Abstract
Objective
Vascular calcification requires the differentiation of vascular smooth muscle cells (VSMCs) into osteoblast-like cells. This phenomenon can be enhanced by inflammation and oxidative stress. Zingerone is one of the active ingredients present in the ginger plant that has anti-inflammatory and antioxidant effects. Other functions include anti-obesity, anti-nausea effects. However, the functions of zingerone on vascular calcification has not yet been elucidated. This study investigated the effect of zingerone on vascular calcification and its molecular mechanism.
Methods
Reverse transcription-polymerase chain reaction (PCR), real-time PCR and Western blot analysis was used to measure expression levels of osteogenic marker genes and to investigate whether calcification was regulated by the expression of AMP-activated protein kinase (AMPK) and tissue inhibitor of metalloproteinase 4 (TIMP4). Alizarin red S staining was used to measure calcium deposition. Studies were carried out in VSMCs.
Results
Zingerone induced the expression of 2 markers of VSMCs differentiation (α-smooth muscle actin (α-SMA) and smooth muscle 22α (SM22α)) and decreased the expression of core-binding factor α-1 (CBFA1). Additionally, zingerone decreased inorganic phosphate (Pi)-induced expression of distal-less homeobox 5 and CBFA1. AMPK phosphorylation and TIMP4 expression were increased by zingerone. Importantly, zingerone protected VSMCs from calcification, and this protective effect was confirmed by increased TIMP4 via overexpression of AMPK, and inhibition of TIMP4 by Compound C. Zingerone upregulated AMPK/TIMP4 expression and recovered Pi-induced inhibition of TIMP4.
Conclusions
Taken together, our results show that zingerone inhibits Pi-induced vascular calcification by regulating the AMPK/TIMP4 signaling cascade in VSMCs. These results suggest that the natural product zingerone could be useful for treating vascular and metabolic diseases.
Keywords: Zingerone, AMP-activated protein kinase, Tissue inhibitor of metalloproteinase-4
INTRODUCTION
Ginger, the root of Zingiber officinale, is one of the most commonly used spices.1 Ginger is used in Asia to treat various diseases such as headache, nausea, rheumatism, cold and diarrhea.2,3,4 Ginger is not an allergenic food, and contains a substantial amount of zingerone (vanillyl acetone). Zingerone, which is the product of the conversion of gingerol by drying or a retro-aldol reaction, has antioxidant, anti-inflammatory, anti-obesity, anti-nausea, and anti-vomiting actions, and is also an oxidative stress antagonist.5,6 Despite showing several potential therapeutic actions, the effects of zingerone on vascular calcification and the potential mechanisms involved in any effect have not yet been defined.6,7
Vascular calcification, which occurs as a result of the deposition of mineral in the arterial wall, is related to an augmented risk of heart disease, stroke and atherosclerotic plaque rupture.8 Calcification arises in both the intimal and medial layers of the arteries. Arterial obstruction and atherosclerotic plaque rupture are associated with intimal calcification, and diseases such as vessel stiffness, systolic hypertension, and heart failure are associated with medial calcification.9 Intimal and medial layer calcification are driven by the differentiation of vascular smooth muscle cells (VSMCs). Vascular calcification is a controlled process regulated by osteo-chondrogenic reprogramming in VSMC. It shares many features with bone mineralization.10 During calcification of VSMCs, several stimuli induces expression of bone formation marker genes, such as distal-less homeobox 5 (Dlx5), core-binding factor α-1 (CBFA1, also known as Runx2 is a key transcription factor associated with osteoblast differentiation.), and alkaline phosphatase (ALP).11,12,13
Metalloproteases exist in all living organisms, and during eukaryotic evolution the number of metalloproteases expanded, so they became the largest type of protease gene in humans.14,15 Metalloproteases are inhibited by members of the tissue inhibitor of metalloproteinase (TIMP) family (TIMP1, 2, 3, 4).16,17 TIMP1, 2, 3, 4 inhibit most of the metzincins, a subfamily of 89 secreted cell surface-binding metalloprotease.18 TIMPs are involved in the development of vascular disease.19
AMP-activated protein kinase (AMPK) is a cellular energy sensor. AMPK is activated by various stimuli that deplete cellular energy levels, such as glucose deficiency, hypoxia and exposure to toxins that inhibit the mitochondrial respiratory chain complex.20,21 AMPK, which is a serine/threonine protein kinase complex, consists of catalytic α-subunit (α1 and α2), a β-subunit (β1 and β2) that acts as a scaffold, and a regulatory γ-subunit (γ1, γ2, and γ3). AMPK activity is related to muscle loss and oxidative stress and regulates vascular calcification by controlling the expression of Dlx5 and CBFA1.
In this study, we hypothesized that zingerone would inhibit inorganic phosphate (Pi)-induced vascular calcification through AMPK/TIMP4 signaling. During calcification of VSMCs, based on data from osteogenic marker genes such as Dlx5 and CBFA1, it was confirmed that zingerone may work an important role in the inhibition of vascular calcification. Even we have shown that AMPK can work as a regulator as an upstream of TIMP4. This study showing complete inhibition of vascular calcification by zingerone will contribute to the study of several vascular diseases.
MATERIALS AND METHODS
1. Primary culture of VSMCs
VSMCs were isolated from aortas of rats by digestion with collagenase and elastase. Cells were maintained in Dulbecco's Modified Eagle's Medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Atlas, Fort Collins, CO, USA), 1% penicillin streptomycin (Gibco). Cells were serially passaged and used between passage 3 and 10. Rats were maintained in accordance with the institutional guidelines of the Committee for Laboratory Animal Care and Use of Daegu University.
2. RNA isolation and quantitative real-time reverse transcription-polymerase chain reaction (PCR) analysis
Total RNA was extracted with TRI-Solution (Bio Science Technology, Seoul, Korea) according to the manufacturer's instructions. RNA concentration was measured using a multi-plate reader according to the manufacturer's instructions. To quantify gene transcription, cDNA was amplified on a LightCycler Nano Instrument (Roche, Mannheim, Germany) using AmpiGene qPCR Green Mix Hi-ROX (Enzo Life Sciences, Farmingdale, NY, USA) and specific primers. Quantitative real-time PCR is included in Table 1.
Table 1. Specific primer for real-time PCR.
| Primer | Sequences (5′→3′) | |
|---|---|---|
| ALP | Forward | AACCCAGACACAAGCATTCC |
| Reverse | GAGAGCGAAGGGTCAGTCAG | |
| Dlx5 | Forward | GCCCACCAACCAGCCAGAGA |
| Reverse | GCGAGGTACTGAGTCTTCTGAAACC | |
| CBFA1 | Forward | AGATGACATCCCCATCCATC |
| Reverse | GTGAGGGATGAAATGCTGG | |
| TIMP1 | Forward | CCAGAGCGATCACTTTGCCT |
| Reverse | GAAAGGTAAACAGTGTTCAGGCT | |
| TIMP2 | Forward | ACGCTTAGCATCACCCAGAAG |
| Reverse | ATAGGGCAGCGTGTGATCTTG | |
| TIMP3 | Forward | ATCCCCAGGATGCCTTCTG |
| Reverse | CCTTCCTTCACCAGCTTCTTT | |
| TIMP4 | Forward | CTAGAAACCAACAGTCAGAAGC |
| Reverse | GTTCTGGTGGTAGTGATGATTC | |
PCR, polymerase chain reaction; ALP, alkaline phosphatase; Dlx5, distal-less homeobox 5; CBFA1, core-binding factor α-1; TIMP, tissue inhibitor of metalloproteinase.
3. Western blotting
After quantifying total protein according to the previously reported method, protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane.13,22 Signals were detected using ECL reagent (Advansta, Menlo Park, CA, USA) according to the manufacturer's protocol. Densitometric analysis of the blotted membrane was performed using a Fusion Solo system (Vilber Lourmat, Eberhardzell, Germany).
4. Alizarin red S (ARS) staining
We investigated whether detect calcium deposits in the extracellular matrix of VSMCs through ARS. Medium was generated by mixing phosphate, then 100 nM zingerone was added and cells were cultured for 2 weeks. Cultured cells were fixed with 10% formaldehyde (Merck KGaA, Darmstadt, Germany) for 5 minutes, and rinsed 3 times with deionized water, then treated with incubated with 2% ARS diluted in deionized water (Sigma-Aldrich, St. Louis, MO, USA) for 20 minutes. After an additional wash with deionized water, samples were imaged with a Perfection V37 (Epson, Suwa, Japan) scanner.
5. Plasmids and small interfering RNA (siRNA) assay
The expression vector encoding TIMP4 (pCMV-TIMP4) were provided from Korea Human Gene Bank, Medical Genomics Research Center, KRIBB (Daejeon, Korea). siRNA targeting TIMP4 and negative control siRNA were purchased from Bioneer (Daejeon, Korea). Total RNA was isolated 48 hours after transfection for use in real-time PCR and Western blotting experiments.
6. Transient transfection assay
VSMCs were seeded into a 35 mm dish and cultured for 24 hours in Dulbecco's Modified Eagle's Medium containing 10% fetal bovine serum. For overexpression, cells were transfected with 2 μg plasmid vector using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). For knock-down, cells were transfected with 100 nM siRNA using Lipofectamine 2000 (Invitrogen). The cells were harvested 24–48 hours after transfection.
7. Statistical analysis
All experiments were repeated at least 3 times. The results are expressed as the mean±standard error of triplicate independent experiments. Statistical analyses were performed using the Student's t-test. Differences between groups were considered significant when p<0.05.
RESULTS
1. Zingerone correlates with calcification of VSMCs
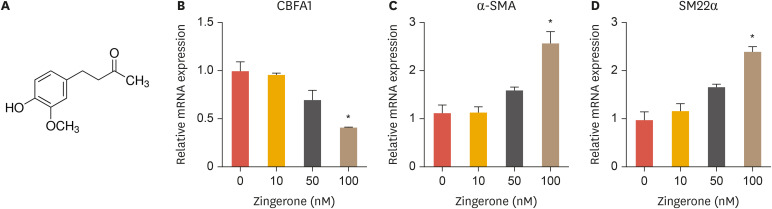
To investigate the effects of zingerone (Fig. 1A, 4-(4-hydroxy-3-methoxyphenyl)-butan-2-one) on VSMCs, we measured the effects of 10, 50, and 100 nM zingerone on the expression of markers of VSMCs differentiation. Zingerone reduced the mRNA expression of CBFA1, which is responsible for inducing calcification, and increased α-smooth muscle actin (α-SMA) and smooth muscle 22α (SM22α) mRNA expression in a concentration-dependent manner (Fig. 1B-D). Zingerone reduced CBFA1 mRNA expression, while increased α-SMA and SM22α mRNA expression in a dose-dependent manner (Fig. 1B-D). These results show that zingerone induces the expression of 2 markers (α-SMA and SM22α) associated with the differentiation of VSMCs.
Fig. 1. Zingerone induces differentiation of VSMCs. The structure of zingerone (A). Aortic VSMCs were treated with 10, 50, or 100 nM zingerone for 12 hours, and then mRNA expression of CBFA1, α-SMA, and SM22α was measured by real-time polymerase chain reaction (B, C). Data represent the mean±standard error of the mean of 3 individual experiments.
VSMC, vascular smooth muscle cell; CBFA1, core-binding factor α-1; α-SMA, α-smooth muscle actin; SM22α, smooth muscle 22α.
*p<0.05; Statistical significance was determined relative to a control by the Student's t-test.
2. Zingerone inhibits Pi-induced calcification of aortic VSMCs
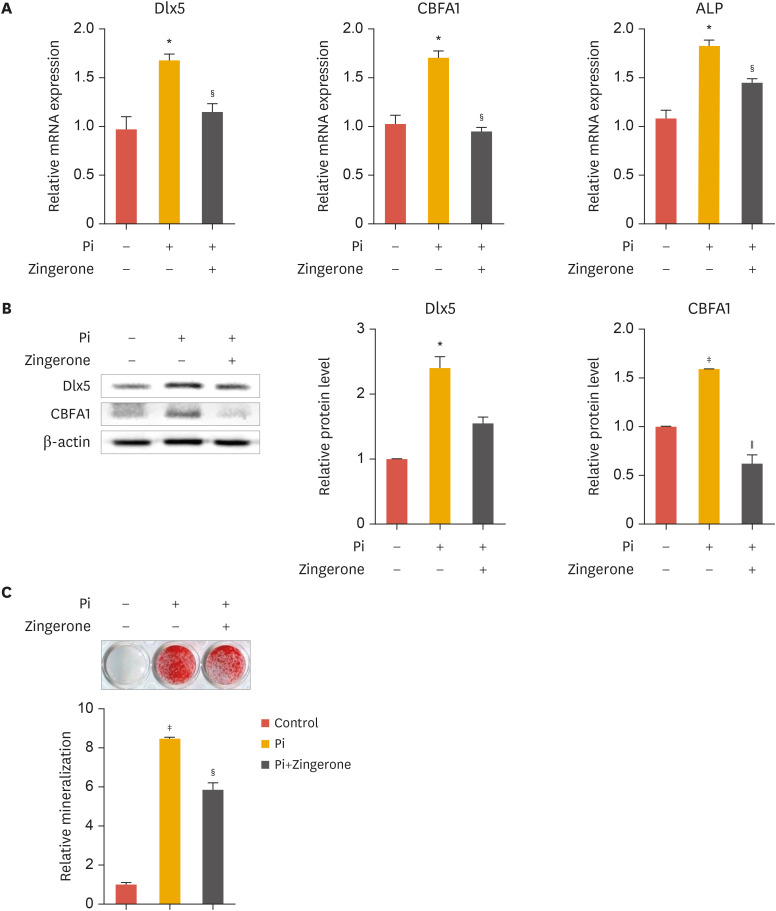
As shown in Fig. 1B-D, zingerone was effective at 100 nM. Therefore, 100 nM zingerone was chosen for further experiments in VSMC. To investigate the effect of zingerone on vascular calcification induced by Pi, VSMCs were cultured in calcification media containing or lacking zingerone. The presence of Pi in the culture media significantly upregulated the expression of osteogenic marker genes in VSMCs. Zingerone significantly suppressed Pi-induced osteogenic marker gene expression, such as Dlx5, CBFA1, and ALP (Fig. 2A). Additionally, the change of Dlx5 and CBFA1 protein levels were same pattern to mRNA expression (Fig. 2B, left panel); Dlx5 and CBFA1 protein bands from Western blot analysis were confirmed by densitometry analysis (Fig. 2B, right panel). We used ARS staining, which stains calcium deposits, to evaluate the effects of zingerone on Pi-induced calcium deposition. After 2 weeks of culture with Pi and/or zingerone, zingerone inhibited Pi-induced staining levels in VSMCs (Fig. 2C). These results suggest that zingerone can suppress Pi-induced calcification of VSMCs.
Fig. 2. Zingerone inhibits Pi-induced calcification of VSMCs. VSMCs were cultured with inorganic phosphate and/or zingerone for 2 days or 2 weeks. (A-C) mRNA expression of Dlx5, CBFA1 and ALP was measured by real-time polymerase chain reaction (A). Protein levels of Dlx5, CBFA1 were measured by Western blot analysis (B, left panel). Densitometry analysis was performed with the indicated antibodies (B, right panel). Alizarin red S staining (C) Data represent the mean±standard error of the mean of 3 individual experiments.
Pi, inorganic phosphate; VSMC, vascular smooth muscle cell; Dlx5, distal-less homeobox 5; CBFA1, core-binding factor α-1; ALP, alkaline phosphatase.
Statistical significance was determined relative to the control (*p<0.05; †p<0.01; ‡p<0.001) or compared with Pi-treated cells (§p<0.05; ∥p<0.01) using the Student's t-test.
3. Zingerone protects VSMCs from calcification via AMPK phosphorylation
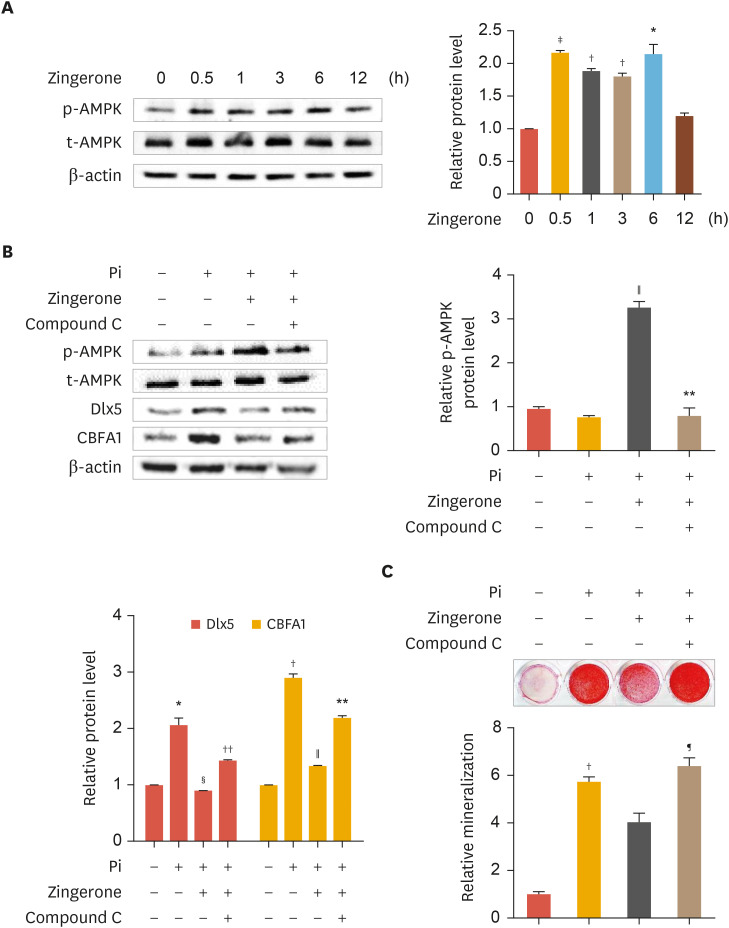
Activation of the AMPK signaling pathway attenuates vascular calcification.23,24 We investigated whether zingerone mediates AMPK. This led us to investigate whether the protective effects of zingerone against Pi-induced calcification are mediated via activation of the AMPK pathway. Zingerone increased AMPK phosphorylation in a time-dependent manner (Fig. 3A, left panel). AMPK protein bands from Western blot analysis were confirmed by densitometry analysis (Fig. 3A, right panel). To further confirm, the AMPK inhibitor Compound C (1 µM) was treated. Protein levels of Dlx5 and CBFA1 were reduced by zingerone, but Compound C upregulated the protein levels of Dlx5 and CBFA1 in zingerone-treated VSMCs (Fig. 3B, left panel). AMPK phosphorylation protein bands from Western blot analysis were confirmed by densitometry analysis (Fig. 3B, right panel). Dlx5 and CBFA1 protein bands were confirmed graph by densitometry analysis (Fig. 3B, lower panel). Also in ARS staining analysis, zingerone decreased protein level of calcification; however, these effects were recovered by Compound C (Fig. 3C). These results suggest that zingerone protects phosphate-induced calcification of VSMC through AMPK activation.
Fig. 3. The protective effect of zingerone on Pi-induced calcification mediated by AMPK. VSMCs were treated with zingerone (100 nM) for 0.5, 1, 3, 6 and 12 hours (A). Cells were treated with or without Pi, zingerone, and Compound C (1 µM) for 2 days or 2 weeks (B, C) Protein levels of p-AMPK, AMPK, Dlx5, and CBFA1 were measured by Western blot analysis (A, B, left panel). Densitometry analysis was performed with the indicated antibodies (A, right panel and B, right panel, down panel). Alizarin red S staining (C). Data represent the mean±standard error of the mean of 3 individual experiments.
Pi, inorganic phosphate; AMPK, AMP-activated protein kinase; p-AMPK, phospho-AMPK; t-AMPK, total AMPK; VSMC, vascular smooth muscle cell; Dlx5, distal-less homeobox 5; CBFA1, core-binding factor α-1.
Statistical significance was determined relative to a control (*p<0.05; †p<0.01; ‡p<0.001) or compared with the Pi-treated group (§p<0.05; ∥p<0.01), Pi and zingerone group (¶p<0.05; **p<0.01; ††p<0.005) using the Student's t-test.
4. TIMP4 is upregulated by zingerone in VSMC
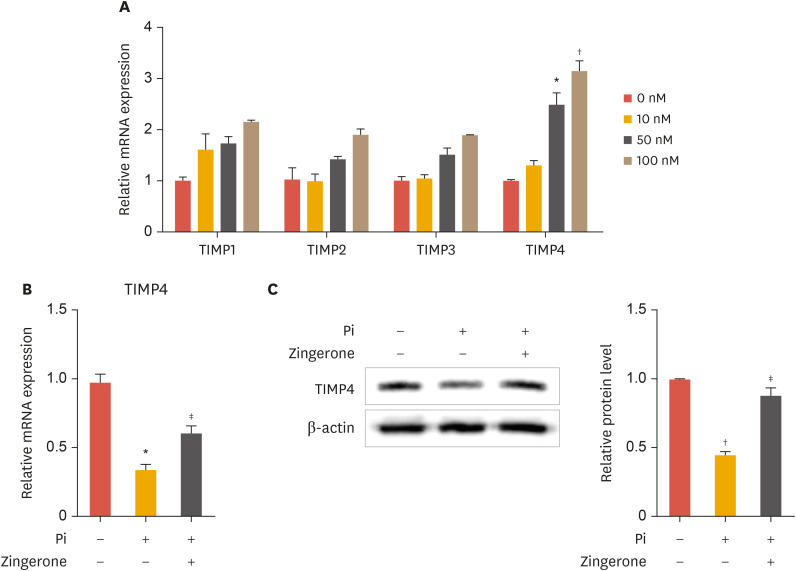
TIMP4 has been reported to regulate abdominal aortic aneurysms in aortic VSMCs.25 Increased levels of TIMP4 lower the risk of abdominal aortic aneurysm. Based on this finding, we investigated whether zingerone modulates the expression of TIMPs. Zingerone concentration-dependently increased TIMP4 mRNA expression, and slightly (but not significantly) increased the expression of TIMP1, TIMP2, and TIMP3 (Fig. 4A). Zingerone recovered the mRNA expression and protein levels of TIMP4 inhibited by Pi (Fig. 4B and C, left panel). The TIMP4 protein bands from Western blot analysis were confirmed by densitometry analysis (Fig. 4C, right panel). These results suggest that zingerone regulates TIMP4 expression in VSMCs.
Fig. 4. Zingerone increases TIMP4 expression. VSMCs were treated with zingerone (100 nM) for 12 hours (A). Cells were treated with or without zingerone and phosphate for 2 days (B, C) mRNA expression of TIMP4 was measured by real-time polymerase chain reaction (A, B). The protein levels of TIMP4 was measured by Western blot analysis (C). Densitometry analysis was measured with the TIMP4 (B, right panel). Data represent the mean±standard error of the mean of 3 individual experiments.
TIMP, tissue inhibitor of metalloproteinase; VSMC, vascular smooth muscle cell; Pi, inorganic phosphate.
Statistical significance was determined relative to a control by the Student's t-test (*p<0.05; †p<0.01) and compared with Pi-treated cells by the Student's t-test (‡p<0.05).
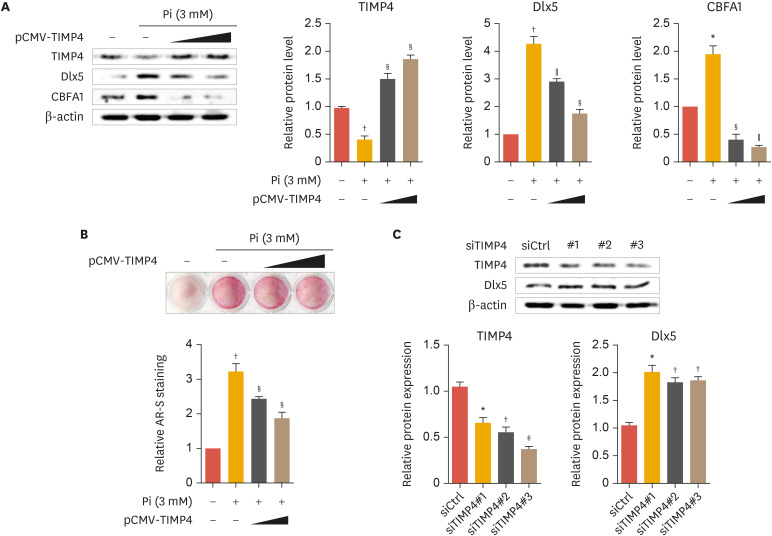
5. TIMP4 upregulation inhibits vascular calcification
Based on our results presented in Fig. 4, we explored the effect of TIMP4 gene overexpression and knock-down on vascular calcification. TIMP4 overexpression concentration-dependently reduced Pi-induced increases in Dlx5 and CBFA1 protein levels whereas increased TIMP4 expressions (Fig. 5A, upper panel). TIMP4, Dlx5, and CBFA1 protein bands from Western blot analysis were confirmed by densitometry analysis (Fig. 5A, lower panel). Furthermore, ARS staining analysis confirmed that Pi-induced calcification was inhibited by overexpression of TIMP4 (Fig. 5B). TIMP4 gene knock-down decreased TIMP4 protein levels, and increased protein expression of Dlx5 (Fig. 5C). TIMP4 and Dlx5 protein bands from Western blot analysis were confirmed by densitometry analysis (Fig. 5C, lower panel). These results indicate that TIMP4 participated in inhibition of vascular calcification.
Fig. 5. TIMP4 represses calcification of VSMCs. VSMCs were transiently transfected with pCMV-TIMP4 and incubated in the presence of 3 mM Pi for 2 days (A, B). VSMCs were transfected with siTIMP4-1, 2 and 3 and incubated for 2 days (C). Protein levels of TIMP4, Dlx5, and CBFA1 measured by Western blot analysis (A, C, upper panel). Protein levels of TIMP4, Dlx5, and CBFA1 were measured by densitometry analysis (A, C, lower panel). Alizarin red S staining (B). Data represent the mean±standard error of the mean of 3 individual experiments.
TIMP, tissue inhibitor of metalloproteinase; VSMC, vascular smooth muscle cell; Pi, inorganic phosphate; Dlx5, distal-less homeobox 5; CBFA1, core-binding factor α-1.
Statistical significance determined relative to a control by the Student's t-test (*p<0.05; †p<0.01; ‡p<0.001), and compared with phosphate-treated cells by the Student's t-test (§p<0.05; ∥p<0.01).
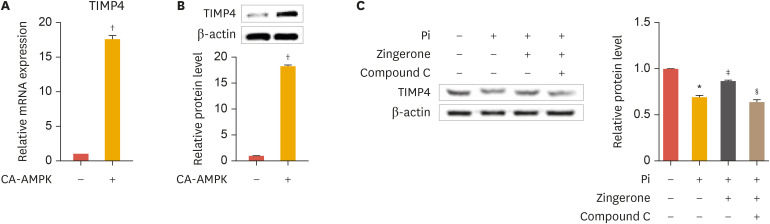
6. Zingerone-mediated AMPK activation upregulates TIMP4
To further investigate the link between the activities of zingerone, AMPK, and TIMP4 in vascular calcification, we transduced VSMCs with a constitutively active form of AMPK (CA-AMPK). The CA-AMPK obviously increased mRNA expression and protein levels of TIMP4 (Fig. 6A and B). Additionally, TIMP4 protein levels were inhibited by Pi, upregulated by zingerone, and reduced by Compound C (1 µM) (Fig. 6C, left panel). TIMP4 protein bands from Western blot analysis were confirmed by densitometry analysis (Fig. 6C, right panel). These results suggest that zingerone reduced vascular calcification through activation of AMPK and TIMP4, and that AMPK is upstream of TIMP4.
Fig. 6. Zingerone enhances TIMP4 expression through AMPK phosphorylation. VSMCs were transfected with pc-DNA3.0-AMPK and incubated for 2 days (A, B). VSMCs were cultured with or without Pi, zingerone, and Compound C (1 µM) for 2 days (C). mRNA expression of TIMP4 was measured by real-time polymerase chain reaction (A). Protein levels of TIMP4 measured by Western blot analysis (B, C). Protein levels of TIMP4 were measured by densitometry analysis (B, lower panel and C, right panel). Data represent the mean±standard error of the mean of 3 individual experiments.
TIMP, tissue inhibitor of metalloproteinase; AMPK, AMP-activated protein kinase; VSMC, vascular smooth muscle cell; Pi, inorganic phosphate.
Statistical significance was determined relative to a control (*p<0.01; †p<0.005) or compared with the Pi-treated group (‡p<0.05) Pi and zingerone group (§p<0.05) using the Student's t-test.
DISCUSSION
In this study, we identified the effect of zingerone on Pi-induced osteogenic trans-differentiation of VSMCs. The process of calcification of VSMCs is similar to that of mineralization of osteoblasts.26,27,28 In vitro, high Pi treatment leads to an increased expression of osteogenic differentiation and calcification markers in VMSCs derived from rat aorta smooth muscle cells.29 In our study, zingerone prevented phosphate-induced calcification (Fig. 2). Zingerone significantly inhibits the cellular production of proinflammatory mediators such as tumor necrosis factor-α in Raw 264.7 macrophages.30 Also, zingerone has anti-angiogenic activity by inhibiting matrix metalloproteases during tumor progression.5 Recently, it was reported that zingerone increases osteogenic marker genes such as Runx2, ALP and Col-I mRNA in mouse mesenchymal stem cells.31
Increased vascular calcification is associated with the development of atherosclerosis and diabetes, as well as certain heredity conditions, especially chronic kidney disease. The presence of Pi is a major inducer of calcification. During the calcification process, osteogenic marker genes increase; zingerone concentration-dependently decreased the mRNA expression of CBFA1 and induced differentiation of VSMCs (Fig. 1B-D).
Piperine, a component of black pepper, modulates the activity of AMPK; specifically, piperine stimulates osteoblast differentiation marker genes through AMPK activation.32 Since the molecular structure of zingerone is similar to piperine and capsaicin (found in chili pepper),33 we hypothesized that AMPK is stimulated by zingerone. Indeed, our experiments confirmed that 100 nM zingerone significantly increased AMPK activity (Fig. 3A). Despite the treatment of zingerone, calcification was deposited by Compound C (Fig. 3C). Results of studies presented in Figs. 1-3 show that zingerone induces AMPK phosphorylation and this effect abolishes vascular calcification.
TIMP4 expression was increased by zingerone, and increased TIMP4 contributed to the suppression of calcification (Fig. 4). AMPK/TIMP4 signaling has several related studies to pulmonary artery, kidney and obesity.34,35,36 Although several studies suggest that AMPK activation modulates TIMP expression, this has not been specifically demonstrated in VSMCs. We used a loss- and gain-of-function approach to investigate the role of AMPK and TIMP4 in VSMC calcification. High Pi induced the expression of osteogenic genes and inhibited TIMP4 expression. We believe that this study is the first to show that Pi inhibits TIMP4 expression. Overexpression of TIMP4 decreased Pi-induced calcification, and knock-down of TIMP4 increased calcification (Fig. 5). TIMPs suppress the activity of metalloproteases such as matrix metalloprotease and disintegrin and metalloproteases. Furthermore, we used a constitutively active form of AMPK to show that AMPK positively regulates TIMP4 mRNA and protein levels; in addition, TIMP4 expression was inhibited by the AMPK inhibitor Compound C (Fig. 6). Therefore, zingerone reverses vascular calcification by activating a pathway involving AMPK and TIMP4, and AMPK is upstream of TIMP4.
To the best of our knowledge, this is the first study to describe a protective effect of zingerone on phosphate-induced calcification of VSMCs. Zingerone can reduce phosphate-induced calcification of aortic VSMCs through activation of AMPK/TIMP4 signaling. These results suggest that the natural product zingerone may be useful for treating vascular and metabolic diseases.
Footnotes
Funding: This study was supported by funding from the Korean Society of Lipid and Atherosclerosis.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Lim YJ, Min HY, Jang WG.
- Investigation: Lim YJ, Min HY.
- Methodology: Min HY, Jang WG.
- Project administration: Jang WG.
- Supervision: Jang WG.
- Validation: Min HY, Jang WG.
- Writing - original draft: Lim YJ, Min HY.
- Writing - review & editing: Lim YJ, Jang WG.
References
- 1.Surh Y. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat Res. 1999;428:305–327. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 2.Daily JW, Zhang X, Kim DS, Park S. Efficacy of ginger for alleviating the symptoms of primary dysmenorrhea: a systematic review and meta-analysis of randomized clinical trials. Pain Med. 2015;16:2243–2255. doi: 10.1111/pme.12853. [DOI] [PubMed] [Google Scholar]
- 3.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 4.Chen JC, Huang LJ, Wu SL, Kuo SC, Ho TY, Hsiang CY. Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice. J Agric Food Chem. 2007;55:8390–8397. doi: 10.1021/jf071460f. [DOI] [PubMed] [Google Scholar]
- 5.Bae WY, Choi JS, Kim JE, Park C, Jeong JW. Zingerone suppresses angiogenesis via inhibition of matrix metalloproteinases during tumor development. Oncotarget. 2016;7:47232–47241. doi: 10.18632/oncotarget.10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alibakhshi T, Khodayar MJ, Khorsandi L, Rashno M, Zeidooni L. Protective effects of zingerone on oxidative stress and inflammation in cisplatin-induced rat nephrotoxicity. Biomed Pharmacother. 2018;105:225–232. doi: 10.1016/j.biopha.2018.05.085. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad B, Rehman MU, Amin I, Mir MU, Ahmad SB, Farooq A, et al. Zingerone (4-(4-hydroxy-3-methylphenyl) butan-2-one) protects against alloxan-induced diabetes via alleviation of oxidative stress and inflammation: probable role of NF-kB activation. Saudi Pharm J. 2018;26:1137–1145. doi: 10.1016/j.jsps.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicoll R, Henein MY. The predictive value of arterial and valvular calcification for mortality and cardiovascular events. Int J Cardiol Heart Vessels. 2014;3:1–5. doi: 10.1016/j.ijchv.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow B, Rabkin SW. The relationship between arterial stiffness and heart failure with preserved ejection fraction: a systemic meta-analysis. Heart Fail Rev. 2015;20:291–303. doi: 10.1007/s10741-015-9471-1. [DOI] [PubMed] [Google Scholar]
- 10.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–2948. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheen CR, Kuss P, Narisawa S, Yadav MC, Nigro J, Wang W, et al. Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J Bone Miner Res. 2015;30:824–836. doi: 10.1002/jbmr.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 13.Min HY, Son HE, Jang WG. Estradiol-induced RORα expression positively regulates osteoblast differentiation. Steroids. 2019;149:108412. doi: 10.1016/j.steroids.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Gomis-Rüth FX. Structural aspects of the metzincin clan of metalloendopeptidases. Mol Biotechnol. 2003;24:157–202. doi: 10.1385/MB:24:2:157. [DOI] [PubMed] [Google Scholar]
- 15.Quesada V, Ordóñez GR, Sánchez LM, Puente XS, López-Otín C. The Degradome database: mammalian proteases and diseases of proteolysis. Nucleic Acids Res. 2009;37:D239–D243. doi: 10.1093/nar/gkn570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert E, Dassé E, Haye B, Petitfrère E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol. 2004;49:187–198. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterchi EE, Stöcker W, Bond JS. Meprins, membrane-bound and secreted astacin metalloproteinases. Mol Aspects Med. 2008;29:309–328. doi: 10.1016/j.mam.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuadrado E, Rosell A, Penalba A, Slevin M, Alvarez-Sabín J, Ortega-Aznar A, et al. Vascular MMP-9/TIMP-2 and neuronal MMP-10 up-regulation in human brain after stroke: a combined laser microdissection and protein array study. J Proteome Res. 2009;8:3191–3197. doi: 10.1021/pr801012x. [DOI] [PubMed] [Google Scholar]
- 20.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KM, Jeon WJ, Kim EJ, Jang WG. CRTC2 suppresses BMP2-induced osteoblastic differentiation via Smurf1 expression in MC3T3-E1 cells. Life Sci. 2018;214:70–76. doi: 10.1016/j.lfs.2018.10.052. [DOI] [PubMed] [Google Scholar]
- 23.Chen WR, Yang JQ, Liu F, Shen XQ, Zhou YJ. Melatonin attenuates vascular calcification by activating autophagy via an AMPK/mTOR/ULK1 signaling pathway. Exp Cell Res. 2020;389:111883. doi: 10.1016/j.yexcr.2020.111883. [DOI] [PubMed] [Google Scholar]
- 24.Li KX, Du Q, Wang HP, Sun HJ. Death-associated protein kinase 3 deficiency alleviates vascular calcification via AMPK-mediated inhibition of endoplasmic reticulum stress. Eur J Pharmacol. 2019;852:90–98. doi: 10.1016/j.ejphar.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Lutshumba J, Liu S, Zhong Y, Hou T, Daugherty A, Lu H, et al. Deletion of BMAL1 in smooth muscle cells protects mice from abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2018;38:1063–1075. doi: 10.1161/ATVBAHA.117.310153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelse MA, Neele JM, Bronckers AL, Pannekoek H, de Vries CJ. Vascular calcification: expression patterns of the osteoblast-specific gene core binding factor α-1 and the protective factor matrix Gla protein in human atherogenesis. Cardiovasc Res. 2001;52:281–289. doi: 10.1016/s0008-6363(01)00375-3. [DOI] [PubMed] [Google Scholar]
- 27.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J Biol Chem. 1998;273:30427–30434. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 28.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Liu Y, Zhang Y, Bi X, Nie L, Liu C, et al. High phosphate-induced downregulation of PPARγ contributes to CKD-associated vascular calcification. J Mol Cell Cardiol. 2018;114:264–275. doi: 10.1016/j.yjmcc.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Woo HM, Kang JH, Kawada T, Yoo H, Sung MK, Yu R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007;80:926–931. doi: 10.1016/j.lfs.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 31.Srinaath N, Balagangadharan K, Pooja V, Paarkavi U, Trishla A, Selvamurugan N. Osteogenic potential of zingerone, a phenolic compound in mouse mesenchymal stem cells. Biofactors. 2019;45:575–582. doi: 10.1002/biof.1515. [DOI] [PubMed] [Google Scholar]
- 32.Kim DY, Kim EJ, Jang WG. Piperine induces osteoblast differentiation through AMPK-dependent Runx2 expression. Biochem Biophys Res Commun. 2018;495:1497–1502. doi: 10.1016/j.bbrc.2017.11.200. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Simon SA. Similarities and differences in the currents activated by capsaicin, piperine, and zingerone in rat trigeminal ganglion cells. J Neurophysiol. 1996;76:1858–1869. doi: 10.1152/jn.1996.76.3.1858. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Han D, Zhang Y, Xie X, Ke R, Zhu Y, et al. Activation of AMPK prevents monocrotaline-induced extracellular matrix remodeling of pulmonary artery. Med Sci Monit Basic Res. 2016;22:27–33. doi: 10.12659/MSMBR.897505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu WW, Guan MP, Zheng ZJ, Gao F, Zeng YM, Qin Y, et al. Exendin-4 alleviates high glucose-induced rat mesangial cell dysfunction through the AMPK pathway. Cell Physiol Biochem. 2014;33:423–432. doi: 10.1159/000358623. [DOI] [PubMed] [Google Scholar]
- 36.Luo T, Nocon A, Fry J, Sherban A, Rui X, Jiang B, et al. AMPK activation by metformin suppresses abnormal extracellular matrix remodeling in adipose tissue and ameliorates insulin resistance in obesity. Diabetes. 2016;65:2295–2310. doi: 10.2337/db15-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]