Abstract
Human interleukin-24 (IL-24) has been found recently to play a tumor-suppressor role in a variety of tumors, including gliomas. However, the exact mechanism of glioma tumor suppression by IL-24 remains unclear. We collected by surgery 30 gliomas at different grades and evaluated IL-24 and double-stranded RNA-activated protein kinase (PKR) expression using fluorescence quantitative real-time PCR and immunohistochemical techniques. Two human glioma cell lines, U87 and U251, were transfected with Ad5F35-IL24 via recombinant adenovirus-mediated gene transfer and apoptosis, as well as PKR and eIF-2α expression analyzed. The results showed that IL-24 and PKR expression decreased with increasing tumor grade. Compared with cells of the control groups, Ad5F35-IL24-infected U87 and U251 cells exhibited a significantly increased apoptosis and elevated PKR, eIF-2α, p-PKR, and p-eIF-2α levels, while the expression of Bcl-2 was decreased. Finally, IL-24 also sensitized apoptosis of glioma cells to temozolomide (TMZ). This study indicates that IL-24 upregulates expression and activation of PKR, further increasing expression and activation of eIF-2α, and decreasing Bcl-2 to promote apoptosis. IL-24 also increases chemosensitivity of glioma cells to TMZ.
Key words: Interleukin-24 (IL-24), RNA-activated protein kinase (PKR), eIF-2α, Glioma
INTRODUCTION
Interleukin-24 (IL-24), also known as melanoma differentiation-associated gene-7 (MDA-7), is a recently discovered IL-10 cytokine family member with antitumor effects. Recent studies have focused on the antitumor effects of IL-24, initially identified by Jiang et al. in a study on malignant melanocytoma (1). IL-24 is a protein that has a dual effect, cytokine-like properties, and a tumor-suppressor function. Normal IL-24 physiological concentrations regulate the immune response, and supraphysiological concentrations show excellent antitumor properties. In recent years, the tumor-suppressor role of IL-24 has been studied in a variety of tumors. It is presently thought that IL-24 plays a tumor-suppressor role mainly through the regulation of apoptotic signaling and the promotion of apoptosis in tumor cells (2–4).
The double-stranded RNA-activated protein kinase PKR is a serine/threonine protein kinase. PKR is one of the major regulatory factors with interferon-mediated antiviral and apoptosis-inducing effects and mediates apoptosis by a variety of stimulatory factors (5). Located on human chromosome 2p21-2, the PKR gene is transcribed by a promoter consisting of a number of potential transcriptional regulatory elements and activated through two double-stranded RNA-binding regions (6). Activated PKR regulates multiple downstream targets including eIF-2α and NF-κB, thereby inhibiting protein synthesis and ultimately suppressing cell growth and promoting apoptosis (7–10).
Temozolomide (TMZ), a DNA alkylating antineoplastic drug used universally in glioma treatment, could inhibit the proliferation and induce apoptosis of glioma cells (11). However, the TMZ-based chemotherapy still remains modest, mainly due to the development of drug resistance (12). Thus, it is essential to develop novel therapeutic strategies to inhibit glioma.
Studies have shown that IL-24 plays a tumor-suppressor role in glioma (13), although the exact mechanism is not clear. Our results indicated that IL-24 expression is related to glioma malignancy grade, and enhanced IL-24 expression of gliomas increased apoptosis by upregulation of expression and phosphorylation of PKR and eIF-2α and reducing Bcl-2 expression. Also, IL-24 shows a synergistic effect with anticancer agent TMZ on apoptosis in glioma cells.
MATERIALS AND METHODS
Glioma Tissue Specimens and Cell Lines
Glioma tissue specimens were collected by surgical resection from 30 patients from January to December 2009 from the Department of Neurosurgery, Cangzhou Central Hospital, after informed consent from adult patients diagnosed with glioma (Hebei, China). All glioma specimens were subjected to hematoxylin and eosin staining, followed by routine pathological examinations, and classified in accordance with the 2007 WHO Classification of Brain Tumors. There were nine glioma specimens at grades I–II, 11 specimens at grade III (anaplastic astrocytoma), and 10 specimens at grade IV (glioblastoma). The patients included 18 males and 12 females (43 ± 19 years old). Normal brain tissue specimens were obtained from traumatic patients via decompression surgery after informed consent (n = 7).
Two human glioma cell lines, U87 and U251, were purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences. Cells were cultured and passaged in Dulbecco’s modified Eagle’s medium at 37°C with 5% CO2.
Recombinant Adenovirus
Recombinant adenovirus carrying the IL-24 gene (Ad5F35-IL24, Ad-IL-24) and control adenovirus (Ad5F35-EGFP, Ad-EGFP) were provided by Beijing Vector Gene Technology Co. Ltd. (Beijing, China). The virus titers of Ad-IL24 and Ad-EGFP were 2.8 × 1011 and 5.8 × 1011 pfu/ml, respectively.
Fluorescence Quantitative Real-Time Polymerase Chain Reaction (qPCR) Assay
Total RNA was extracted from glioma tissue specimens and cell cultures and quantified by spectrophotometry (required OD260/OD280 ratio, 1.8–2.0). After reverse transcription, cDNA was combined with GAPDH (internal control), IL-24, or PKR primers on a 7300 HT Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) for amplification. Relative gene expression levels were calculated using ABI 7300 System SDS Software v1.3.2.10.
Primers were synthesized by Shanghai Biological Engineering Co., Ltd. (Shanghai, China). The primer sequences were as follows: GAPDH (upstream primer 5′-TGAACGGGAAGCTCACTGG-3′ and downstream primer 5′-GCTTCACCACCTTCTTGATGTC-3′); IL-24 (upstream primer 5′-CAGGCGGTTTCTGCTATTCC-3′ and downstream primer 5′-GGCGTGAAGTGTCCAGTGAA-3′); PKR (upstream primer 5′-GGAAAGCGAACAAGGAGTAAGG-3′ and downstream primer 5′-CCAAAGCGTAGAGGTCCACT-3′).
Immunohistochemical Assay
Glioma and normal brain tissue specimens were paraffin-embedded and sectioned for detection of IL-24 and PKR expression. After conventional dewaxing and dehydration, specimen sections were incubated with primary antibodies for IL-24 and PKR (1:100; Promega, USA) at 4°C for 24 h, followed by secondary antibodies (1:100; Sigma-Aldrich, USA) at 37°C for 30 min. Negative controls contained phosphate-buffered saline in place of primary antibody. Specimens were developed using avidin–biotin–HRP complex with diaminobenzidine (DAB), restained with hematoxylin, and mounted for microscopic examination.
Comprehensive scoring evaluated the positive cell count (percentage) and intensity of the staining reaction. The scoring criteria for positive cell percentages were as follows: no positive cells, 0 points; <20%, 1 point; 20–39%, 2 points; 40–69%, 3 points; and ≥70%, 4 points. The staining intensity referred to the majority of cells, and the scoring criteria were as follows: negative stain, 0 points; light brownish yellow, 1 point; dark brownish yellow, 2 points; and dark brown, 3 points. Multiplication of the intensity and the percentage scores gave rise to the final staining score: 0 (negative), + (1–4), ++ (5–8), and +++ (9–12). For each specimen, the mean total score of three sections was calculated to obtain the mean expression value of the indicator and used for statistical analysis.
Apoptosis Detection
Ad-IL-24 or Ad-EGFP-transfected glioma cells and control cells were harvested, washed, and double stained with annexin V and propidium iodide (PI). Apoptosis was detected using flow cytometry. Cells negative for both annexin V and PI were considered normal, whereas those positive for annexin V and negative for PI were apoptotic, and those positive for both were considered necrotic.
Western Blot Detection
Total protein was extracted from tumor cells using conventional methods, measured using the bicinchoninic acid method, and concentrations adjusted to equal levels. Protein (100 µg) was separated by electrophoresis on 10–12% SDS-PAGE gels and electrotransferred to PVDF membranes. After blocking, membranes were incubated with primary antibodies for IL-24 (1:1,000; Santa Cruz, USA), PKR, p-PKR, eIF-2α, and p-eIF-2α (all 1:500) using GAPDH as an internal control. After incubation with HRP-labeled secondary antibody, membranes were fixed, developed, and photographed. Semiquantitative analysis was performed using the UVP-Lab work 4.60 image analysis system (UVP Inc., Upland, CA, USA).
Statistical Analysis
The experimental data were analyzed using SPSS 11.5 (SPSS Inc., Chicago, IL, USA). Measurement data were expressed as the mean ± standard error of the mean. Multiple group comparisons of the means were performed using one-way ANOVA. Pairwise comparison of values with significant differences was performed using the least significant difference test. A value of p < 0.05 was considered statistically significant.
RESULTS
Decreased IL-24 and PKR Expression in Glioma
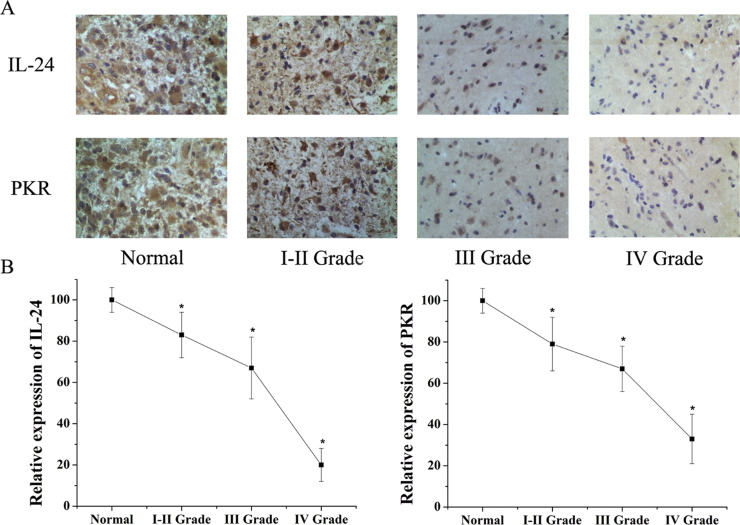
We analyzed IL-24 and PKR expression in 30 human gliomas at different grades and seven normal brain tissue specimens. Immunohistochemical results showed that IL-24 and PKR expression decreased in glioma compared with normal brain. IL-24 and PKR expression levels decreased with increasing grade (Fig. 1A, Table 1). Similar results were obtained for IL-24 and PKR mRNA expression in all specimens, as detected using qPCR (Fig. 1B). These results indicate that IL-24 and PKR expression is lower in higher-grade gliomas.
Figure 1.
(A) Immunohistochemistry was used to test the expression of IL-24 and PKR in normal brain tissues and Grades I–II, III, and IV glioma tissues. Results showed expression of IL-24 and PKR was lower in glioma tissues than in normal brain tissues. Both IL-24 and PKR were decreased with increasing glioma grade in human glioma tissues. (B) qPCR results also showed IL-24 and PKR expression levels decreased with increasing glioma grade. *Significant difference (p < 0.05).
Table 1.
Expression of IL-24 and PKR in Glioma Tissues Tested by Immunohistochemical Assay
| Tissues (No.) | Expression of IL-24 | Expression of PKR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | Positive Rate (%) | − | + | ++ | +++ | Positive Rate (%) | |
| Normal (7) | 0 | 0 | 5 | 2 | 100 | 0 | 0 | 6 | 1 | 100 |
| I–II Grade (9) | 1 | 2 | 4 | 2 | 88.9 | 2 | 2 | 4 | 1 | 77.8 |
| III Grade (11) | 3 | 4 | 3 | 1 | 72.7 | 3 | 5 | 3 | 0 | 72.7 |
| IV Grade (10) | 6 | 2 | 2 | 0 | 40 | 5 | 3 | 2 | 0 | 50 |
IL-24 Promotes Apoptosis in Glioma Cells
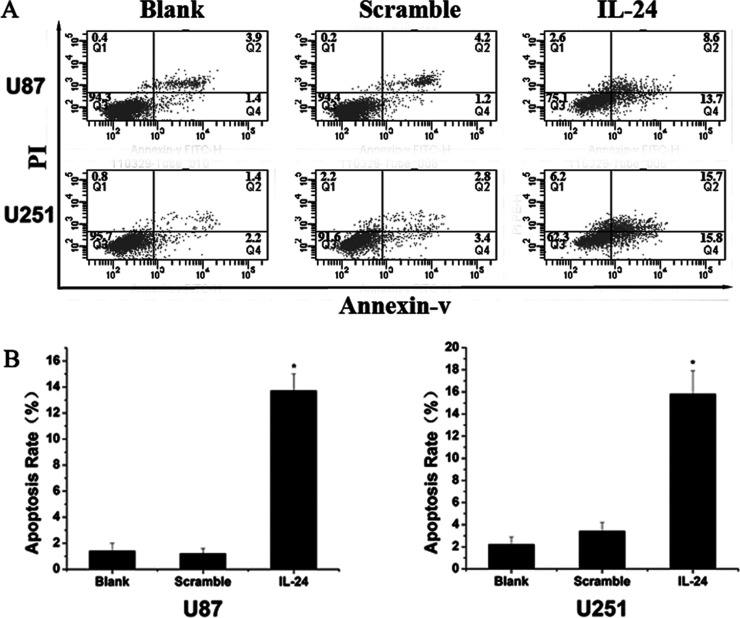
The glioma cell lines U87 and U251 were transfected with Ad-IL-24 with Ad-EGFP as a control and the effect of elevated IL-24 expression examined using flow cytometry. As in Figure 2, the results showed that in U87 cells, 12.3% of Ad-IL-24 transfected cells underwent apoptosis, significantly higher than those observed in the Ad-EGFP (4%) and control (3%) groups. In U251 cells, 13% of Ad-IL-24 cells underwent apoptosis, higher than those in the Ad-EGFP (5.4%) and control (3.6%) groups. These results demonstrate that elevated IL-24 expression effectively promotes apoptosis in glioma cells.
Figure 2.
(A) The apoptosis rate using flow cytometry of glioma cells transfected with Ad-IL-24 was higher than the scramble group and control group in U87 and U251. The scramble group was transfected with Ad-EGFP. (B) Graphical representation of (A). *Significant difference (p < 0.05).
IL-24 Promotes Apoptosis in Glioma by Regulation of PKR, eIF-2α, and Bcl-2
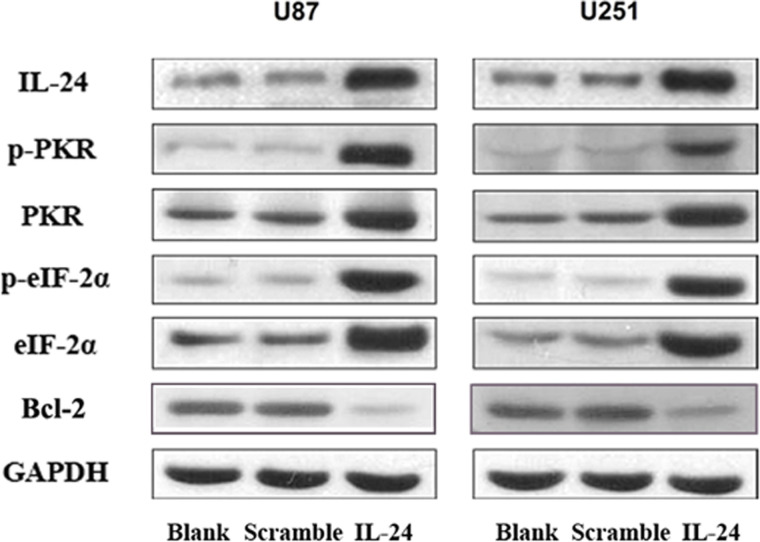
To further explore the mechanism by which IL-24 promotes apoptosis in glioma cells, we examined PKR, p-PKR, eIF-2α, p-eIF-2α, and Bcl-2 expression in U87 and U251 cells transfected with Ad-IL-24, Ad-EGFP, or control. Interestingly, PKR and p-PKR expression levels in U87 and U251 glioma cells were higher in the Ad-IL-24 group than in the Ad-EGFP and control groups (Fig. 3). Additionally, the downstream signaling protein eIF-2α and its activated form p-eIF-2α were expressed at increased levels in Ad-IL-24-transfected U87 and U251 compared with the other two groups (Fig. 4). As an antiapoptosis protein, Bcl-2 was decreased in Ad-IL-24-transfected U87 and U251 cells. These results suggest that overexpression of IL-24 likely upregulates the expression and activation of PKR and eIF-2α and decreases Bcl-2 expression to promote apoptosis.
Figure 3.
Western blotting analysis of IL-24, PKR, p-PKR, eIF-2α, p-eIF-2α, and Bcl-2 in U87 and U251 untransfected cells or cells transfected with scramble EGFP or IL-24. GAPDH serves as a loading control.
Figure 4.
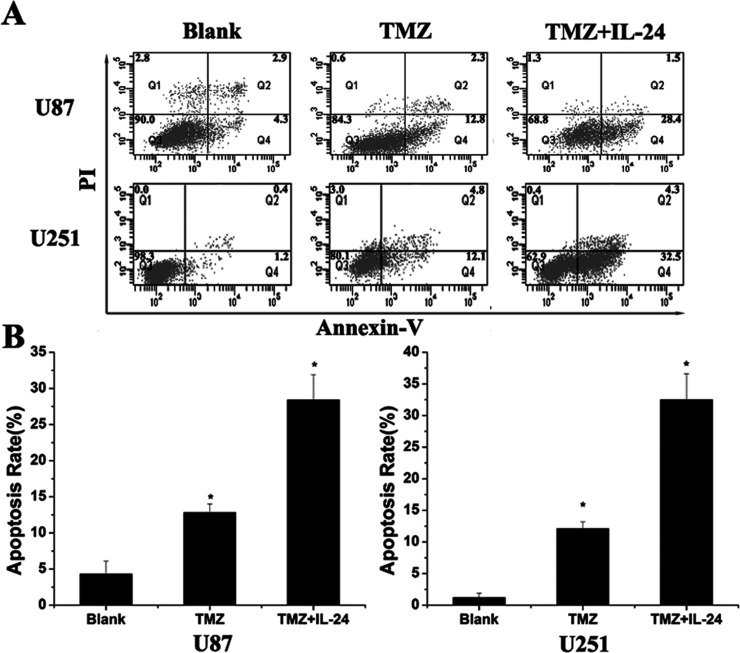
(A) Flow cytometry tested the apoptosis of glioma cells in U87 and U251, including blank cells, cells treated with TMZ (100 µM), and cells transfected with IL-24 after being treated with TMZ (100 µM). The apoptosis rate of cells treated with both TMZ and IL-24 was significant higher than those treated with TMZ only. *Significant difference (p < 0.05).
IL-24 Enhances TMZ-Induced Apoptosis in Glioma Cells
To test whether IL-24 exhibits a synergistic effect in apoptosis of glioma cells with the anticancer agent TMZ, U87 and U251 cells were treated with 100 µM of TMZ. After transfection with Ad-IL-24, apoptosis rates of glioma cells were tested with flow cytometry. Our results showed that the apoptosis rates of glioma cells treated with TMZ were 12.8% in U87 and 12.1% in U251, and the apoptosis of glioma cells were significantly increased (28.4% in U87; 32.5% in U251) after being treated together with TMZ and IL-24. This means IL-24 renders glioma cells more sensitive to TMZ treatment.
DISCUSSION
Glioma is the most common type of primary central nervous system malignancy in the brain. Gliomas show infiltrative growth with no clear boundaries. At present, surgical treatment combined with radiotherapy and chemotherapy is the primary method used to treat gliomas. However, complete surgical resection of this type of tumor remains difficult in the clinic, whereas chemotherapy and radiotherapy have poor therapeutic effects because of a lack of specificity for killing glioma cells. Additionally, chemotherapy and radiotherapy may exhibit toxic side effects that cannot be tolerated by the central nervous system or other areas of the body. Existing studies have shown that IL-24 selectively kills many types of tumors (including lung, breast, and melanoma) without damaging normal cells (14–16). Recently, Yacoub et al. (17,18) found that IL-24 plays a certain role in suppressing gliomas and improving their susceptibility to radiation. However, the relationship between IL-24 and the development and progression of human gliomas as well as the exact mechanism of action by IL-24 on gliomas is not clear. We found that IL-24 and PKR expression decreased in gliomas, and the higher the grade of malignancy, the lower the levels of IL-24 and PKR expression. Our results also indicated that IL-24 promoted apoptosis in glioma cells by upregulating PKR/eIF-2α expression and phosphorylation and decreasing Bcl-2 expression.
IL-24 is a tumor-suppressing factor that has been extensively studied, and its anticancer properties have been verified in a variety of tumors. The occurrence and development of gliomas are associated with abnormal expression of various cytokines. It is presently thought that tumor-suppressor gene mutations in glial cells lead to reduced expression of tumor-suppressor genes, thereby causing changes in a series of cell signaling pathways and ultimately leading to conversion of glial cells into glioma cells. Our results demonstrate that IL-24 expression decreases in human glioma tissues compared with normal brain tissues and that its expression level gradually declines with increasing grade, suggesting that IL-24 may be involved in the occurrence and development of gliomas. We also found that glioma PKR expression decreased compared with that in normal brain. Interestingly, PKR is a protein molecule regulating apoptosis, and its activation has been found to play an antitumor role by mediating increased apoptosis in a variety of tumor cells (19). Together these findings reveal that IL-24 and PKR may be associated and jointly play roles in suppressing the occurrence and development of glioma.
We transfected IL-24 into two glioma cell lines and found significant increases in apoptosis of IL-24-infected cells, suggesting that IL-24 promotes glioma apoptosis. Additional studies have shown that IL-24 upregulates PKR expression and increases apoptosis in lung cancer, thus playing a role in inhibiting lung cancer (20).We asked whether IL-24 also promotes glioma apoptosis by regulating PKR. Our study demonstrated that expression of both PKR and p-PKR increased in glioma cells exhibiting elevated IL-24 expression, similar to what was observed for eIF-2α and p-eIF-2α. Because eIF-2α is an important signaling protein that regulates apoptosis downstream of PKR, we propose that IL-24 likely upregulates PKR expression and activation, thereby increasing downstream eIF-2α expression and activation, and ultimately promoting apoptosis. In addition, IL-24 could also regulate the expression of antiapoptosis protein to promote apoptosis in glioma cells, such as Bcl-2.
TMZ treatment is one of the major chemotherapies in treating glioma. But the resistance to TMZ often causes the failure of treatment for glioma. Therefore, a new strategy to increase the effectiveness of TMZ is necessary. Some RNA or protein had been reported to enhance chemotherapy sensitivity of glioma, such as MiR-143 and LRIG1 (12,21). Our results showed that overexpression of IL-24 significantly enhanced apoptosis sensitivity to treatment of TMZ. Thus, the IL-24 restoration approach may offer a new modulation strategy to overcome chemoresistance to TMZ treatment in glioma.
In summary, we indicate that decreased IL-24 expression is related to glioma occurrence and development. Overexpression of IL-24 could promote apoptosis of glioma cells by increasing PKR/eIF-2α expression and phosphorylation and decreasing Bcl-2 expression. IL-24 enhanced chemoresistance to TMZ treatment in glioma.
ACKNOWLEDGMENTS
Cangzhou City Science and Technology Project. Item No.: 131 302 137.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Jiang H.; Su Z. Z.; Lin J. J.; Goldstein N. I.; Young C. S.; Fisher P. B. The melanoma differentiation associated gene MDA-7 suppresses cancer cell growth. Proc. Natl. Acad. Sci. USA 93:9160–9165; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang C. J.; Zhang H.; Chen K.; Zheng J. W.; Xiao C. W.; Ji W. W.; Yu Y.; Hu H. Y.; Li Y.; Xue X. B. Ad.MDA-7 (IL-24) selectively induces apoptosis in hepatocellular carcinoma cell lines, suppresses metastasis, and enhances the effect of doxorubicin on xenograft tumors. Oncol. Res. 18(11–12):561–574; 2010. [DOI] [PubMed] [Google Scholar]
- 3. Park M. A.; Hamed H. A.; Mitchell C.; Cruickshanks N.; Dash R.; Allegood J.; Dmitriev I. P.; Tye G.; Ogretmen B.; Spiegel S.; Yacoub A.; Grant S.; Curiel D. T.; Fisher P. B.; Dent P. A serotype 5/3 Adenovirus expressing MDA-7/IL-24 infects renal carcinoma cells and promotes toxicity of agents that increase Ros and ceramide levels. Mol. Pharmacol. 79(3):368–380; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Panneerselvam J.; Munshi A.; Ramesh R. Molecular targets and signaling pathways regulated by interleukin (IL)-24 in mediating its antitumor activities. J. Mol. Signal. 8:15; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garcia M. A.; Meurs E. F.; Esteban M. The dsRNA protein kinase PKR: Virus and cell control. Biochimie 89:799–811; 2007. [DOI] [PubMed] [Google Scholar]
- 6. Lee S. B.; Esteban M. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology 199:491–496; 1994. [DOI] [PubMed] [Google Scholar]
- 7. Saelens X.; Kalai M.; Vandenabeele P. Translation inhibition in apoptosis: Caspase-dependent PKR activation and eIF2-alpha phosphorylation. J. Biol. Chem. 276:41620–41628; 2001. [DOI] [PubMed] [Google Scholar]
- 8. Sudhakar A.; Ramachandran A.; Ghosh S.; Hasnain S. E.; Kaufman R. J.; Ramaiah K. V. Phosphorylation of serine 51 in initiation factor 2 alpha (eIF2 alpha) promotes complex formation between eIF2 alpha(P) and eIF2B and causes inhibition in the guanine nucleotide exchange activity of eIF2B. Biochemistry 39:12929–12938; 2000. [DOI] [PubMed] [Google Scholar]
- 9. Der S. D.; Yang Y. L.; Weissmann C.; Williams B. R. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:3279–3283; 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friedrich I.; Ben-Bassat H.; Levitzki A. Activation of dsRNA dependent protein kinase PKR in Karpas299 does not lead to cell death. Cancer Biol. Ther. 4:734–739; 2005. [DOI] [PubMed] [Google Scholar]
- 11. Prasad G.; Sottero T.; Yang X.; Mueller S.; James C. D.; Weiss W.A.; Polley M. Y.; Ozawa T.; Berger M. S.; Aftab D. T.; Prados M. D.; Haas-Kogan D. A. Inhibition of PI3K/mTOR pathways in glioblastoma and implications for combination therapy with temozolomide. Neuro. Oncol. 13:384–392; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang L.; Shi Z. M.; Jiang C. F.; Liu X.; Chen Q. D.; Qian X.; Li D. M.; Ge X.; Wang X. F.; Liu L. Z.; You Y. P.; Liu N.; Jiang B. H. MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget 5(14):5416–5427; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Q.; Zhu Y.; Yang P. Is MDA-7/IL-24 a potential target and biomarker for enhancing drug sensitivity in human glioma U87 cell line? Anat. Rec (Hoboken) 296(8):1154–1160; 2013. [DOI] [PubMed] [Google Scholar]
- 14. Mhashilkar A. M.; Schrock R. D.; Hindi M.; Liao J.; Sieger K.; Kourouma F.; Zou-Yang X. H.; Onishi E.; Takh O.; Vedvick T. S.; Fanger G.; Stewart L.; Watson G. J.; Snary D.; Fisher P. B.; Saeki T.; Roth J. A.; Ramesh R.; Chada S. Melanoma differentiation associated gene-7 (MDA-7): A novel anti-tumor gene for cancer gene therapy. Mol. Med. 7(4):271–282; 2001. [PMC free article] [PubMed] [Google Scholar]
- 15. Su Z. Z.; Madireddi M. T.; Lin J. J.; Young C. S.; Kitada S.; Reed J. C.; Goldstein N. I.; Fisher P. B. The cancer growth suppressor gene MDA-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc. Natl. Acad. Sci. USA 95:14400–14405; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saeki T.; Mhashilkar A.; Chada S.; Branch C.; Roth J. A.; Ramesh R. Tumor-suppressive effects by adenovirus-mediated MDA-7 gene transfer in non-small cell lung cancer cell in vitro. Gene. Ther. 7:2051–2057; 2000. [DOI] [PubMed] [Google Scholar]
- 17. Yacoub A.; Mitchell C.; Lebedeva I. V.; Sarkar D.; Su Z. Z.; McKinstry R.; Gopalkrishnan R. V.; Grant S.; Fisher P. B.; Dent P. MDA-7 (IL-24) inhibits growth and enhances radiosensitivity of glioma cells in vitro via JNK signaling. Cancer Biol. Ther. 2:347–353; 2003. [DOI] [PubMed] [Google Scholar]
- 18. Yacoub A.; Mitchell C.; Hong Y.; Gopalkrishnan R. V.; Su Z. Z.; Gupta P.; Sauane M.; Lebedeva I. V.; Curiel D. T.; Mahasreshti P. J.; Rosenfeld M. R.; Broaddus W. C.; James C. D.; Grant S.; Fisher P. B.; Dent P. MDA-7 regulates cell growth and radiosensitivity in vitro of primary (non-established) human glioma cells. Cancer Biol. Ther. 3:739–751;2004. [DOI] [PubMed] [Google Scholar]
- 19. Jagus R.; Joshi B.; Barber G. N. PKR, apoptosis and cancer. Int. J. Biochem. Cell. Biol. 31:123–138; 1999. [DOI] [PubMed] [Google Scholar]
- 20. Pataer A.; Vorburger S. A.; Barber G. N.; Chada S.; Mhashilkar A. M.; Zou-Yang H.; Stewart A. L.; Balachandran S.; Roth J. A.; Hunt K. K.; Swisher S. G. Adenoviral transfer of the melanoma differentiation-associated gene 7 (MDA7) induces apoptosis of lung cancer cells via up-regulation of the double-stranded RNA-dependent protein kinase (PKR). Cancer. Res. 62:2239–2243; 2002. [PubMed] [Google Scholar]
- 21. Wang X.; Xiao Q.; Xing X.; Tian C.; Zhang H.; Ye F.; Wan F.; Wang B.; Guo D.; Lei T. LRIG1 enhances cisplatin sensitivity of glioma cell lines. Oncol. Res. 20(5–6):205–211; 2012. [DOI] [PubMed] [Google Scholar]