Abstract
Antiangiogenic therapy is a promising strategy for cancer therapy. However, antiangiogenic therapy can induce intratumor hypoxia and hypoxia-inducible factor-1 (HIF-1) expression, which slows cancer progression. In our present study, we found that antiangiogenic therapy with sunitinib plus HIF-1 dimerization inhibitor acriflavine retarded tumor growth in a murine model of breast cancer. The combination of sunitinib with acriflavine significantly decreased vascular endothelial growth factor and TGF-β expression and reduced tumor vasculature followed by increased intratumor necrosis and apoptosis. Moreover, decreased accumulation of myeloid-derived suppressor cells in the spleen was observed after the combinational therapy. In conclusion, the combination of HIF-1 inhibition and antiangiogenic therapy may represent a novel strategy for cancer patients.
Key words: Hypoxia-inducible factor-1 (HIF-1), Acriflavine, Breast cancer, Antiangiogenic therapy
INTRODUCTION
Antiangiogenic therapy has been shown to hold great promise for the treatment of various cancers (1). Antiangiogenic drugs can serve as chemosensitizing agents (2,3). However, antiangiogenic therapy has limited effectiveness for patients with breast cancer. Vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor sunitinib and VEGF-neutralizing antibody Avastin (bevacizumab) induce intratumor hypoxia in xenografts of human breast cancer and increase the population of cancer stem cells, which is primarily mediated by hypoxia-inducible factor-1α (HIF-1α) (4). Intratumor hypoxia is a hallmark of tumors. Poor oxygenation in tumor is associated with a more aggressive phenotype (5). There is a pressing need to develop efficacious targeted therapies for these adverse effects.
HIF-1, a basic helix–loop–helix-PAS heterodimer, is composed of HIF-1α and HIF-1β subunits and regulated by cellular oxygen tension (6). HIF-1β subunits are constitutively expressed, whereas HIF-1α is induced in response to hypoxia. At normoxia, HIF-1α subunit is constantly synthesized and hydroxylated, which is required for binding of von Hippel-Lindau protein and the following proteasomal degradation (7). Under hypoxic conditions, hydroxylation and degradation of HIF decreases and HIF-1α accumulates and dimerizes with HIF-1β to form a transcription factor (7). HIF-1 upregulates the expression of VEGF, GLUT1, and hexokinase, which are involved in angiogenesis and tumor metabolism (8).
Hypoxic conditions in tumors promote angiogenesis by mediating recruitment of bone marrow-derived cells in a HIF-dependent manner (9). Loss of HIF-1α activity in various tumors blocks angiogenesis and results in retarded tumor mass and reduced metastasis (10,11). HIF-1α maintains murine lymphoma stem cells by the cross talk with notch pathway, and targeting HIF-1α can eliminate cancer stem cells in hematological malignancies (12). The importance of HIF-1α raises the general question as to how to target HIF-1α for cancer therapy. Drugs that inhibit HIF-1 activity show antitumor activity. Lee et al. have identified an inhibitor of HIF-1 dimerization, acriflavine, which shows anticancer efficacy in vivo (13). Acriflavine binds to HIF-1α and HIF-2α directly, and thus it inhibits HIF-1 dimerization and transcriptional activity (13). Treatment of prostate cancer xenografts with acriflavine inhibits angiogenic cytokine expression and proangiogenic cell mobilization and eventually retards tumor growth (13).
We asked whether HIF-1 inhibition would potentiate the antitumor effect of antiangiogenic therapy. In this study, we investigated the synergistic antitumor effect of sunitinib and HIF-1 inhibitor acriflavine. We demonstrated, by using the 4T1 breast cancer model, that sunitinib in combination with acriflavine induced tumor retardation with high efficiency.
MATERIALS AND METHODS
Cells and Reagents
Murine breast cancer cells were cultured in standard RPMI-1640-based media supplemented with 10% FBS (Gibco) and antibiotics. Sunitinib was purchased from Pfizer and acriflavine from Sigma-Aldrich.
Animal Model and Treatment
4T1 cells (1 × 106) were inoculated subcutaneously into the right flanks of 6- to 8-week-old BALB/c mice. At day 7 after tumor inoculation, animals were randomly assigned into four groups and treated daily with vehicle, sunitinib (10 mg/kg PO), acriflavine (2 mg/kg IP), sunitinib (10 mg/kg PO), and acriflavine (2 mg/kg IP), respectively. Tumor burden was measured by the gross wet weight of tumors.
Immunohistochemistry and Immunofluorescence
At day 12 after treatment, tumors were harvested and fixed with 4% paraformalin. Tumor tissues were embedded in paraffin and sectioned and then evaluated by H&E staining and immunohistochemistry. After sunitinib treatment, tumors were stained with antibody against HIF-1 (Novus Biologicals). The tumor vasculature was evaluated by CD31-FITC (BioLegend) staining.
RT-PCR
Total RNAs were isolated with reagent (Axygen) and reverse transcribed (TaKaRa). cDNAs were amplified by RT-PCR (TaKaRa). Cycle thresholds were normalized to an internal control 18S rRNA. The sequence of primer pairs is as follows: TGF-β forward: CTCCCGTGGCTTCTAGTGC, reverse: GCCTTAGTTTGGACAGGATCTG; VEGF forward: CCACGTCAGAGAGCAACATCA, reverse: TCATCTCTCCTATGTGCTGGCTTT; HIF-1α forward: ACAAGTCACCACAGGACAG, reverse: AGGGAGAAAATCAAGTCG; 18S RNA forward: CGCCGCTAGAGGTGAAATTCT, reverse: CGAACCTCCGACTTTCGTTCT. The amount of RNA was calculated using the ΔΔCT method; the level of expression of RNA was normalized to the adapted internal control.
TUNEL Assay
TUNEL assay was performed to detect the apoptosis according to the manufacturer’s instruction (Promega).
Flow Cytometry
At 12 days after treatment, spleens were harvested and erythrocytes were lysed. Spleen cells were stained with CD11b-PE and Gr-1-FITC antibodies (BD Pharmingen). For flow cytometry analysis, cells were acquired with a FACSCalibur flow cytometer and analyzed with CellQuest software (BD Biosciences).
Statistical Analysis
Statistical significance was analyzed by Student’s t-test when compared between two groups and one-way ANOVA when compared among more than three groups. Results were expressed as the means ± SD. A value of p < 0.05 was considered as statistically significant.
RESULTS
Antiangiogenic Therapy With Sunitinib Enhanced HIF-1 Expression
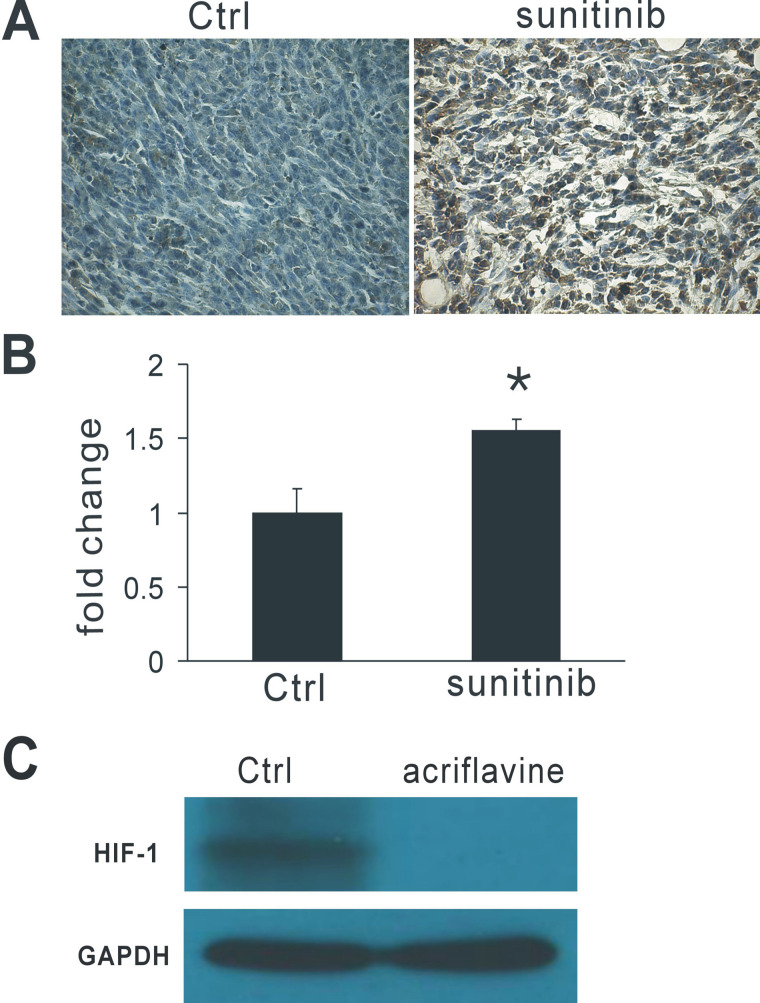
To assess whether the HIF-1 expression could be induced by antiangiogenic therapy, we treated 4T1 murine breast cancer-bearing mice with the multireceptor tyrosine kinase inhibitor sunitinib. The administration of sustained and low dose of sunitinib increased HIF-1α expression in tumor tissues as analyzed by immunohistochemistry (Fig. 1A) and RT-PCR assay (Fig. 1B). To test whether HIF-1 dimerization inhibitor acriflavine decreased HIF-1 in vivo, we performed Western blot after acriflavine therapy. We found that acriflavine markedly inhibited HIF-1 in tumor tissue compared with control (Fig. 1C). These results show that antiangiogenic therapy with sunitinib results in enhanced HIF-1α expression and that acriflavine can obviously decrease HIF-1 protein in tumor tissues.
Figure 1.
Antiangiogenic therapy with sunitinib increased HIF-1 expression in tumor tissues. (A) Representative images of immunohistochemical analysis for HIF-1 in tumor sections. (B) RT-PCR analysis of HIF-1α levels in tumors treated with sunitinib. (C) Western blot analysis of HIF-1 protein levels in tumor tissues. Acriflavine obviously decreased HIF-1 in tumor tissues. GAPDH was the loading control. *p < 0.05 versus control.
Sunitinib Together With Acriflavine Inhibited Tumor Growth
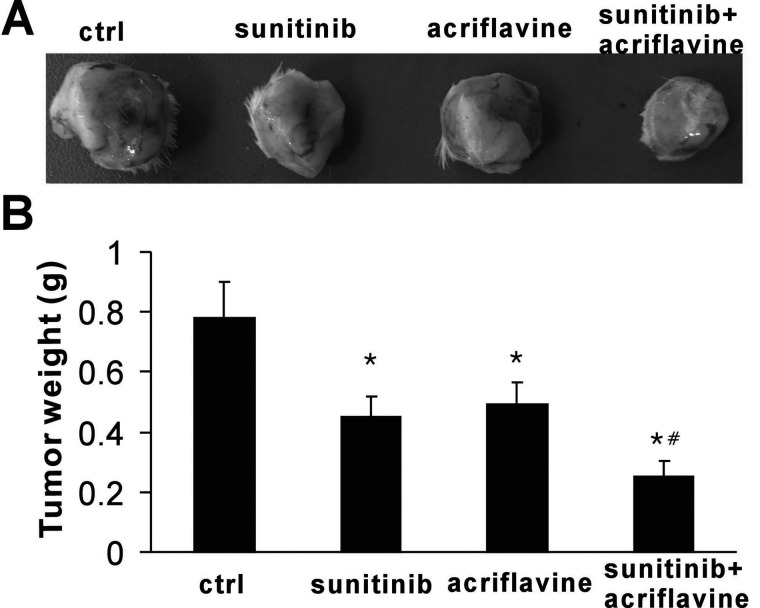
To evaluate whether HIF-1 inhibition could enhance the efficacy of antiangiogenic agents, we analyzed the effects of sunitinib and acriflavine in vivo in a syngenic model of 4T1 murine breast cancer. We found that both acriflavine and sunitinib were effective for 4T1 murine cancers (Fig. 2A, B). After 12 days of treatment, tumor weight in the vehicle group reached about 0.78 ± 0.12 g, whereas it was 0.45 ± 0.07 and 0.49 ± 0.07 g in the sunitinib and acriflavine monotherapy groups, respectively (p < 0.01). A dramatic inhibition of tumor growth was observed after treatment with sunitinib plus acriflavine compared with vehicle and monotherapy groups (p < 0.01), with the tumor weight only 0.25 ± 0.05 g (Fig. 2B). These results reveal that combination of HIF-1 inhibition with antiangiogenic therapy shows a synergistic and robust antitumor response.
Figure 2.
Sunitinib together with acriflavine inhibited tumor growth. (A) Representative images of tumor engraftments after treatment. (B) The combination therapy with sunitinib plus acriflavine showed synergistic antitumor effects (n = 5). *p < 0.01 versus control, #p < 0.01 versus sunitinib or acriflavine.
Sunitinib Plus Acriflavine Reduced VEGF and TGF-β Expression and Tumor Vasculature
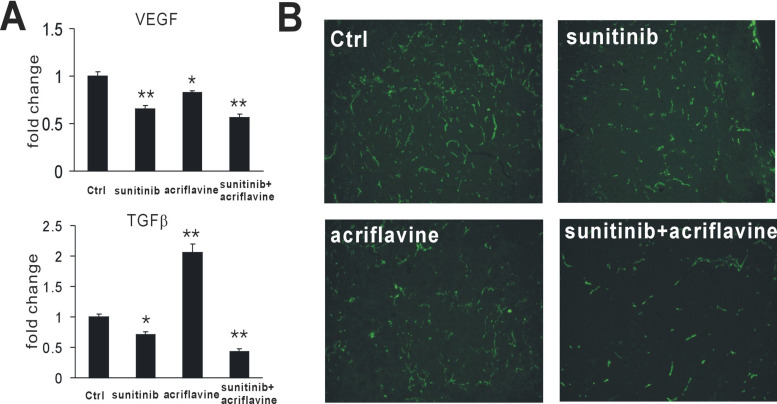
To assess whether sunitinib and acriflavine therapy reduced the expression of TGF-β and VEGF in tumors, RT-PCR was performed to assess those cytokine levels. Tumors treated with sunitinib showed reduced TGF-β and VEGF expression. Notably, although acriflavine reduced VEGF, it also upregulated TGF-β. However, strikingly, the combination of sunitinib and acriflavine resulted in a more pronounced inhibition of VEGF and TGF-β (Fig. 3A), suggesting their altered immunosuppressive phenotype and angiostatic microenvironment. Furthermore, we performed immunohistochemical analysis for CD31 in tumor sections. As expected, sunitinib or acriflavine decreased tumor microvessels (Fig. 3B). Treatment of 4T1 breast tumors with sunitinib plus acriflavine in combination showed increased antiangiogenic effects compared with treatment with sunitinib or acriflavine alone (Fig. 3B). These results show that HIF-1 inhibition enhances the antiangiogenic effect of sunitinib.
Figure 3.
Sunitinib plus acriflavine decreased VEGF and TGF-β expression and tumor vasculature. (A) RT-PCR analysis of VEGF and TGF-β levels in tumors after treatment. *p < 0.05, **p < 0.01 versus control. (B) Representative images of immunohistochemical analysis for CD31 in tumor sections.
Sunitinib Together With Acriflavine Resulted in Increased Necrosis and Apoptosis
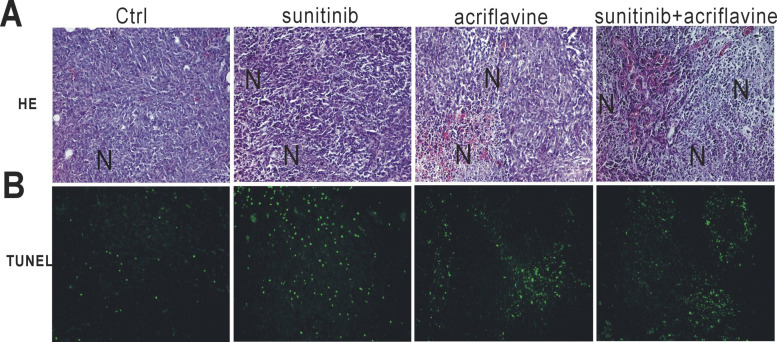
To assess whether sunitinib and acriflavine therapy induced tumor necrosis and apoptosis, we evaluated histologically the tumor sections. More necrotic areas and apoptotic cells were found after treatment with sunitinib or acriflavine compared with the control group (Fig. 4A, B), whereas the two drugs in combination exhibited the most necrotic areas and apoptotic cells in the tumor beds (Fig. 4A, B), suggesting that HIF-1 inhibition plus antiangiogenic therapy can induce more tumor cell death.
Figure 4.
Sunitinib plus acriflavine resulted in increased necrosis and apoptosis. (A) Representative images of H&E stain. N indicates necrosis. (B) Representative images of TUNEL on tumor sections.
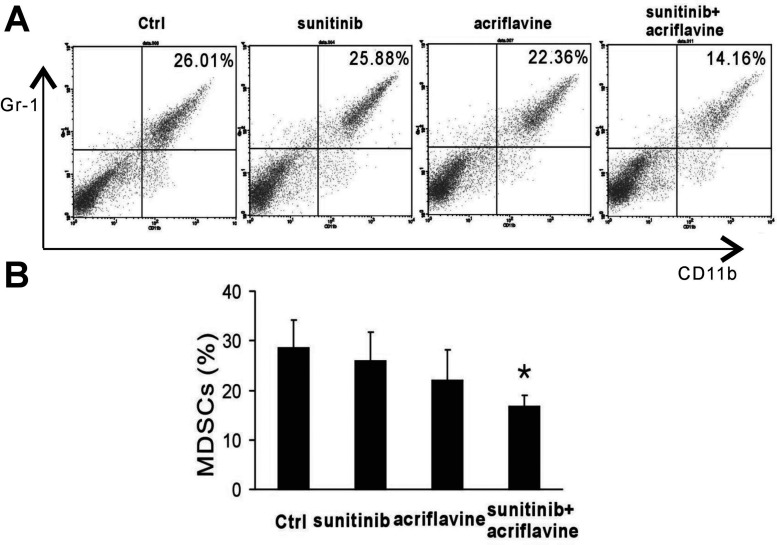
Combinational Therapy Reduced Myeloid-Derived Suppressor Cells (MDSCs) in Spleen of Tumor-Bearing Mice
To investigate whether the combinational therapy could reduce the systemic suppressive MDSCs, the number of MDSCs in the spleen was evaluated by flow cytometry. Flow cytometry analysis on day 12 after treatment demonstrated that sunitinib or acriflavine was not effective at reducing the number of MDSCs in the spleen. We observed that, once in combination, sunitinib and acriflavine showed a dramatic reduction in the percentage of splenic MDSCs (Fig. 5A, B). These results show that HIF-1 inhibition with acriflavine can reverse the immunosuppressive status in spleens of tumor-bearing mice.
Figure 5.
Sunitinib together with acriflavine decreased MDSCs in spleen. (A, B) Flow cytometry analysis of CD11b+Gr1+ MDSCs in the spleen of tumor-bearing mice. *p < 0.05 versus control.
DISCUSSION
Hypoxia is a major hallmark of the tumor microenvironment (7). Antiangiogenic therapy can induce hypoxia, which slows the cancer progression (14). Targeting hypoxia represents a promising strategy for cancer therapy (15). Several HIF inhibitors, such as anthracycline (16), acriflavine (13), PX-478 (17), and YC-1 (18), show potent antitumor effect. We show in this study that in a murine model of breast cancer, HIF-1 inhibition enhanced the antitumor effect of antiangiogenic therapy.
In this study, tumors treated with sunitinib, at a sustained and low dose, showed high levels of HIF-1α expression, one of the principal adaptive factors to low oxygen levels. HIF-1α is associated with almost every aspect of cancer progression. Multiple HIF-regulated genes are preferentially expressed in glioma stem cells compared with non-stem tumor cells or normal neural progenitors and then regulate the tumorigenic capacity of glioma stem cells (19). Targeting HIFs attenuates the tumor initiation potential of glioma stem cells (19) and depletes lymphoma stem cells (12) in vivo. HIF is also known for angiogenesis regulation (20,21). Disruption of HIF in mouse causes embryonic lethality and is associated with vascular regression due to extensive endothelial cell death (22). HIF-1-responsive genes, such as VEGF, ANGPT2, platelet-derived growth factor-B (PDGF-B), placental growth factor-1 (PlGF), erythropoietin (EPO), and stromal-derived growth factor 1 (SDF-1) have been demonstrated to participate in several neovascularization-related diseases (22,23). We found that acriflavine could reduce tumor vasculature, matching the previously described results (13). The antiangiogenic effect of this drug was due to decreased VEGF, SCF, and SDF1 and reduced circulating angiogenic cells in the peripheral blood (13). In this study, we found that sunitinib plus acriflavine markedly reduced VEGF levels and the resultant tumor vasculature. Active angiogenesis is important for cancer growth and progression. Antiangiogenic therapy disturbs the balance between oxygen consumption and delivery. We could see more necrosis and apoptosis after potent antiangiogenic treatment with sunitinib and acriflavine in combination.
We also found that HIF-1 inhibition by acriflavine partially reversed the immunosuppressive status. A plethora of researches indicate the association between hypoxia and immunosuppression. Hypoxia augmented T cell suppression induced by macrophages in vitro, which was dependent on macrophage expression of HIF-1α (24). In addition, HIF-1α-regulated enzymes, such as arginase-1 and inducible nitric oxide synthase (iNOS), are common factors of immunosuppression in T cells (25,26). HIF-1α is also essential for myeloid cell-mediated inflammation (27) and controls the Th17/Treg balance via regulating Th17 signature genes and targeting Foxp3 for proteasomal degradation (28). The important role of CD11b+Gr1+ MDSCs in the modulation of immune responses in tumor-bearing patients or mice has been established, which are a heterogeneous group of myeloid cells representing a heterogeneous population composed of myeloid-derived suppressor cells, immature myeloid cells, and monocytes with potent immunosuppressive and proangiogenic activity (29). In patients with breast cancers, the proportion of CD45+CD13+CD33+CD14–CD15– MDSCs significantly increased in the peripheral blood and primary cancer tissues (30). In pancreatic ductal adenocarcinoma-bearing KPC mice, MDSCs accumulated in both the spleen and the tumor, comprising up to 20–30% of all leukocytes in these tissues (31). MDSCs inhibit the function of cytotoxic T lymphocytes via expression of iNOS and arginase and generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS), which are involved in induction of antigen-specific T cell tolerance and evasion of antitumor immunity (32). An enhanced GM-CSF production is observed in PanIN-harboring human and mouse pancreata, which promote cancer growth via the recruitment of Gr1+CD11b+ myeloid cells and the following evasion of CD8+ T cell-driven antitumor immunity (33). In our present study, we found that combination of sunitinib with acriflavine could result in a significant reduction of accumulation of MDSCs in the spleen, indicating the reversion of systemic immunosuppression. We also demonstrated that the combination could reduce TGF-β mRNA levels, a known tumor immunosuppressive cytokine.
Interestingly, it is considered by the classical theory that HIF-1α was induced by hypoxia, which was associated with the inhibition of oxygen sensor PHDs (34). Moreover, NO, ROS, TNF-α, IL-1β, LPS, and viruses can also regulate HIF-1α (35). Interestingly, apoptotic cells could upregulate HIF-1α expression in a NFAT-dependent manner (35). During the antiangiogenic process, hypoxia and resultant apoptotic cells can stimulate HIF-1α expression. We here consider that in the presence of acriflavine, the function of HIF stimulated by hypoxia and apoptosis after antiangiogenic therapy is antagonized, and then the synergistic antitumor effect followed.
In conclusion, HIF-1 inhibition with acriflavine can potentiate the antitumor effect of sunitinib. The combination of HIF-1 inhibition and antiangiogenic therapy may represent a promising strategy for cancer patients.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Funds (81272523).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ferrara N.; Kerbel R. S. Angiogenesis as a therapeutic target. Nature 438:67–974; 2005. [DOI] [PubMed] [Google Scholar]
- 2. Kerbel R. S. Antiangiogenic therapy: A universal chemosensitization strategy for cancer? Science 312:1171–1175; 2006. [DOI] [PubMed] [Google Scholar]
- 3. Shaked Y.; Henke E.; Roodhart J. M. L.; Mancuso P.; Langenberg M. H. G.; Colleoni M.; et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: Implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 14:263–273; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conley S. J.; Gheordunescu E.; Kakarala P.; Newman B.; Korkaya H.; Heath A. N.; et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc. Natl. Acad. Sci. USA 109:2784–2789; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ebos J. M. L.; Lee C. R.; Cruz-Munoz W.; Bjarnason G. A.; Christensen J. G.; Kerbe R. S. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15:232–239; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang G. L.; Jiang B. H.; Rue E. A.; Semenza G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92:5510–5514; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Keith B.; Simon M. C. Hypoxia-inducible factors, stem cells, and cancer. Cell 129:465–472; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Semenza G. L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer 3:721–731; 2003. [DOI] [PubMed] [Google Scholar]
- 9. Du R.; Lu K. V.; Petritsch C.; Liu P.; Ganss R.; Passegué E.; et al. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13:206–220; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liao D.; Corle C.; Seagroves T. N.; Johnson R. S. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 67:563–572; 2007. [DOI] [PubMed] [Google Scholar]
- 11. Blouw B.; Song H.; Tihan T.; Bosze J.; Ferrara N.; Gerber H. P.; et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell 4:133–146; 2003. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y.; Liu Y.; Malek S. N.; Zheng P.; Liu Y. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell 8:399–411; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee K. A.; Zhang H.; Qian D. Z.; Rey S.; Liu J. O.; Semenza G. L. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc. Natl. Acad. Sci. USA 106:17910–17915; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Pennacchietti S.; Michieli P.; Galluzzo M.; Mazzone M.; Giordano S.; Comoglio P. M. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 3:347–361; 2003. [DOI] [PubMed] [Google Scholar]
- 15. Brown J. M.; Giaccia A. J. The unique physiology of solid tumors: Opportunities (and problems) for cancer therapy. Cancer Res. 58:1408–1416; 1998. [PubMed] [Google Scholar]
- 16. Lee K. A.; Qian D. Z.; Rey S.; Wei H.; Liu J. O.; Semenza G. L. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc. Natl. Acad. Sci. USA 106:2353–2358; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Macpherson G. R.; Figg W. D. Small molecule-mediated anti-cancer therapy via hypoxia-inducible factor-1 blockade. Cancer Biol. Ther. 3:503–504; 2004. [DOI] [PubMed] [Google Scholar]
- 18. Welsh S.; Williams R.; Kirkpatrick L.; Paine-Murrieta G.; Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1α. Mol. Cancer Ther. 3:233–244; 2004. [PubMed] [Google Scholar]
- 19. Li Z.; Bao S.; Wu Q.; Wang H.; Eyler C.; Sathornsumetee S.; et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 15:501–513; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carmeliet P.; Dor Y.; Herbert J. M.; Fukumura D.; Brusselmans K.; Dewerchin M.; et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394:485–490; 1998. [DOI] [PubMed] [Google Scholar]
- 21. Forsythe J. A.; Jiang B. H.; Iyer N. V.; Agani F.; Leung S. W.; Koos R. D.; et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16:4604–4613; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly B. D.; Hackett S. F.; Hirota K.; Oshima Y.; Cai Z.; Berg-Dixon S.; et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ. Res. 93:1074–1081; 2003. [DOI] [PubMed] [Google Scholar]
- 23. Yoshida T.; Zhang H.; Iwase T.; Shen J.; Semenza G. L.; Campochiaro P. A. Digoxin inhibits retinal ischemia-induced HIF-1α expression and ocular neovascularization. FASEB J. 24:1759–1767; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doedens A. L.; Stockmann C.; Rubinstein M. P.; Liao D.; Zhang N.; DeNardo D. G.; et al. Macrophage expression of hypoxia-inducible factor-1α suppresses T-cell function and promotes tumor progression. Cancer Res. 70:7465–7475; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bronte V.; Serafini P.; Santo C. D.; Marigo I.; Tosello V.; Mazzoni A.; et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 170:270–278; 2003. [DOI] [PubMed] [Google Scholar]
- 26. Matlack R.; Yeh K.; Rosini L.; Gonzalez D.; Taylor J.; Silberman D.; et al. Peritoneal macrophages suppress T-cell activation by amino acid catabolism. Immunology 117:386–395; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cramer T.; Yamanishi Y.; Clausen B. E.; Förster I.; Pawlinski R.; Mackman N.; et al. HIF-1α is essential for myeloid cell-mediated inflammation. Cell 112:645–657; 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dang E. V.; Barbi J.; Yang H. Y.; Jinasena D.; Yu H.; Zheng Y.; et al. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell 146:772–784; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ostrand-Rosenberg S.; Sinha P. Myeloid-derived suppressor cells: Linking inflammation and cancer. J. Immunol. 182:4499–4506; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu J.; Du W.; Yan F.; Wang Y.; Li H.; Cao S.; et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J. Immunol. 190:3783–3797; 2013. [DOI] [PubMed] [Google Scholar]
- 31. Bayne L. J.; Beatty G. L.; Jhala N.; Clark C. E.; Rhim A. D.; Stanger B. Z.; et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T Cell immunity in pancreatic cancer. Cancer Cell 21:822–835; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu T.; Gabrilovich D. I. Molecular pathways: Tumor-infiltrating myeloid cells and reactive oxygen species in regulation of tumor microenvironment. Clin. Cancer Res. 18:4877–4882; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pylayeva-Gupta Y.; Lee K. E.; Hajdu C. H.; Miller G.; Bar-Sagi D.; et al. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21:836–847; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cummins E. P.; Berra E.; Comerford K. M.; Ginouves A.; Fitzgerald K. T.; Seeballuck F.; et al. Prolyl hydroxylase-1 negatively regulates IκB kinase-β, giving insight into hypoxia-induced NFκB activity. Proc. Natl. Acad. Sci. USA 103:18154–18159; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dehne N.; Brüne B. HIF-1 in the inflammatory microenvironment. Exp. Cell Res. 315:1791–1797; 2009. [DOI] [PubMed] [Google Scholar]