Abstract
BACKGROUND/OBJECTIVES
It is difficult to consistently demonstrate the health effects of soy isoflavones owing to the multitude of factors contributing to their bioavailability. To accurately verify these health effects, dietary isoflavone intake should be measured using a biologically active dose rather than an intake dose. This concept has been expanded to the development of new exposure biomarkers in nutrition research. This review aims to provide an overview of the development of exposure biomarkers and suggest a novel research strategy for identifying the health effects of soy isoflavone intake.
MATERIALS/METHODS
We cover recent studies on the health effects of soy isoflavones focusing on isoflavone metabolites as exposure biomarkers.
RESULTS
Compared to non-fermented soy foods, fermented soy foods cause an increased concentration of isoflavones in the biofluid immediately following ingestion. The correlation between exposure biomarkers in blood and urine and the food frequency questionnaire was slightly lower than that of corresponding 24-h dietary recalls. Urinary and blood isoflavone levels did not show a consistent association with chronic disease and cancer risk.
CONCLUSION
It is crucial to understand the variable bioavailabilities of soy isoflavones, which may affect evaluations of soy isoflavone intake in health and disease. Further studies on the development of valid exposure biomarkers are needed to thoroughly investigate the health effects of isoflavone.
Keywords: Soybeans, fermentation, dietary exposure, metabolomics, biological availability, clinical study
INTRODUCTION
The importance of diet for improving health and preventing chronic disease is widely recognized [1]. Notably, there is an increasing interest in studying the mechanism of action involved in the effects of food constituents, such as phytochemicals, in biological systems. However, because individual responses (e.g., bioavailability) to the diet are different, identifying the link between food intake and physiological outcome is not sufficient to solve the complex characteristics of diet-health associations [2].
To understand the health effects of certain foods, scientists have utilized new biomarkers to assess dietary intake and its biological effects [3]. Advances in analytical and data processing technologies have made it possible to use the metabolites present in biological fluids, such as blood and urine, as biomarkers of intake and exposure. Exposure biomarkers of various foods have been used to support associations between dietary exposure and disease risk [4,5].
Soy foods, the primary source of isoflavone exposure, are commonly consumed in their fermented form in Korea. Isoflavone patterns in soy foods differ based on the method of processing, such as fermentation. An accurate determination of dietary isoflavone intake is limited by the absence of a common database establishing the isoflavone composition of various food sources [6]. Additionally, although circulating and excreted levels of isoflavones have been reported to vary between individuals, this has not been studied extensively. Therefore, this review aims to: 1) summarize factors affecting the bioavailability of soy isoflavone and recent studies on its health effects, and 2) suggest a new research strategy for identifying an exposure biomarker of soy isoflavone intake by providing an overview of the recent development of exposure biomarkers.
EXPOSURE BIOMARKERS FOR VALIDATION OF FOOD INTAKE
Classification of biomarkers
Biomarkers provide objective measurements used to assess the exposure, effects, or susceptibility of humans or animals [3]. Gao et al. [3] suggested the following principles for the classification of dietary and health biomarkers based on their intended use: 1) exposure and intake biomarkers represent food intake, food compounds or nutrients, and dietary patterns in biological fluids; 2) effect (efficacy) biomarkers monitor changes in biochemical, physiological, or psychological states as a response to nutritional exposures; 3) Susceptibility (health state) biomarkers can be expressed as personal resilience, and are linked to various host factor-related risks based on their food and nutrition status. Among these 3 groups, exposure biomarkers are essential for linking dietary compounds to health outcomes and provide early signals of biological effects [7]. To apply biomarkers to future nutrition research, we should focus on the development of appropriate biomarkers by understanding the bioavailability and mode of action of bioactive food components, as well as conducting extensive population studies [7].
Research on exposure biomarkers
Biomarkers of food intake are mainly small molecules derived from the digestion and biotransformation of either the food itself, or of certain food-derived compounds [8]. Advances in metabolomics and statistical techniques have allowed exposure biomarkers to be used as objective indicators of specific food intake. For example, intervention studies have identified biomarkers of specific foods of interest, including coffee [9], fish and meat [10], cruciferous vegetables [11], and wine [12]. Exposure biomarkers have also been identified in cohort studies and correlated with a self-reported intake of meat [13], whole grain bread [14,15] and a vegetarian diet [16]. Exposure biomarkers can provide evidence for associations between food intake and potential health effects.
Shi et al. [4] reported that a principal component analysis-derived metabolite pattern was correlated with fish intake (ρ = 0.37; P < 0.001), but was not associated with type 2 diabetes (T2D) risk. However, after removing the counteractive effects of co-exposure to persistent organic pollutants present in fish, the study showed that fish intake in fact lowered the risk of T2D (OR, 0.75; 95% CI, 0.54–1.02; P = 0.07) [4]. Metabolite markers of coffee exposure have been suggested to be strong predictors of liver cancer and liver disease mortality [5]. Trigonelline, a known coffee biomarker, was inversely associated with liver disease mortality. In contrast, 2 bile acids, namely glycochenodeoxycholic acid and glycocholic acid, were inversely associated with coffee intake but positively associated with both liver cancer and liver disease mortality. In a study that identified metabolites associated with high adherence to the Alternative Healthy Eating Index, circulating metabolites such as specific fatty acids were inversely correlated to the risk of cardiovascular disease (CVD) [17].
SOY ISOFLAVONE METABOLISM
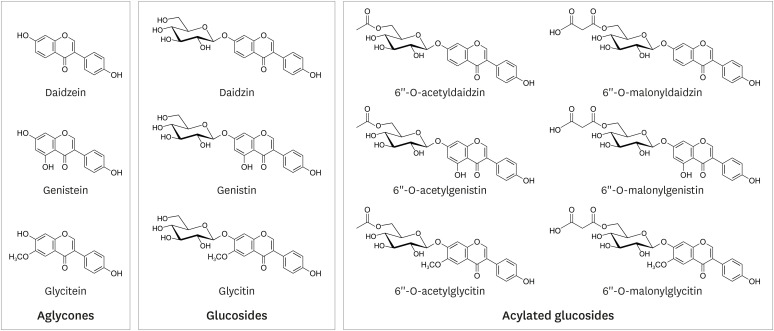
Soybean isoflavones consist of 3 aglycones (daidzein, genistein, and glycitein), 3 simple glucosides (daidzin, genistin, and glycitin), and 6 acylated glucosides (Fig. 1). The compositions of the acyl groups vary depending on the processing method, such as heating or fermentation [18]. Therefore, the absorption of soy isoflavones varies significantly between subjects or the provided food matrix [18,19].
Fig. 1. Chemical structure of soybean isoflavones.
Isoflavone glucosides are not absorbed intact across the enterocyte, as they must first be deglycosylated [20]. Deglycosylation of isoflavone glucoside is carried out by the enterocyte brush boundary enzyme, lactase phlorizin hydrolase, before being absorbed into the small intestine, as shown by the appearance of a weak plasma peak after approximately 1 h [21]. Aglycones are conjugated in the intestine and liver during first-pass absorption and are then predominantly circulated in the plasma and urine as glucuronide conjugates, or to a lesser extent, sulfate conjugates [20,22].
Isoflavone glucosides are hydrolyzed and metabolized by microflora in the colon. These microflora may also further degrade the isoflavone and its conjugates to reductive metabolites and ring-fission products [23]. For example, daidzein is metabolized by microbes into O-desmethylangolensin and equol. Dietary isoflavones are almost entirely conjugated with sulfates and glucuronides after intestinal absorption, whereas unconjugated isoflavones, the biologically active form, make up only 1–5% of the total isoflavone in the blood [24]. Recently, several studies have suggested that the conjugates may either have biological activity themselves [25] or serve as excellent sources of biologically active compounds within target cells through deconjugation [26,27].
In order to establish conclusive evidence for the health benefits of isoflavones, it is essential to accurately determine the type and bioavailability of isoflavone in soy foods. Generally, released aglycone was quantified by the presence of glucuronidase and sulfatase enzymes in plasma and urine samples, rather than the presence of metabolites. Although this technique provided valuable insights, the results it produced were indirect and inaccurate [28].
FACTORS AFFECTING THE BIOAVAILABILITY OF ISOFLAVONES
The bioavailability of dietary foods is affected by both food-related and human biological factors [28]. Food matrices, interactions with other compounds, and chemical structures all directly influence bioavailability. Following ingestion, bioavailability is affected by host-related factors including intestinal factors, such as enzyme activity or intestinal microflora, and systemic factors, such as gender, age, genetics, and physical condition [28].
Isoflavones have different intestinal absorption rates based on their chemical structure. In an animal study, acylated glucosides, such as malonyl glucoside, were hydrolyzed only in colon homogenates with high bacterial concentrations, whereas hydroxylation of simple glucoside occurred in jejunum, ileum, and colon homogenates [29]. This study also reported that although aglycone and simple glucosides are absorbed faster than malonyl glucoside, the absorption of malonyl-rich isoflavone in the colon contributes to a significant increase in plasma levels [29]. This result suggests that the gut microbiome produces variability of isoflavone absorption rates in the colon among different individuals.
In addition, the fermentation process can cause structural changes in soy isoflavone, which consequently affects its bioavailability and possibly optimizes its biological effect [22]. These results may help explain the discrepancies between clinical findings regarding soy foods in Asia versus the West. However, few studies have been conducted to develop biomarkers for fermented soybean (FS) intake.
Here, we outline the effects of fermentation on the bioavailability of soy isoflavone from 3 human clinical studies, as shown in Table 1. In the first study, Okabe et al. [30] reported that the isoflavone of aglycon-rich FS powder was absorbed faster and in higher amounts than those of glucoside-rich non-fermented soybean (NFS) powder in postmenopausal women. The serum concentrations of total isoflavone (calculated as the sum of genistein, daidzein, and glycitein) 1–4 h after oral consumption was significantly higher in the aglycone-rich FS than the glucoside-rich NFS group; however, there was no difference between the 2 groups 6–24 h after administration. The total isoflavone concentration in urine samples was significantly increased after intake of FS compared to that after intake of NFS in samples collected at 2–4 h and 24–48 h after administration. Genistein, daidzein, and glycitein had trends similar to that of total isoflavone in blood and urine samples, but the gut-mediated metabolite, equol, did not. A similar study by Kano et al. [31] also investigated the effects of fermentation on bioavailability of isoflavones after ingesting soymilk. The initial absorption rate of total isoflavone was greater after ingesting FS milk than after ingesting NFS milk. However, concentrations of isoflavone did not differ between the 2 kinds of milk in both blood and urine after 6–24 h and 12–48 h, respectively [31]. A third study revealed that the consumption of FS milk did not enhance urinary isoflavone excretion at any isoflavone dosage (from low to high) on any of the 3 collection days (day 4, 13, and 14) over a period of 2 weeks [32].
Table 1. Studies on the bioavailability of isoflavone after intakes of FS and NFS.
| Subject/design | Source | Isoflavone dose | Sampling time | Blood (FS vs. NFS) | Urine (FS vs. NFS) | Ref. |
|---|---|---|---|---|---|---|
| 11 women (PostMeno)/cross-over | Soy powder with water | FS (95% aglycone): 95 µmol | Serum: 0–24 h | Total isoflavone | Total isoflavone FS > NFS | Okabe et al. [30] |
| NFS (86% glucoside): 95 µmol | Urine: 0–48 h | Cmax: 2.8 vs. 1.7 µmol/L | ||||
| AUC: 23.8 vs. 20.0 µmol/L | ||||||
| Tmax: 1 vs. 5 h | ||||||
| 12 healthy adults (75% men)/cross-over | Soymilk | FS (93% aglycone): 99.8 µmol | Serum: 0–24 h | Total isoflavone | Total isoflavone FS > NFS until 8 h | Kano et al. [31] |
| NFS (99% glucoside): 101.3 µmol | Urine: 0–48 h | Cmax:2.0 vs. 1.0 µmol/L | No difference during 24 h of 48 h | |||
| AUC: 17.3 vs. 9.6 µmol/L | ||||||
| Tmax: 1 vs. 6 h | ||||||
| 16 women (PostMeno)/cross-over | Soymilk | FS: 64, 102, 172 mg | Serum: 0–24 h | - | No difference during 24 h at any of dose and day | Tsangalis et al. [32] |
| NFS: 68, 100, 169 mg | Urine: 0–24 h at day 4, 13, 14 |
FS, fermented soybean; NFS, non-fermented soybean; PostMeno, post menopause; Cmax, maximum plasma concentration; AUC, area under the curve; Tmax, time of the maximal plasma concertation.
In summary, fermentation was observed to increase the initial concentration of isoflavones in the blood after soy food intake. However, urinary total isoflavone was not affected by fermentation at 24 or 48 h after ingestion. Isoflavones are absorbed in the form of aglycone in the small intestine, and the composition of various glucosides can affect the absorption and metabolism of isoflavones. Unfortunately, the effects of chemical structure on bioavailability and biological benefits have not yet been assessed in detail. Thus, further studies are needed to develop isoflavone exposure biomarkers.
STUDIES ON ISOFLAVONE EXPOSURE BIOMARKERS
Exposure biomarkers provide objective exposure measures that explain bioavailability and metabolism after ingestion and are not vulnerable to bias reporting. Therefore, biomarkers can be used to improve the accuracy of assessments of isoflavone exposure [33]. Typically, high isoflavone levels in urine and blood are associated with soy consumption, indicating dietary isoflavone intake.
Table 2 shows recent studies on isoflavone exposure biomarkers in blood and urine. Recent isoflavone intakes estimated by 24-h recall showed a high correlation (r = 0.46–0.97) [34,35,36,37,38,38] with urinary isoflavones. However, the food frequency questionnaire (FFQ), which reflects habitual dietary intake, showed a weak correlation (r = 0.29; P < 0.01) with isoflavone concentrations in urine [36]. Chávez-Suárez et al. [37] reported that total isoflavone and individual isoflavone intakes estimated by FFQ showed no correlation to urinary levels (r = 0.002; P = 0.983), whereas 24-h recalls of total isoflavones (r = 0.460; P < 0.001) and genistein (r = 0.374; P = 0.002) were correlated to their urinary levels. Exposure biomarkers of isoflavones, as well as those of carotenoids, folate, cruciferous vegetables, and fruits showed moderately valued correlations (r = 0.30–0.49) with dietary intake [39]. The correlations between biomarkers and FFQ were slightly lower than those of the corresponding 24-h dietary recalls [39]. Further, the concentrations of isoflavone in urine or blood can be used to provide relative validation of isoflavone intake estimates from a self-reported FFQ [39,40,41,42,43].
Table 2. Studies on dietary isoflavones as biomarkers in blood or urine.
| Subject | Specimen | Biomarker | Dietary data (mg/day) | Correlation | Ref. |
|---|---|---|---|---|---|
| 24 pubertal girls | 12 h urine | ISO, DAI, GEN, GLY, Equol | 3 day 24 h recall | lSO: r = 0.72; P < 0.001 | Kim et al. [34] |
| O-DMA, DHDE, DHGE | ISO: 3.0–13.3 | DAI: r = 0.64; P < 0.01 | |||
| DAI: 1.2–5.3 | GEN: r = 0.62; P < 0.01 | ||||
| GEN: 1.4–6.2 | GLY: r = 0.57; P < 0.01 | ||||
| GLY: 0.3–1.8 | |||||
| 256 premenopausal women | 12 h urine | ISO | FFQ | r = 0.51; P < 0.001 | Morimoto et al. [35] |
| Low: 0.1–2.3 | |||||
| High: 49.8–74.6 | |||||
| 360 women | 2 × overnight urine (48 h apart) | ISO | 2 day 24 h recall | 24 h recall | Atkinson et al. [36] |
| DAI (ug): 5.0–6.4 | r = 0.52; P = 0.001 | ||||
| GEN (ug): 7.3–9.3 | FFQ: r = 0.29; P < 0.01 | ||||
| FFQ | |||||
| DAI (ug): 3.4–5.6 | |||||
| GEN (ug): 5.2–8.5 | |||||
| 100 healthy women | 12 h urine | ISO DAI, GEN, GLY | ISO | 24 h recall | Chávez-Suárez et al. [37] |
| Equol, Biochanin A | 24 h recall: 0.57 (GEN: 0.26) | ISO: r = 0.460; P < 0.001 | |||
| Formononetin | FFQ: 1.17 | GEN: r = 0.374; P = 0.002 | |||
| No sig. in FFQ | |||||
| 14 adults (14% men) | 24 h urine | ISO | 24 h weighed food record: 11.0 | Urine: r = 0.97; P < 0.001 | Ritchie et al. [38] |
| Plasma | 24 h recall: 12.3 | Plasma: r = 0.92; P < 0.001 | |||
| Measured diet vs. estimated: r = 0.98, P < 0.001 |
ISO, total isoflavone; GEN, genistein; DAI, daidzein; GLY, glycitein; O-DMA, O-desmethylangolensin; DHDE, dihydrodaidzein; DHGE, dihydrogenistein; FFQ, food frequency questionnaire.
Although the association between dietary isoflavone intake and urinary isoflavone excretion is weak at a low intake, urinary isoflavones can be used to discriminate between low- and high-soy diets across the population [35]. Following the study of relative validity and reproducibility of FFQ using 24-h dietary recalls and urinary biomarkers, the correlation between soy food intake and isoflavone levels in urine was different among ethnic groups in Asia [43]. Depending on the source and processing methods of soy food that contribute to isoflavone exposure, bioavailability may vary, leading to differences in urine or blood isoflavone levels.
RECENT STUDIES ON THE HEALTH EFFECTS OF SOY ISOFLAVONES
Five studies investigated the association of the biomarkers for dietary isoflavone with chronic disease and cancer risk (Table 3). Urinary isoflavone levels were not associated with CVD-related markers such as low-density lipoprotein-, high-density lipoprotein-cholesterol, and blood pressure [44]. A null association was also observed between urinary isoflavone and the risk of ischemic stroke [45]. The authors determined this was because urinary isoflavone excretion reflected short-term exposure, and participants showed differences in biological metabolism such as gut microbiota [45]. On the other hand, the levels of total isoflavone in urine showed no association with T2D, whereas higher daidzein levels in urine were associated with decreased risk of T2D (OR, 0.71; 95% CI, 0.55–0.93) [46]. Plasma isoflavone levels have been used as exposure biomarkers to predict the risk of prostate or liver cancer. Levels higher than the median of plasma genistein (> 640.2 nmol/L) were associated with a low risk of prostate cancer (OR, 0.31; 95% CI, 0.13–0.71) [47]. However, plasma genistein, daidzein, glycitein, and equol showed no association with primary liver cancer [48].
Table 3. The observational studies on the disease risk of dietary isoflavones by using level in biological samples as biomarker.
| Outcome | Subjects | Type of specimen | Biomarkers | Association1) | Study name2)/design | Ref. |
|---|---|---|---|---|---|---|
| CVD | 303 adults | 24 h urine | ISO | ↔ | CARDIAC/CS | Yamori et al. [44] |
| Ischemic stroke | 1,422 case; 1,422 control | Spot urine | GEN, DAI, GLY, O-DMA, DHGE, DHDE, equol | ↔ | SWHS/NCC | Yu et al. [45] |
| Type 2 diabetes | 1,111 case; 1,111 control | Spot urine | ISO, GEN, DAI, O-DMA, DHGE, DHDE | ↓ (only DAI) | NHS/NCC | Ding et al. [46] |
| Prostate cancer | 46 case; 54 control | Plasma | GEN | ↓ | CC | Wu et al. [47] |
| Liver cancer | 18,628 (34% men) | Plasma | GEN, DAI, GLY, equol | ↔ | JPHC/NCC | Michikawa et al. [48] |
| 90 case; 175 control (69% men) |
CVD, cardiovascular disease; ISO, total isoflavone; GEN, genistein; DAI, daidzein; GLY, glycitein; CS, cross-sectional; FFQ, food frequency questionnaire; Q, quartile or quintile; NCC, nested case-control; HR, hazard ratio; CI, confidence interval; O-DMA, O-desmethylangolensin; DHGE, dihydrogenistein; DHDE, dihydrodaidzein; WHO, World Health Organization; CARDIAC, Cardiovascular Disease and Alimentary Comparison; SWHS, The Shanghai Women's Health Study; NHS, Nurses' Health Study; JPHC, The Japan Public Health Center-based prospective.
1)↑ positive association, ↓ inversely association, ↔ no association; 2)WHO-coordinated CARDIAC, SWHS, NHS, JPHC.
The exposure biomarkers for isoflavones proposed in recent studies were presented as total isoflavone or aglycone (parent isoflavone compound) equivalent doses. Most of the conjugated forms of dietary soy isoflavones in blood or urine after absorption are sulfates or glucuronides [24]. Circulating isoflavone conjugates may act as a source of aglycone at specific target tissues [26]. However, the health effects and mechanisms of action of conjugated metabolites in humans remain unclear. In addition, recent studies may be limited due to possible measurement errors, variable food sources, and differing levels of isoflavone intake among populations. The effects of isoflavone on chronic disease and cancer risk merit further study using both biomarkers and data on dietary intake.
CONCLUSION
The composition of isoflavone in soy foods differs based on the method of processing, fermentation conditions, and the microorganisms present. Additionally, bioavailability may vary depending on the soy food source, processing methods, and individual differences in biological metabolism. These factors lead to differences in urine or blood isoflavone concentrations. Human metabolites of isoflavone can be used as biomarkers to improve the accuracy of exposure assessments for dietary isoflavone. However, the effects of chemical structures on bioavailability and biological benefits have not yet been assessed in detail. Therefore, these factors are rarely considered in epidemiological and clinical studies, and their health impacts remain unclear.
Most studies on isoflavone showed that the biologically active molecules are either aglycones or their unconjugated metabolites. However, the conjugates may be considered either biologically active themselves or serve as excellent sources of biologically active compounds within target cells by deconjugation. Data on human metabolites without enzymatic hydrolysis of conjugates in blood and urine are not yet sufficient to understand the exposure biomarkers of soy isoflavone intake. However, direct quantification of conjugates in biological samples without enzymatic hydrolysis would pose a major challenge. It will be valuable to develop exposure biomarkers in future studies on soy isoflavones for different types of soy foods in order to fully understand the association between various soy foods and risks of disease.
Footnotes
Funding: This research was supported by a research program for Agricultural Science & Technology Development from National Institute of Agricultural Science (NAS)-Rural Development Administration (RDA), Korea (grant number: PJ 01308801).
Conflict of Interest: The authors declare no potential conflicts of interests.
- Writing - original draft: Jang HH.
- Writing - review & editing: Lee YM, Choe JS, Kwon O.
References
- 1.Praticò G, Gao Q, Scalbert A, Vergères G, Kolehmainen M, Manach C, Brennan L, Pedapati SH, Afman LA, Wishart DS, Vázquez-Fresno R, Andres-Lacueva C, Garcia-Aloy M, Verhagen H, Feskens EJ, Dragsted LO. Guidelines for Biomarker of Food Intake Reviews (BFIRev): how to conduct an extensive literature search for biomarker of food intake discovery. Genes Nutr. 2018;13:3. doi: 10.1186/s12263-018-0592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon O. A big picture view of precision nutrition: from reductionism to holism. J Nutr Health. 2019;52:1–5. [Google Scholar]
- 3.Gao Q, Praticò G, Scalbert A, Vergères G, Kolehmainen M, Manach C, Brennan L, Afman LA, Wishart DS, Andres-Lacueva C, Garcia-Aloy M, Verhagen H, Feskens EJ, Dragsted LO. A scheme for a flexible classification of dietary and health biomarkers. Genes Nutr. 2017;12:34. doi: 10.1186/s12263-017-0587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi L, Brunius C, Bergdahl IA, Johansson I, Rolandsson O, Donat Vargas C, Kiviranta H, Hanhineva K, Åkesson A, Landberg R. Joint analysis of metabolite markers of fish intake and persistent organic pollutants in relation to type 2 diabetes risk in Swedish adults. J Nutr. 2019;149:1413–1423. doi: 10.1093/jn/nxz068. [DOI] [PubMed] [Google Scholar]
- 5.Loftfield E, Rothwell JA, Sinha R, Keski-Rahkonen P, Robinot N, Albanes D, Weinstein SJ, Derkach A, Sampson J, Scalbert A, Freedman ND. Prospective investigation of serum metabolites, coffee drinking, liver cancer incidence, and liver disease mortality. J Natl Cancer Inst. 2020;112:286–294. doi: 10.1093/jnci/djz122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lampe JW. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J Nutr. 2003;133(Suppl 3):956S–964S. doi: 10.1093/jn/133.3.956S. [DOI] [PubMed] [Google Scholar]
- 7.Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008;19:73–82. doi: 10.1016/j.copbio.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Maruvada P, Lampe JW, Wishart DS, Barupal D, Chester DN, Dodd D, Djoumbou-Feunang Y, Dorrestein PC, Dragsted LO, Draper J, Duffy LC, Dwyer JT, Emenaker NJ, Fiehn O, Gerszten RE, Hu FB, Karp RW, Klurfeld DM, Laughlin MR, Little AR, Lynch CJ, Moore SC, Nicastro HL, O'Brien DM, Ordovás JM, Osganian SK, Playdon M, Prentice R, Raftery D, Reisdorph N, Roche HM, Ross SA, Sang S, Scalbert A, Srinivas PR, Zeisel SH. Perspective: dietaryiomarkers of intake and exposure-exploration with omics approaches. Adv Nutr. 2020;11:200–215. doi: 10.1093/advances/nmz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinzmann SS, Holmes E, Kochhar S, Nicholson JK, Schmitt-Kopplin P. Correction to 2-Furoylglycine as a Candidate Biomarker of Coffee Consumption. J Agric Food Chem. 2016;64:8958. doi: 10.1021/acs.jafc.6b04833. [DOI] [PubMed] [Google Scholar]
- 10.Cheung W, Keski-Rahkonen P, Assi N, Ferrari P, Freisling H, Rinaldi S, Slimani N, Zamora-Ros R, Rundle M, Frost G, Gibbons H, Carr E, Brennan L, Cross AJ, Pala V, Panico S, Sacerdote C, Palli D, Tumino R, Kühn T, Kaaks R, Boeing H, Floegel A, Mancini F, Boutron-Ruault MC, Baglietto L, Trichopoulou A, Naska A, Orfanos P, Scalbert A. A metabolomic study of biomarkers of meat and fish intake. Am J Clin Nutr. 2017;105:600–608. doi: 10.3945/ajcn.116.146639. [DOI] [PubMed] [Google Scholar]
- 11.Andersen MB, Reinbach HC, Rinnan Å, Barri T, Mithril C, Dragsted LO. Discovery of exposure markers in urine for Brassica-containing meals served with different protein sources by UPLC-qTOF-MS untargeted metabolomics. Metabolomics. 2013;9:984–997. [Google Scholar]
- 12.Urpi‐Sarda M, Boto‐Ordóñez M, Queipo‐Ortuño MI, Tulipani S, Corella D, Estruch R, Tinahones FJ, Andres‐Lacueva C. Phenolic and microbial‐targeted metabolomics to discovering and evaluating wine intake biomarkers in human urine and plasma. Electrophoresis. 2015;36:2259–2268. doi: 10.1002/elps.201400506. [DOI] [PubMed] [Google Scholar]
- 13.Mitry P, Wawro N, Rohrmann S, Giesbertz P, Daniel H, Linseisen J. Plasma concentrations of anserine, carnosine and pi-methylhistidine as biomarkers of habitual meat consumption. Eur J Clin Nutr. 2019;73:692–702. doi: 10.1038/s41430-018-0248-1. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Aloy M, Llorach R, Urpi-Sarda M, Tulipani S, Salas-Salvadó J, Martínez-González MA, Corella D, Fitó M, Estruch R, Serra-Majem L, Andres-Lacueva C. Nutrimetabolomics fingerprinting to identify biomarkers of bread exposure in a free-living population from the PREDIMED study cohort. Metabolomics. 2015;11:155–165. [Google Scholar]
- 15.Zhu Y, Wang P, Sha W, Sang S. Urinary biomarkers of whole grain wheat intake identified by non-targeted and targeted metabolomics approaches. Sci Rep. 2016;6:36278. doi: 10.1038/srep36278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miles FL, Lloren JI, Haddad E, Jaceldo-Siegl K, Knutsen S, Sabate J, Fraser GE. Plasma, urine, and adipose tissue biomarkers of dietary intake differ between vegetarian and non-vegetarian diet groups in the Adventist Health Study-2. J Nutr. 2019;149:667–675. doi: 10.1093/jn/nxy292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbaraly T, Würtz P, Singh-Manoux A, Shipley MJ, Haapakoski R, Lehto M, Desrumaux C, Kähönen M, Lehtimäki T, Mikkilä V, Hingorani A, Humphries SE, Kangas AJ, Soininen P, Raitakari O, Ala-Korpela M, Kivimäki M. Association of circulating metabolites with healthy diet and risk of cardiovascular disease: analysis of two cohort studies. Sci Rep. 2018;8:8620. doi: 10.1038/s41598-018-26441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setchell KD. Absorption and metabolism of soy isoflavones-from food to dietary supplements and adults to infants. J Nutr. 2000;130:654S–655S. doi: 10.1093/jn/130.3.654S. [DOI] [PubMed] [Google Scholar]
- 19.de Pascual-Teresa S, Hallund J, Talbot D, Schroot J, Williams CM, Bugel S, Cassidy A. Absorption of isoflavones in humans: effects of food matrix and processing. J Nutr Biochem. 2006;17:257–264. doi: 10.1016/j.jnutbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Hendrich S, Murphy PA. Glucuronides are the main isoflavone metabolites in women. J Nutr. 2003;133:399–404. doi: 10.1093/jn/133.2.399. [DOI] [PubMed] [Google Scholar]
- 21.Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer. 2007;57:1–10. doi: 10.1080/01635580701267677. [DOI] [PubMed] [Google Scholar]
- 22.Setchell KD. The history and basic science development of soy isoflavones. Menopause. 2017;24:1338–1350. doi: 10.1097/GME.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 23.Zeng M, Sun R, Basu S, Ma Y, Ge S, Yin T, Gao S, Zhang J, Hu M. Disposition of flavonoids via recycling: Direct biliary excretion of enterically or extrahepatically derived flavonoid glucuronides. Mol Nutr Food Res. 2016;60:1006–1019. doi: 10.1002/mnfr.201500692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol. 2010;8:89–98. doi: 10.1089/lrb.2009.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee DH, Kim MJ, Ahn J, Lee SH, Lee H, Kim JH, Park SH, Jang YJ, Ha TY, Jung CH. Nutrikinetics of isoflavone metabolites after fermented soybean product (cheonggukjang) ingestion in ovariectomized mice. Mol Nutr Food Res. 2017;61:1700322. doi: 10.1002/mnfr.201700322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002;76:588–594. doi: 10.1093/ajcn/76.3.588. [DOI] [PubMed] [Google Scholar]
- 27.Ronis MJ, Little JM, Barone GW, Chen G, Radominska-Pandya A, Badger TM. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J Med Food. 2006;9:348–355. doi: 10.1089/jmf.2006.9.348. [DOI] [PubMed] [Google Scholar]
- 28.D'Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R. Bioavailability of the polyphenols: status and controversies. Int J Mol Sci. 2010;11:1321–1342. doi: 10.3390/ijms11041321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yonemoto-Yano H, Maebuchi M, Fukui K, Tsuzaki S, Takamatsu K, Uehara M. Malonyl isoflavone glucosides are chiefly hydrolyzed and absorbed in the colon. J Agric Food Chem. 2014;62:2264–2270. doi: 10.1021/jf404378r. [DOI] [PubMed] [Google Scholar]
- 30.Okabe Y, Shimazu T, Tanimoto H. Higher bioavailability of isoflavones after a single ingestion of aglycone-rich fermented soybeans compared with glucoside-rich non-fermented soybeans in Japanese postmenopausal women. J Sci Food Agric. 2011;91:658–663. doi: 10.1002/jsfa.4228. [DOI] [PubMed] [Google Scholar]
- 31.Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006;136:2291–2296. doi: 10.1093/jn/136.9.2291. [DOI] [PubMed] [Google Scholar]
- 32.Tsangalis D, Wilcox G, Shah NP, Stojanovska L. Bioavailability of isoflavone phytoestrogens in postmenopausal women consuming soya milk fermented with probiotic bifidobacteria. Br J Nutr. 2005;93:867–877. doi: 10.1079/bjn20041299. [DOI] [PubMed] [Google Scholar]
- 33.Rienks J, Barbaresko J, Nöthlings U. Association of isoflavone biomarkers with risk of chronic disease and mortality: a systematic review and meta-analysis of observational studies. Nutr Rev. 2017;75:616–641. doi: 10.1093/nutrit/nux021. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Kim HJ, Joung H, Park MK, Li S, Song Y, Franke AA, Paik HY. Overnight urinary excretion of isoflavones as an indicator for dietary isoflavone intake in Korean girls of pubertal age. Br J Nutr. 2010;104:709–715. doi: 10.1017/S0007114510000978. [DOI] [PubMed] [Google Scholar]
- 35.Morimoto Y, Beckford F, Franke AA, Maskarinec G. Urinary isoflavonoid excretion as a biomarker of dietary soy intake during two randomized soy trials. Asia Pac J Clin Nutr. 2014;23:205–209. doi: 10.6133/apjcn.2014.23.2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkinson C, Skor HE, Fitzgibbons ED, Scholes D, Chen C, Wähälä K, Schwartz SM, Lampe JW. Overnight urinary isoflavone excretion in a population of women living in the United States, and its relationship to isoflavone intake. Cancer Epidemiol Biomarkers Prev. 2002;11:253–260. [PubMed] [Google Scholar]
- 37.Chávez-Suárez KM, Ortega-Vélez MI, Valenzuela-Quintanar AI, Galván-Portillo M, López-Carrillo L, Esparza-Romero J, Saucedo-Tamayo MS, Robles-Burgueño MR, Palma-Durán SA, Gutiérrez-Coronado ML, Campa-Siqueiros MM, Grajeda-Cota P, Caire-Juvera G. Phytoestrogen Concentrations in Human Urine as Biomarkers for Dietary Phytoestrogen Intake in Mexican Women. Nutrients. 2017;9:1078. doi: 10.3390/nu9101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ritchie MR, Morton MS, Deighton N, Blake A, Cummings JH. Plasma and urinary phyto-oestrogens as biomarkers of intake: validation by duplicate diet analysis. Br J Nutr. 2004;91:447–457. doi: 10.1079/BJN20031062. [DOI] [PubMed] [Google Scholar]
- 39.Fraser GE, Jaceldo-Siegl K, Henning SM, Fan J, Knutsen SF, Haddad EH, Sabaté J, Beeson WL, Bennett H. Biomarkers of dietary intake are correlated with corresponding measures from repeated dietary recalls and food-frequency questionnaires in the Adventist Health Study-2. J Nutr. 2016;146:586–594. doi: 10.3945/jn.115.225508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heald CL, Bolton-Smith C, Ritchie MR, Morton MS, Alexander FE. Phyto-oestrogen intake in Scottish men: use of serum to validate a self-administered food-frequency questionnaire in older men. Eur J Clin Nutr. 2006;60:129–135. doi: 10.1038/sj.ejcn.1602277. [DOI] [PubMed] [Google Scholar]
- 41.Tseng M, Olufade T, Kurzer MS, Wähälä K, Fang CY, van der Schouw YT, Daly MB. Food frequency questionnaires and overnight urines are valid indicators of daidzein and genistein intake in U.S. women relative to multiple 24-h urine samples. Nutr Cancer. 2008;60:619–626. doi: 10.1080/01635580801993751. [DOI] [PubMed] [Google Scholar]
- 42.French MR, Thompson LU, Hawker GA. Validation of a phytoestrogen food frequency questionnaire with urinary concentrations of isoflavones and lignan metabolites in premenopausal women. J Am Coll Nutr. 2007;26:76–82. doi: 10.1080/07315724.2007.10719588. [DOI] [PubMed] [Google Scholar]
- 43.Whitton C, Ho JC, Tay Z, Rebello SA, Lu Y, Ong CN, van Dam RM. Relative validity and reproducibility of a food frequency questionnaire for assessing dietary intakes in a multi-ethnic Asian population using 24-h dietary recalls and biomarkers. Nutrients. 2017;9:1059. doi: 10.3390/nu9101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamori Y, Sagara M, Arai Y, Kobayashi H, Kishimoto K, Matsuno I, Mori H, Mori M. Soy and fish as features of the Japanese diet and cardiovascular disease risks. PLoS One. 2017;12:e0176039. doi: 10.1371/journal.pone.0176039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu D, Shu XO, Li H, Yang G, Cai Q, Xiang YB, Ji BT, Franke AA, Gao YT, Zheng W, Zhang X. Dietary isoflavones, urinary isoflavonoids, and risk of ischemic stroke in women. Am J Clin Nutr. 2015;102:680–686. doi: 10.3945/ajcn.115.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ding M, Franke AA, Rosner BA, Giovannucci E, van Dam RM, Tworoger SS, Hu FB, Sun Q. Urinary isoflavonoids and risk of type 2 diabetes: a prospective investigation in US women. Br J Nutr. 2015;114:1694–1701. doi: 10.1017/S0007114515003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu Y, Zhang L, Na R, Xu J, Xiong Z, Zhang N, Dai W, Jiang H, Ding Q. Plasma genistein and risk of prostate cancer in Chinese population. Int Urol Nephrol. 2015;47:965–970. doi: 10.1007/s11255-015-0981-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michikawa T, Inoue M, Sawada N, Tanaka Y, Yamaji T, Iwasaki M, Shimazu T, Sasazuki S, Mizokami M, Tsugane S The Japan Public Health Center-based Prospective Study Group. Plasma isoflavones and risk of primary liver cancer in Japanese women and men with hepatitis virus infection: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2015;24:532–537. doi: 10.1158/1055-9965.EPI-14-1118. [DOI] [PubMed] [Google Scholar]