Abstract
Doxorubicin plays a major role in the treatment of osteosarcoma disorders. The Notch signaling pathway exerts various biological functions, including cell proliferation, differentiation, and apoptosis. In the present study, we investigated the effects of different doses of doxorubicin on proliferation and apoptosis of osteosarcoma cells with or without Notch signaling. Results found that cellular viability was downregulated while caspase 3 activity and expression were promoted in osteosarcoma cells following treatment with various doses of doxorubicin for 24, 48, and 72 h, and the effects showed a dose- and time-dependent manner. Furthermore, it was found that various doses of doxorubicin activated the Notch signaling pathway, shown by the elevated expression of Notch target genes NOTCH1, HEY1, HES1, AND HES5. It was further proved that, after small interfering RNA (siRNA)-mediated knockdown of Notch, the effects of doxorubicin on the viability and apoptosis of osteosarcoma cells were significantly reduced. It was indicated that doxorubicin treatment reduced the proliferation and promoted the apoptosis of osteosarcoma cells, and this effect was mediated by the Notch signaling pathway.
Key words: Doxorubicin, Notch signaling, Osteosarcoma cell, Proliferation, Apoptosis
INTRODUCTION
Osteosarcoma is the most common primary bone tumor in children and young adults, and appropriate chemotherapy for osteosarcoma can increase limb sparing and overall survival (1,2). Doxorubicin is a common chemotherapeutic drug with a wide spectrum of anticancer activity against several malignancies, including breast cancer, ovarian cancer, peripheral T-cell lymphomas, intra-abdominal desmoid tumors, multiple myeloma, and AIDS-related Kaposi’s sarcoma (3–5). However, the recent findings suggested that the most common side effects associated with doxorubicin include acute and chronic toxicities, such as myelosuppression, hepatotoxicity, cardiotoxicity, and testicular damage (6–8). Ouyang and Li even proved that doxorubicin alone was able to inhibit the proliferation of the MG63 human osteosarcoma cell line (9). However, little is known about the cellular and molecular mechanisms of doxorubicin function in this district.
The Notch signaling pathway plays a pivotal role in a variety of biological processes, including cell proliferation and apoptosis, stem cell maintenance, and differentiation (10–12). Furthermore, deregulated Notch activity has been reported in skeletal development, tumorigenesis, and chemoresistance (13,14). Notch signaling was also reported to contribute to the carcinogenesis of osteosarcoma (15). There is increasing evidence that tumor-initiating cells are dependent on Notch signaling (16,17). So unraveling the complex circuitry of Notch signaling in both normal and malignant tissue is of great importance in cancer therapy. In this study, we assessed the effect of doxorubicin on proliferation and apoptosis of osteosarcoma cells with or without the Notch signaling pathway for exploring the mechanism of doxorubicin function.
MATERIALS AND METHODS
Cell Culture
The human osteosarcoma cell lines 143B obtained from the China Center for Type Culture Collection (CCTCC; Wuhan, China) were cultured in RPMI-1640 containing 10% (v/v) fetal bovine serum and 1% (v/v) penicillin/streptomycin (Life Technologies, Carlsbad, CA, USA). Cells were propagated in a humidified environment at 37°C with 5% CO2 and 100% humidity.
Doxorubicin Treatment
The human osteosarcoma cell lines 143B were divided into three groups according to the incubation time (24, 48, and 72 h). According to the clinical serum concentrations of the drug, each group was treated with 0.1, 0.5, 1, or 10 µM doxorubicin (First Pharmaceuticals, Hangzhou, China), and cell viability, apoptosis, and Notch pathway activity were investigated.
MTT Methods
The endogenous effects of doxorubicin on cell viability were evaluated by the methyl thiazolyl tetrazolium (MTT) method. In brief, human osteosarcoma cells were seeded in 96-well plates at a density of 1.0 × 106/ml; cell viability was then evaluated by adding 15 µl 5 mg/ml MTT (Sigma-Aldrich, St. Louis, MO, USA) solution to each well of a one-cell culture plate and further incubated for 4 h. After the medium was removed, 150 µl of dimethyl sulfoxide was added to each well, and the plate was agitated for 10 min on a shaker to dissolve the formazan. The 490-nm absorbance was determined using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Caspase 3 Activity Assay
A caspase 3 colorimetric activity assay was performed according to the manufacturer’s instructions. In brief, the reaction buffer and the specific enzyme DEVD-pNA were added to each sample and incubated for 1–2 h at 37°C. The developed colorimetric reaction was measured at 405 nm in a 96-well microplate reader (Bio-Rad model) and values plotted as arbitrary units.
Real-Time RT-PCR
Expression of caspase 3, NOTCH1, HEY1, HES1, and HES5 in osteosarcoma cells was determined at indicated times by RNA preparation and quantitative reverse transcription polymerase chain reaction (RT-PCR). Briefly, total cellular RNA was isolated from cells on six-well plates using TRIzol reagent following the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). RNA quality was assessed by agarose gel electrophoresis, and complementary DNA (cDNA) was synthesized with random hexamer (TaKaRa, Osaka, Japan). The real-time RT-PCR analysis was carried out using the QuantiTect SYBR Green RT-PCR Kit (Qiagen, Valencia, CA, USA) under the ABI Prism 7500 Sequence Detector (Applied Biosystems, Foster City, CA, USA), according to the manufacturer’s instructions. The reaction was run at one cycle of 50°C for 2 min and 95°C for 15 min, followed by 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. We used β-actin expression as an internal control. Specific primer sequences were synthesized in Biosune Biological Technology Corp (Shanghai, China), and the sequences of the primers are shown in Table 1.
Table 1.
Primers Used in This Study
| Gene | Serial Number | Primers |
|---|---|---|
| β-Actin | NM_031144.3 | Sense: 5′-GACATGCCGCCTGGAGAAAC-3′ Antisense: 5′-AGCCCAGGATGCCCTTTAGT-3′ |
| Caspase 3 | NM_004346.3 | Sense: 5′-GTAAGAGATTGCTAATCC-3′ Antisense: 5′-CCGGAGCTGCTGATTG-3′ |
| NOTCH1 | NM_017617.3 | Sense: 5′-GCGCTTGACGCAGGCTTAGG-3′ Antisense: 5′-CGGTAGGTCTGATGTGTGCTG-3′ |
| HEY1 | NM_001040708.1 | Sense: 5′-GCGGTAGCAGCTTTGCAGGA-3′ Antisense: 5′-ATTCGGCTGTGTATTATGTG-3′ |
| HES1 | NM_005524.3 | Sense: 5′-AGCCCAGGATGCCCTTTAG-3′ Antisense: 5′-AGCCCAGGATGCCCTTTAG-3′ |
| HES5 | NM_001010926.3 | Sense: 5′-AGTCCCTTCTCTAACTGTCG-3′ Antisense: 5′-AGGCTATTCAGCGAGGTTTC-3′ |
Western Blot
Western blot analysis was performed as previously reported (18). Briefly, proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to PVDF membranes. Then the membranes were blocked in 5% nonfat milk for 2 h at room temperature and incubated at 4°C overnight with antibodies Notch (1:1,000 diluted; Cell Signaling Technology, Berkeley, CA, USA). After overnight incubation, the membranes were washed and immunoblotted with HRP-conjugated anti-rabbit IgG antibody (diluted 1:1,000; Amersham Biosciences, Piscataway, NJ, USA) at 37°C for 1 h. The membranes were then developed with enhanced chemiluminescence (ECL) substrate (Beyotime, China) and exposed to X-ray film. β-Actin (monoclonal anti-β-actin, 1:1,000; Beyotime) was used to ensure adequate sample loading for all Western blots. Band density was quantitated using ImageJ software.
Plasmids and Small Interference RNA (siRNA)
Notch-specific siRNA was as follows: GATCCAGGAAGAGTGTTCCTGATTTTCAAGA (sense), GAAATCAGGAACACTCTTCCTTTTTTTGGAAA (antisense). For transient transfections, cells in the exponential growth phase were grown to 65% confluence and then transfected with 6 µg of Notch-specific siRNA construct or nontargeting siRNA using HiPerFect (Qiagen) according to the manufacturer’s protocols. After cells were cultured in medium for 48 h, the efficiency of Notch siRNA was determined by Western blot analysis. Furthermore, after cells in the exponential growth phase were grown to 65% confluence and transfected with Notch siRNA for 48 h, then 10 µM of doxorubicin was added in the medium for 24 h, and cell viability and apoptosis were investigated.
Statistical Analysis
Statistical analysis was carried out with one-way analysis of variance (ANOVA) using SPSS17.0 software. Values are expressed as means ± standard deviation (SD). The mean values and standard deviations were calculated from three independent experiments. Differences were considered statistically significant at p < 0.05.
RESULTS
Doxorubicin Inhibited Osteosarcoma Cell Growth
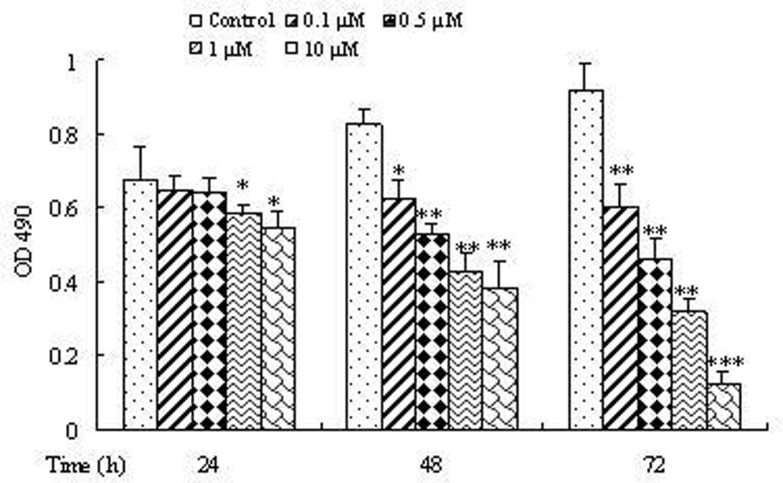
To determine the influence of doxorubicin on osteosarcoma cell growth, cell proliferation was detected by MTT colorimetry. Our data showed that, compared to the control, different concentrations of doxorubicin used in this study resulted in statistically significant cell growth inhibition (p < 0.05), and this effect showed a time- and dose-dependent manner (Fig. 1).
Figure 1.
Effect of various concentrations and treatment time of doxorubicin on the proliferation of osteosarcoma cells. 143B cells were treated with 0.1, 0.5, 1, or 10 µM doxorubicin for 24, 48, and 72 h, and the proliferation of osteosarcoma cells was measured by MTT method. Data are expressed as mean ± SD of three independent experiments in triplicate. *p < 0.05, **p < 0.01, or p > 0.05 versus control.
Doxorubicin Increased Osteosarcoma Cell Apoptosis
To determine the effect of doxorubicin on cell apoptosis, mRNA expression and activity of caspase 3, which is an apoptosis indicator, were examined in doxorubicin-treated osteosarcoma cells. It suggested that, compared with the control group, both mRNA expression (Fig. 2A) and the activity of caspase 3 (Fig. 2B) were significantly higher in doxorubicin-treated groups (p < 0.05), and the effects were both time and dose dependent. It indicated that doxorubicin increased osteosarcoma cell apoptosis.
Figure 2.
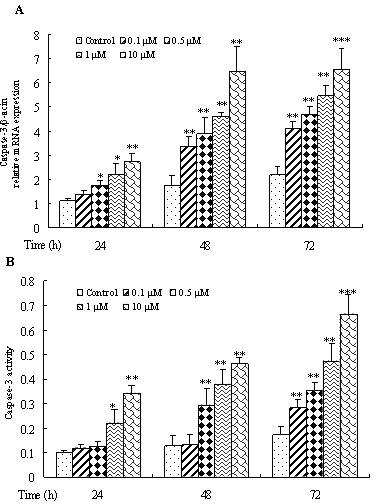
Effect of various concentrations and treatment time of doxorubicin on the apoptosis of osteosarcoma cells. 143B cells were treated with 0.1, 0.5, 1, or 10 µM doxorubicin for 24, 48, and 72 h, and mRNA expression (A) and activity (B) of caspase 3 in osteosarcoma cells were determined by RT-PCR and caspase 3 kit. Data are expressed as mean ± SD of three independent experiments in triplicate. *p < 0.05, **p < 0.01, or p > 0.05 versus control.
Activation of Notch Signaling Pathway in Doxorubicin-Treated Osteosarcoma Cells
To understand the molecular mechanism involved in doxorubicin function, the alterations in the expression of Notch target genes NOTCH1, HEY1, HES1, and HES5 in 143B cells treated with 0.1, 0.5, 1.0, and 10 µM doxorubicin for 24, 48, and 72 h were assessed using real-time RT-PCR analysis. It found that there was a marked increase in the mRNA expression of NOTCH1 (Fig. 3A), HEY1 (Fig. 3B), HES1 (Fig. 3C), and HES5 (Fig. 3D) following doxorubicin treatments (p < 0.05), and the increase was both dose and time dependent. It indicated that doxorubicin could activate the Notch signaling pathway in osteosarcoma cells.
Figure 3.
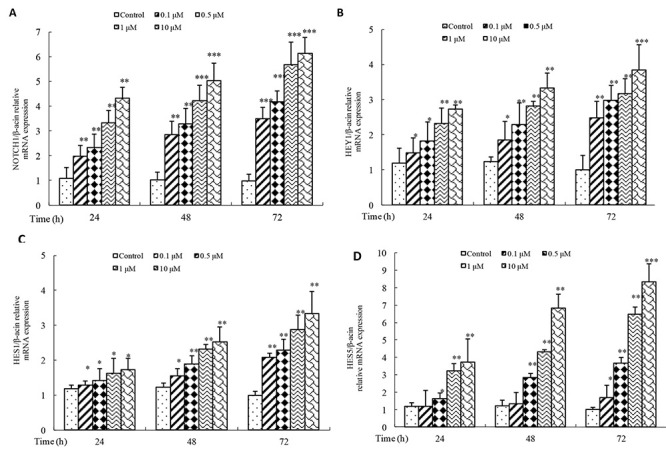
Effect of various concentrations of doxorubicin on the activation of Notch signaling pathway in osteosarcoma cells. 143B cells were treated with 0.1, 0.5, 1, or 10 µM doxorubicin for 24, 48, and 72 h, and mRNA expression level of NOTCH1 (A), HEY1 (B), HES1 (C), and HES5 (D) was detected by real-time PCR. Data are expressed as mean ± SD of three independent experiments in triplicate. *p < 0.05, **p < 0.01, or p > 0.05 versus control.
Role of the Notch Signaling Pathway in Doxorubicin-Induced Proliferation and Apoptosis of Osteosarcoma Cells
To assess whether doxorubicin exerted its influences by altering the Notch signaling pathway, cell proliferation and apoptosis were investigated following the block of the Notch signaling pathway activation by Notch inhibition using siRNA. It found that Notch protein expression was significantly downregulated by siRNA (p < 0.05), indicating the good efficiency of Notch siRNA (Fig. 4A). Furthermore, the inhibition of Notch signaling promoted osteosarcoma cell proliferation and inhibited apoptosis in control cells (p < 0.01). However, compared to the control group, doxorubicin together with Notch siRNA treatment showed little changes in cell proliferation (Fig. 4B) and apoptosis (p > 0.05) (Fig. 4C, D). Together, these results strongly indicated that Notch signaling pathway activation was required for doxorubicin to trigger the proliferation inhibition and the apoptosis increase in osteosarcoma cells.
Figure 4.
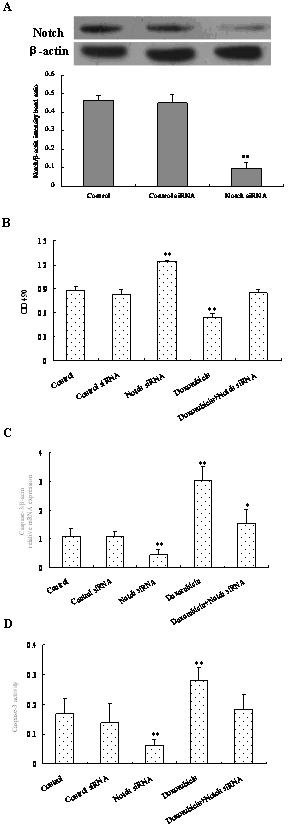
Effect of doxorubicin on cell viability and apoptosis following the block of Notch signaling pathway. Cells were seeded in six-well plates and then treated with 6 µg nontarget (negative control) or Notch siRNA in osteosarcoma cells for 48 h. Western blot was performed to determine the efficiency of the siRNA knockdown of Notch (A). Cells attaining 65% confluency were transfected with Notch siRNA for 48 h, and 10 µM of doxorubicin was added for 24 h, then cell viability was determined by the MTT method (B), and mRNA expression (C) and activity of caspase 3 (D) were measured. Data are expressed as mean ± SD of three independent experiments in triplicate. *p < 0.05, **p < 0.01, or p > 0.05 versus control.
DISCUSSION
Osteosarcoma, one of the most common malignant bone tumors in childhood and adolescence, leads the causes of disability and mortality in osteosarcoma patients (19,20). Doxorubicin is an effective chemotherapeutic drug used in the treatment of diverse types of cancer, such as osteosarcoma (14,21). Previous study suggested that doxorubicin was able to enhance the killing of tumor cells through the inhibition of cell proliferation (22). However, the mechanism through which it inhibits the proliferation of cancer cells remains unclear. In the present study, we evaluate the effect and mechanism of doxorubicin in the proliferation and apoptosis of osteosarcoma cells.
Aberrant proliferation is an essential feature of cancer cells. Previous studies have reported that doxorubicin blocked the proliferation of cancer cells through proteolytic activation of CREB3L1 (23). Doxorubicin may be used for the management of hepatocarcinoma (24). Furthermore, doxorubicin alone was able to inhibit the proliferation of the MG63 human osteosarcoma cell line (9). In this study, we also proved that different concentrations of doxorubicin lead to statistically significant cell growth inhibition in a time- and dose-dependent manner.
Apoptosis is a fundamental and complex biological process that enables an organism to kill and remove unwanted cells during animal development, normal homeostasis, and disease, and caspases are proven to be crucial for this process in diverse metazoan organisms (25). Although there are at least 14 caspases in humans, only a subset of these enzymes is detectably proteolytically activated by various distinct death stimuli in different cell types (26,27). In animals, principally caspase 3 appears to be activated in a protease cascade that leads to inappropriate activation or rapid disablement of key structural proteins and important signaling, homeostatic, and repair enzymes (28,29). Therefore, caspase 3 is regarded as a crucial component of the proteolytic cascade during apoptosis. Doxorubicin has been shown to induce the promotion of cardiac myocyte apoptosis (30). The activity and mRNA expression of caspase 3 were assessed in this study, and it suggested that doxorubicin could increase the apoptosis of osteosarcoma cells by promoting caspase 3 expression and activity.
Notch is an evolutionarily conserved transmembrane receptor involved in development and diseases, and Notch activation appears to be important in the proliferation and differentiation of stem and progenitor cells (30). Several malignancies that are resistant to therapy have been closely associated with upregulation of Notch signaling (31). Deregulated Notch activity has been reported to contribute to the carcinogenesis of osteosarcoma (32). Notch-1 showed a crucial role in osteosarcoma invasion and metastasis (33). Inhibition of the Notch pathway suppressed osteosarcoma growth both in vitro and in vivo (34–36). In this study, we provided evidence that doxorubicin could activate the Notch signaling pathway in osteosarcoma cells, indicating the involvement of Notch activation in doxorubicin functions. To further elucidate the involvement of the Notch pathway in doxorubicin function, the effects of doxorubicin on the proliferation and apoptosis of osteosarcoma cells were investigated following the block of Notch signaling. It indicated that the Notch signaling pathway was involved in the changes of the proliferation and apoptosis of osteosarcoma cells induced by doxorubicin.
In conclusion, the results demonstrated that different doses of doxorubicin could activate the Notch signaling pathway. Furthermore, doxorubicin inhibited proliferation while it promoted apoptosis of osteosarcoma cells through upregulation of the Notch signaling pathway.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81341078) and Hubei Natural Science Foundation of China (2011CHB039).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Hansen A. R.; Hughes B. G.; Paul S.; Steadman P.; Sommerville S.; Dickinson I. C.; Walpole E. T.; Thomson D. B.; Mar Fan H. G.; Joubert W. L. Single institution retrospective review of perioperative chemotherapy in adult and adolescent patients with operable osteosarcoma. Asia. Pac. J. Clin. Oncol.; 2014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2. He H.; Ni J.; Huang J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol. Lett. 7:1352–1362; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duggan S. T.; Keating G. M. Pegylated liposomal doxorubicin: A review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs 71(18):2531–2558; 2011. [DOI] [PubMed] [Google Scholar]
- 4. Vici P.; Pizzuti L.; Gamucci T.; Sergi D.; Conti F.; Zampa G.; Del Medico P.; De Vita R.; Pozzi M.; Botti C.; Di Filippo S.; Tomao F.; Sperduti I.; Di Lauro L. Non-pegylated liposomal Doxorubicin-cyclophosphamide in sequential regimens with taxanes as neoadjuvant chemotherapy in breast cancer patients. J. Cancer 5(6):398–405; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamamoto H.; Oshiro R.; Nishimura J.; Uemura M.; Haraguchi N.; Hata T.; Takemasa I.; Mizushima T.; Sekimoto M.; Doki Y.; Mori M. Low-dose dacarbazine-doxorubicin therapy against intra-abdominal desmoid tumors. Oncol. Rep. 29(5):1751–1755; 2013. [DOI] [PubMed] [Google Scholar]
- 6. Damodar G.; Smitha T.; Gopinath S.; Vijayakumar S.; Rao Y. An evaluation of hepatotoxicity in breast cancer patients receiving injection doxorubicin. Ann. Med. Health Sci. Res. 4(1):74–79; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pecoraro M.; Sorrentino R.; Franceschelli S.; Del Pizzo M.; Pinto A.; Popolo A. Doxorubicin-mediated cardiotoxicity: Role of mitochondrial connexin 43. Cardiovasc. Toxicol.; 2015. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8. Türedi S.; Yuluğ E.; Alver A.; Kutlu Ö.; Kahraman C. Effects of resveratrol on doxorubicin induced testicular damage in rats. Exp. Toxicol. Pathol. 67(3):229–235; 2015. [DOI] [PubMed] [Google Scholar]
- 9. Ouyang Z. X.; Li X. A. Inhibitory effects of tamoxifen and doxorubicin, alone and in combination, on the proliferation of the MG63 human osteosarcoma cell line. Oncol. Lett. 6(4):970–976; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ai X.; Jia Z.; Liu S.; Wang J.; Zhang X. Notch-1 regulates proliferation and differentiation of human bladder cancer cell lines by inhibiting expression of Krüppel-like factor 4. Oncol. Rep. 32(4):1459–1464; 2014. [DOI] [PubMed] [Google Scholar]
- 11. Guruharsha K. G.; Kankel M. W.; Artavanis-Tsakonas S. The Notch signaling system: Recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 13:654–666; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwon O. J.; Valdez J. M.; Zhang L.; Zhang B.; Wei X.; Su Q.; Ittmann M. M.; Creighton C. J.; Xin L. Increased Notch signaling inhibits anoikis and stimulates proliferation of prostate luminal epithelial cells. Nat. Commun. 5:4416; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lobry C.; Oh P.; Mansour M. R.; Look A. T.; Aifantis I. Notch signaling: Switching an oncogene to a tumor suppressor. Bloo. 123(16):2451–2459; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merino H.; Singla D. K. Notch-1 mediated cardiac protection following embryonic and induced pluripotent stem cell transplantation in doxorubicin-induced heart failure. PLoS One 9(7):e101024; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma Y.; Ren Y.; Han E. Q.; Li H.; Chen D.; Jacobs J. J.; Gitelis S.; O’Keefe R. J.; Konttinen Y. T.; Yin G.; Li T. F. Inhibition of the Wnt-β-catenin and Notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem. Biophys. Res. Commun. 431(2):274–279; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Groeneweg J. W.; DiGloria C. M.; Yuan J.; Richardson W. S.; Growdon W. B.; Sathyanarayanan S.; Foster R.; Rueda B. R. Inhibition of notch signaling in combination with Paclitaxel reduces platinum-resistant ovarian tumor growth. Front. Oncol. l4:171; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan B.; Liu L.; Zhao Y.; Xiu L. J.; Sun D. Z.; Liu X.; Lu Y.; Shi J.; Zhang Y. C.; Li Y. J.; Wang X. W.; Zhou Y. Q.; Feng S. H.; Lv C.; Wei P. K.; Qin Z. F. Xiaotan Sanjie decoction attenuates tumor angiogenesis by manipulating Notch-1-regulated proliferation of gastric cancer stem-like cells. World. J. Gastroenterol. 20(36):13105–13118; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang S. Q.; Dong X. Y.; Zhang W. Obestatin changes proliferation, differentiation and apoptosis of porcine preadipocytes. Ann. Endocrinol. (Paris) 75(1):1–9; 2014. [DOI] [PubMed] [Google Scholar]
- 19. Geller D. S.; Gorlick R. Osteosarcoma. Clin. Adv. Hematol. Oncol. 8:705–718; 2010. [PubMed] [Google Scholar]
- 20. Ritter J. Bielack S. S.. Osteosarcoma. Ann. Oncol. 21S:320–325; 2010. [DOI] [PubMed] [Google Scholar]
- 21. Oliveira M. S.; Melo M. B.; Carvalho J. L.; Melo I. M.; Lavor M. S. Doxorubicin cardiotoxicity and cardiac function improvement after stem cell therapy diagnosed by strain echocardiography. J. Cancer Sci. Ther. 5:52–57; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng X.; Ou Y.; Li X. A. Inhibitory effects of tamoxifen and doxorubicin, alone and in combination, on the proliferation of the MG63 human osteosarcoma cell line. Oncol. Lett. 6(4):970–976; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Denard B.; Lee C.; Ye J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. eLife 1:e00090; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garg M.; Madan J.; Pandey R. S.; Sardana S.; Katyal A.; Chandra R. Galactosylated gelatin nanovectors of doxorubicin inhibit cell proliferation and induce apoptosis in hepatocarcinoma cells. Anticancer Drugs 23(8):836–845; 2012. [DOI] [PubMed] [Google Scholar]
- 25. Jacobson M. D.; Weil M.; Raff M. C. Programmed cell death in animal development. Cell 88:347–354; 1997. [DOI] [PubMed] [Google Scholar]
- 26. Polverino A. J.; Patterson S. D. Selective activation of caspases during apoptotic induction in HL-60 cells. Effects of a tetrapeptide inhibitor. J. Biol. Chem. 272:7013–7021; 1997. [DOI] [PubMed] [Google Scholar]
- 27. Thornberry N. A.; Lazebnik Y. Caspases: Enemies within. Science 281:1312–1316; 1998. [DOI] [PubMed] [Google Scholar]
- 28. Martins L. M.; Kottke T.; Mesner P. W.; Basi G. S.; Sinha S.; Frigon N. J. Activation of multiple interleukin-1beta converting enzyme homologues in cytosol and nuclei of HL-60 cells during etoposide-induced apoptosis. J. Biol. Chem. 272:7421–7430; 1997. [DOI] [PubMed] [Google Scholar]
- 29. Nicholson D. W.; Thornberry N. A. Caspases: Killer proteases. Trends Biochem. Sci. 22:299–306; 1997. [DOI] [PubMed] [Google Scholar]
- 30. Niessen K.; Karsan A. Notch signaling in cardiac development. Circ. Res. 102:1169–1181; 2008. [DOI] [PubMed] [Google Scholar]
- 31. Meng R. D.; Shelton C. C.; Li Y. M.; Qin L. X.; Notterman D. gamma-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 69: 573–582; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McManus M. M.; Weiss K. R.; Hughes D. P. Understanding the role of notch in osteosarcoma. Adv. Exp. Med. Biol. 804:67–92; 2014. [DOI] [PubMed] [Google Scholar]
- 33. Hughes D. P. How the Notch pathway contributes to the ability of osteosarcoma cells to metastasize. Cancer Treat. Res. 152:479–496; 2009. [DOI] [PubMed] [Google Scholar]
- 34. Haddox C. L.; Han G.; Anijar L. Osteosarcoma in pediatric patients and young adults: a single institution retrospective review of presentation, therapy, and outcome. Sarcoma 2014:402509; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rampias T.; Vgenopoulou P.; Avgeris M.; Polyzos A.; Stravodimos K.; Valavanis C.; Scorilas A.; Klinakis A. A new tumor suppressor role for the Notch pathway in bladder cancer. Nat. Med. 20(10):1199–1205; 2014. [DOI] [PubMed] [Google Scholar]
- 36. Tanaka M.; Setoguchi T.; Hirotsu M. Inhibition of Notch pathway prevents osteosarcoma growth by cell cycle regulation. Br. J. Cancer 100:1957–1965; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]