Abstract
Objective
To evaluate the utility of sentinel lymph node mapping (SLN) in endometrial cancer (EC) patients in comparison with lymphadenectomy (LND).
Methods
Comprehensive search was performed in MEDLINE, EMBASE, CENTRAL, OVID, Web of science databases, and three clinical trials registration websites, from the database inception to September 2020. The primary outcomes covered operative outcomes, nodal assessment, and oncological outcomes. Software Revman 5.3 was used. Trial sequential analysis (TSA) and Grading of Recommendations Assessment, Development, and Evaluation (GRADE) were performed.
Results
Overall, 5,820 EC patients from 15 studies were pooled in the meta-analysis: SLN group (N = 2,152, 37.0%), LND group (N = 3,668, 63.0%). In meta-analysis of blood loss, SLN offered advantage over LND in reducing operation bleeding (I2 = 74%, P<0.01). Z-curve of blood loss crossed trial sequential monitoring boundaries though did not reach TSA sample size. There was no difference between SLN and LND in intra-operative complications (I2 = 7%, P = 0.12). SLN was superior to LND in detecting positive pelvic nodes (P-LN) (I2 = 36%, P<0.001), even in high risk patients (I2 = 36%, P = 0.001). While no difference was observed in detection of positive para-aortic nodes (PA-LN) (I2 = 47%, P = 0.76), even in high risk patients (I2 = 62%, P = 0.34). Analysis showed no difference between two groups in the number of resected pelvic nodes (I2 = 99%, P = 0.26). SLN was not associated with a statistically significant overall survival (I2 = 79%, P = 0.94). There was no difference in progression-free survival between SLN and LND (I2 = 52%, P = 0.31). No difference was observed in recurrence. Based on the GRADE assessment, we considered the quality of current evidence to be moderate for P-LN biopsy, low for items like blood loss, PA-LN positive.
Conclusion
The present meta-analysis underlines that SLN is capable of reducing blood loss during operation in regardless of surgical approach with firm evidence from TSA. SLN mapping is more targeted for less node dissection and more detection of positive lymph nodes even in high risk patients with conclusive evidence from TSA. Utility of SLN yields no survival detriment in EC patients.
Keywords: endometrial cancer, sentinel node mapping, lymphadenectomy, operation, lymph node assessment, oncological outcome
Highlights
SLN is capable of reducing blood loss during operation in regardless of surgical approach with firm evidence from TSA.
SLN mapping is more targeted for less node dissection and more detection of positive lymph nodes even in high risk patients with conclusive evidence from TSA.
Utility of SLN yields no survival detriment in EC patients.
Introduction
Endometrial cancer (EC) is the most common gynecological malignancy in developed countries, and an estimated 65,620 new cases in United States in 2020 (1). The disease incidence has been climbing by 1.5 times over the last 10 years, and the death cases have increased by 58.4% according to latest statistics (1, 2). Though 5-year overall survival (OS) has reached at 80%, it has not made any progress since 1985, estimated in the US Surveillance, Epidemiology, and End Results (SEER) database (3).
Surgical staging is the step of final diagnosis and first treatment in most EC patients, and the standard operation includes hysterectomy, bilateral salpingo-oophorectomy, and lymph node assessment, allowing prognostic stratification and potentially benefited patients identification (3).
Lymph node status is a definite prognostic factor, albeit clinical trials showed no survival benefit in patients with nodal examination versus those not (4, 5). Ongoing controversy remains the extent of nodal dissection to tailor post-operation therapy. Traditional lymph node assessment contains systematic pelvic ± para-aortic lymphadenectomy (LND), and given low lymph nodal involvement rate, LND is prone to cause overtreatment and thus more surgery-related complications like lymphedema (6).
Sentinel lymph node mapping (SLN) has emerged as a reliable alternative in EC nodal assessment. Accumulating studies have demonstrated SLN was equal to LND in low- and high-risk EC patients and oncological outcomes were similar in both SLN and LND groups (7, 8). It has been recommended in low- and high-risk EC patients for surgical staging procedures in 2020 National Comprehensive Cancer Network (NCCN) guidelines (9). The superiority of SLN lies in pathological ultra-staging to avoid overtreatment and undertreatment.
A previous meta-analysis indicated SLN was superior to LND in nodal assessment (10), but given its limited data, further discussion about operative and oncological outcomes is still needed. The aim of this meta-analysis was to systematically review current evidence in comparison of two nodal assessment technologies, SLN and LND, in EC patients. The main outcomes contain surgery-related outcomes, nodal assessment, and oncological outcomes.
Materials and Methods
This analysis has been registered in the International Prospective Register of Systematic Reviews (PROSPERO, https://www.crd.york.ac.uk/prospero, ID: CRD42020175099). And this meta-analysis was completed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11).
Search Strategy
Comprehensive search was performed in MEDLINE, EMBASE, CENTRAL, OVID, Web of science databases, from the database inception to September 2020. The key words included “endometrial cancer,” “sentinel node,” and “lymphadenectomy”. And three clinical trials registration websites, the Clinical trials.gov (www.clinicaltrials.com), WHO trial website (https://apps.who.int/trialsearch), and the Controlled Trials meta Register (www.controlled-trials.com), were searched as well. Details of search strategy is shown in Supplement Files S1.
Inclusion and Exclusion Criteria
Two independent reviewers (YG and HC) conducted selection of studies based on a protocol defined priorly. Studies were included if they met the following criteria: 1) patients diagnosed with endometrial cancer; 2) clinical trials concerning the comparison of sentinel node mapping and lymphadenectomy; 3) reported operative outcomes like operative time, blood loss, operative complications; lymph nodes assessment like the number of positive pelvic lymph nodes; oncological outcomes like overall survival and recurrence, but not limited to these above. The exclusion criteria as: 1) <10 patients; 2) review, case report, comment, and other types without original data; 3) full text could not be obtained; 4) written other than in English. At first screening, titles and abstracts of articles were assessed according to inclusion and exclusion criteria. Then full texts were read to identify eligibility. Consensus was made by discussion when disagreement occurring.
Data Extraction
Data were extracted using a modified form based on the Cochrane reviews handbook. The following information was collected: author, year of publication, study design, patients’ characteristics, surgical approach, SLN technique, operative outcomes, nodal assessment and oncological outcomes, and so forth. Two reviewers (YG and LZ) conducted date extraction independently, and inconformity was resolved by discussion.
Quality Assessment
The Newcastle-Ottawa scale for cohort study was used for article quality assessment. This scale is comprised of three parts (selection, comparability, and outcome). With a maximum of nine stars, articles reaching six stars were included finally. Two reviewers (YG and YK) assessed articles independently, and consensus was reached by discussion in the event of disparity (Supplement Files S2).
Statistical Analysis
Software Revman 5.3 was used to pool data and generate forest plots. Mantel-Haenszel method was used in dichotomous data and the odds ratio (OR) was calculated. And for continuous data inverse variance and mean difference (MD) were applied. Random-effect model was used in analysis. Heterogeneity of included studies was assessed by I2 and I2>50% was defined as high heterogeneity. Subgroup analysis by SLN procedure or patients risk stratification was introduced when meeting high heterogeneity. When necessary, data, like operative time or blood loss, in form of (median, range) were transformed into (mean, standard difference) according to recommended methods (12). Hazard ratio (HR) and 95% confidence interval (CI) of overall survival (OS) and progression-free survival (PFS) were extracted from Kaplan-Meier curve using Engauge Digitizer software (10.7) and recommended methods (13). And for data failing to conducting meta-analysis, a narrative systemic review was performed. Trial sequential analysis (TSA) was performed by TSA software (version 0.9β) and we calculated sample size adjusted for this meta-analysis to testify whether the evidence is confirmed and conclusive. Pooled analysis was graded by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach, and the certainty of evidence was assessed as high, moderate, low, or very low, using GRADE pro website (https://gdt.gradepro.org).
Results
A total of 2,048 articles were screened through the search strategy, and 21 articles were included after full text reading. Four were excluded for low quality assessment score (<6) (14–17), two were excluded for patients overlapping (18, 19), thus leaving 15 articles eligible for final analysis (7, 20–33) (Figure 1). Characteristics of the 15 studies are summarized in Supplement Table S1. Overall, 5,820 EC patients were pooled in the meta-analysis: SLN group (N = 2,152, 37.0%), LND group (N = 3,668, 63.0%), respectively.
Figure 1.
Selection of studies for inclusion in the systematic review.
Operative Outcomes
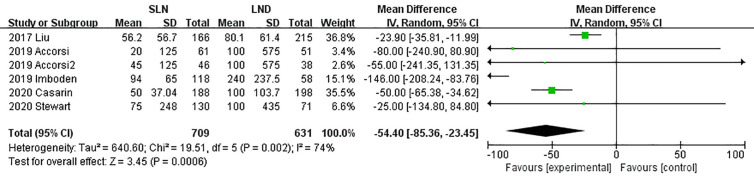
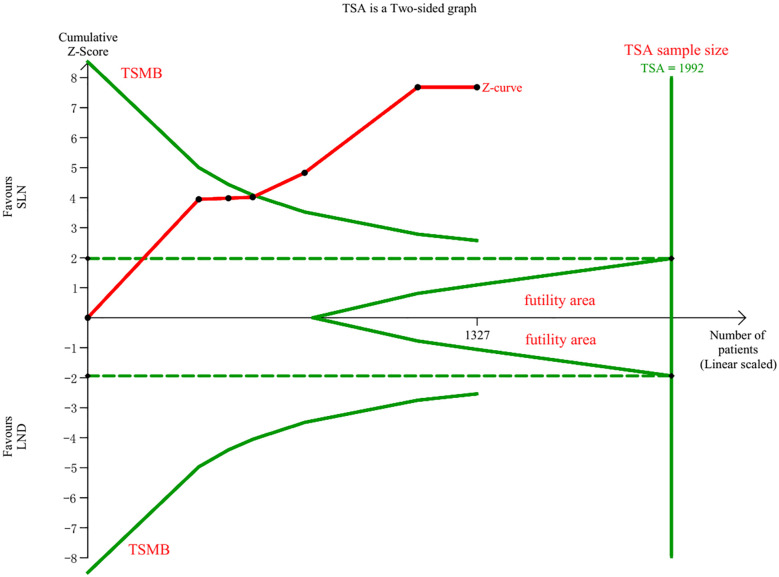
Data regarding operation related outcomes were available in seven studies (Supplement Table S2). In meta-analysis of blood loss, SLN offered advantage over LND in reducing operation bleeding; the MD was −54.40, 95% CI −85.36~−23.45 (I2 = 74%, P<0.001; Figure 2). Z-curve of blood loss crossed trial sequential monitoring boundaries (TSMB) though did not reach TSA sample size, and indicating the result was true-positive (Figure 3). When intra-operative complications were measured, there was no difference between SLN and LND (I2 = 7%, P=0.11, Figure 4).
Figure 2.
Meta-analysis of blood loss.
Figure 3.
TSA of blood loss, α = 0.05, β = 0.8, two-sided test.
Figure 4.
Meta-analysis of intra-operative complications.
When operative time was pooled, subgroup analysis failed to identify high heterogeneity (I2 = 99%, P<0.01, Supplement Figure 1A), but a tendency of shorter operative time in SLN group was shown in Supplement Table S2. And TSA of operative time showed Z-curve crossed TSMB and highly surpassed TSA sample size (Supplement Figure 1B). Post-operative complications were assessed by Accordion Severity Grading System, Clavien-Dindo scale and MSKCC’s Surgical Secondary Events Grading System. It seemed that SLN group had lower post-operative complications but more data are needed to conduct further analysis. When considering conversion rate, re-admission, re-operation, length of stay and frozen utility, potential advantage of SLN could be seen in shortening length of stay and frozen utility (Supplement Figures 1C, D). And TSA of length of stay showed inconclusive result for insufficient sample size (Supplement Figure 1E). Additionally, TSA of post-operative complications, conversate rate, re-admission, re-operation, and frozen utility were available for low sample size.
Lymph Node Assessment
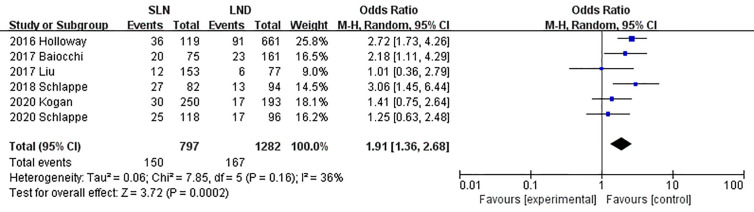
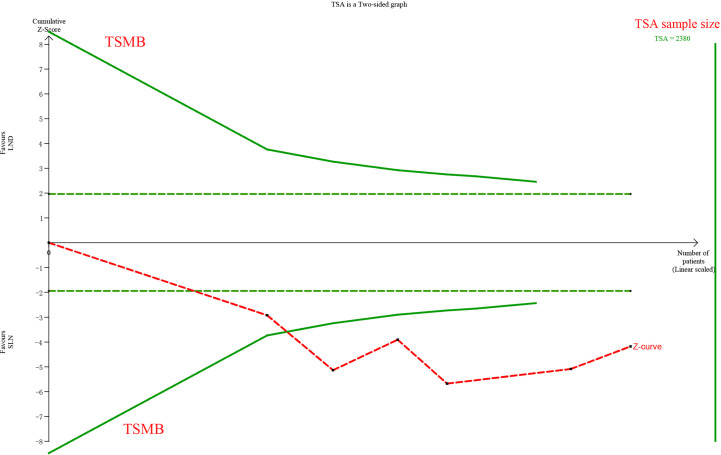
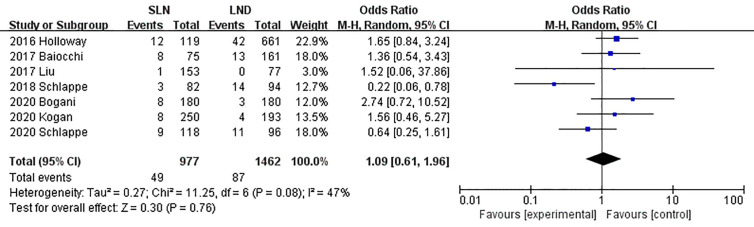
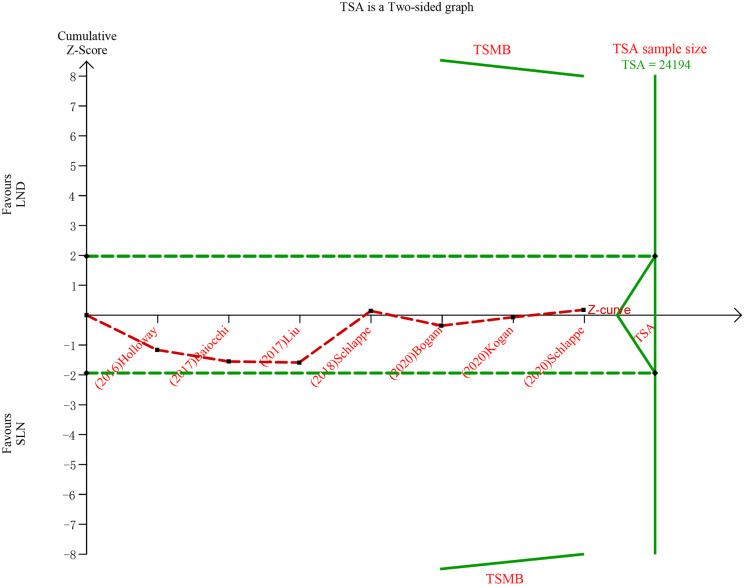
The meta-analysis of nodal assessment was based on 10 trials (Supplement Table S3). SLN was superior to LND in detecting positive pelvic lymph nodes (I2 = 36%, P<0.001, Figure 5). The Z-curve crossed TSMB and did not reach TSA sample size, and indicating the result was conclusive (Figure 6). While no difference was observed in detection of positive para-aortic nodes between two groups (I2 = 47%, P = 0.76, Figure 7); and Z-curve did not cross TSMB and did not reach TSA sample size, and indicating the result was under discussion (Figure 8). In high risk patients, SLN had a higher pelvic nodes detection rate (OR 2.00, 95% CI 1.21~3.32, I2 = 36%, P = 0.007, Supplement Figure 2A) and showed no difference in para-aortic nodes detection (OR 0.62, 95% CI 0.24~1.64, I2 = 62%, P = 0.34, Supplement Figure 2B).
Figure 5.
Meta-analysis of pelvic lymph nodes positive.
Figure 6.
TSA of pelvic lymph nodes, α = 0.05, β = 0.8, relative risk reduction = −73.8%, incidence in control group = 16.4%, two-sided test.
Figure 7.
Meta-analysis of para-aortic lymph nodes positive.
Figure 8.
TSA of para-aortic lymph nodes, α = 0.05, β = 0.8, relative risk reduction = 15.4%, incidence in control group = 9.2%, two-sided test.
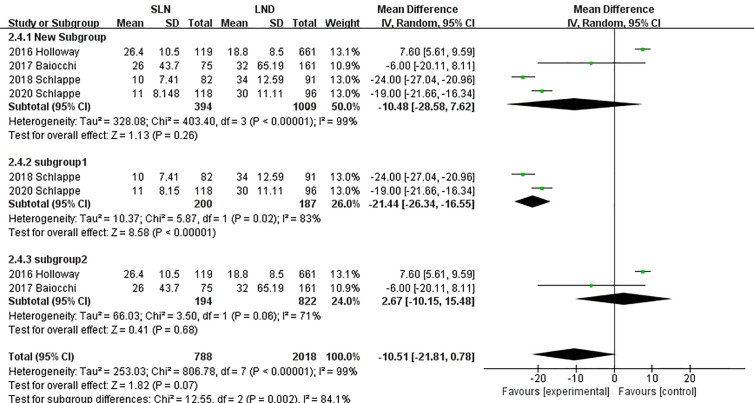
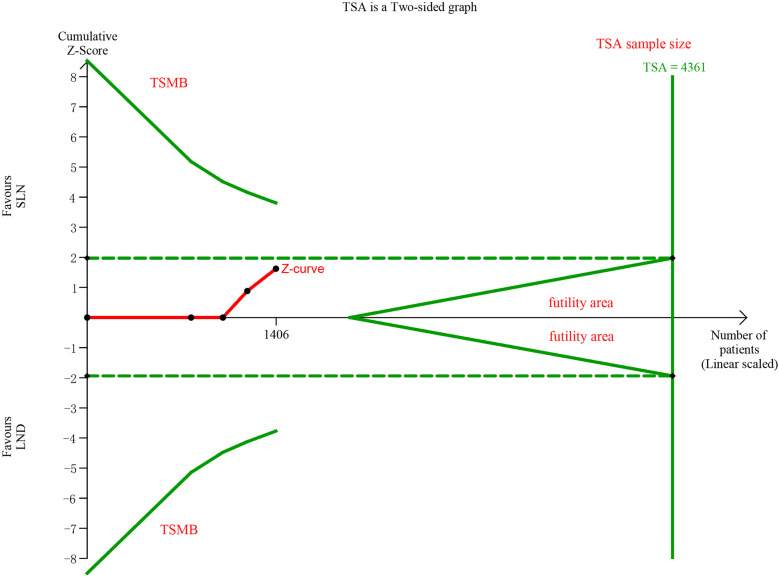
In pooling data of resected pelvic nodes, analysis showed no difference between two groups (I2 = 99%, P = 0.26). Considering two SLN algorithm (subgroup1 SLN ± P-LND ± PA-LND; subgroup2 SLN+P-LND ± PA-LND) existed, subgroup analysis by SLN procedure was conducted and indicated that SLN procedure (SLN ± P-LND ± PA-LND) removed less pelvic nodes than LND(I2 = 83%, P<0.01, Figure 9), and Z-curve did not cross TSMB and did not reach TSA sample size, and indicating more studies were needed (Figure 10). The same subgroup analysis was undergone in pooling data of resected para-aortic nodes as well, and similar result was observed that SLN procedure removed less para-aortic nodes (I2 = 0%, P<0.001, Figure 11), and Z-curve did not cross TSMB and did not reach TSA sample size, and indicating more studies were needed (Figure 12).
Figure 9.
Meta-analysis of pelvic lymph nodes removed.
Figure 10.
TSA of pelvic lymph nodes removed, α = 0.05, β = 0.8, two-sided test.
Figure 11.
Meta-analysis of para-aortic lymph nodes removed.
Figure 12.
TSA of para-aortic lymph nodes removed, α = 0.05, β = 0.8, two-sided test.
Oncological Outcomes
Supplement Table S4 reports data concerning disease characteristics. SLN was not associated with a statistically significant OS (I2 = 79%, P = 0.81, Supplement Figure 3A). There was no difference in PFS between SLN and LND groups (I2 = 52%, P = 0.31, Supplement Figure 3B). No difference was observed in overall recurrence (all sites, I2 = 75%, P = 0.41, Supplement Figure 3C), and Z-curve did not cross TSMB and did not reach TSA sample size, and indicating more studies were needed (Supplement Figure 3D).
In terms of recurrence pattern, analysis of nodal recurrence, locoregional recurrence, and multifocal recurrence showed no differences between SLN and LND (P>0.05); TSA showed further studies were needed (Supplement Figures 4A–E). Data on death of disease was available for two trials, and meta-analysis showed no difference between two groups (P>0.05, Supplement Figure 5).
GRADE Assessment
Based on the GRADE assessment, we considered the quality of current evidence to be moderate for P-LN biopsy, low for items like blood loss, PA-LN positive. We postulated that because of basis on cohort studies, the grading hardly reached higher (Table 1).
Table 1.
GRADE evidence profile: Quality assessment.
| Certainty assessment | Number of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | SLN(n) | LND(n) | Relative(95% CI) | Absolute(95% CI) | ||
| Operation time | ||||||||||||
| 8 | Observational studies | Not serious | Seriousa | Not serious | Not serious | None | 959 | 2,243 | – | MD 75.37 lower (106.36 lower to 44.38 lower) |
⨁

 VERY LOW |
CRITICAL |
| Blood loss | ||||||||||||
| 6 | Observational studies | Not serious | Not serious | Not serious | Not serious | None | 709 | 631 | – | MD 54.4 lower (85.36 lower to 23.45 lower) |
⨁⨁
 LOW |
CRITICAL |
| P-LN positive | ||||||||||||
| 6 | Observational studies | Not serious | Not serious | Not serious | Not serious | strong associationb | 150/797 (18.8%)c | 167/1,282 (13.0%) | OR 1.91 (1.36 to 2.68) |
92 more per 1,000 (from 39 more to 156 more) |
⨁⨁⨁ MODERATE |
CRITICAL |
| PA-LN positive | ||||||||||||
| 7 | Observational studies | Not serious | Not serious | Not serious | Not serious | None | 49/977 (5.0%) | 87/1,462 (6.0%) | OR 1.09 (0.61to 1.96) |
5 fewer per 1,000 (from 21 fewer to 51 more) |
⨁⨁
 LOW |
CRITICAL |
GRADE, Grading of recommendation assessment, development, and evaluation; n, number of patients; CI, Confidence interval; MD, Mean difference; RR, Risk ratio; OR, Odds ratio; HR, Hazard Ratio; aHeterogeneity = 99%; bRR>2; c[n/total n (%)].
Discussion
The analysis reviewed present evidence in comparison of SLN and LND in EC patients, and the main findings covered operation-related outcomes, nodal assessment, and oncological outcomes. To our knowledge, this is the first meta-analysis discussing surgery-related outcomes, and found SLN was capable to reduce blood loss with firm evidence from TSA. The pooled date validates that SLN allows an accurate detection of positive lymph nodes in circumstance of less node removal, especially with conclusive evidence from TSA of P-LN positive patients. Additionally, no difference is observed in OS, PFS, and recurrence between two procedures, and TSA of recurrence showed further investigation are needed.
SLN has been gaining popularity in gynecological cancer staging over the past decades. Initial exploration of SLN by gynecological oncologist started at vulvar cancer (34) and subsequent studies validated its feasibility (35). It has experienced two stages of SLN employment in EC staging, and in the first stage multiple researches were focusing on the feasibility and reliability of SLN. Abu-Rustum et al. (36) identified a 100% sensitivity and low false-negative rate in grade 1 EC patients, with the methods of SLN procedure followed by systematic pelvic and para-aortic LND. After accumulating studies indicating the accuracy of SLN in EC staging, SLN has been evolved as a more targeted alternative for nodal assessment (37, 38). In 2014, SLN was firstly recommended by NCCN guidelines to stage I patients (39). A meta-analysis reported a>80% overall detection rate of SLN (40). A consensus from the Society of Gynecological Oncology (SGO) in 2017, approved the execution of SLN in low-risk patients (41). Till 2018, NCCN guidelines began to support SLN application in all EC patients including those with high risk (42). At present the discussion about SLN vs. LND is still going on (43). A recent meta-analysis highlighted the safety and effectiveness of SLN (10), but given its limitation we conducted this analysis with latest evidence, bigger sample size and border outcomes measures.
SLN is introduced into EC staging with the aim of reducing LND-related morbidity and gaining prognostic factors of lymph node status (41). SLN techniques has been evolving during gynecologic oncology application. Three different tracers, patent blue, technetium 99, and indocyanine green (ICG), are the mainstay of SLN mapping (41). Considering the unreliability and radiation, the SGO recommended ICG dye with infrared imaging to EC patients for its high success and technical ease (41). The optimal site for tracer injection has been investigated in precious studies (44–46), out of common sites like myometrium, cervix injection is regarded as the most effective way to trace SLN (41).
Recent studies focusing on operation-related outcomes indicated that SLN procedure could reduce operative time of minimal invasive surgery and laparotomy (23, 25, 26, 28, 30, 33). Stewart et al. (33) observed significant decrease in operative time (210 vs 170 min, P = 0.007) taking account of the surgery approach. And Valerio G. et al. (47) discussed robotic surgery in elderly gynecological cancer patients and demonstrated that minimally invasive could considered for older patients (even over 75 years old); and this illustrated SLN procedure during minimal invasive surgery could benefit patients more, especially these over 75 years old. The meta-analysis indicated that blood loss was significantly lower in SLN group. In terms of complications, more evidence is needed for supporting intra- and post-operation complications declining. Accordion Severity Grading System and Clavien-Dindo scale are used to assess post- operation complications; two studies by Accordion Severity Grading System (30, 33) and two by Clavien-Dindo scale (26, 28) indicated SLN group occurred less post-operation complications. A retrospective study reported lower-limb lymphedema could only been seen in LND group (25). Leitao et al. (48) concluded that SLN was independently related to lower self-reported lower-extremity lymphedema rate than LND.
The meta-analysis indicates that SLN is more targeted for less node dissection and more detection of positive lymph nodes. The FIRES trial, enrolling stage I patients, yielded a high sensitivity of 97% and a negative predictive value of 99.6% by SLN mapping, and prevented more people from the morbidity of LND (38). Accumulating data indicated SLN was significantly associated with accurate detection of pelvic lymph nodes and was non-inferior to LND in para-aortic nodes assessment in high risk EC patients (7, 18, 21–23, 32, 41). The pathologic ultra-staging technique adopted by SLN mapping, defines positive lymph node as macro-metastasis (≥2 mm), micro-metastasis (≥0.2 mm), and isolated tumor cells (≤0.2 mm) (41). Ultra-staging could upgrade 10–40% patients in previous studies for identification of low volume metastasis in lymph nodes (49, 50).
This meta-analysis showed no survival and recurrence detriment in SLN mapping compared with LND, in accordance with the previous meta-analysis (10). Experience from a study with 1,135 low risk patients indicated that 3-year OS and PFS were similar in two groups (P>0.07) (15). A multicenter study in high risk patients showed HR for association of staging approach (SLN and LND) with progression and death was 3.12 (95% CI 1.02–9.57) and 0.69 (95% CI 0.24–1.95) respectively (32). Similarly, Multinu et al (18). reported the risk of progression and death were not significantly different between SLN vs. LND (HR 1.27, 95% CI 0.6–2.67; HR 2.10, 95% CI 0.79–5.58, respectively). Additionally, no difference in recurrence pattern was observed between two groups; this meta-analysis showed there was no difference in overall recurrence (all sites), nodal recurrence, locoregional recurrence, and multifocal recurrence. Multiple studies reported distant/multifocal recurrence was predominant; Schiavone et al. (24) found 74% patients occurred multifocal recurrence and 16% endured nodal recurrence. A 56% (19/34) of multifocal recurrence in all patients with recurrence was reported in retrospective cohort study (18).
The imitations of the meta-analysis are as follows. First, most of pooled studies are retrospective cohort studies, futured prospective studies comparing SLN and LND are warranted. Second, some included studies did not provide the needed data directly, therefore some statistical methods were utilized to obtain proper data, which may decrease inaccuracy. Third, this meta-analysis is based on observational studies, and fails to reach high GRADE assessment.
In conclusion, the present meta-analysis reviewed current evidence on SLN mapping in comparison of LND. SLN is capable of reducing blood loss during operation in regardless of surgical approach with firm evidence from TSA. Future studies on operation time and complications are needed for further analysis. SLN mapping is more targeted for less node dissection and more detection of positive lymph nodes even in high risk patients with conclusive evidence from TSA. Utility of SLN yields no survival detriment in EC patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conceptualization: YG. Methodology: All authors. Project administration: YX. Supervision: YX. Writing—original draft: YG. Writing—review and editing: all authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No.81772783 and No. 81472446) and the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (CAMS-2017-I2M-1-002) to YX.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.580128/full#supplementary-material
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin (2010) 60(5):277–300. 10.3322/caac.20073 [DOI] [PubMed] [Google Scholar]
- 3. Brooks RA, Fleming GF, Lastra RR, Lee NK, Moroney JW, Son CH, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin (2019) 69(4):258–79. 10.3322/caac.21561 [DOI] [PubMed] [Google Scholar]
- 4. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): a randomised study. Lancet (2009) 373(9658):125–36. 10.1016/s0140-6736(08)61766-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst (2008) 100(23):1707–16. 10.1093/jnci/djn397 [DOI] [PubMed] [Google Scholar]
- 6. Euscher ED, Malpica A. Gynaecological malignancies and sentinel lymph node mapping: an update. Histopathology (2020) 76(1):139–50. 10.1111/his.14017 [DOI] [PubMed] [Google Scholar]
- 7. Schlappe BA, Weaver AL, Ducie JA, Eriksson AGZ, Dowdy SC, Cliby WA, et al. Multicenter study comparing oncologic outcomes between two nodal assessment methods in patients with deeply invasive endometrioid endometrial carcinoma: A sentinel lymph node algorithm versus a comprehensive pelvic and paraaortic lymphadenectomy. Gynecol Oncol (2018) 151(2):235–42. 10.1016/j.ygyno.2018.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim CH, Soslow RA, Park KJ, Barber EL, Khoury-Collado F, Barlin JN, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer (2013) 23(5):964–70. 10.1097/IGC.0b013e3182954da8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American National comprehensive cancer network. Uterine neoplasms(2020 v1) (2020). Available at: www.nccn.org, [updated 4th March, 2020; cited 2020 4th March].
- 10. Bogani G, Murgia F, Ditto A, Raspagliesi F. Sentinel node mapping vs. lymphadenectomy in endometrial cancer: A systematic review and meta-analysis. Gynecol Oncol (2019) 153(3):676–83. 10.1016/j.ygyno.2019.03.254 [DOI] [PubMed] [Google Scholar]
- 11. Vrabel M. Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Oncol Nurs Forum (2015) 42(5):552–4. 10.1188/15.Onf.552-554 [DOI] [PubMed] [Google Scholar]
- 12. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol (2005) 5):13. 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8):16. 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raimond E, Ballester M, Hudry D, Bendifallah S, Darai E, Graesslin O, et al. Impact of sentinel lymph node biopsy on the therapeutic management of early-stage endometrial cancer: Results of a retrospective multicenter study. Gynecol Oncol (2014) 133(3):506–11. 10.1016/j.ygyno.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 15. Zahl Eriksson AG, Ducie J, Ali N, McGree ME, Weaver AL, Bogani G, et al. Comparison of a sentinel lymph node and a selective lymphadenectomy algorithm in patients with endometrioid endometrial carcinoma and limited myometrial invasion. Gynecol Oncol (2016) 140(3):394–9. 10.1016/j.ygyno.2015.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buda A, Di Martino G, Restaino S, De Ponti E, Monterossi G, Giuliani D, et al. The impact on survival of two different staging strategies in apparent early stage endometrial cancer comparing sentinel lymph nodes mapping algorithm and selective lymphadenectomy: An Italian retrospective analysis of two reference centers. Gynecol Oncol (2017) 147(3):528–34. 10.1016/j.ygyno.2017.09.033 [DOI] [PubMed] [Google Scholar]
- 17. Ducie JA, Eriksson AGZ, Ali N, McGree ME, Weaver AL, Bogani G, et al. Comparison of a sentinel lymph node mapping algorithm and comprehensive lymphadenectomy in the detection of stage IIIC endometrial carcinoma at higher risk for nodal disease. Gynecol Oncol (2017) 147(3):541–8. 10.1016/j.ygyno.2017.09.030 [DOI] [PubMed] [Google Scholar]
- 18. Multinu F, Ducie JA, Eriksson AGZ, Schlappe BA, Cliby WA, Glaser GE, et al. Role of lymphadenectomy in endometrial cancer with nonbulky lymph node metastasis: Comparison of comprehensive surgical staging and sentinel lymph node algorithm. Gynecol Oncol (2019) 155(2):177–85. 10.1016/j.ygyno.2019.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Basaran D, Bruce S, Aviki EM, Mueller JJ, Broach VA, Cadoo K, et al. Sentinel lymph node mapping alone compared to more extensive lymphadenectomy in patients with uterine serous carcinoma. Gynecol Oncol (2020) 156(1):70–6. 10.1016/j.ygyno.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holloway RW, Gupta S, Stavitzski NM, Zhu X, Takimoto EL, Gubbi A, et al. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol Oncol (2016) 141(2):206–10. 10.1016/j.ygyno.2016.02.018 [DOI] [PubMed] [Google Scholar]
- 21. Baiocchi G, Mantoan H, Kumagai LY, Goncalves BT, Badiglian-Filho L, de Oliveira Menezes AN, et al. The Impact of Sentinel Node-Mapping in Staging High-Risk Endometrial Cancer. Ann Surg Oncol (2017) 24(13):3981–7. 10.1245/s10434-017-6132-8 [DOI] [PubMed] [Google Scholar]
- 22. How J, Gauthier C, Abitbol J, Lau S, Salvador S, Gotlieb R, et al. Impact of sentinel lymph node mapping on recurrence patterns in endometrial cancer. Gynecol Oncol (2017) 144(3):503–9. 10.1016/j.ygyno.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 23. Liu CY, Elias KM, Howitt BE, Lee LJ, Feltmate CM. Sentinel lymph node mapping reduces practice pattern variations in surgical staging for endometrial adenocarcinoma: A before and after study. Gynecol Oncol (2017) 145(2):248–55. 10.1016/j.ygyno.2017.03.012 [DOI] [PubMed] [Google Scholar]
- 24. Schiavone MB, Scelzo C, Straight C, Zhou Q, Alektiar KM, Makker V, et al. Survival of Patients with Serous Uterine Carcinoma Undergoing Sentinel Lymph Node Mapping. Ann Surg Oncol (2017) 24(7):1965–71. 10.1245/s10434-017-5816-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Accorsi GS, Paiva LL, Schmidt R, Vieira M, Reis R, Andrade C. Sentinel Lymph Node Mapping vs Systematic Lymphadenectomy for Endometrial Cancer: Surgical Morbidity and Lymphatic Complications. J Minim Invasive Gynecol (2019) S1553-4650(19):30374–7. 10.1016/j.jmig.2019.07.030 [DOI] [PubMed] [Google Scholar]
- 26. Imboden S, Mereu L, Siegenthaler F, Pellegrini A, Papadia A, Tateo S, et al. Oncological safety and perioperative morbidity in low-risk endometrial cancer with sentinel lymph-node dissection. Eur J Surg Oncol (2019) 45(9):1638–43. 10.1016/j.ejso.2019.05.026 [DOI] [PubMed] [Google Scholar]
- 27. Le T, McDonald S, Samant R, Fung Kee Fung M. Robotic sentinel node mapping in clinical stage 1 endometrial cancer using methylene blue dyes using the robotic platform. Int J Med Robot (2019) 15(4):e2006. 10.1002/rcs.2006 [DOI] [PubMed] [Google Scholar]
- 28. Polan RM, Rossi EC, Barber EL. Extent of lymphadenectomy and postoperative major complications among women with endometrial cancer treated with minimally invasive surgery. Am J Obstet Gynecol (2019) 220(3):263.e1–.e8. 10.1016/j.ajog.2018.11.1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bogani G, Casarin J, Leone Roberti Maggiore U, Ditto A, Pinelli C, Dell’acqua A, et al. Survival outcomes in endometrial cancer patients having lymphadenectomy, sentinel node mapping followed by lymphadectomy and sentinel node mapping alone: Long-term results of a propensity-matched analysis. Gynecol Oncol (2020) 158(1):77–83. 10.1016/j.ygyno.2020.04.691 [DOI] [PubMed] [Google Scholar]
- 30. Casarin J, Multinu F, Tortorella L, Cappuccio S, Weaver AL, Ghezzi F, et al. Sentinel lymph node biopsy for robotic-assisted endometrial cancer staging: further improvement of perioperative outcomes. Int J Gynecol Cancer (2020) 30(1):41–7. 10.1136/ijgc-2019-000672 [DOI] [PubMed] [Google Scholar]
- 31. Kogan L, Matanes E, Wissing M, Mitric C, How J, Amajoud Z, et al. The added value of sentinel node mapping in endometrial cancer. Gynecol Oncol (2020) 158(1):84–91. 10.1016/j.ygyno.2020.04.687 [DOI] [PubMed] [Google Scholar]
- 32. Schlappe BA, Weaver AL, McGree ME, Ducie J, Zahl Eriksson AG, Dowdy SC, et al. Multicenter study comparing oncologic outcomes after lymph node assessment via a sentinel lymph node algorithm versus comprehensive pelvic and paraaortic lymphadenectomy in patients with serous and clear cell endometrial carcinoma. Gynecol Oncol (2020) 156(1):62–9. 10.1016/j.ygyno.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stewart KI, Eska JS, Harrison RF, Suidan R, Abraham A, Chisholm GB, et al. Implementation of a sentinel lymph node mapping algorithm for endometrial cancer: surgical outcomes and hospital charges. Int J Gynecol Cancer (2020) 30(3):352–7. 10.1136/ijgc-2019-000941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stehman FB, Bundy BN, Thomas G, Varia M, Okagaki T, Roberts J, et al. Groin dissection versus groin radiation in carcinoma of the vulva: a Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys (1992) 24: (2):389–96. 10.1016/0360-3016(92)90699-i [DOI] [PubMed] [Google Scholar]
- 35. Oonk MH, van Hemel BM, Hollema H, de Hullu JA, Ansink AC, Vergote I, et al. Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol (2010) 11(7):646–52. 10.1016/s1470-2045(10)70104-2 [DOI] [PubMed] [Google Scholar]
- 36. Abu-Rustum NR, Khoury-Collado F, Pandit-Taskar N, Soslow RA, Dao F, Sonoda Y, et al. Sentinel lymph node mapping for grade 1 endometrial cancer: is it the answer to the surgical staging dilemma? Gynecol Oncol (2009) 113(2):163–9. 10.1016/j.ygyno.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Touhami O, Gregoire J, Renaud MC, Sebastianelli A, Plante M. Performance of sentinel lymph node (SLN) mapping in high-risk endometrial cancer. Gynecol Oncol (2017) 147(3):549–53. 10.1016/j.ygyno.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 38. Rossi EC, Kowalski LD, Scalici J, Cantrell L, Schuler K, Hanna RK, et al. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol (2017) 18(3):384–92. 10.1016/s1470-2045(17)30068-2 [DOI] [PubMed] [Google Scholar]
- 39. Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Chan J, et al. Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw (2014) 12(2):248–80. 10.6004/jnccn.2014.0025 [DOI] [PubMed] [Google Scholar]
- 40. Bodurtha Smith AJ, Fader AN, Tanner EJ. Sentinel lymph node assessment in endometrial cancer: a systematic review and meta-analysis. Am J Obstet Gynecol (2017) 216(5):459–76.e10. 10.1016/j.ajog.2016.11.1033 [DOI] [PubMed] [Google Scholar]
- 41. Holloway RW, Abu-Rustum NR, Backes FJ, Boggess JF, Gotlieb WH, Jeffrey Lowery W, et al. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol Oncol (2017) 146(2):405–15. 10.1016/j.ygyno.2017.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2018) 16(2):170–99. 10.6004/jnccn.2018.0006 [DOI] [PubMed] [Google Scholar]
- 43. Grassi T, Mariani A, Cibula D, Soliman PT, Suman VJ, Weaver AL, et al. A prospective multicenter international single-arm observational study on the oncological safety of the sentinel lymph node algorithm in stage I intermediate-risk endometrial cancer (SELECT, SEntinel Lymph node Endometrial Cancer Trial). Int J Gynecol Cancer (2020) 30(10):1627–32. 10.1136/ijgc-2020-001698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tanner EJ, Sinno AK, Stone RL, Levinson KL, Long KC, Fader AN. Factors associated with successful bilateral sentinel lymph node mapping in endometrial cancer. Gynecol Oncol (2015) 138(3):542–7. 10.1016/j.ygyno.2015.06.024 [DOI] [PubMed] [Google Scholar]
- 45. Sawicki S, Lass P, Wydra D. Sentinel Lymph Node Biopsy in Endometrial Cancer–Comparison of 2 Detection Methods. Int J Gynecol Cancer (2015) 25(6):1044–50. 10.1097/igc.0000000000000447 [DOI] [PubMed] [Google Scholar]
- 46. Favero G, Pfiffer T, Ribeiro A, Carvalho JP, Baracat EC, Mechsner S, et al. Laparoscopic sentinel lymph node detection after hysteroscopic injection of technetium-99 in patients with endometrial cancer. Int J Gynecol Cancer (2015) 25(3):423–30. 10.1097/igc.0000000000000387 [DOI] [PubMed] [Google Scholar]
- 47. Gallotta V, Conte C, D’Indinosante M, Federico A, Biscione A, Vizzielli G, et al. Robotic Surgery in Elderly and Very Elderly Gynecologic Cancer Patients. J Minim Invasive Gynecol (2018) 25(5):872–7. 10.1016/j.jmig.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 48. Leitao MM, Jr., Zhou QC, Gomez-Hidalgo NR, Iasonos A, Baser R, Mezzancello M, et al. Patient-reported outcomes after surgery for endometrial carcinoma: Prevalence of lower-extremity lymphedema after sentinel lymph node mapping versus lymphadenectomy. Gynecol Oncol (2020) 156(1):147–53. 10.1016/j.ygyno.2019.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Touhami O, Trinh XB, Gregoire J, Sebastianelli A, Renaud MC, Grondin K, et al. Predictors of non-sentinel lymph node (non-SLN) metastasis in patients with sentinel lymph node (SLN) metastasis in endometrial cancer. Gynecol Oncol (2015) 138(1):41–5. 10.1016/j.ygyno.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 50. Koskas M, Chereau E, Ballester M, Dubernard G, Lecuru F, Heitz D, et al. Accuracy of a nomogram for prediction of lymph-node metastasis detected with conventional histopathology and ultrastaging in endometrial cancer. Br J Cancer (2013) 108(6):1267–72. 10.1038/bjc.2013.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.