Abstract
Cholangiocarcinomas are neoplasms that involve the epithelial cells of the bile duct, also known as cholangiocytes. This disease is difficult to diagnose early, as most symptoms present late in the disease. In addition, the specific anatomic position can cause periductal extension and result in a very low radical excision rate and a very poor prognosis. Improved understanding of the features underlying the onset of cholangiocarcinoma and its carcinogenic mechanism may lead to early diagnosis and better prognosis. With the development of molecular biology, much has been learned about oncogenes, tumor-suppressor genes, DNA methylation, microRNAs, and the molecular mechanisms of tumor invasion and metastasis. Based on our research and others, this review article will discuss the current status and prospects of early diagnosis of cholangiocarcinoma.
Key words: Cholangiocarcinoma, Epidemiology, Diagnosis, Oncogene, Tumor-suppressor gene, Methylation, Serum tumor markers, MicroRNAs
INTRODUCTION
In recent years, with the development of imaging diagnostics and clinical reconstruction, the incidence and mortality rate of cholangiocarcinoma (CCA) has been increasing yearly (1,2). In China, CCA is the fifth most common disease among digestive tract malignant neoplasms and the most rapidly increasing alimentary canal tumor. In the US, there are about 3,000 new patients diagnosed with CCA every year. At present, CCA is the second most common cancer of the hepatobiliary system (3–5). As CCA has no special clinical manifestation or specific tumor markers, some biliary diseases are thought to be risk factors or precursors for cholangiocarcinoma. To improve the diagnostic rate of CCA, it is imperative to understand and define its prognosis.
EPIDEMIOLOGY AND RISK FACTORS FOR CCA
The autopsy record from the Japanese Association for Pathology catalogs the cause of death from CCA to be 0.31% from 1976 to 1977, which increased to 0.58% from 1996 to 1997 (6). In China, the documentation of 680 cases of CCA in eight hospitals from the Chinese Medical Association demonstrated that the occurrence of the biliary tumor was highest in the 60- to 65-year-old age group (20.7%) with an average age onset of 55.1 years. The youngest patient to be diagnosed with CCA was 26 years old, while the oldest patient was 90 years old. The male-to-female ratio was 1.39:1, suggesting the male incidence of disease to be greater than the female incidence (7). Globally, the incidence of CCA varies, reflecting differences in both genetic and environmental risk factors. The highest incidence of CCA is found in northeast Thailand (with 80–90 cases per 100,000 people), whereas Australia reports the lowest incidence (with 0.4 cases per 100,000 people). However, the global incidence of CCA is increasing for unknown reasons (8).
Risk factors for the disease are many, including primary sclerosing (PSC) (9), intestinal infection, congenital choledochal cysts (10), Clonorchis sinensis and Opisthorchis viverrini (liver flukes), and chronic intrahepatic lithiasis. In recent years, much attention has been paid to a link between hepatitis virus infection, especially to the hepatotrophic viruses hepatitis B virus (HBV) and hepatitis C virus (HCV). Viral replication and cellular injury in the hepatitis virus infections are not only confined to the liver, but could also lead to bile duct damage and loss, as liver and bile ducts develop homologously during embryogenesis. Hilar and intrahepatic bile ducts are easily infected by HBV and HCV. From 1990 to 1997, the Japan Liver Cancer Research Class demonstrated that CCA patients tested positive for the HCV antibody; 28.3% of male CCA patients (among 982 cases) and 26.6% of female CCA patients (among 509 cases) had positive HCV antibody titers (11). Independently, our study found 6 cases (8.8%) to be positive for the HBV protein and 24 cases (35%) for the HCV-C protein, respectively, among 68 cases of proximal CCA. Both the HBV and HCV-C protein localized to the cytoplasm of cholangiocytes (12,13). Therefore, recent evidence suggests a risk factor link between hepatitis virus infection and development of CCA.
CLINICAL PRESENTATION
The leading symptom for diagnosis of CCA is patient jaundice. However, other nonspecific manifestations, such as loss of appetite, atony, and abdominal swelling, should also be considered. The clinical presentation for diagnosis of CCA by the Chinese Medical Association is shown in Table 1 (14).
Table 1.
Clinical Presentation of Cholangiocarcinoma
| Clinical Presentation | Cases | (%) |
|---|---|---|
| Jaundice | 650 | 95.6 |
| Abdominal swelling | 337 | 49.5 |
| Atony | 208 | 30.6 |
| Emaciation | 184 | 27.1 |
| Loss of appetite | 89 | 13.1 |
Diagnosis of CCA becomes evident once patients appear with jaundice, but development of jaundice occurs late in the advancement of CCA, resulting in loss of the chance for radical excision. Thus, before emerging jaundice, early diagnosis is very important for patient outcome. During the early stages of tumor progression, the depletion of bile is hindered, and the inner pressure of the biliary passage is increased. If the inner pressure of the biliary passage does not exceed the liver secreting pressure, patients do not present with jaundice, but instead present with nonspecific symptoms such as dilation of the biliary passage, abdominal swelling, atony, and loss of appetite. This is also known as “occult” jaundice (15). Combined increased understanding of these manifestations with molecular and imaging techniques may increase detection of dilation of the biliary passage and CCA at a very early stage of the cancer.
LABORATORY EXAMINATION
Bilirubin
All 680 cases of patients with CCA reporting to the Chinese Medical Association had elevated total serum bilirubin (26.3–536.4 mmol/L), with a mean of 276.4 mmol/L as compared to a healthy control. The amount of direct bilirubin was 17.7–308.4 mmol/L (mean 170.2 mmol/L), which accounted for more than half of the total bilirubin. Among the patients, 650 patients had total serum bilirubin greater than 34.2 mmol/L (a healthy control 3.2–23.5 mmol/L). Therefore, assessing an increase in total serum bilirubin may be an early marker for CCA.
Serum Enzyme
Serum γ-glutamyl transpeptidase (γ-GT) and alkaline phosphatase (AKP) are important markers for obstructive jaundice (16,17). The percentage of patient serum positive for γ-GT was 100% (680/680) and 93.7% (637/680) for AKP positivity according to the Chinese Medical Association. γ-GT and AKP are also associated with liver parenchymatous disease, although γ-GT is more sensitive to CCA than AKP (14). However, the drastic increase of both AKP and γ-GT enzymes above the amount often seen in liver parenchymatous disease suggests the possibility of γ-GT and AKP as markers of cholangiocarcinoma.
Serum Tumor Markers
Both pan-serum tumor markers CEA and CA19-9 were increase significantly in CCA patients (18). The positive rate of CEA was 16.0% (109/680) and 68.4% (465/680) for CA19-9 as compared to healthy controls according to the Chinese Medical Association (p < 0.001). The serum tumor marker CA19-9 is reported to be more specific than CEA for the diagnosis of CCA (14). Thus, testing serum levels of AKP and γ-GT together with CA19-9 may be useful for the diagnosis of the bile duct carcinoma, CCA.
IMAGING EXAMINATION
B-Ultrasonography
B-ultrasonography is the first choice for examination of an obstruction because of its duplicity, simplicity, and inexpensiveness. In CCA, B-ultrasonography can identify an obstruction of the extrahepatic bile duct. Together with the recent arrival of color Doppler ultrasonography, it is now possible to differentiate between biliary sludge and carcinoma by studying fine vessel patterns. Moreover, it can predict the depth of tumor involvement with accuracy (19). Therefore, B-ultrasonography may be a useful tool for identifying CCA.
Computed Tomography (CT)
The main purpose of CT is to diagnose the extent of tumor growth and ascertain whether there is direct infiltration into adjacent tissues or vessels or nodal or distant metastases. CT has been proven extremely useful for assessing the extent of resection necessary in patients with advanced disease (20). Knowing tumor size may aid in the decision to resect the tumor in advance stage CCA.
Magnetic Resonance Imaging (MRI) and Magnetic Resonance Cholangio-Pancreaticography (MRCP)
The morphological appearance of CCA seen in an MRI is similar to that obtained with CT. Tumors appear hypointense on T1-weighted images and hyperintense on T2-weighted images. MRI is particularly useful for visualizing invasion of the hepatoduodenal ligament, portal-vein encasement, and lymph node involvement. However, MRCP can provide more detailed information than ultrasonography or CT, and this technique has replaced the more invasive cholangiography at many hospitals (21).
Cholangiography
Direct cholangiography, such as endoscopic retrograde cholangio-pancreatography (ERCP) and percutaneous transhepatic cholangiography (PTC), is helpful in planning surgical procedures. These procedures indicate tumors in adjacent intrahepatic ducts or in the extrahepatic bile ducts, and for the investigation of advanced tumors with obstructive jaundice, can also assist in the planning of palliative management. With the development of image diagnosis, the application of invasive techniques, such as PTC and ERCP, has decreased dramatically due to complications they cause, such as cholangitis, pancreatitis, and bleeding. Nevertheless, some early stage cancers can be confirmed by ERCP, which may therefore still be useful for early diagnosis of difficult-to-diagnose tumors (7,21).
Selective Angiography
Selective angiography is very accurate and can display encasement of vessels or neovascularization, which can be used to confirm a carcinoma diagnosis. Although rarely used for diagnostic purposes, angiography is valuable for assessing the extent of tumor growth and providing information about the resectability of the mass, similar to CT and MRI (22,23).
Positron Emission Tomography (PET)
A PET scan is a noninvasive imaging modality that provides functional images by detecting uptake of the radiotracer 18F-fluorodeoxyglucose (FDG) in neoplastic cells. PET is currently considered to be a standard modality for the display of many malignancies (24). In the last decade, integrated PET and CT imaging systems (PET/CT) have allowed for the acquisition of both anatomical and functional images. PET and PET/CT have been proven useful in the diagnosis and staging of CCA. In a recent study, PET showed a 90% sensitivity and 78% specificity in cases of CCA (25).
NEW DETECTING MOLECULARMETHODS IN CCA
Flow Cytometry (FCM) Analysis of DNA Content in Cholangiocarcinoma
Abnormal DNA content is a common characteristic of several malignancy neoplasms. Detection of DNA content in tumor cells by FCM may provide a new method for diagnosis. Krishnamurthy et al. (26) examined the DNA content of shedding bile duct cells by ERCP, and found increased DNA content in diagnosed cholangiocarcinomas. Additionally, Ryan and Baldfauf (27) found an increase in sensitivity after detecting DNA content by ERCP or PTC brushing bile duct epithelial cells. These studies show that DNA content analysis could offer a new way for early diagnosis of CCA.
Fluorescence In Situ Hybridization (FISH)
FISH analysis increases the sensitivity of cytology in diagnosing CCA. FISH can detect polysomy or amplification of at least two chromosomes, tetrasomy, and trisomy 7. Of those, polysomy in the presence of a dominant stricture is considered sufficient for diagnosis of CCA, especially if the polysomy can be confirmed over time. In a recent study, patients with primary sclerosing cholangitis (PSC) who had polysomy and a level of CA19-9 greater than 129 U/ml all went on to develop cancer (28).
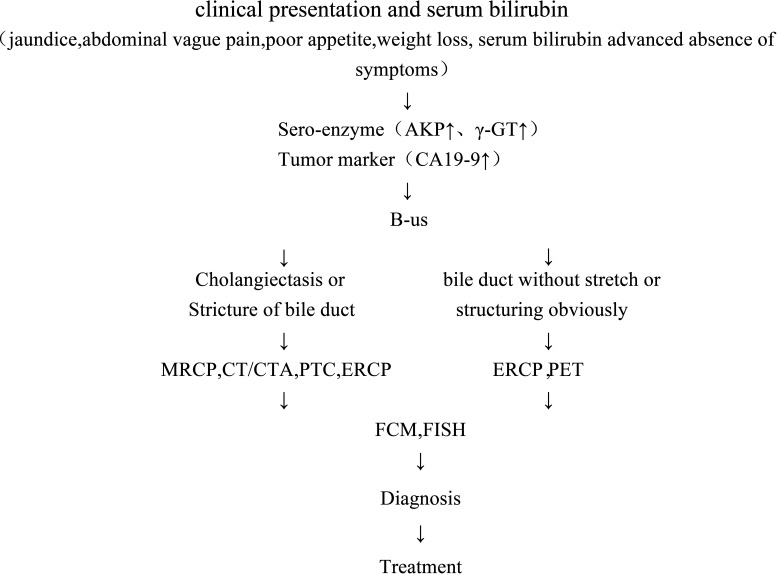
To summarize, we have created the following diagnostic system for CCA based on the patients’ clinical presentation (jaundice, vague abdominal pain, poor appetite, and weight loss) and lab results (serum bilirubin, serum enzyme, and advanced tumor marker). To begin, we chose imaging studies using B-ultrasonography. If we detect cholangiectasis or stricture of the bile duct, other imaging studies (MRCP, CT/CTA, PTC, ERCP) will be used for the definite diagnosis of CCA. If neither imaging studies can be used, then FCM or FISH should be used to preclude diagnosis of cholangiocarcinoma (Fig. 1).
Figure 1.
The diagnosis system for CCA.
RECENT DISCOVERY IN THE MECHANISM AND DIAGNOSIS OF CHOLANGIOCARCINOMA
Genetics and Epigenetics
As with most cancers, development of CCA is believed to be a multistep accumulation of genetic and epigenetic alterations in regulatory genes, leading to the activation of oncogenes and inactivation or loss of tumor suppressor genes (29).
Ras Gene
The Ras proteins are pivotal regulators of cellular proliferation, differentiation, motility, and apoptosis. Mutations of the K-ras gene in CCA have been reported by several investigators. Caldas et al. (30) detected two cases of a point mutation in codon 12 of K-ras among three cases of stool samples of CCA. In addition, Itoi et al. (31) analyzed the bile of CCA patients by PCR-RFLP, and also found the 12th codon point mutation of K-ras. These findings indicate that detecting the K-ras gene mutation could provide useful information for the early diagnosis of CCA.
c-ErbB-2 Gene
The c-ErbB-2 gene is also known as HER-2 gene. Aishina et al. (32) detected c-ErbB-2 in 80% of CCA patients with lymph node metastasis, suggesting it may be related to carcinogenesis and metastasis of CCA.
Bcl-2 Gene
Bcl-2 is often thought of as a survival or apoptosis-inhibiting gene. Ito et al. (33) found Bcl-2 protein positivity to be 31.7% in intrahepatic bile ducts, 44.7% in extrahepatic bile ducts in patients with CCA. Overexpression of the Bcl-2 gene has also been related to the invasiveness of the cholangiocarcinoma. Like c-ErbB-2, detecting Bcl-2 gene expression levels could be used as an index to measure the extent of malignancy in CCA.
p53 Gene
We examined the p53 gene status (exon 5-8) by automated sequencing of 36 randomly selected frozen samples of CCA. p53 gene mutations were found in 22 of 36 patients (61.1%) (34). These data demonstrate that mutations of the p53 gene in CCA are very common; therefore, detecting the mutations of p53 would be a useful molecular tool for early diagnosis.
DPC4/Smad Gene
The tumor-suppressor gene Smad4 (DPC4) mediates the TGF-β signaling pathway known for suppressing epithelial cell growth and is associated with several different human cancers. Hahn et al. (35) examined 32 cases of CCA tissues and found that the DPC4 gene was inactivated in 80% of the common bile duct carcinomas. In addition, Argani et al. (36) found DPC4 gene inactivation in 55% of distant CCA cases (among 88 cases) as compared to 15% DPC4 gene inactivation in hilar cholangiocarcinomas (among 28 cases). Therefore, DPC4 may demonstrate different mechanisms of carcinogenesis between superior and distant CCA.
CpG Island Methylation in Cholangiocarcinoma
Mammalian cells possess the capacity to epigenetically modify their genomes via the covalent addition of a methyl group to the five-position of the cytosine ring within the context of the CpG dinucleotide. Certain regions of the genome, which are often clustered at the five-ends of genes possess the expected CpG frequency and have been termed CpG islands. CpG island methylation has been shown to be essential for normal development, X-chromosome inactivation, imprinting and the suppression of parasitic DNA sequences. However, hypermethylation of CpG islands can cause inactivation of genes and relate to tumorigenesis and progress. We studied the methylation status of several genes in the p53–Bax mitochondrial apoptosis pathway in CCA by methylation-specific PCR. The frequency of tumor-suppressor gene methylation in CCA was P14 (24%), DAPK (30.6%), TMS1/ASC (36.1%). Gene methylation in tissues surrounding the cancer was DAPK (5.6%), TMS1/ASC (8.3%) (37,38). Our study indicates that methylation of the p53–Bax mitochondrial apoptosis pathway in CCA is a common epigenetic event and may be significant for early diagnosis.
MicroRNAs
miRNAs are small noncoding RNAs (approximately 22 nucleotides) that regulate the expression of multiple genes by binding to complementary sites of targeted mRNAs, causing translational repression (imperfect target duplexes) or degradation (perfect matches). They participate in the regulation of multiple cell types under physiological and pathological conditions and are fundamental in different cellular processes, such as development, proliferation, apoptosis, metabolism, morphogenesis, and in diseases. Several miRNAs are upregulated (miR-141, miR-200b, miR-21, let-7a, miR-370, miR-29b (39), miR-148a, miR-152, and miR-301) (40) in CCA. Mechanistic understanding suggests that these changes in miRNA expression increase CCA proliferation and survival: for example, miR-141 decreases CLOCK expression, which disinhibits cell proliferation.
Tumor Microenvironment
Carcinogenesis in CCA includes alterations in the stroma, recruitment of fibroblasts, remodeling of the extracellular matrix (ECM), changing patterns of immune cell migration, and promotion of angiogenesis. The tumor stroma surrounds the malignant ducts and glands and comprises most of the tumor mass. The stroma promotes tumor progression, via reciprocal communication between the stromal cells and cancer cells (41).
EMT
Epithelial–mesenchymal transition (EMT) is a key process in the development of many cancers, resulting in cellular rearrangements and a motile fibroblastic phenotype that is adapted to invasion. EMT has recently been demonstrated in IHCC, and mirrors that seen in other malignancies, with cells having an aggressive, dedifferentiated phenotype and increased motility. Epithelial markers E-cadherin and α- and β-catenin are downregulated in these cells, whereas markers such as N-cadherin, S100A4, and vimentin are upregulated. These changes correlate with increased invasiveness in vitro and with metastasis and poorer prognosis in vivo (42).
MMPs Gene
In addition to EMT, several other processes might promote cell motility and invasiveness in CCA. In particular, CCA is associated with increased levels of matrix metalloproteinases (MMPs), which break down the ECM to allow tumor spread. Immunohistochemical analysis found that 48% and 76% of surgically resected specimens expressed MMP-9 and MMP-7, respectively (43).
VEGF Gene
CCA requires a rich vascular bed. Although minimal information is available on angiogenesis in CCA, it is known that high levels of the vascular endothelial growth factor (VEGF) are expressed by various human CCA cell lines (KMC-1, KMC-2, KMBC, and KMG-C) and tumor tissues. Studies of xenografted cells have shown that endothelin 1 (ET-1) inhibits VEGF-A and VEGF-C expression or release, which reduces cell proliferation and increases fibrosis and apoptosis in tumor tissue. Therefore, VEGF may play a role in increasing tumor vasculature in CCA (44).
Developmental Pathways
The below signaling pathways may be required for the development of cholangiocarcinoma.
The Notch Signaling Pathway
The Notch signaling pathway regulates embryonic development and proliferation of the biliary tree (45). Two recent studies in mice have demonstrated that Notch activation is required for conversion of normal adult hepatocytes to biliary cells that are precursors of intrahepatic CCA. Overexpression of intracellular domain of the Notch 1 receptor in liver cells of mice resulted in formation of intrahepatic CCA (46).
Wnt Signaling
Wnt signaling is also required for intrahepatic bile duct development and proliferation. Wnt-inducible signaling pathway protein 1v is overexpressed in stroma nests around CCA, and levels of WISP 1v are associated with reduced survival times of patients (47).
Hedgehog Signaling
Hedgehog signaling is deregulated in many types of tumors, including CCA. Inhibition of hedgehog signaling with cyclopamine impedes CCA cell migration, proliferation, and invasion (48).
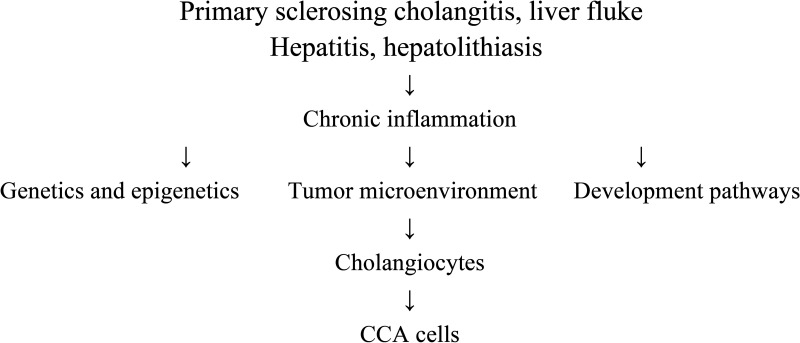
In conclusion, Figure 2 illustrates the molecular mechanisms for CCA.
Figure 2.
The overview of molecular mechanisms of CCA.
SUMMARY
The development and growth of a malignant tumor is an extremely complicated event, which must coordinate with multiple genes and multiple risk factors. Recent years have seen an expansion in basic research on CCA, and the pathways of CCA are now beginning to be elucidated. However, we are still unable to provide an early and definitive diagnosis of CCA to predict which patients with high-risk conditions will go on to develop the disease or to provide effective targeted therapy for any patient group. Thus, more work is urgently needed to identify diagnostic biomarkers and to design effective pharmacy therapies using existing and new models. With the development of modern molecular biology, we could study the pathogenesis in more detail by using molecular biological techniques to seek key components at the development of stages. At present, increased attention to clinical presentation and diagnostic procedure combined with molecular biological techniques may greatly advance the progress in early diagnosis of CCA.
ACKNOWLEDGMENTS
Written informed consent was obtained from the patient for publication of this report. Authors’ contributions: L.X.F. performed the literature review. All authors have read and approved the final version of the manuscript. The work was supported by grants from the Natural Science Foundation of Shandong Province, China (No. ZR2014HM052). Ethical approval: Not needed.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in United States. Hepatology 33:1353–1357; 2001. [DOI] [PubMed] [Google Scholar]
- 2. Khan S. A.; Thomas H. C.; Davidson B. R.; Taylor-Robinson S. D. Cholangiocarcinoma. Lancet 366:1303–1314; 2005. [DOI] [PubMed] [Google Scholar]
- 3. Taylor-Robinson S. D.; Toledano M. B.; Arora S.; Keegan T. J.; Hargreaves S.; Beck A.; Khan S. A.; Elliott P.; Thomas H. C. Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut 48:816–820; 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malhi H.; Gores G. J. Cholangiocarcinoma: Modern advances in understanding a deadly old disease. J. Hepatol. 45:856–867; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh P.; Patel T. Advances in the diagnosis, evaluation and management of cholangiocarcinoma. Curr. Opin. Gastroenterol. 22:294–299; 2006. [DOI] [PubMed] [Google Scholar]
- 6. Okuda K.; Nakanuma Y.; Miyazaki M. Cholangiocarcinoma: Recent progress. Part 1: Epidemiology and etiology. J. Gastroenterol. Hepatol. 17:1049–1055; 2002. [DOI] [PubMed] [Google Scholar]
- 7. Liu X. F.; Zhou X. T.; Zou S. Q. An analysis of 680 cases of cholangiocarcinoma from 8 hospitals. Hepatobiliary Pancreat. Dis. Int. 4:585–588; 2005. [PubMed] [Google Scholar]
- 8. Emadossadaty S. A.; Ladep N. G.; Thomas H. C.; Elliott P.; Taylor-Robinson S. D.; Toledano M. B. Rising trends in cholangiocarcinoma: Is the ICD classification system misleading us? J. Hepatol. 56:848–854; 2012. [DOI] [PubMed] [Google Scholar]
- 9. LaRusso N. F.; Shneider B. L.; Black D.; Gores G. J.; James S. P.; Doo E.; Hoofnagle J. H. Primary scleresing cholangitis: Summary of a workshop. Hepatology 44:746–764; 2006. [DOI] [PubMed] [Google Scholar]
- 10. Tyson G. L.; EI-Serag H. B. Risk factors for cholangiocarcinoma. Hepatology 54:173–184; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kobayashi M.; Ikeda K.; Saitoh S.; Suzuki F.; Tsubota A.; Suzuki Y.; Arase Y.; Murashima N.; Chayama K.; Kumada H. Incidence of primary cholangio- cellular carcinoma of the liver in Japanese patients with hepatitis C virus-related cirrhosis. Cancer 88:2471–2477; 2000. [DOI] [PubMed] [Google Scholar]
- 12. Zou S. Q.; Liu X. F.; Guo R. X.; Li C. L.; Zhou X. S.; Zhu X. G.; Huang Z. Q. The retrospective analysis of HBV and HCV infection in cholangiocarcinoma. Zhonghua Wai Ke Za Zhi 41:417–419; 2003. [PubMed] [Google Scholar]
- 13. Liu X. F.; Zou S. Q.; Qiu F. Z. Pathogenesis of cholangiocarcinoma in the porta hepatitis and infection of hepatitis virus. Hepatobiliary Pancreat. Dis. Int. 2:285–289; 2003. [PubMed] [Google Scholar]
- 14. Liu X. F.; Zhou X. T.; Xu Z.; Song Z. W.; Tian Y. L.; Zou S. Q. Diagnosis of extrahepatic bile duct carcinoma Chinese J. Pract. Surg. 27:224–225; 2007. [Google Scholar]
- 15. Cueni-Heer E.; Cueni B.; Schmid M. Early diagnosis of hepatic duct carcinoma. Schweiz. Med. Wochenschr. 107:534–537; 1977. [PubMed] [Google Scholar]
- 16. Bhudhisawasdi V.; Muisuk K.; Areejitranusorn P.; Kularbkaew C.; Khampitak T.; Saeseow O. T.; Wongkham S. Clinical value of biliary alkaline phosphatase in non-jaundiced cholangiocarcinoma. J. Cancer Res. Clin. Oncol. 130:87–92; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haratake J.; Kasai T.; Makino H. Diffuse mucosal carcinoma of intrahepatic and extrahepatic bile ducts including gallbladder. Pathol. Int. 52:784–788; 2002. [DOI] [PubMed] [Google Scholar]
- 18. Qin X. L.; Wang Z. R.; Shi J. S.; Lu M.; Wang L.; He Q. R. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: In comparison with CEA. World J. Gastroenterol. 10:427–432; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bloom C. M.; Langer B.; Wilson S. R. Role of US in the detection, characterization, and staging of cholangiocarcinoma. Radiographics 19:1199–1218; 1999. [DOI] [PubMed] [Google Scholar]
- 20. Valls C. Obstructive jaundice: Diagnostic and therapeutic management. J. Radiol. 87:460–478; 2006. [DOI] [PubMed] [Google Scholar]
- 21. Stroszczynski C.; Hunerbein M. Malignant biliary obstruction: Value of imaging findings. Abdom. Imaging 30:314–323; 2005. [DOI] [PubMed] [Google Scholar]
- 22. Yalcin S. Diagnosis and management of cholangiocarcinomas: A comprehensive review. Hepatogastroenterology 51:43–50; 2004. [PubMed] [Google Scholar]
- 23. Ortner M. A. Klatskin tumors—Diagnostic and interventional therapy. Schweiz. Rundsch. Med. Prax. 95:1637–1642; 2006. [DOI] [PubMed] [Google Scholar]
- 24. Iglehart J. K. The new era of medical imaging—Progress and pitfalls. N. Engl. J. Med. 354:2822–2828; 2006. [DOI] [PubMed] [Google Scholar]
- 25. Wakabayashi H.; Akamoto S.; Yachida S.; Okano K.; Izuishi K.; Nishiyama Y.; Maeta H. Significance of fluorodeoxyglucose PET imaging in the diagnosis of malignancies in patients with biliary stricture. Eur. J. Surg. Oncol. 31:1175–1179; 2005. [DOI] [PubMed] [Google Scholar]
- 26. Krishnamurthy S.; Katz R. L.; Shumate A.; Strohlein K.; Khanna A.; Tucker S. L.; Raijman I.; Lahoti S. DNA image analysis combined with routine cytology improves diagnostic sensitivity of common bile duct brushing. Cancer 93:229–235; 2001. [DOI] [PubMed] [Google Scholar]
- 27. Ryan M. E.; Baldauf M. C. Comparison of flow cytometry for DNA content and brush cytology for detection of malignancy in pancreaticobiliary strictures. Gastrointest. Endosc. 40:133–139; 1994. [DOI] [PubMed] [Google Scholar]
- 28. Barr Fritcher E. G.; Kipp B. R.; Voss J. S.; Clayton A. C.; Linder K. D.; Halling K. C.; Gores G. J. Primary sclerosing cholangitis patients with serial polysomy fluorescence in situ hybridization results are at increased risk of cholangiocarcinoma. Am. J. Gastroenterol. 106:2023–2028; 2011. [DOI] [PubMed] [Google Scholar]
- 29. Kosei M.; Shoji N.; Sonshin T. Molecular mechanism of cholangiocarcinoma carcinogenesis. J Hepatobiliary Pancreat. Sci. 21:754–760; 2014. [DOI] [PubMed] [Google Scholar]
- 30. Caldas C.; Hahn S. A.; Hruban R. H.; Redston M. S.; Yeo C. J.; Kern S. E. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res. 54:3568–3573; 1994. [PubMed] [Google Scholar]
- 31. Itoi T.; Takei K.; Shinohara Y.; Takeda K.; Nakamura K.; Horib T.; Sanada A.; Ohno H.; Matsubayashi H.; Saito T.; Watanabe H. K-ras codon 12 and P53 mutations in biopsy specimens and bile from biliary tract cancers. Pathol. Int. 49:30–37; 1999. [DOI] [PubMed] [Google Scholar]
- 32. Aishima S. I.; Taguchi K. I.; Sugimachi K.; Shimada M.; Sugimachi K.; Tsuneyoshi M. c-erbB-2 and c-Met expression relates to cholangiocarcinogenesis and progression of intrahepatic cholangiocarcinoma. Histopathology 40:269–278; 2002. [DOI] [PubMed] [Google Scholar]
- 33. Ito Y.; Takeda T.; Sasaki Y.; Sakon M.; Monden M.; Yamada T.; Ishiguro S.; Imaoka S.; Tsujimoto M.; Matsuura N. Bcl-2 expression in cholangiocellular carcinoma is inversely correlated with biologically aggressive phenotypes. Oncology 59:63–67; 2000. [DOI] [PubMed] [Google Scholar]
- 34. Liu X. F.; Zhang H.; Zhu S. G.; Zhou X. T.; Su H. L.; Xu Z.; Li S. J. Correlation of p53 gene mutation and expression of P53 protein in cholangiocarcinoma. World J. Gastroenterol. 12:4706–4709; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hahn S. A.; Bartsch D.; Schroers A.; Galehdari H.; Becker M.; Ramaswamy A.; Schwarte-Waldhoff I.; Maschek H.; Schmiegel W. Mutations of the DPC4 Smad4 gene in biliary tract carcinoma. Cancer Res. 58:1124–1126; 1998. [PubMed] [Google Scholar]
- 36. Argani P.; Shaukat A.; Kaushal M.; Wilentz R. E.; Su G. H.; Sohn T. A.; Yeo C. J.; Cameron J. L.; Kern S. E.; Hruban R. H. Differing rates of loss of DPC4 expression and of p53 overexpression among carcinomas of the proximal and distal bile ducts. Cancer 91:1332–1341; 2001. [PubMed] [Google Scholar]
- 37. Liu X. F.; Jiang H.; Zhang C. S; Yu S. P.; Wang Z. Q.; Su H. L. Targeted drug regulation on methylation of p53-Bax mitochondrial apoptosis pathway effects the growth of cholangiocarcinoma cells. J. Int. Med. Res. 40:67–75; 2012. [DOI] [PubMed] [Google Scholar]
- 38. Liu X. F.; Tang K.; Yu S. P.; Wang Z. Q.; Su H. L. Correlation between promoter of methylation p14, TMS1/ASC, and DAPK, and p53 mutation with prognosis in cholangiocarcinoma. World J. Surg. Oncol. 10:5–15; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meng F.; Wehbe-Janek H.; Henson R.; Smith H.; Patel T. Epigenetic regulat- ion of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene 27:378–386; 2008. [DOI] [PubMed] [Google Scholar]
- 40. Braconi C.; Huang N.; Patel T. MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology 51:881–890; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siria A. E. The role of cancer-associated hyofibroblasts in intrahepatic cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 9:44–54; 2012. [DOI] [PubMed] [Google Scholar]
- 42. Yao X.; Wang X.; Wang Z.; Dai L.; Zhang G.; Yan Q.; Zhou W. Clinicopathological and prognostic significance of epithelial mesenchymal transition-related protein expression in intrahepatic cholangiocarcinoma. Onco. Targets Ther. 5:255–261; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Itatsu K.; Sasaki M.; Yamaguchi J.; Ohira S.; Ishikawa A.; Ikeda H.; Sato Y.; Harada K.; Zen Y.; Sato H. Cyclooxygenase-2 is involved in the up-regulation of matrix metalloproteinase-9 in cholangiocarcinoma induced by tumor necrosis factor-α. Am. J. Pathol. 174:829–841; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fava G.; Demorrow S.; Gaudio E.; Franchitto A.; Onori P.; Carpino G.; Glaser S.; Francis H.; Coufal M.; Marucci L. Endothelin inhibits cholangiocarcinoma growth by a decrease in the vascular endothelial growth factor expression. Liver Int. 29:1031–1042; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hofmann J. J.; Zovein A. C.; Koh H.; Radtke F.; Weinmaster G.; Iruela-Arispe M. L. Jaggedl in the portal vein mesenchyme regulates intrahepatic bile duct development: Insights into Alagille syndrome. Development 137:4061–4072; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sekiya S.; Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J. Clin. Invest. 122:3914–3918; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sirica A. E.; Nathanson M. H.; Gores G. J.; Larusso N. F. Pathobiology of biliary epithelia and cholangiocarcinoma: Proceedings of the Henry M. and Lillian Stratton Basic Research Single-Topic Conference. Hepatology 48:2040–2046; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. El Khatib M.; Kalnytska A.; Palagani V.; Kossatz U.; Manns M. P.; Malek N. P.; Wilkens L.; Plentz R. R. Inhibition of hedgehog signaling attenuates carcinogenesis in vitro and increases necrosis of cholangiocellular carcinoma. Hepatology 57:1035–1045; 2013. [DOI] [PubMed] [Google Scholar]