Abstract
Berberine is a clinically important natural isoquinoline alkaloid found in many medicinal herbs. Berberine has been shown to have many pharmacological effects including antimicrobial, antitumor, and anti-inflammatory activities. However, the effects and mechanism of action of berberine have not been studied in chondrosarcoma. Therefore, the effects of berberine on proliferation in a human chondrosarcoma cell line (HTB-94) were investigated. Berberine inhibited cell proliferation in a concentration-dependent manner. We also determined that inhibition of cell proliferation by berberine occurred via G2/M phase arrest in HTB-94 cells. Berberine induced cell cycle arrest at the G2/M phase by upregulation of p53 and p21 expression and suppressed cyclin B1, cyclin-dependent kinase 1 (cdc2), cdc25c, and phosphorylated retinoblastoma tumor-suppressor protein (pRb) expression. In addition, berberine stimulated phosphorylation of protein kinase B (Akt) and p38 kinase. Inhibition of phosphatidylinositol 3-kinase (PI3K)/Akt with LY294002 (LY) and p38 kinase with SB203580 (SB), respectively, decreased berberine-induced p53 and p21 expression and restored cell proliferation and expression of cyclin B1, cdc2, cdc25c, and pRb cell cycle progression proteins. These results suggest that berberine-induced inhibition of cell proliferation by cell cycle arrest at the G2/M phases was regulated through PI3K/Akt and p38 kinase pathways in HTB-94 chondrosarcoma cells.
Key words: Berberine, Chondrosarcoma, Proliferation, Cell cycle arrest
INTRODUCTION
Chondrosarcoma, which develops in cartilage cells, is the second most common bone malignancy. Chondrosarcoma primarily affects the pelvis and the long bones of the arms and legs (1). Clinically, surgical resection is the primary and most successful means of treating chondrosarcomas. However, there is a high incidence of mortality associated with this malignancy due to the lack of an effective adjuvant therapy. Therefore, it is important to explore novel adjuvant treatments (2,3).
Many adjuvant chemotherapy agents affect the progression of cancer cells through the cell cycle. Cell cycle progression is controlled via a network of cell cycle regulatory proteins. Cyclin B1/cyclin-dependent kinase 1 (cdc2) complex is an important regulator for progression through the G2/M phase checkpoint (4). Cdc25c controls progression through the G2 phase and entry into mitosis. In the late G2 phase, the cdc25c phosphatase dephosphorylates the cyclin B1/cdc2 complex leading to the activation of the cyclin B1/cdc2 complex and to entry into mitosis (5).
The retinoblastoma tumor-suppressor protein (Rb) is known to be active in the G1 phase of the cell cycle. Rb appears to suppress transcription of E2F genes through interaction with E2F and thereby suppresses cell growth. Rb activity, including the ability to interact with E2F, is regulated by phosphorylation, primarily by cyclin D-dependent kinases. Cyclin D/cyclin-dependent kinase 4 (cdk4) activity is induced by growth stimulation and initiates the cascade of events that causes E2F accumulation and S phase entry (6,7).
These cell cycle regulatory molecules are modulated by some upstream proteins such as p53. p53 mediates either apoptosis or cell cycle arrest in response to DNA damage. p53 can regulate the expression of p21, an inhibitor of most of the cyclin-dependent kinases that directly binds to some cyclin-dependent kinase–cyclin complexes to inhibit their kinase activity. Since p21 inhibits both the cyclin-dependent G1 kinases and the G2/M-specific cdc2 kinase, p53 may be able to control both the G1 and the G2/M checkpoints (4,8).
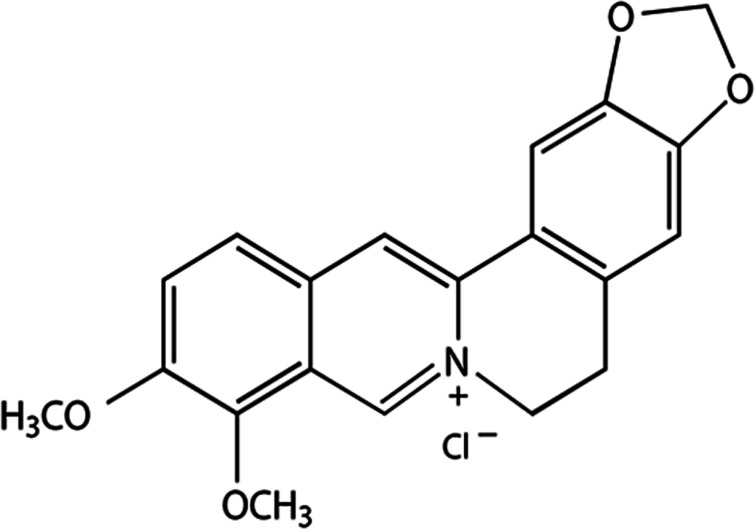
Berberine (Fig. 1) is an isoquinoline alkaloid component found in several Chinese herbal medicines. It is present in several medicinal plants such as Berberis aquifolium, Berberis vulgaris, Berberis aristata, Coscinium fenestratum, and Coptis chinensis (9,10). Berberine has been reported to exert a variety of pharmacological activities such as antibacterial (11), antihypertensive (12), anti-inflammatory (13), antidiabetic (14), and antihyperlipidemic effects (15). A recent study showed berberine had potential chemotherapeutic efficacy against cancer (16). In addition, considerable in vitro evidence has indicated that berberine inhibited growth in several human cancer cell lines (10).
Figure 1.
Chemical structure of berberine (molecular weight = 371.8). Berberine is an isoquinoline alkaloid component found in several traditional Chinese medicines including those isolated from Berberis aquifolium, Berberis vulgaris, Berberis aristata, Coscinium fenestratum, and Coptis chinensis (9,10).
However, the effects of berberine on the proliferation of human chondrosarcoma cells have not been studied. Therefore, the aim of this study was to investigate the effect of berberine on proliferation of HTB-94 human chondrosarcoma cells and the underlying molecular mechanisms. These data could provide experimental evidence for developing effective drugs for the clinical treatment of human chondrosarcoma.
MATERIALS AND METHODS
Materials
Berberin was purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Invitrogen (Burlington, ON, Canada). Stereptomycin and penicillin were obtained from Sigma-Aldrich. SB203580 and LY294002 were purchased from Calbiochem (San Diego, CA, USA).
Cell Culture
HTB-94 chondrosarcoma cells were obtained from American Type Culture Collection (ATCC; Rockville, MD, USA). HTB-94 cells were grown in DMEM (Invitrogen) supplemented with 10% (v/v) bovine calf serum (Invitrogen), 50 µg/ml streptomycin (Sigma-Aldrich), and 50 units/ml penicillin (Sigma-Aldrich). Cell cultures were incubated at 37°C in a humidified atmosphere of 5% CO2.
MTT Cell Proliferation Assay
HTB-94 cells were plated on a 96-well plate and incubated overnight to allow attachment. The next day, the medium was replaced. The cells were treated with berberine (0, 10, 30, 50, or 100 μM) and incubated for 6, 12, or 24 h. Four hours before the end of the incubation period, 10 µl of methyl thiazole tetrazolium (MTT) reagent I (10 mg/ml) was added to each well. After completing the incubation, 100 µl of MTT reagent II (solubilization buffer, 10% SDS with 0.01 N HCl, dimethyl sulfoxide) was added to each well, and the cells were incubated overnight at 37°C under 5% CO2. Finally, absorbance was measured at 595 nm using an ELISA plate reader.
Flow Cytometry Analysis of Cell Cycle Distribution
HTB-94 cells were harvested, washed once with phosphate-buffered saline (PBS), fixed in ice-cold 70% ethanol, and then stored at 4°C. The cells were washed again with PBS, suspended in 1 ml of a propidium iodide (PI; Sigma-Aldrich) solution containing 50 µg/ml RNase A (Sigma-Aldrich), 50 µg/ml PI, and 0.1% (v/v) NP-40, and then incubated in a 37°C water bath for 20 min in the dark. Flow cytometric analyses were conducted using a flow cytometer. Cell Quest software (Partec, Munich, Germany) was used to determine the relative DNA content based on fluorescence.
Western Blot Analysis
Proteins were isolated from whole cell lysates using a buffer containing 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, and 0.1% sodium dodecyl sulfate (SDS) supplemented with protease inhibitors and phosphatase inhibitors. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The nitrocellulose membrane was blocked with 5% nonfat dry milk in Tris-buffered saline and incubated with the following primary antibodies: anti-p53, anti-p21, anti-cyclin B1, anti-cdc2, anti-cdc25c, anti-pRb, anti-Rb, anti-pAkt, anti-phosphorylated p38 (pp38), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Santa Cruz Biotechnologies, CA, USA). Western blots were developed using peroxidase-conjugated secondary antibodies (Sigma-Aldrich) and a chemiluminescence system (LAS-4000 imager, Fujifilm Corp., Tokyo, Japan). The bands were quantified by densitometric analysis using the ImageJ software package (Software Inquiry, Quebec, Canada).
Data Analysis and Statistics
The results are expressed as the mean ± the standard deviation. These values were calculated from the specified number of determinations. The data were tested for overall significance with an analysis of variance (ANOVA), and Tukey’s test was used to analyze pairwise differences. A value of p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
Berberine Inhibited Cell Proliferation and Caused G2/M Arrest in HTB-94 Human Chondrosarcoma Cells
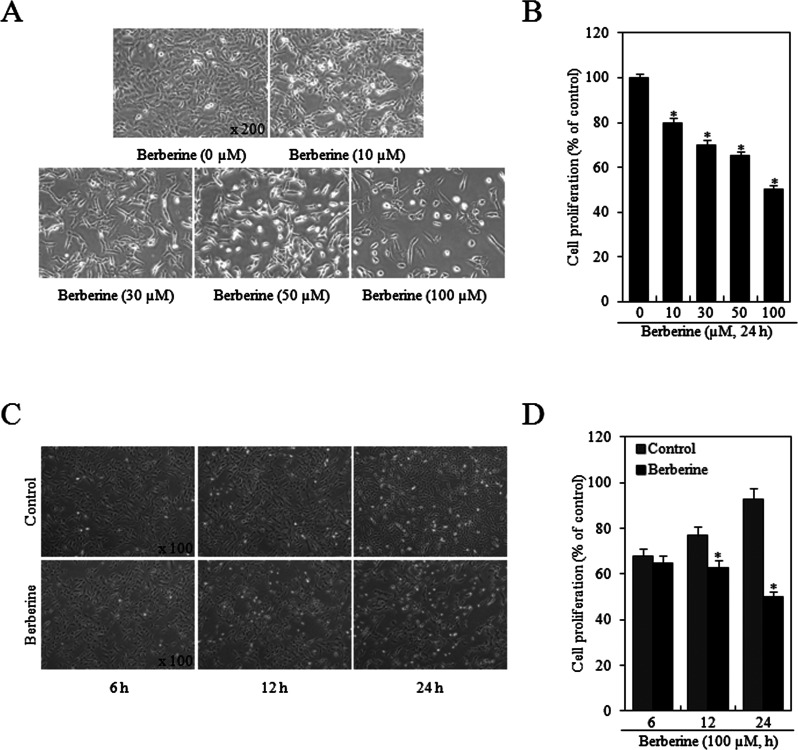
HTB-94 cells were treated with various concentrations of berberine (0, 10, 30, 50, or 100 μM) for 24 h or 100 μM berberine for specific times (6, 12, or 24 h), and the effects on cell proliferation were determined using the MTT assay. The effects of berberine on cell growth in HTB-94 cells were detected by phase-contrast microscopy and the MTT assay (Fig. 2A, C). As shown in Figure 2B and D, berberine inhibited the growth of HTB-94 cells in a concentration- and time-dependent manner with the maximal effect observed at a concentration of 100 μM and 24 h treatment.
Figure 2.
Treatment of human chondrosarcoma HTB-94 cells with berberine inhibited cell proliferation. HTB-94 cells were treated with the indicated concentrations of berberine (0–100 mM) for 24 h or 100 µM berberine for various time periods (6, 12, or 24 h). (A and C) Photographs of HTB-94 cells were taken with a phase-contrast microscope (magnification, A: 200×, B: 100×). (B and D) Cell proliferation was estimated using the methyl thiazole tetrazolium (MTT) assay. The photomicrograph data are presented as the results of a typical experiment, and the graphical data are expressed as mean values ± standard deviation. *p < 0.05 compared to the control.
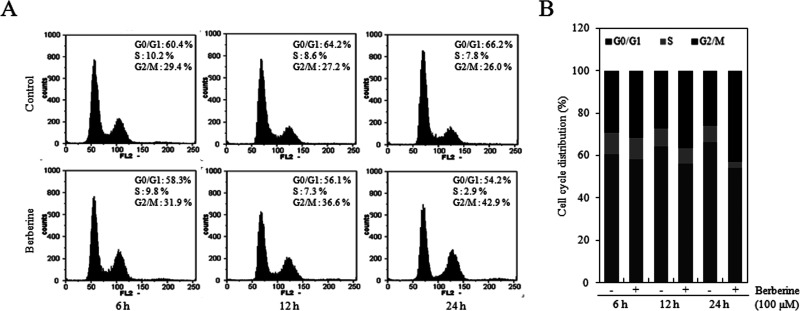
To further examine the effects on cell proliferation, cell cycle phase distribution of HTB-94 cells treated with berberine was analyzed by flow cytometry. HTB-94 cells were treated with 0 or 100 μM berberine for 6, 12, or 24 h. The results showed that berberine increased the proportion of cells at the G2/M phase in a time-dependent manner compared with the untreated control cells (Fig. 3A). The proportion of cells at S phases was reduced by approximately fivefold (Fig. 3B). These results suggested that berberine inhibits cell proliferation through induction of G2/M phase cell cycle arrest in HTB-94 cells.
Figure 3.
Treatment of human chondrosarcoma HTB-94 cells with berberine induced cell cycle arrest at the G2/M phase. HTB-94 cells were treated with 100 µM berberine for various times (6, 12, or 24 h). (A) Using flow cytometry, we demonstrated the effect of berberine on cell cycle distribution of HTB-94 cells. (B) The percentage of the cell population in each phase (G1, S, and G2/M) of the cell cycle was determined. The flow cytometry data are presented as results of a typical experiment, and the graphical data are expressed as mean values.
Berberine Inhibited Expression of Cell Cycle Proteins Involved in Regulation of G2/M Phase in HTB-94 Cells
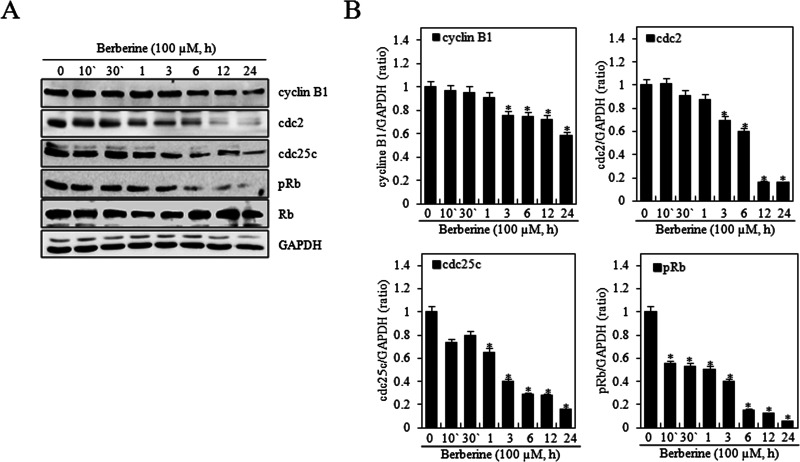
To determine the molecular mechanism underlying berberine-induced cell cycle arrest, proteins involved in G2/M phase progression in HTB-94 cells were investigated. HTB-94 cells were treated with 0 or 100 μM berberine for 10 or 30 min or 1, 3, 6, 12, or 24 h. Western blot analysis was performed using antibodies against cell cycle-related proteins (cyclin B1, cdc2, and cdc25c) (Fig. 4). Berberine significantly decreased the levels of cyclin B1, cdc2, and cdc25c proteins (Fig. 4A).
Figure 4.
Berberine inhibited expression of cell cycle proteins involved in regulation of the G2/M phase in HTB-94 cells. HTB-94 cells were untreated (0) or treated with 100 µM berberine for the indicated times (10 or 30 min or 1, 3, 6, 12, or 24 h). (A) Expression of cyclin B1, cdc2, cdc25c, phosphorylated retinoblastoma tumor-suppressor protein (pRb), Rb, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was determined by Western blot analysis. GAPDH was used as loading control. (B) The relative amounts of cyclin B1, cdc2, cdc25c, and pRb were quantified by a densitometric analysis (ImageJ). The Western blot data represent the results of a typical experiment, and the graphical data are expressed as mean values ± standard deviation. *p < 0.05 compared to the control.
Rb is a tumor suppressor that plays an inhibitory role in the control of the cell cycle and in tumor progression. It has been shown that Rb protein is responsible for inhibiting a primary G1 phase, thereby blocking S phase entry and cell growth (17). However, previous studies also suggested that Rb protein interacts with some components of cell cycle regulation mechanisms during G2 (18). Therefore, we examined phosphorylation of Rb protein by berberine. The phosphorylation of Rb protein was decreased by berberine treatment in a time-dependent manner as determined by Western blot analysis. Rb expression was not changed by berberine (Fig. 4A). Quantification of protein content identified a reduction of cyclin B1, cdc2, cdc25c, and pRb in a time-dependent manner (Fig. 4B). These results indicated that berberine induced cell cycle arrest at the G2/M phase by inhibition of G2/M phase-related protein in HTB-94 cells.
Berberine Induced p53/p21-Dependent G2/M Phase Arrest in HTB-94 Cells
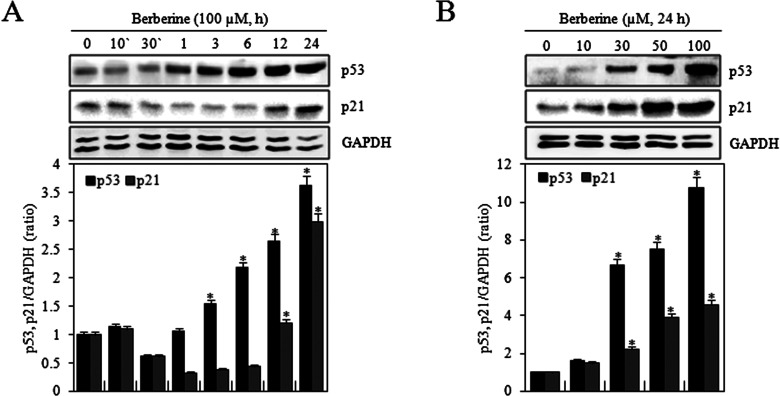
Berberine inhibited cell proliferation and G2/M phase progression. p53 mediates either apoptosis or cell cycle arrest in response to DNA damage, and p21 is an inhibitor of most of the cyclin-dependent kinases (8). Therefore, the effects of berberine on expression of p53 and p21 in HTB-94 cells were examined. Cells were treated with 0 or 100 μM berberine for indicated times (10 or 30 min or 1, 3, 6, 12, or 24 h) or treated with various concentrations of berberine (0, 10, 30, 50, or 100 μM) for 24 h. Berberine significantly increased the expression of p53 and p21 in a time- and concentration-dependent manner, as determined by Western blot analysis (Fig. 5A, B). These results suggested that berberine-induced G2/M phase arrest might involve increased expression of p53 and p21 in HTB-94 cells.
Figure 5.
Berberine induced p53/p21-dependent G2/M phase arrest in HTB-94 cells. HTB-94 cells were untreated (0) or treated with 100 µM of berberine for the indicated times (10 or 30 min or 1, 3, 6, 12, or 24 h). (A) Expression of p53, p21, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was detected using Western blot analysis. GAPDH was used as loading control (upper panel). The relative amounts of p53 and p21 were quantified by a densitometric analysis (ImageJ, lower panel). HTB-94 cells were treated with the indicated various concentrations of berberine (0, 10, 30, 50, or 100 mM) for 24 h. (B) Expression of p53, p21, and GAPDH was detected using Western blot analysis. GAPDH was used as loading control (upper panel). The relative amounts of p53 and p21 were quantified by a densitometric analysis (ImageJ, lower panel). Western blot data are presented as results of a typical experiment, and graphical data are expressed as the mean ± standard deviation. *p < 0.05 compared to the control.
Berberine Inhibited Proliferation and G2/M Phase Progression via Phosphatidylinositol 3-Kinase (PI3K)/Protein Kinase B (Akt) and p38 Kinase Pathways in HTB-94 Cells
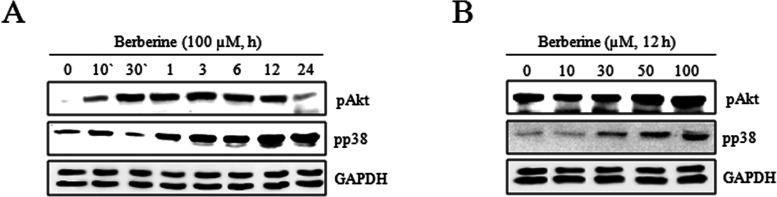
To determine the underlying signaling pathway that was involved, the molecular mechanism of the effects of berberine on the proliferation of HTB-94 cells was further investigated. Because these pathways are related to the inflammation, differentiation, and proliferation of cancers, the effects of berberine on the activation of PI3K/Akt and p38 kinase were examined. HTB-94 cells were treated with 0 or 100 μM berberine for indicated times (10 or 30 min or 1, 3, 6, 12, or 24 h) or treated with various concentrations of berberine (0, 10, 30, 50, or 100 μM) for 12 h. Berberine induced the activation of Akt from 30 min to 12 h and activation of p38 kinase from 1 h to 24 h. Berberine also induced activation of Akt and p38 kinase in a concentration-dependent manner as determined by Western blot analysis of phosphorylated Akt and p38 (Fig. 6A, B).
Figure 6.
Berberine activated PI3K/Akt and p38 kinase in HTB-94 cells. HTB-94 cells were untreated (0) or treated with 100 µM of berberine for the indicated times (10 or 30 min or 1, 3, 6, 12, or 24 h). (A) Expression of phosphorylated protein kinase B (pAkt), phosphorylated p38 (pp38), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was detected using Western blot analysis. GAPDH was used as loading control. HTB-94 cells were untreated or treated with the indicated various concentrations of berberine (0, 10, 30, 50, or 100 mM) for 12 h. (B) Expression of pAkt, pp38, and GAPDH was detected using Western blot analysis. GAPDH was used as loading control. The data present results of typical experiments from at least three independent experiments.
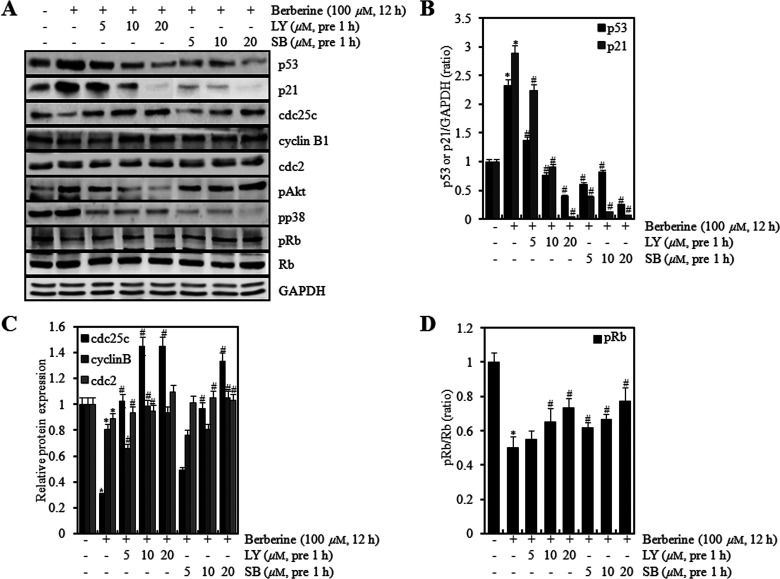
To test the functions of PI3K/Akt and p38 kinase on proliferation by berberine in HTB-94 cells, we pretreated the cells with LY294002 (LY) and SB203580 (SB) and for 1 h and then treated with berberine. LY and SB inhibited berberine-induced expression of p53 and p21 in a concentration-dependent manner and restored berberine-reduced expression of cyclin B1, cdc2, cdc25c, and pRb as shown by Western blot analysis (Fig. 7A). Quantification of protein expression was performed using densitometric analysis (ImageJ) (Fig. 7B–D).
Figure 7.
Berberine regulated expression of p53, p21, Cyclin B1, cdc2, cdc25c, and pRb via PI3K/Akt and p38 kinase pathway in HTB-94 cells. HTB-94 cells were untreated or treated with 100 µM of berberine in the absence or presence of the indicated concentrations of LY294002 or SB203580 (5, 10, or 20 mM). (A) Expression of p53, p21, cyclin B1, cdc2, cdc25c, phosphorylated retinoblastoma tumor-suppressor protein (pRb), phosphorylated protein kinase B (pAkt), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was determined with Western blot analysis. Expression of GAPDH was used as a loading control. (B–D) The relative amounts of p53, p21, Cyclin B1, cdc2, cdc25c, and pRb were quantified by a densitometric analysis (ImageJ). The Western blot data present results of typical experiments from at least three independent experiments, and graphical data are expressed as the mean ± values with standard deviation. *p < 0.05 compared to the control; # < 0.05 compared to the berberine.
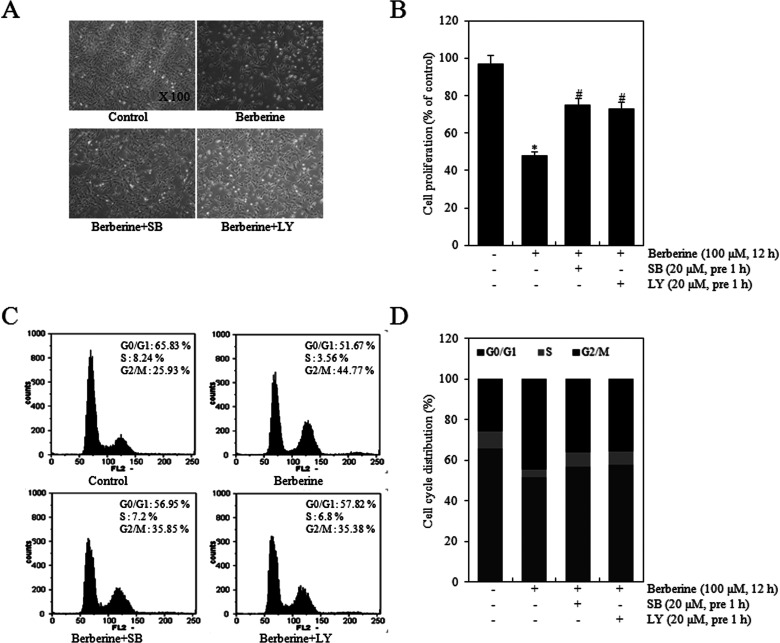
HTB-94 cells were treated with 0 or 100 μM of berberine in the absence or presence of 20 μM LY or 20 μM SB for 12 h. Whether cell proliferation study results coincided with cell cycle arrest results was investigated by cell phase-contrast microscopy and the MTT assay. Treatment with LY or SB prior to berberine reversed berberine-induced suppression of cell proliferation (Fig. 8A, B). As shown in Figure 8C–D, compared with berberine treatment alone, SB and LY blocked the berberine-induced increase in the proportion of cells in the G2/M phase (Fig. 8C) and decreased the percentage of cells in the G phase by 1.2-fold (Fig. 8D). These results indicated that PI3K/Akt and p38 kinase activation played an important role on berberine-induced inhibition of proliferation and G2/M phase progression in HTB-94 cells. Taken together, the results suggested that the antiproliferative effects and G2/M phase arrest by berberine are modulated through PI3K/Akt and p38 kinase pathways in chondrosarcoma cells.
Figure 8.
Berberine modulated proliferation and G2/M phase arrest through PI3K/Akt and p38 kinase pathways in HTB-94 cells. HTB-94 cells were untreated or treated with 100 µM berberine in the absence or presence of 20 µM LY294002 or 20 µM SB203580 for 12 h. (A) Photographs of HTB-94 cells were taken by phase-contrast microscope (magnification, 100×). (B) Cell proliferation was estimated using the methyl thiazole tetrazolium (MTT) assay. (C) Cell cycle arrest was determined using flow cytometric analysis. (D) The percentage of the cells in phases G0/G1 (dark gray), S (light gray), G2/M (black) was determined. Photomicrograph and flow cytometry data are presented as results of a typical experiment and cell proliferation data as the mean ± standard deviation. *p < 0.05 compared to the control. # < 0.05 compared to the berberine.
DISCUSSION
Induction of cell cycle arrest is regulated by a large number of molecules. In our study, we found that activation of PI3K/Akt and p38 kinase mediated the effect of berberine on cell cycle arrest and induction of p53 and p21 expression. Consistent with this, the role of p38 kinase pathway in mediating the cancer cell growth inhibition and induction of apoptosis has been established and reported. Inactivation of the p38 pathway enhanced cellular transformation and rendered mice prone to tumor development with concurrent disruption of induction of senescence. Conversely, persistent activation of p38 inhibited tumorigenesis, suggesting a tumor-suppressing function of the p38 pathway (19,20). Phosphatidylinositol 3-kinases (PI3K) are a family of enzymes involved in cellular functions. PI3K/Akt pathway is common in human cancer, and there is increasing evidence of PI3K/Akt being involved in the development of many types of cancers (19).
Cell cycle checkpoints play a significant role in the control mechanisms that ensure the proper progression of the cell cycle. One of these checkpoints, the G2/M checkpoint, blocks entry into mitosis when DNA is damaged (21). p53 and p21 proteins play an important role in cell cycle progression. p21 is an inhibitor of cyclin/cyclin-dependent kinase complexes and interacts with other regulators of signal transduction (22). The induction of p21 is mediated by both p53-dependent and p53-independent mechanisms and is essential for the onset of both G1 and G2 cell cycle arrest in damage response and cell senescence (23,24).
Berberine has been reported to be cytotoxic to various cancer cell lines. Other results indicate that berberine suppressed growth of HepG2 hepatoma cells (25), colon tumor cells (26,27), epithelial ovarian carcinoma cells (28), and breast cancer cells (29). In our studies, berberine inhibited proliferation of human chondrosarcoma cells. Thus, berberine has been shown to inhibit the growth of a wide variety of cancer cells.
In addition, our results suggested that berberine-induced antiproliferative effects were produced through G2/M phase arrest in human chondrosarcoma cells. In another study, berberine inhibited human colorectal adenocarcinoma cell growth via G2/M phase arrest (30). Berberine induced G2/M arrest in the human promyelocytic leukemia HL-60 cells and murine myelomonocytic leukemia WEHI-3 cells via the inhibition of cyclin B1 and the promotion of Wee1 (24). However, berberine induced accumulation of cells not only in the G2/M phase but also in the G1 phase in several types of cancer cells. Other studies found G1 cell cycle arrest induced by berberine in Bel7402 cells and cholangiocarcinoma QBC939 cells (10,31).
Other studies reported that Huanglian, a Chinese herbal extract containing berberine, inhibited cell proliferation by suppression of cyclin B1 and cdc2 expression in human cancer cells (32). In another study, berberine decreased the expression of cyclin B1 in LoVo cells (30). Our study also showed that berberine decreased expression of cyclin B1 and cdc2 in human chondrosarcoma cells. These results suggest that berberine might be one of the effective components of Huanglian extract exerting anticancer effects.
In conclusion, our data demonstrated the antiproliferative effect of berberine in human chondrosarcoma cells. Berberine inhibited cell growth by inducing G2/M phase arrest and decreasing the expression of the G2/M phase regulatory proteins such as cyclin B1, cdc2, cdc25c, and pRb. In addition, berberine-induced inhibition of G2/M phase cell cycle progression was dependent on functional p53 and p21 in HTB-94 cells. Berberine also activated the phosphorylation of Akt and p38. Inhibition of PI3K/Akt and p38 decreased berberine-induced p53 and p21 expression and increased expression of cyclin B1, cdc2, cdc25c, and pRb. Our results suggested that berberine modulated proliferation and G2/M phase cell cycle arrest through the PI3K/Akt and p38 kinase pathways in human chondrosarcoma HTB-94 cells. The antiproliferative ability of berberine could make it a potentially useful therapeutic agent against human chondrosarcoma.
ACKNOWLEDGMENTS
This work was supported by a grant from the Korean Health Technology R&D project, Ministry of Health and Welfare, Republic of Korea (A120960) as well as the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST) (2014-R1A1A3049653). Eo, Seong Hui: Conducted the research and wrote the manuscript; Kim, Ju Hee: Conducted the research; Kim, Song Ja: Designed the experiments, conducted the research and analyzed the data, wrote the manuscript.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Galoian K. A.; Guettouche T.; Issac B.; Qureshi A.; Temple H. T. Regulation of onco and tumor suppressor MiRNAs by mTORC1 inhibitor PRP-1 in human chondrosarcoma. Tumour Biol. 35:2335–2341; 2013. [DOI] [PubMed] [Google Scholar]
- 2. Sun Y.; Guo W.; Ren T.; Liang W.; Zhou W.; Lu Q.; Jiao G.; Yan T. Gli1 inhibition suppressed cell growth and cell cycle progression and induced apoptosis as well as autophagy depending on ERK1/2 activity in human chondrosarcoma cells. Cell Death Dis. 5:e979; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu C. M.; Li T. M.; Tan T. W.; Fong Y. C.; Tang C. H. Berberine reduces the metastasis of chondrosarcoma by modulating the αvβ3 integrin and the PKCδ, c-Src, and AP-1 signaling pathways. Evidence Based. Complement. Alternat. Med. 2013:423164; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ding L.; Huang Y.; Dai M.; Zhao X.; Du Q.; Dong F.; Wang L.; Huo R.; Zhang W.; Xu X.; Tong D. Transmissible gastroenteritis virus infection induces cell cycle arrest at S and G2/M phases via p53-dependent pathway. Virus Res. 178:241–251; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruppenthal S. L.; Noll A.; Gotz C.; Montenarh M. Interference between p53 and cdc25C in cell cycle regulation. Int. J. Oncol. 31:345–352; 2007. [PubMed] [Google Scholar]
- 6. Harbour J. W.; Dean D. C. The Rb/E2F pathway: Expanding roles and emerging paradigms. Genes. Dev. 14:2393–2409; 2000. [DOI] [PubMed] [Google Scholar]
- 7. Nevins J. R. The Rb/E2F pathway and cancer. Hum. Mol. Genet. 10:699–703; 2001. [DOI] [PubMed] [Google Scholar]
- 8. Agarwal M. L.; Agarwal A.; Taylor W. R.; Stark G. R. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc. Natl. Acad. Sci. USA 92:8493–8497; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kulkarni S. K.; Dhir A. Berberine: A plant alkaloid with therapeutic potential for central nervous system disorders. Phytother. Res. 24:317–324; 2010. [DOI] [PubMed] [Google Scholar]
- 10. Brents L. K.; Medina-Bolivar F.; Seely K. A.; Nair A.; Bratton S. M.; Nopo-Olazabal L.; Patel R. Y.; Liu H.; Doerksen R. J.; Prather P. L.; Radominska-Pandya A. Natural prenylated resveratrol analogs arachidin-1 and -3 demonstrate improved glucuronidation profiles and have affinity for cannabinoid receptors. Xenobiotica 42:139–156; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amin A. H.; Subbaiah T. V.; Abbasi K. M. Berberine sulfate: Antimicrobial activity, bioassay, and mode of action. Can. J. Microbiol. 15:1067–1076; 1969. [DOI] [PubMed] [Google Scholar]
- 12. Bova S.; Padrini R.; Goldman W. F.; Berman D. M.; Cargnelli G. On the mechanism of vasodilating action of berberine: Possible role of inositol lipid signaling system. J. Pharmacol. Exp. Ther. 261:318–323; 1992. [PubMed] [Google Scholar]
- 13. Akhter M. H.; Sabir M.; Bhide N. K. Anti-inflammatory effect of berberine in rats injected locally with cholera toxin. Indian. J. Med. Res. 65:133–141; 1977. [PubMed] [Google Scholar]
- 14. Lee Y. S.; Kim W. S.; Kim K. H.; Yoon M. J.; Shen Y.; Ye J. M.; Lee C. H.; Oh W. K.; Kim C. T.; Hohnen-Behrens C.; Gosby A.; Kraegen E. W.; James D. E.; Kim J. B. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55:2256–2264; 2006. [DOI] [PubMed] [Google Scholar]
- 15. Kong W.; Wei J.; Abidi P.; Lin M.; Inaba S.; Wang Y.; Wang Z.; Si S.; Pan H.; Wang S.; Wu J.; Wang Y.; Li Z.; Liu J.; Jiang J. D. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 10:1344–1351; 2004. [DOI] [PubMed] [Google Scholar]
- 16. Diogo C. V.; Machado N. G.; Barbosa I. A.; Serafim T. L.; Burgeiro A.; Oliveira P. J. Berberine as a promising safe anti-cancer agent—Is there a role for mitochondria? Curr. Drug Targets 12:850–859; 2011. [DOI] [PubMed] [Google Scholar]
- 17. Giacinti C.; Giordano A. RB and cell cycle progression. Oncogene 25:5220–5227; 2006. [DOI] [PubMed] [Google Scholar]
- 18. Karantza V.; Maroo A.; Fay D.; Sedivy J. M. Overproduction of Rb protein after the G1/S boundary causes G2 arrest. Mol. Cell. Biol. 13:6640–6652; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li B.; Li J.; Xu W. W.; Guan X. Y.; Qin Y. R.; Zhang L. Y.; Law S.; Tsao S. W.; Cheung A. L. Suppression of esophageal tumor growth and chemoresistance by directly targeting the PI3K/AKT pathway. Oncotarget 30:11576–11587; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng F.; Tang Q.; Wu J.; Zhao S.; Liang Z.; Li L.; Wu W.; Hann S. p38alpha MAPK-mediated induction and interaction of FOXO3a and p53 contribute to the inhibited-growth and induced-apoptosis of human lung adenocarcinoma cells by berberine. J. Exp. Clin. Res. 33:36; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor W. R.; Stark G. R. Regulation of the G2/M transition by p53. Oncogene 20:1803–1815; 2001. [DOI] [PubMed] [Google Scholar]
- 22. Roninson I. B. Oncogenic functions of tumour suppressor p21 (Waf1/Cip1/Sdi1): Association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett. 179:1–14; 2002. [DOI] [PubMed] [Google Scholar]
- 23. Adriaenssens E.; Lemoine J.; El Yazidi-Belkoura I.; Hondermarck H. Growth signaling in breast cancer cells: Outcomes and promises of proteomics. Biochem. Pharmacol. 64:797–803; 2002. [DOI] [PubMed] [Google Scholar]
- 24. Lin C. C.; Lin S. Y.; Chung J. G.; Lin J. P.; Chen G. W.; Kao S. T. Down-regulation of cyclin B1 and up-regulation of Wee1 by berberine promotes entry of leukemia cells into the G2/M-phase of the cell cycle. Anticancer Res. 26:1097–1104; 2006. [PubMed] [Google Scholar]
- 25. Chi C. W.; Chang Y. F.; Chao T. W.; Chiang S. H.; P`eng F. K.; Lui W. Y.; Liu T. Y. Flow cytometric analysis of the effect of berberine on the expression of glucocorticoid receptors in human hepatoma HepG2 cells. Life Sci. 54:2099–2107; 1994. [DOI] [PubMed] [Google Scholar]
- 26. Wang L.; Cao H.; Lu N.; Lui L.; Wang B.; Hu T.; Israel D. A.; Peek R. M. Jr.; Polk D. B.; Yan F. Berberine inhibits proliferation and down-regulates epidermal growth factor receptor through activation of Cbl in colon tumor cells. PLoS One 8:e56666; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu K., Yang Q.; Mu Y.; Zhou L.; Liu Y.; Zhou Q.; He B. Berberine inhibits the proliferation of colon cancer cells by inactivating Wnt/beta-catenin signaling. Int. J. Oncol. 41:292–298; 2012. [DOI] [PubMed] [Google Scholar]
- 28. Park K. S.; Kim J. B.; Lee S. J.; Bae J. Berberine-induced growth inhibition of epithelial ovarian carcinoma cell lines. J. Obstet. Gynaecol. Res. 38:535–540; 2012. [DOI] [PubMed] [Google Scholar]
- 29. Wen C. J.; Wu L. X.; Fu L. J.; Zhang Y. W.; Zhang X.; Zhou H. H. Genomic screening for targets regulated by berberine in breast cancer cells. Asian Pac. J. Cancer Prev. 14:6089–6094; 2013. [DOI] [PubMed] [Google Scholar]
- 30. Cai Y.; Xia Q.; Luo R.; Huang P.; Sun Y.; Shi Y.; Jing W. Berberine inhibits the growth of human colorectal adenocarcinoma in vitro and in vivo. J. Nat. Med. 68:53–62; 2014. [DOI] [PubMed] [Google Scholar]
- 31. Ma C.; Tang K.; Liu Q.; Zhu R.; Cao Z. Calmodulin as a potential target by which berberine induces cell cycle arrest in human hepatoma Bel7402 cells. Chem. Biol. Drug Des. 81:775–783; 2013. [DOI] [PubMed] [Google Scholar]
- 32. Li X. K.; Motwani M.; Tong W.; Bornmann W.; Schwartz G. K. Huanglian A. Chinese herbal extract, inhibits cell growth by suppressing the expression of cyclin B1 and inhibiting CDC2 kinase activity in human cancer cells. Mol. Pharmacol. 58:1287–1293; 2000. [DOI] [PubMed] [Google Scholar]