Abstract
For non-small cell lung cancer (NSCLC) patients at stage IB, adjuvant chemotherapy does not improve survival. Evidence suggests that dendritic cell (DC)-activated cytokine-induced killer (DC-CIK) cell therapy in addition to chemotherapy improves survival for stage I–IIIA NSCLC patients after surgery, but there are not enough data to confirm this benefit specifically for those at stage IB. Herein, we retrospectively evaluated the efficacy and safety of this therapy administered to stage IB NSCLC patients. Sixty-six patients were treated with four-cycle adjuvant chemotherapy initiated 3 weeks after surgical resection. In addition, 28 of these patients underwent DC-CIK therapy on a trimonthly basis (average 3.1 times, range 1–6) beginning 1 month after chemotherapy. The disease-free survival (DFS) rates of the two groups were statistically similar, although patients who received DC-CIK therapy showed slightly higher 1- and 2-year DFS rates (100.0% and 96.4%, respectively, compared with 81.6% and 76.3%). More importantly, patients in the DC-CIK therapy group had significantly longer overall survival (p = 0.018). For patients who received treatment after recurrence, the DC-CIK therapy group had longer progression-free survival compared with the chemotherapy-only group. In addition, patients given DC-CIK therapy experienced less fatigue and appetite loss. The rate of adverse side effects was similar between the two groups. In conclusion, for these stage IB NSCLC patients, DC-CIK therapy significantly improved 2-year DFS rates compared with those who received chemotherapy only. DC-CIK therapy also benefited patients’ quality of life, and adverse events were acceptable.
Key words: Chemotherapy, Cytokine-induced killer (CIK) cells, Natural killer cells, Non-small cell lung cancer (NSCLC)
INTRODUCTION
Lung cancer is one of the most common tumors in the world and a primary cause of death. In China in 2008, there were 2.8 million reported lung cancer patients and almost two million deaths caused by the disease (1). Surgery remains the standard therapeutic approach for lung cancer, but tumor recurrence is the major challenge: for patients who received systematic adjuvant chemotherapy and radiotherapy, the recurrence rate ranged from 15% for stage IA to 60% for stage IIIA (2). Non-small cell lung cancer (NSCLC) patients at phase I have an overall better prognosis, but 30% of patients experience recurrence eventually.
Cytokine-induced killer (CIK) cells are immune cells that can seek out and kill tumor cells (3). After their discovery in 1991, further in-depth research was conducted at the Stanford University Medical Center. Subsequently, therapy using CIK cells has been extensively used in multiple types of cancer (4).
The ability of CIK cells to kill tumor cells depends on the presence of dendritic cells (DCs), which recognize antigens and trigger an immune response with the activation of both DC-mediated MHC (major histocompatibility complex)-restricted and MHC-unrestricted cytotoxicity (5). In addition, cluster of differentiation 40 (CD40) can stimulate the proliferation and maturation of CIK cells after secretion of multiple cytokines (6). DC-CIK cell therapies combined with chemotherapy have been reported beneficial in leukemia, renal tumor, liver cancer, and gastric cancer (6–11).
Multiple studies have shown the efficacy of CIK cell therapy in lung cancer patients. For instance, a study based on 59 cases of advanced lung cancer showed that CD-CIK combined with chemotherapy prolonged progression-free survival (PFS) and overall survival (12,13), while another study found that such treatment resulted in superior PFS (6.9 months) compared to chemotherapy alone (5.2 months) but did not alter survival rates at 1, 2, and 5 years (14). It was also reported that DC-CIK as maintenance treatment after chemotherapy prolonged PFS from 2.56 months to 3.20 months (15).
As for use as adjuvant therapy after surgery in NSCLC patients, a study with 84 NSCLC subjects that compared chemotherapy alone with DC-CIK + chemotherapy found that the latter was associated with a marked increase in 2-year survival (78.8% and 94.7%, respectively, p < 0.05) (16). However, more than 50% of the patients enrolled in that study were at phases II–III, and the analysis did not address whether phase I NSCLC patients, with a lower risk of relapse, could benefit from treatment of CIK + chemotherapy.
Adjuvant chemotherapy in stage IB NSCLC remains controversial. It is generally believed that patients with low tumor burden may benefit from CIK therapy. Given that adjuvant chemotherapy does not improve survival of stage IB NSCLC patients (12), we investigated whether sequential immunotherapy after adjuvant chemotherapy could improve survival of this lung cancer subgroup.
Recurrence after radical resection of lung cancer could be due to incomplete resection, dissemination during surgery, angiogenic growth factors secreted by the tumor, and other reasons. It can also be associated with postoperative immunosuppression. A previous study showed that CIK therapy could markedly improve antitumor immunity in tumor patients (2). Thus, we compared the efficacy and safety of postoperative sequential CIK therapy after adjuvant chemotherapy with that of adjuvant chemotherapy only in postoperative NSCLC patients.
MATERIALS AND METHODS
Patient Selection
The Institutional Review Board of our hospital approved the study, which was conducted in accordance with the ethics principles of the Declaration of Helsinki and consistent with good clinical practices and applicable laws and regulations. All patients provided written informed consent prior to enrollment.
All the participants in this retrospective study were NSCLC patients at stage IB, with an Eastern Cooperative Oncology Group (ECOG) performance score of less than 1 (i.e., all were fully active) and were at least 18 years of age (Table 1). These patients had undergone wedge resection, and as inclusion criteria for the study, their tumors were poorly differentiated (including neuroendocrine carcinoma), >4 cm, with vascular invasion and visceral pleural involvement, a cutting edge <1 cm, and insufficient clearance of lymph nodes. The patients had functional bone marrow, liver, and kidneys and therefore were considered able to tolerate chemotherapy. There were no limitations placed on adjuvant chemotherapy regimens, which included gemcitabine plus platinum, taxane plus platinum, vinorelbine plus platinum, and pemetrexed combined with platinum. Adjuvant radiotherapy was also allowed.
Table 1.
Patient Demographics and Clinicopathology
| DC-CIK Therapy | Chemotherapy | p | |||
|---|---|---|---|---|---|
| Gender | 0.013 | ||||
| Men | 13 | 46.4% | 29 | 76.3% | |
| Women | 15 | 53.6% | 9 | 23.7% | |
| Age (years) | 0.051 | ||||
| ≤65 | 20 | 71.4% | 18 | 47.4% | |
| >65 | 8 | 28.6% | 20 | 52.6% | |
| Type | 0.607 | ||||
| Squamous cell carcinoma | 6 | 21.4% | 14 | 36.8% | |
| Adenocarcinoma | 17 | 60.7% | 19 | 50.0% | |
| Large cell carcinoma | 2 | 7.1% | 2 | 5.3% | |
| Other | 3 | 10.7% | 3 | 7.9% | |
| Size | 0.879 | ||||
| <4 cm | 16 | 57.1% | 21 | 55.3% | |
| ≥4 cm | 12 | 42.9% | 17 | 44.7% | |
| Involvement of visceral pleura | 0.12 | ||||
| No | 22 | 78.6% | 23 | 60.5% | |
| Yes | 6 | 21.4% | 15 | 39.5% | |
| Degree of differentiation | 0.018 | ||||
| High | 5 | 17.9% | 1 | 2.6% | |
| Medium | 12 | 42.9% | 26 | 68.4% | |
| Low | 11 | 39.3% | 8 | 21.1% | |
| No data | 0 | 0.0% | 3 | 7.9% | |
| Cutting edge | 0.504 | ||||
| ≥1cm | 28 | 100.0% | 36 | 94.7% | |
| <1cm | 0 | 0.0% | 2 | 5.3% | |
| Vessel involvement | 0.256 | ||||
| No | 28 | 100.0% | 35 | 92.1% | |
| Yes | 0 | 0.0% | 3 | 7.9% | |
| Cleared lymph nodes | 0.159 | ||||
| ≤6 | 12 | 42.9% | 10 | 26.3% | |
| >6 | 16 | 57.1% | 28 | 73.7% | |
| Chemotherapy regimen | 0.998 | ||||
| Gemcitabine + carboplatin/cisplatin | 5 | 17.9% | 7 | 18.4% | |
| Paclitaxel + carboplatin/cisplatin | 4 | 14.3% | 5 | 13.2% | |
| Vinorelbine + carboplatin/cisplatin | 7 | 25% | 9 | 23.7% | |
| Pemetrexed + carboplatin/cisplatin | 12 | 42.9% | 17 | 44.7% | |
Exclusion criteria were clinical stage beyond stage IB, had received neoadjuvant chemotherapy, were pregnant or lactating, or complicated with a second primary tumor. Also excluded were patients who were receiving ongoing immunosuppressive therapy at the time of resection or had received immunotherapy previously, patients who had undergone partial or whole lung surgery, and those with immune dysfunction or allergy to protein and cell products.
Treatment
Each patient received either adjuvant chemotherapy only or adjuvant chemotherapy and dendritic cell (DC) cytokine-induced killer (CIK) cell therapy based on the patients’ own informed decision, and these comprised the treatment groups of the study. Four cycles of adjuvant chemotherapy were given starting within 3 weeks after radical resection. Dosages were based on National Comprehensive Cancer Network (NCCN) guidelines (17).
Patients in the DC-CIK therapy group received CIK therapy 1 month after adjuvant chemotherapy for at most 1 year on a trimonthly basis. Patients were monitored by follow-up observation. T-cell subsets [CD3+, CD3+CD4+, CD3+CD8+, natural killer (NK), and CD3+CD56+] were monitored before, 1 day after four cycles, and 3 months after chemotherapy. Quality of life was evaluated before and after therapy based on the results of the European Organization for Research and Treatment of Cancer (EORTC) core QLQ-C30 questionnaire.
Dendritic Cell-CIK (DC-CIK) Culture
One hundred and eight peripheral blood mononuclear cells were obtained from patients using a Cobe Spectra Apheresis System (Lakewood, CO, USA). Peripheral blood mononuclear cells were separated via Ficoll separating medium and were maintained at 37°C in a 5% CO2 humidified atmosphere for 3 h to allow attachment of DCs. AIM-V liquid tissue culture medium (GIBCO Invitrogen) supplemented with 1,000 U/ml granulocyte macrophage colony-stimulating factor (GM-CSF; Miltenyi Biotec, Germany) and 1,000 U/ml interleukin (IL)-4 (Miltenyi Biotec, Germany) was added to the adherent cells, and the medium was changed every 2 days. Tumor necrosis factor-α (TNF-α; MiltenyiBiotec, Germany), phytohemagglutinin (PHA), and IL-1β (e-Bioscience, USA) were added on the sixth day to the CIK cells, which were cocultured for 6–8 days upon maturation, verified under the microscope. After 10–14 days’ culture, the cells were collected and washed twice using normal saline. The cells were then suspended in 100 ml normal saline containing 20 g/L human serum albumin and intravenously infused to the patients.
Evaluation of Adverse Events
Adverse events were evaluated using the National Cancer Institute common toxicity criteria (NCI CTC, version 3.0) (18).
Statistical Method
All the data were processed using SPSS 17.0 software. Quantitative data were analyzed using ANOVA, and the chi-squared test was used for categorical data. The t test was used to compare differences between the two groups; before-and-after comparisons were performed using the paired t test. Survival analysis was performed with the Kaplan–Meier method. A value of p < 0.05 was considered statistically significant.
RESULTS
Demographic Data and Clinical Pathological Characteristics
Sixty-six NSCLC patients at stage IB (42 men and 24 women) who received radical resection between March 2009 and March 2011 were enrolled in this study (Table 1). All cases were pathologically confirmed. The ages of these patients ranged from 35 to 82 years, with a median age of 64 years. Cancers comprised 20 squamous cell carcinomas, 36 adenocarcinomas, 4 large cell carcinomas, and 6 other pathological types, including neuroendocrine carcinoma.
Among the 66 patients, 29 (adenocarcinoma) received pemetrexed combined with platinum regimens, 16 received vinorelbine plus platinum, 12 received gemcitabine plus platinum, and 9 received taxane plus platinum, in accordance with the doctors’ recommendation and patients’ choice. Thirty-eight patients underwent chemotherapy only (the chemotherapy group), and 28 received chemotherapy and DC-CIK therapy (the DC-CIK therapy or CIK + chemotherapy group). Most of the patients in the chemotherapy plus DC-CIK group (24 patients) received DC-CIK therapy for more than 6 months, while 4 patients discontinued DC-CIK therapy for financial or personal reasons. Patients in the DC-CIK therapy group were treated with DC-CIK for 3 to 12 months (average, 9.3 months).
The date of the last follow-up visit was April 1, 2013, and the average follow-up time was 36.8 months (range, 4.1–77.5 months).
Disease-Free Survival (DFS) and 2-Year DFS
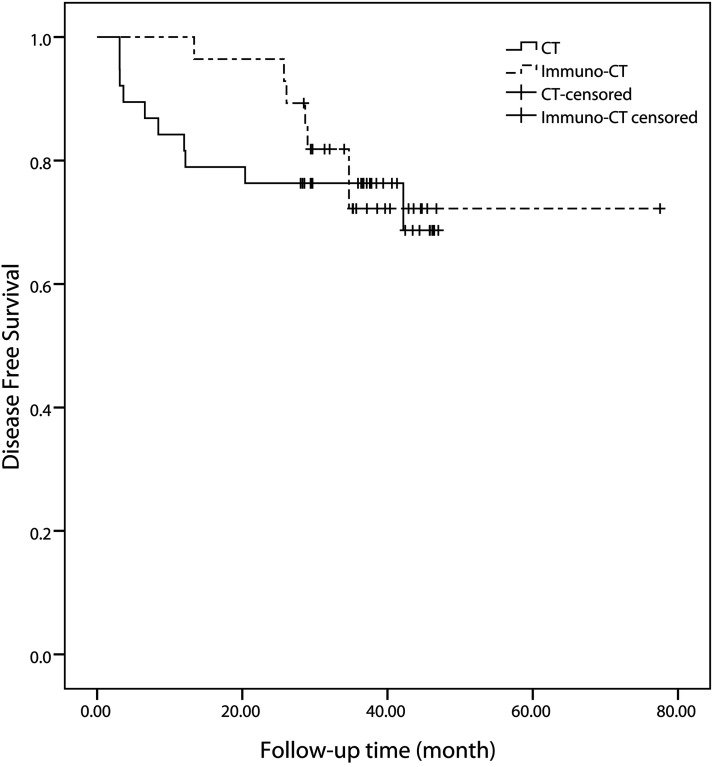
At the last follow-up date, disease progression was found in seven patients of the DC-CIK therapy group (25.0%) and 10 patients in the chemotherapy group (26.3%), a difference that was not significant based on the log-rank test (p = 0.715). However, the mean 1- and 2-year DFS rates of the DC-CIK therapy group were significantly better than those of the chemotherapy group (p = 0.018 and 0.036, respectively): The 1- and 2-year DFS rates of the chemotherapy group were 81.6% and 76.3%, while those of the DC-CIK therapy group were 100.0% and 96.4% (Fig. 1). In the DC-CIK group, the 1-year DFS rates of those who received DC-CIK for more than 6 months or less than 6 months were both 100%. The 2-year DFS of those who received DC-CIK for more than 6 months or less than 6 months were 100% and 75.0%, respectively (p = 0.299).
Figure 1.
Kaplan–Meier survival plots of the DFS rates of the DC-CIK group and chemotherapy (CT) group.
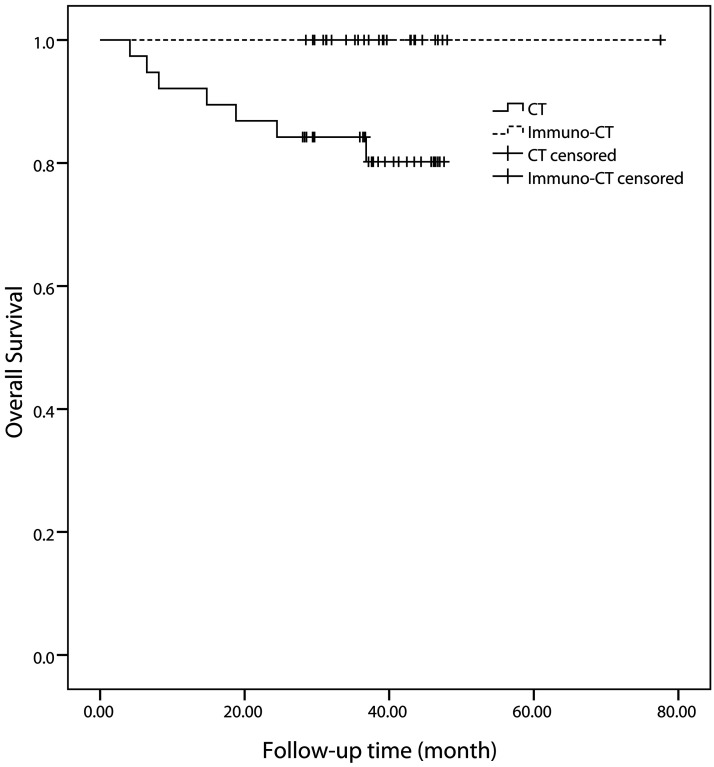
At the last follow-up date, no deaths had occurred in the DC-CIK therapy group, but seven (18.4%) had occurred in the chemotherapy group. This was a significant difference, according to log rank analysis (p = 0.018) (Fig. 2). One-year survival rates for the DC-CIK therapy and chemotherapy groups were 100% and 92.1%, respectively (p = 0.256), and the 2-year survival rates were 100% and 86.8% (p = 0.07).
Figure 2.
Kaplan–Meier survival plots of the overall survival rates of the DC-CIK group and chemotherapy (CT) group.
PFS of the Patients With Recurrence After Retreatment
Of the entire study group, 17 patients experienced recurrence, including 7 patients in the DC-CIK therapy group and 10 patients in the chemotherapy group. All 7 patients with recurrence in the DC-CIK therapy group received retreatment with chemotherapy, radiotherapy, or target therapy. One of these 7 patients suffered further disease progression, but her PFS after the above retreatment reached 381 days. As of the last follow-up date, PFS of the DC-CIK therapy group was >143 days.
Recurrence was found in 10 patients of the chemotherapy group, and 9 patients received retreatment. All 9 of these patients had disease progression. The median progression-free time was 101 days. Log rank analysis showed a significant difference between the two groups in terms of PFS (p = 0.026).
Change of T-Cell Subsets
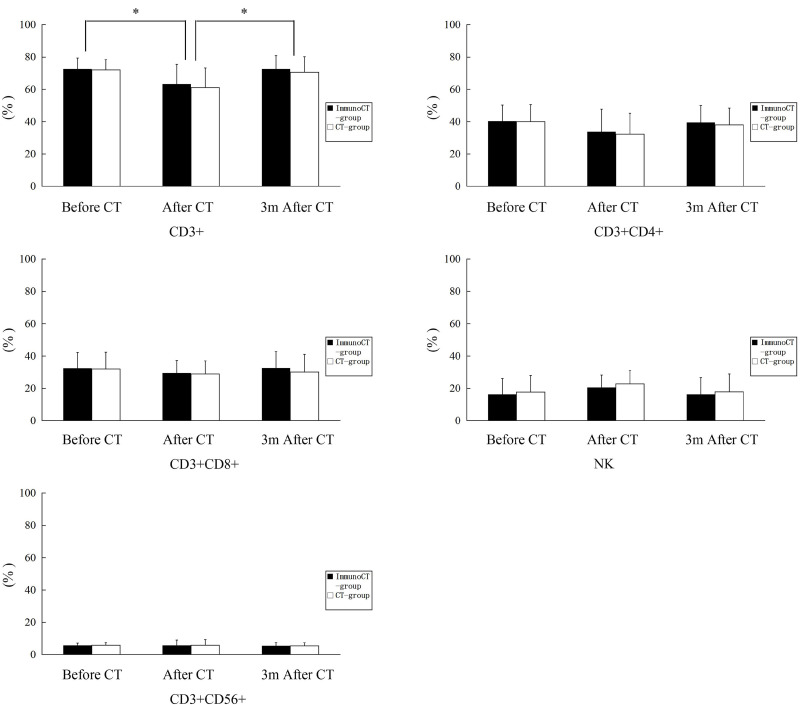
In both treatment groups, the percentage of CD3+ T-cells was markedly reduced after chemotherapy and was restored to normal levels 3 months after chemotherapy. There was no difference between the groups in terms of subsets of T-cells at any time point (Fig. 3).
Figure 3.
T-cell subsets: (A) CD3+, (B) CD3+CD4+, (C) CD3+CD8+, (D) NK, and (E) CD3+CD56+ were monitored before, 1 day after chemotherapy, and 3 months after chemotherapy using flow cytometry.
Quality of Life
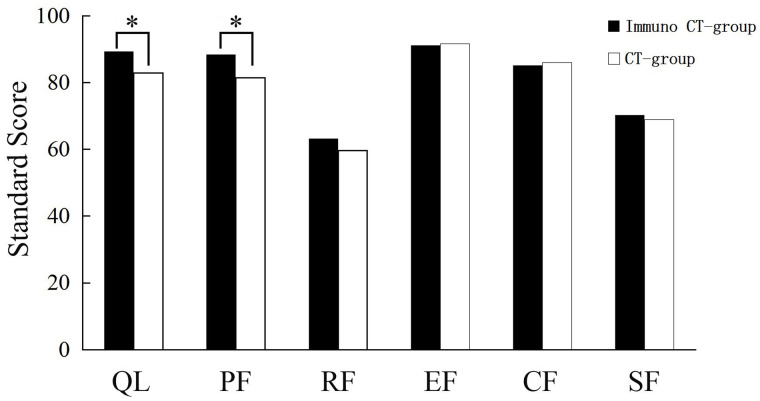
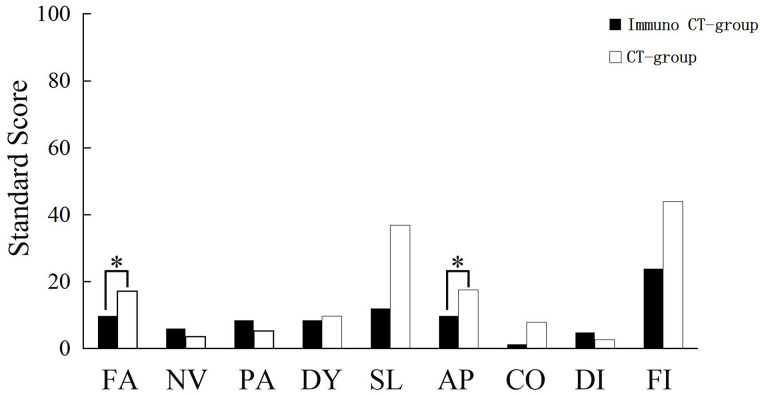
A quality of life survey was conducted using the Chinese version of QLQ-C30. The raw scores were calculated and converted to standard scores, with a range of 0–100 in accordance with the user guideline (19). The results revealed a significant improvement in the overall health status of patients in the DC-CIK group relative to that of the chemotherapy group after treatment (p = 0.023). There was also a significant improvement in physical function (p = 0.000) but not in emotional, cognitive, social, or role functioning (Fig. 4). A comparison of the symptom scale results showed that patients of the DC-CIK therapy group were superior to the chemotherapy group with regard to fatigue and appetite loss (p = 0.000 and p = 0.009, respectively) (Fig. 5.).
Figure 4.
Evaluation of the overall health status and quality of life between the DC-CIK group and chemotherapy group based on the QLQ-C30 questionnaire. *Statistical significance (p < 0.05). CT, chemotherapy.
Figure 5.
Evaluation of the quality of life (symptoms) between the DC-CIK group and chemotherapy group based on the QLQ-C30 questionnaire. *Statistical significance (p < 0.05). CT, chemotherapy.
Adverse Events
Four patients had temporary fever and chills during immunotherapy, which were relieved 12 h after treatment. Comparisons of the incidence of myelosuppression, liver toxicity, and renal toxicity revealed no significant differences between the two groups. There was also no significant difference between the two groups with regard to vomiting or peripheral nerve toxicity (Table 2).
Table 2.
Adverse Event Rates
| DC-CIK + Chemotherapy [n (%)] |
Chemotherapy Only [n (%)] |
|
|---|---|---|
| Bone marrow suppression | ||
| I–II degrees | 13 (65) | 25 (65.8) |
| III–IV degrees | 2 (10) | 4 (10.2) |
| Nausea | 7 (35) | 13 (34.2) |
| Peripheral nerve toxicity | 3 (15) | 6 (15.8) |
| Liver dysfunction | 3 (15) | 5 (13.2) |
| Kidney dysfunction | 3 (15) | 4 (10.2) |
| Fever | 4 (20) | 0 (0) |
DISCUSSION
Our present study specifically investigated the efficacy of DC-CIK therapy + chemotherapy in phase I NSCLC patients. Follow-up over 2.9 years revealed that, compared with chemotherapy alone, 1-year DFS increased from 81.6% to 100.0% (p = 0.018) and 2-year DFS increased from 76.3% to 96.4% (p = 0.036), indicating that DC-CIK + chemotherapy may reduce the occurrence of relapse. Patients in the DC-CIK therapy group experienced fewer adverse side effects, with pyrexia being the most common. This could be due to an improved immune response in these patients. We also found that DC-CIK combined with chemotherapy was superior to chemotherapy alone with regard to life quality and physical functioning. DC-CIK therapy was also associated with less fatigue and better appetite compared with chemotherapy only.
Beneficial effects have been demonstrated in CIK cell-treated advanced cancers, such as liver cancer, gastric cancer, lung cancer, and diffuse large B-cell lymphoma (13,18–20). Previous studies suggest that CIK therapy as adjuvant therapy is particularly beneficial to patients with low tumor burden at advanced stages, such as after resection (4). It is believed that the tumor burden of lung cancer patients is low after surgery, which makes tumor cells more vulnerable to immune cells. Thus, DC-CIK as adjuvant therapy is reasonable to perform after surgery, since it may help to locate and eliminate micrometastatic sites (20). Although, in general, stage IB NSCLC patients have a good prognosis, it is very difficult to improve their survival rate further. The results of the present study for the first time show that DC-CIK therapy improved both DFS and overall survival. This may be because the low tumor burden of stage IB NSCLC patients facilitated the immunoactivity of DC-CIK cells at micrometastatic sites.
We found that for patients treated with CIK + chemotherapy, the disease progression rate was significantly lower than for patients treated with chemotherapy alone, even after recurrence. Among seven patients whose disease recurred and were retreated, only one had disease progression, and PFS was 381 days. However, for patients who received only chemotherapy, nine among nine with recurrence had disease progression. A previous study suggested that CIK cells have a long half-life in vivo (21), which may explain the long-lasting effects of CIK cells, so that even if the disease progresses, the remaining active CIK cells can eliminate tumor cells and slow down the progression of the disease.
Chemotherapy agents such as fluorouracil can cause immunosuppression (22). However, DC-CIK cells may reverse these suppressive effects. Chemotherapy normally reduces the number of CD3+ T-cells and the activity of natural killer cells. DC-CIK may release inflammatory cytokines such as IL-2 and interferon-γ (IFN-γ), which not only kill tumor cells but also mediate the tumor immune response to prevent recurrence (23). In the present study, there was no difference in the T-cell subsets of the patients in the DC-CIK and chemotherapy groups. However, the number of CD3+ T-cells decreased after therapy and were restored 3 months later.
The effects and safety of adjuvant DC-CIK compared with chemotherapy needs to be investigated further. So far, there has only been one trial study that verified the beneficial effect of CIK in adjuvant therapy of NSCLC, but the treatment consequences at stage I NSCLC were inconclusive in subgroup analysis (16). Therefore, the benefit of adjuvant chemotherapy to stage I NSCLC after complete resection remains controversial. However, high-risk stage IB NSCLC can benefit from adjuvant chemotherapy (24). Considering the potential benefit to patients, a well-designed prospective randomized clinical trial to compare chemotherapy and CIK after tumor excision is warranted.
The results of the current study are limited in that it is a nonrandomized retrospective, and the differences in gender and age distribution between the groups may have biased the results. In addition, the sample size is small. Evaluations of sequential treatment as opposed to concurrent treatment, CIK treatment time, and CIK cell number remain subjects for further study.
Our study showed that for high-risk stage IB NSCLC patients, sequential DC-CIK cell therapy after chemotherapy improved clinical outcomes (2-year DFS and life quality) compared with chemotherapy only. The adverse side effects during DC-CIK therapy were acceptable. These results warrant a future randomized study with a larger sample size to evaluate CIK treatment in NSCLC patients.
REFERENCES
- 1. Shin H. R.; Carlos M. C.; Varghese C. Cancer control in the Asia Pacific region: Current status and concerns. Jpn. J. Clin. Oncol. 42:867–881; 2012. [DOI] [PubMed] [Google Scholar]
- 2. Pisters K. M.; Le Chevalier T. Adjuvant chemotherapy in completely resected non-small-cell lung cancer. J. Clin.Oncol. 23:3270–3278, 2005. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt-Wolf I. G.; Negrin R. S.; Kiem H. P.; Blume K. G.; Weissman I. L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 174:139–149; 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ma Y.; Zhang Z.; Tang L.; Xu Y. C.; Xie Z. M.; Gu X. F.; Wang H. X. Cytokine-induced killer cells in the treatment of patients with solid carcinomas: A systematic review and pooled analysis. Cytotherapy 14:483–493; 2012. [DOI] [PubMed] [Google Scholar]
- 5. Yang L.; Ren B.; Li H.; Yu J.; Cao S.; Hao X.; Ren X. Enhanced antitumor effects of DC-activated CIKs to chemotherapy treatment in a single cohort of advanced non-small-cell lung cancer patients. Cancer Immunol. Immunother. 62:65–73; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen H. W.; Liao C. H.; Ying C.; Chang C. J.; Lin C. M. Ex vivo expansion of dendritic-cell-activated antigen-specific CD4+ T cells with anti-CD3/CD28, interleukin-7, and interleukin-15: Potential for adoptive T cell immunotherapy. Clin. Immunol. 119:21–31; 2006. [DOI] [PubMed] [Google Scholar]
- 7. Linn Y. C.; Yong H. X.; Niam M.; Lim T. J.; Chu S., Choong A.; Chuah C.; Goh Y. T.; Hwang W.; Loh Y.; Ng H. J.; Suck G., Chan M.; Koh M. A phase I/II clinical trial of autologous cytokine-induced killer cells as adjuvant immunotherapy for acute and chronic myeloid leukemia in clinical remission. Cytotherapy 14:851–859; 2012. [DOI] [PubMed] [Google Scholar]
- 8. Liu L.; Zhang W.; Qi X.; Li H.; Yu J.; Wei S.; Hao X.; Ren X. Randomized study of autologous cytokine-induced killer cell immunotherapy in metastatic renal carcinoma. Clin. Cancer Res. 18:1751–1759; 2012. [DOI] [PubMed] [Google Scholar]
- 9. Shi L.; Zhou Q.; Wu J.; Ji M.; Li G.; Jiang J.; Wu C. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol. Immunother. 61:2251–2259; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Z. M.; Zhuang R. Y.; Chen Y.; Feng Y.; Li Q.; Liu T. S. A pilot study of chemotherapy combined with intraperitoneal perfusion of cytokine-induced killer cells for advanced gastric cancer patients with ascites. Zhonghua Wei Chang Wai Ke Za Zhi 16:28–31; 2013. [PubMed] [Google Scholar]
- 11. Zhou P.; Liang P.; Dong B.; Yu X.; Han Z.; Xu Y. Phase clinical study of combination therapy with microwave ablation and cellular immunotherapy in hepatocellular carcinoma. Cancer Biol. Ther. 11:450–456; 2011. [DOI] [PubMed] [Google Scholar]
- 12. Pignon J. P.; Tribodet H.; Scagliotti G. V.; Douillard J. Y.; Shepherd F. A.; Stephens R. J.; Dunant A.; Torri V.; Rosell R.; Seymour L.; Spiro S. G.; Rolland E.; Fossati R.; Aubert D.; Ding K.; Waller D.; Le Chevalier T.; Group L. C. Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE Collaborative Group. J. Clin. Oncol. 26:3552–3559; 2008. [DOI] [PubMed] [Google Scholar]
- 13. Wu C.; Jiang J.; Shi L.; Xu N. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res. 28:3997–4002; 2008. [PubMed] [Google Scholar]
- 14. Zhong R.; Teng J.; Han B.; Zhong H. Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol. Immunother. 60:1497–1502; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi S. B.; Ma T. H.; Li C. H.; Tang X. Y. Effect of maintenance therapy with dendritic cells: Cytokine-induced killer cells in patients with advanced non-small cell lung cancer. Tumori 98:314–319; 2012. [DOI] [PubMed] [Google Scholar]
- 16. Li H.; Wang C.; Yu J.; Cao S.; Wei F.; Zhang W.; Han Y.; Ren X. B. Dendritic cell-activated cytokine-induced killer cells enhance the anti-tumor effect of chemotherapy on non-small cell lung cancer in patients after surgery. Cytotherapy 11:1076–1083; 2009. [DOI] [PubMed] [Google Scholar]
- 17. Ettinger D. S.; Cox J. D.; Ginsberg R. J.; Komaki R.; Kris M. G.; Livingston R. B.; Sugarbaker D. J. NCCN non-small-cell lung cancer practice guidelines. The National Comprehensive Cancer Network. Oncology 10:81–111; 1996. [PubMed] [Google Scholar]
- 18. Trotti A.; Colevas A. D.; Setser A.; Rusch V.; Jaques D.; Budach V.; Langer C.; Murphy B.; Cumberlin R.; Coleman C. N.; Rubin P. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin. Radiat. Oncol. 13:176–181; 2003. [DOI] [PubMed] [Google Scholar]
- 19. Aaronson N. K.; Ahmedzai S.; Bergman B.; Bullinger M.; Cull A.; Duez N. J.; Filiberti A.; Flechtner H.; Fleishman S. B.; de Haes J. C. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer. Inst. 85:365–376; 1993. [DOI] [PubMed] [Google Scholar]
- 20. Tucker Z. C.; Laguna B. A.; Moon E.; Singhal S. Adjuvant immunotherapy for non-small cell lung cancer. Cancer Treat. Rev. 38:650–661; 2012. [DOI] [PubMed] [Google Scholar]
- 21. Mesiano G.; Todorovic M.; Gammaitoni L.; Leuci V.; Giraudo Diego L.; Carnevale-Schianca F.; Fagioli F.; Piacibello W.; Aglietta M.; Sangiolo D. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin. Biol. Ther. 12:673–684; 2012. [DOI] [PubMed] [Google Scholar]
- 22. Shebzukhov Y. V.; Koroleva E. P.; Khlgatian S. V.; Lagarkova M. A.; Meshcheryakov A. A.; Lichinitser M. R.; Karbach J.; Jager E.; Kuprash D. V.; Nedospasov S. A. Humoral immune response to thymidylate synthase in colon cancer patients after 5-FU chemotherapy. Immunol. Lett. 100:88–93; 2005. [DOI] [PubMed] [Google Scholar]
- 23. Rijavec M.; Volarevic S.; Osolnik K.; Kosnik M.; Korosec P. Natural killer T cells in pulmonary disorders. Respir. Med. 105( Suppl. 1):S20–25; 2011. [DOI] [PubMed] [Google Scholar]
- 24. Maeda R.; Yoshida J.; Ishii G.; Hishida T.; Nishimura M.; Nagai K. Risk factors for tumor recurrence in patients with early-stage (stage I and II) non-small cell lung cancer: Patient selection criteria for adjuvant chemotherapy according to the seventh edition TNM classification. Chest 140:1494–1502; 2011. [DOI] [PubMed] [Google Scholar]