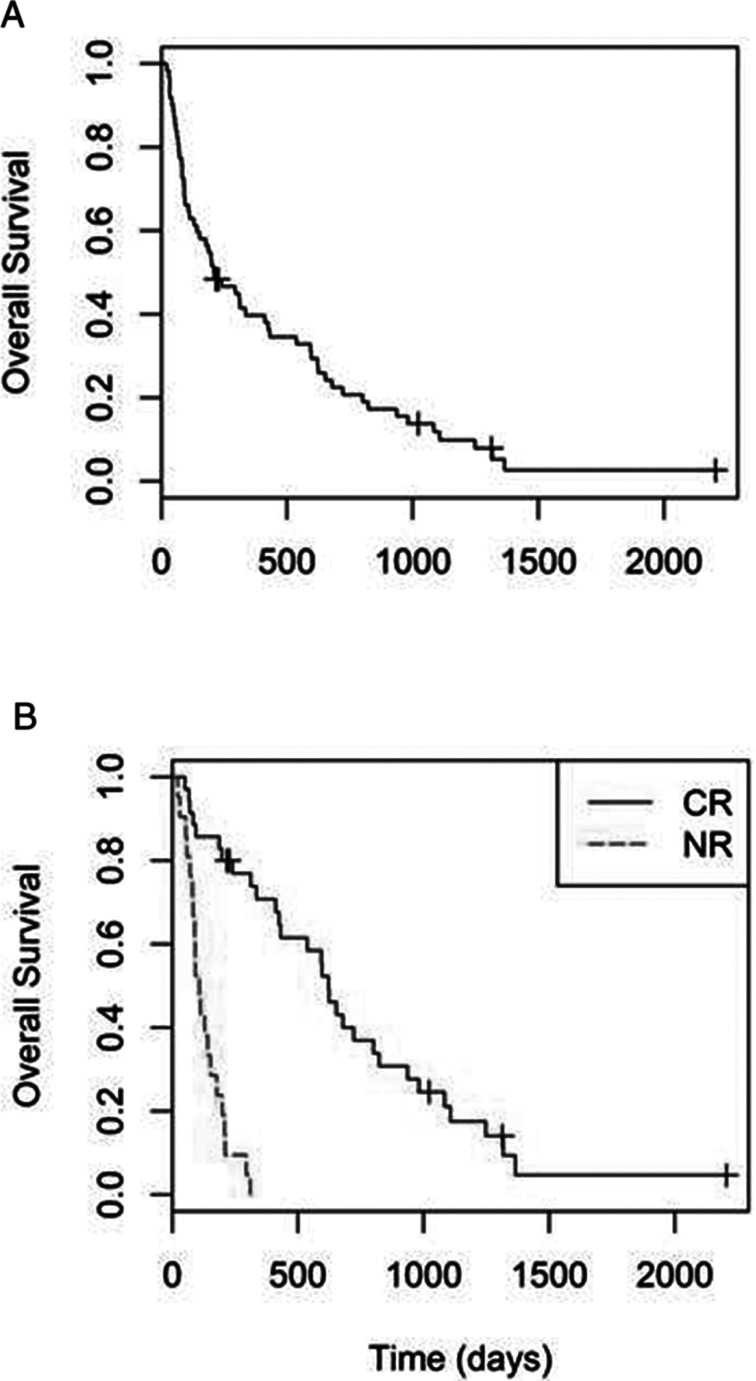
Fifty-seven of the 62 patients had died by the time of analysis. The median OS for all patients was 6.85 months (95% CI 3.7–13.5 months) (Fig. 1). The median OS for patients who achieved remission after induction chemotherapy was significantly higher than those who did not respond to therapy (20.4 months vs. 3.5 months, p < 0.001). Cox regression analysis indicated that unfavorable cytogenetics at diagnosis (p = 0.004) and persistent leukemia after induction therapy (p < 0.001) were independently associated with lower overall survival. The median duration of RFS for patients who achieved CR was 12.4 months (95% CI 7.2–17.4 months).
Figure 1.
(A) Overall survival. (B) Comparison of survival between patients in complete remission (CR) and patients not responsive to induction chemotherapy (NR).
Toxicity
The all-cause 4-week mortality rate was 11%, and the all-cause 8-week mortality rate was 17.7%. Infectious complications were the most common cause of death. The causes of death by 8 weeks included sepsis (nine patients), intracranial hemorrhage (one patient), and progressive disease (one patient). All patients developed grade 4 neutropenia and thrombocytopenia. Complete responders required a median of 34 days (range 18–55 days) from the completion of treatment to neutrophil recovery and a median of 38 days (range 21–65 days) for platelet recovery. All patients had fever associated with neutropenia; 10 patients developed Clostridium difficile colitis, 24 patients had bacteremia, 4 patients had fungemia, 11 patients had bacterial pneumonia, and 9 patients had fungal pneumonia.
DISCUSSION
AML can occur in people of all ages, but it is primarily a disease of older adults, with a median age of diagnosis of 67 years (1). It is estimated that more than 50% of AML patients are over the age of 65 years at time of diagnosis, and these patients have worse outcomes compared to younger individuals. Only 10% to 15% of older AML patients enjoy prolonged RFS (3).
The treatment of AML in older patients presents a number of challenges, and treatment strategies remain debatable. As a consequence, an important decision is which patient should receive intensive chemotherapy to achieve CR and which patient would be better off receiving less intensive options or supportive care only.
Support for the use of intensive chemotherapy in older AML patients is derived from trials designed for older adults and from the extrapolation of data from trials in younger adults. There is evidence from earlier studies that the outcome in elderly AML patients after intensive chemotherapy is superior to less intense therapies. In a prospective study reported by Löwenberg et al., AML patients over 65 years of age (median age 72 years, range 65–82) received one or two courses of daunorubicin, vincristine, and cytarabine for remission induction versus supportive care and mild cytoreductive chemotherapy only for relief of progressive AML-related symptoms (10). The patients that received chemotherapy achieved higher CR rates (58% vs. 0%) and had better OS (21 weeks vs. 11 weeks, p = 0.015). Tilly et al. compared low-dose cytarabine with intensive chemotherapy (rubidazone, a daunorubicin-derived agent, and cytarabine) in 87 AML patients (median age 72 years, range 65–83) (11). The number of CRs was higher in the intensive chemotherapy group, while partial remissions and failures were more frequent in the low-dose cytarabine group. However, infectious complications during induction treatment were more numerous and more severe in the intensive chemotherapy group (p < 0.01), and survival was similar in both groups (8.8 months with low-dose cytarabine vs. 12.8 months with intensive chemotherapy).
Clinical trials have also investigated various doses of cytarabine and anthracyclines as well as different anthracyclines (e.g., daunorubicin, mitoxantrone, or idarubicin) for the treatment of older adults with AML (12–15). Higher doses of anthracyclines resulted in superior CR rates without an apparent increase in toxicity. In a recent phase 3 clinical trial, patients (median age 67 years, range 60–83) were randomly assigned to receive cytarabine plus daunorubicin for 3 days, either at the conventional dose of 45 mg/m2 (411 patients) or at an escalated dose of 90 mg/m2 (402 patients) (14). The CR rate was 64% in the group that received the escalated dose of daunorubicin and 54% in the group that received the conventional dose (p = 0.002). There was no significant difference between the two groups in the incidence of hematologic toxic effects, 30-day mortality (11% and 12% in the two groups, respectively), or the incidence of moderate, severe, or life-threatening adverse events (p = 0.08). Survival endpoints in the two groups did not differ significantly overall. Collectively, these studies demonstrated relatively high CR rates with the use of intensive chemotherapy in elderly AML patients.
The M. D. Anderson Cancer Center analyzed 446 patients ≥70 years of age with AML who were treated with cytarabine-based intensive chemotherapy between 1990 and 2008 (16). The overall complete response rate was 45%, 4-week mortality was 26%, and 8-week mortality was 36%. The median survival was 4.6 months, and the 1-year survival rate was 28%. A multivariate analysis of prognostic factors for 8-week mortality identified the following to be independently adverse: age ≥80 years, complex karyotype, poor performance status, and elevated creatinine >1.3 mg/dl.
Despite poor outcomes, several arguments can be made in favor of intensive chemotherapy in elderly AML patients in regard to improved survival. The Swedish Acute Leukemia Registry reported that intensive chemotherapy decreases rather than increases early death rates and is a prerequisite for long-term survival in most patients up to 80 years of age (17–19). For patients between 70 and 79 years old with a performance status (PS) of 0–II, the 8-week mortality rate with intensive treatment for AML was 8% for those with intermediate-risk genetics and 22% with high-risk genetics. In comparison, with palliative treatment, the 8-week mortality was 23% and 47%, respectively. Long-term survivors were found among the elderly who were given intensive treatment despite their poor initial PS. Also, total survival of elderly AML patients was better in the geographic regions where most of them were given standard intensive therapy. The median survival for patients 70–79 years old with a PS of 0–II who received intensive treatment was 16 months with intermediate genetic risk and 9 months with high genetic risk.
In cytogenetically normal de novo AML patients with an age >60 years, Becker et al. reported that patients with nucleophosmin (NPM1) gene mutation had higher CR rates (84% vs. 48%, p < 0.001), longer disease-free survival (23% vs. 10%, 3-year rates, p < 0.047), and longer overall survival (35% vs. 8%, 3-year rates, p < 0.001) than NPM1 wild-type patients (20). The prognostic impact of NPM1 gene mutations was mainly observed in patients >70 years. Prebet et al. evaluated the characteristics and outcomes of 147 AML patients (median age of 67 years, range 60–82) with translocations associated with rearrangements of the core-binding factor (CBF) genes CBFA and CBFB (21). Sixty patients had t(8;21), and 87 patients had inv(16). A total of 129 patients (88%) achieved CR after one or two induction courses, and 15 patients (10%) died early (i.e., during the 8 weeks after induction). During a median follow-up of 48 months, the 5-year probabilities of OS and leukemia-free survival were 31% and 27%, respectively.
In the current study, adverse cytogenetics and secondary AML were found to influence outcomes including response rates and survival. These predictive factors have also been validated among patients who received intensive induction chemotherapy by other groups (7,22). The OS did not differ from other studies. There was a significant survival benefit in patients who achieved CR, confirming that achievement of CR could be a prerequisite for long-term survival. Despite the presence of comorbidities in the majority of the patients, the mortality was not significantly higher compared to previous reports of older patients with AML who received intensive chemotherapy. This may reflect the improvement in supportive care in the current era, including the routine use of antimicrobial agents and intensive care support.
An inherent limitation of this single institution retrospective study is the basis for the use of intensive induction chemotherapy, as opposed to alternative options. Selection of patients by the treating physician of patients with PS able to tolerate intensive chemotherapy could potentially introduce selection biases and influenced the results of this study. In addition, patients who refused intensive induction chemotherapy or those who were deemed unsuitable for therapy are not accounted for in our analysis. Molecular studies were only performed for a few patients (data not shown) since the study included patients from 2000, when molecular studies were not routinely performed.
Predictive scores that may be used as decision criteria for older AML patients who are treated with intensive chemotherapy have been investigated by several groups (16,23–26). Cytogenetics, age, white blood cell count at diagnosis, PS, type of AML, and organ dysfunction were variables identified in different scoring systems that were significantly related to prognosis.
Over the last decade, new therapeutic approaches have been used in elderly AML patients (Table 4). These options include, among others, the use of low-dose cytarabine (27), hypomethylating agents (28,29), gemtuzumab ozogamicin (GO) (30,31), clofarabine (32,33), farnesyl transferase inhibitors (34,35), FMS-like tyrosine kinase 3 (FLT-3) inhibitors (36), and sapacitabine (37). Among these agents, the hypomethylating agents, decitabine and azacitidine, are approved by the United States Food and Drug Administration (US FDA) for the treatment of myelodysplastic syndromes. GO is a targeted antineoplastic agent consisting of a recombinant anti-CD33 humanized antibody linked to N-acetyl-γ-calicheamicin. GO was approved in 2000 by the US FDA for use in patients age 60 or older with CD33+ AML in first relapse (38,39). GO has also been used in elderly AML patients as frontline therapy. However, in 2011, GO was withdrawn from the US market.
Table 4.
Selected Agents Used in Elderly AML Patients
| Agent | Mechanism of Action |
|---|---|
| 5-azacytidine (azacitidine), 5-aza-2′-deoxycytidine (decitabine) | Inhibitors of DNA methylation |
| Gemtuzumab ozogamicin | Recombinant anti-CD33 humanized antibody linked to N-acetyl-γ- calicheamicin |
| Clofarabine | Second-generation purine nucleoside analog |
| Tipifarnib | Farnesyltransferase inhibitor |
| Lestaurtinib | FMS-like tyrosine kinase 3 (FLT-3) inhibitor |
| Sapacitabine | Cytosine nucleoside analog |
With the development of these new novel agents, it is even more challenging to decide when to treat newly diagnosed elderly AML patients with intensive induction chemotherapy, especially when there are no prospective comparisons of these agents to standard cytarabine-based intensive chemotherapies. Elderly AML patients that have a high risk of treatment-related mortality due to comorbidities and poor PS, a low likelihood of achieving CR due to disease biology (e.g., poor cytogenetic risk) should be offered new investigational agents and be enrolled in clinical trials. Patients without major comorbidities, a higher likelihood of achieving CR, and maintaining long-term remission can be treated with intensive therapies, preferably enrolling in clinical trials that not only investigate remission-induction therapies but novel strategies to prevent disease relapse.
In conclusion, a subgroup of elderly patients may benefit more from initial intensive induction chemotherapy, specifically those patients with PS able to tolerate induction chemotherapy and favorable cytogenetic status. However, despite high rates of initial CR, relapse rates are still high, suggesting that alternative strategies of postremission therapy are warranted.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Dores G. M.; Devesa S. S.; Curtis R. E.; Linet M. S.; Morton L. M. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood 119:34–43; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pollyea D. A.; Kohrt H. E.; Medeiros B. C. Acute myeloid leukaemia in the elderly: A review. Br. J. Haematol. 152:524–542; 2011. [DOI] [PubMed] [Google Scholar]
- 3. Ferrara F.; Schiffer C. A. Acute myeloid leukaemia in adults. Lancet 381:484–495; 2013. [DOI] [PubMed] [Google Scholar]
- 4. Burnett A.; Wetzler M.; Lowenberg B. Therapeutic advances in acute myeloid leukemia. J. Clin. Oncol. 29:487–494; 2011. [DOI] [PubMed] [Google Scholar]
- 5. Rowe J. M.; Tallman M. S. How I treat acute myeloid leukemia. Blood 116:3147–3156; 2010. [DOI] [PubMed] [Google Scholar]
- 6. Teuffel O.; Leibundgut K.; Lehrnbecher T.; Alonzo T. A.; Beyene J.; Sung L. Anthracyclines during induction therapy in acute myeloid leukaemia: A systematic review and meta-analysis. Br. J. Haematol. 161:192–203; 2013. [DOI] [PubMed] [Google Scholar]
- 7. Byrd J. C.; Mrozek K.; Dodge R. K.; Carroll A. J.; Edwards C. G.; Arthur D. C.; Pettenati M. J.; Patil S. R.; Rao K. W.; Watson M. S.; Koduru P. R.; Moore J. O.; Stone R. M.; Mayer R. J.; Feldman E. J.; Davey F. R.; Schiffer C. A.; Larson R. A.; Bloomfield C. D. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461). Blood 100:4325–4336; 2002. [DOI] [PubMed] [Google Scholar]
- 8. Slovak M. L.; Kopecky K. J.; Cassileth P. A.; Harrington D. H.; Theil K. S.; Mohamed A.; Paietta E.; Willman C. L.; Head D. R.; Rowe J. M.; Forman S. J.; Appelbaum F. R. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 96:4075–4083; 2000. [PubMed] [Google Scholar]
- 9. Cheson B. D.; Bennett J. M.; Kopecky K. J.; Buchner T.; Willman C. L.; Estey E. H.; Schiffer C. A.; Doehner H.; Tallman M. S.; Lister T. A.; Lo-Coco F.; Willemze R.; Biondi A.; Hiddemann W.; Larson R. A.; Lowenberg B.; Sanz M. A.; Head D. R.; Ohno R.; Bloomfield C. D. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J. Clin. Oncol. 21:4642–4649; 2003. [DOI] [PubMed] [Google Scholar]
- 10. Lowenberg B.; Zittoun R.; Kerkhofs H.; Jehn U.; Abels J.; Debusscher L.; Cauchie C.; Peetermans M.; Solbu G.; Suciu S. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: A randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J. Clin. Oncol. 7:1268–1274; 1989. [DOI] [PubMed] [Google Scholar]
- 11. Tilly H.; Castaigne S.; Bordessoule D.; Casassus P.; Le Prise P. Y.; Tertian G.; Desablens B.; Henry-Amar M.; Degos L. Low-dose cytarabine versus intensive chemotherapy in the treatment of acute nonlymphocytic leukemia in the elderly. J. Clin. Oncol. 8:272–279; 1990. [DOI] [PubMed] [Google Scholar]
- 12. Arellano M.; Winton E.; Pan L.; Lima L.; Tighiouart M.; Bhalla K.; Heffner L. T.; Neely J.; Hutcherson D.; McLemore M.; Langston A.; Khoury H. J. High-dose cytarabine induction is well tolerated and active in patients with de novo acute myeloid leukemia older than 60 years. Cancer 118:428–433; 2012. [DOI] [PubMed] [Google Scholar]
- 13. Burnett A. K.; Milligan D.; Goldstone A.; Prentice A.; McMullin M. F.; Dennis M.; Sellwood E.; Pallis M.; Russell N.; Hills R. K.; Wheatley K.; United Kingdom National Cancer Research Institute Haematological Oncology Study G. The impact of dose escalation and resistance modulation in older patients with acute myeloid leukaemia and high risk myelodysplastic syndrome: The results of the LRF AML14 trial. Br. J. Haematol. 145:318–332; 2009. [DOI] [PubMed] [Google Scholar]
- 14. Lowenberg B.; Ossenkoppele G. J.; van Putten W.; Schouten H. C.; Graux C.; Ferrant A.; Sonneveld P.; Maertens J.; Jongen-Lavrencic M.; von Lilienfeld-Toal M.; Biemond B. J.; Vellenga E.; van Marwijk Kooy M.; Verdonck L. F.; Beck J.; Dohner H.; Gratwohl A.; Pabst T.; Verhoef G. High-dose daunorubicin in older patients with acute myeloid leukemia. N. Engl. J. Med. 361:1235–1248; 2009. [DOI] [PubMed] [Google Scholar]
- 15. Lowenberg B.; Suciu S.; Archimbaud E.; Haak H.; Stryckmans P.; de Cataldo R.; Dekker A. W.; Berneman Z. N.; Thyss A.; van der Lelie J.; Sonneveld P.; Visani G.; Fillet G.; Hayat M.; Hagemeijer A.; Solbu G.; Zittoun R. Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy—The value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly: Final report. European Organization for the Research and Treatment of Cancer and the Dutch-Belgian Hemato-Oncology Cooperative Hovon Group. J. Clin. Oncol. 16:872–881; 1998. [DOI] [PubMed] [Google Scholar]
- 16. Kantarjian H.; Ravandi F.; O’Brien S.; Cortes J.; Faderl S.; Garcia-Manero G.; Jabbour E.; Wierda W.; Kadia T.; Pierce S.; Shan J.; Keating M.; Freireich E. J. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood 116:4422–4429; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Juliusson G. Most 70- to 79-year-old patients with acute myeloid leukemia do benefit from intensive treatment. Blood 117:3473–3474; 2011. [DOI] [PubMed] [Google Scholar]
- 18. Juliusson G. Older patients with acute myeloid leukemia benefit from intensive chemotherapy: An update from the Swedish Acute Leukemia Registry. Clin. Lymphoma Myeloma Leuk. 11(Suppl. 1):S54–59; 2011. [DOI] [PubMed] [Google Scholar]
- 19. Juliusson G.; Antunovic P.; Derolf A.; Lehmann S.; Mollgard L.; Stockelberg D.; Tidefelt U.; Wahlin A.; Hoglund M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 113:4179–4187; 2009. [DOI] [PubMed] [Google Scholar]
- 20. Becker H.; Marcucci G.; Maharry K.; Radmacher M. D.; Mrozek K.; Margeson D.; Whitman S. P.; Wu Y. Z.; Schwind S.; Paschka P.; Powell B. L.; Carter T. H.; Kolitz J. E.; Wetzler M.; Carroll A. J.; Baer M. R.; Caligiuri M. A.; Larson R. A.; Bloomfield C. D. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: A Cancer and Leukemia Group B study. J. Clin. Oncol. 28:596–604; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prebet T.; Boissel N.; Reutenauer S.; Thomas X.; Delaunay J.; Cahn J. Y.; Pigneux A.; Quesnel B.; Witz F.; Thepot S.; Ugo V.; Terre C.; Recher C.; Tavernier E.; Hunault M.; Esterni B.; Castaigne S.; Guilhot F.; Dombret H.; Vey N. Acute myeloid leukemia with translocation (8;21) or inversion (16) in elderly patients treated with conventional chemotherapy: A collaborative study of the French CBF-AML intergroup. J. Clin. Oncol. 27:4747–4753; 2009. [DOI] [PubMed] [Google Scholar]
- 22. Grimwade D.; Walker H.; Harrison G.; Oliver F.; Chatters S.; Harrison C. J.; Wheatley K.; Burnett A. K.; Goldstone A. H. Medical Research Council Adult Leukemia Working P. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): Analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood 98:1312–1320; 2001. [DOI] [PubMed] [Google Scholar]
- 23. Giles F. J.; Borthakur G.; Ravandi F.; Faderl S.; Verstovsek S.; Thomas D.; Wierda W.; Ferrajoli A.; Kornblau S.; Pierce S.; Albitar M.; Cortes J.; Kantarjian H. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br. J. Haematol. 136:624–627; 2007. [DOI] [PubMed] [Google Scholar]
- 24. Kantarjian H.; O’Brien S.; Cortes J.; Giles F.; Faderl S.; Jabbour E.; Garcia-Manero G.; Wierda W.; Pierce S.; Shan J.; Estey E. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer 106:1090–1098; 2006. [DOI] [PubMed] [Google Scholar]
- 25. Malfuson J. V.; Etienne A.; Turlure P.; de Revel T.; Thomas X.; Contentin N.; Terre C.; Rigaudeau S.; Bordessoule D.; Vey N.; Gardin C.; Dombret H.; Acute Leukemia French Association. Risk factors and decision criteria for intensive chemotherapy in older patients with acute myeloid leukemia. Haematologica 93:1806–1813; 2008. [DOI] [PubMed] [Google Scholar]
- 26. Wheatley K.; Brookes C. L.; Howman A. J.; Goldstone A. H.; Milligan D. W.; Prentice A. G.; Moorman A. V.; Burnett A. K.; United Kingdom National Cancer Research Institute Haematological Oncology Clinical Studies Group and Acute Myeloid Leukaemia Subgroup. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br. J. Haematol. 145:598–605; 2009. [DOI] [PubMed] [Google Scholar]
- 27. Burnett A. K.; Milligan D.; Prentice A. G.; Goldstone A. H.; McMullin M. F.; Hills R. K.; Wheatley K. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer 109:1114–1124; 2007. [DOI] [PubMed] [Google Scholar]
- 28. Cashen A. F.; Schiller G. J.; O’Donnell M. R.; DiPersio J. F. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J. Clin. Oncol. 28:556–561; 2010. [DOI] [PubMed] [Google Scholar]
- 29. Fenaux P.; Mufti G. J.; Hellstrom-Lindberg E.; Santini V.; Gattermann N.; Germing U.; Sanz G.; List A. F.; Gore S.; Seymour J. F.; Dombret H.; Backstrom J.; Zimmerman L.; McKenzie D.; Beach C. L.; Silverman L. R. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. 28:562–569; 2010. [DOI] [PubMed] [Google Scholar]
- 30. Burnett A. K.; Hills R. K.; Hunter A. E.; Milligan D.; Kell W. J.; Wheatley K.; Yin J.; McMullin M. F.; Dignum H.; Bowen D.; Russell N. H.; UK National Cancer Research Institute AML Working Group. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: Results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia 27:75–81; 2013. [DOI] [PubMed] [Google Scholar]
- 31. McHayleh W.; Foon K.; Redner R.; Sehgal R.; Raptis A.; Agha M.; Luong M.; Schlesselman J. J.; Boyiadzis M. Gemtuzumab ozogamicin as first-line treatment in patients aged 70 years or older with acute myeloid leukemia. Cancer 116:3001–3005; 2010. [DOI] [PubMed] [Google Scholar]
- 32. Burnett A. K.; Russell N. H.; Kell J.; Dennis M.; Milligan D.; Paolini S.; Yin J.; Culligan D.; Johnston P.; Murphy J.; McMullin M. F.; Hunter A.; Das-Gupta E.; Clark R.; Carr R.; Hills R. K. European development of clofarabine as treatment for older patients with acute myeloid leukemia considered unsuitable for intensive chemotherapy. J. Clin. Oncol. 28:2389–2395; 2010. [DOI] [PubMed] [Google Scholar]
- 33. Kantarjian H. M.; Erba H. P.; Claxton D.; Arellano M.; Lyons R. M.; Kovascovics T.; Gabrilove J.; Craig M.; Douer D.; Maris M.; Petersdorf S.; Shami P. J.; Yeager A. M.; Eckert S.; Abichandani R.; Faderl S. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J. Clin. Oncol. 28:549–555; 2010. [DOI] [PubMed] [Google Scholar]
- 34. Harousseau J. L.; Martinelli G.; Jedrzejczak W. W.; Brandwein J. M.; Bordessoule D.; Masszi T.; Ossenkoppele G. J.; Alexeeva J. A.; Beutel G.; Maertens J.; Vidriales M. B.; Dombret H.; Thomas X.; Burnett A. K.; Robak T.; Khuageva N. K.; Golenkov A. K.; Tothova E.; Mollgard L.; Park Y. C.; Bessems A.; De Porre P.; Howes A. J. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood 114:1166–1173; 2009. [DOI] [PubMed] [Google Scholar]
- 35. Lancet J. E.; Gojo I.; Gotlib J.; Feldman E. J.; Greer J.; Liesveld J. L.; Bruzek L. M.; Morris L.; Park Y.; Adjei A. A.; Kaufmann S. H.; Garrett-Mayer E.; Greenberg P. L.; Wright J. J.; Karp J. E. A phase 2 study of the farnesyltransferase inhibitor tipifarnib in poor-risk and elderly patients with previously untreated acute myelogenous leukemia. Blood 109:1387–1394; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knapper S.; Burnett A. K.; Littlewood T.; Kell W. J.; Agrawal S.; Chopra R.; Clark R.; Levis M. J.; Small D. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood 108:3262–3270; 2006. [DOI] [PubMed] [Google Scholar]
- 37. Kantarjian H.; Faderl S.; Garcia-Manero G.; Luger S.; Venugopal P.; Maness L.; Wetzler M.; Coutre S.; Stock W.; Claxton D.; Goldberg S. L.; Arellano M.; Strickland S. A.; Seiter K.; Schiller G.; Jabbour E.; Chiao J.; Plunkett W. Oral sapacitabine for the treatment of acute myeloid leukaemia in elderly patients: A randomised phase 2 study. Lancet Oncol. 13:1096–1104; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bross P. F.; Beitz J.; Chen G.; Chen X. H.; Duffy E.; Kieffer L.; Roy S.; Sridhara R.; Rahman A.; Williams G.; Pazdur R. Approval summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 7:1490–1496; 2001. [PubMed] [Google Scholar]
- 39. Larson R. A.; Sievers E. L.; Stadtmauer E. A.; Lowenberg B.; Estey E. H.; Dombret H.; Theobald M.; Voliotis D.; Bennett J. M.; Richie M.; Leopold L. H.; Berger M. S.; Sherman M. L.; Loken M. R.; van Dongen J. J. M.; Bernstein I. D.; Appelbaum F. R. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 104:1442–1452; 2005. [DOI] [PubMed] [Google Scholar]