Abstract
This review focuses on the role of adipose tissue in obese individuals in the development of metabolic diseases, and their consequences for metabolic and functional derangements in the heart. The general idea is that the expansion of adipocytes during the development of obesity gives rise to unhealthy adipose tissue, characterized by low-grade inflammation and the release of proinflammatory adipokines and fatty acids (FAs). This condition, in turn, causes systemic inflammation and elevated FA concentrations in the circulation, which links obesity to several pathologies, including impaired insulin signaling in cardiac muscle and a subsequent shift in myocardial substrate oxidation in favor of FAs and reduced cardiac efficiency. This review also argues that efforts to prevent obesity-related cardiometabolic disease should focus on anti-obesogenic strategies to restore normal adipose tissue metabolism.
Keywords: Visceral adipose tissue, Inflammation, Lipid metabolism, Heart, Oxygen consumption
INTRODUCTION
Obesity causes adverse metabolic effects and is a major risk factor for metabolic diseases, such as type 2 diabetes and fatty liver disease, which increase the risk of coronary heart disease (CHD) and ischemic stroke. Obesity is a growing health problem in both developed and developing countries, and in the last 20 years the world has witnessed an alarming increase in obesity.1 Obesity has nearly tripled worldwide since 1975, and according to the World Health Organization, more than 1.9 billion adults (18 years and older) were overweight in 2016.2 Of these, over 650 million were obese (defined as a body mass index above 30 kg/m2). It should also be noted that 38 million children under the age of 5 were overweight or obese in 2019. In China, the world's most populous country, obesity has also increased at an alarmingly rapid rate, and during the past decade the prevalence of obesity in the country has tripled, while that of abdominal obesity has increased by more than 50%.3 These numbers are expected to rise in the future unless effective actions are taken to prevent such a development.2 The current rise in human obesity is primarily linked to increased energy intake and decreased energy expenditure, resulting in excess fat deposition in adipose tissue.4
ADIPOSE TISSUE: AN ENERGY RESERVOIR WITH THE CAPACITY TO CHANGE ITS DIMENSIONS IN RESPONSE TO NUTRITIONAL STATUS
Storage of extra energy obtained during food abundance in adipose tissue is an essential physiological activity in living organisms, especially in free-ranging animals who have to deal with marked seasonal alterations in food availability.5 Fat storage is also important in humans in order to maintain metabolic homeostasis during the post-prandial period, and even more importantly, in humans undergoing extended periods of starvation. The adipose tissue is distributed throughout the body and has a large capacity to expand to accommodate excess energy in the form of lipids. White adipose tissue comprises two major depots, subcutaneous and visceral adipose tissue, the latter of which is found within the abdominal cavity and stored around important internal organs. Anatomically, it is further subdivided into mesenteric, omental, perirenal, and peritoneal depots.6,7 Although adipose tissue historically has been regarded as an energy storage depot, research over the last few decades has revealed that adipose tissue also acts as an endocrine organ. Thus, several cytokines, hormones, and peptides secreted by adipocytes, collectively termed as “adipokines” (e.g. leptin, resistin, adiponectin, tumor necrosis factor alpha [TNF-α], and interleukin [IL]-6) have been identified and intensively investigated to elucidate their roles in the control of energy homeostasis.8,9
Subcutaneous adipose tissue is the largest fat depot in the body. The expansion of subcutaneous adipose tissue occurs through the recruitment and differentiation of adipose precursor cells, resulting in healthy adipose tissue.10 However, when the storage capacity of the subcutaneous depot is exceeded, excess energy intake leads to fat accumulation in undesirable locations, such as the intra-abdominal depots, as well as in ectopic tissues such as the liver, skeletal muscle, and heart. Over time, this situation creates a condition commonly referred to as “lipotoxicity,” as described in more detail below.
LOW-GRADE INFLAMMATION IN OBESE ADIPOSE TISSUE
Adipose tissue is considered to be a pathogenic site of obesity-induced insulin resistance.11 This is due to the fact that adipose tissue in obese individuals, particularly those with abdominal obesity, is associated with a chronic, local low-grade inflammatory response involving the production of pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) and chemokines.4,12,13,14 Numerous studies have shown that cellular stress is a major factor contributing to inflammation in adipose tissue.4,15 Thus, in response to nutrient excess, adipocytes expand and become hypertrophic. At the same time, the distance between blood-bearing vessels increases and oxygen diffusion becomes insufficient,16 leading to local hypoxia, which triggers the increased secretion of inflammatory markers.17 Characteristically, the adipose tissue of obese individuals shows lower blood flow, higher vasoconstriction, and lower capillary density than adipose tissue in non-obese individuals.15
Macrophage infiltration is another characteristic of adipose tissue in obese individuals. After initial rolling and attachment of monocytes to activated endothelial cells, monocytes then extravasate through the endothelial cell layer and differentiate into macrophages. It has been reported that monocyte chemoattractant protein-1 (MCP-1) secretion is markedly enhanced locally and in the plasma of obese rodents and humans.18,19 At the onset of an inflammatory process, the macrophages that are usually present in the adipose tissue switch from an anti-inflammatory (M2) state to a pro-inflammatory (M1) state.20 More than 90% of M1-type macrophages are localized to dead adipocytes and form so-called "crown-like structures," which are a characteristic component of the immuno-histological picture of adipose tissue in both obese mice and humans.16 Cross-talk between adipocytes, macrophages, and endothelial cells may enhance the inflammatory state by increasing the secretion of pro-inflammatory cytokines and chemokines, which in turn can develop into local and/or systemic insulin resistance in a paracrine and/or endocrine fashion.
Sun et al.17 documented increased interstitial fibrosis in white adipose tissue during the development of obesity, which may reduce extracellular matrix flexibility and decrease the tissue plasticity, ultimately leading to adipocyte dysfunction. Abnormal collagen deposition, which characterizes fibrosis development in adipose tissue, is paralleled by the infiltration of macrophages and other immune cells.21 Under these conditions, fibrotic response genes are markedly up-regulated, and classically activated pro-inflammatory M1 macrophages are attracted by dead adipocytes,17 reinforcing the inflammatory process and altering adipose tissue metabolism. Thus, the development of hypertrophic adipose tissue (in response to excess energy intake), macrophage infiltration, and fibrosis are major factors initiating the local low-grade inflammatory response in adipose tissue. On the molecular level, this process includes activation of the c-Jun N-terminal kinase (JNK) and IκB kinase (IKK) β/nuclear factor kappa light chain enhancer of activated B cells inflammatory signaling pathways,22 which in turn regulate protein phosphorylation and cellular transcriptional events leading to the secretion of proinflammatory cytokines (TNF-α, IL-6, and IL-1β) and chemokines, such as MCP-1.23
INFLAMMATION AND LIPID OVERLOAD CAUSE DYSREGULATION OF MYOCARDIAL METABOLISM AND VENTRICULAR FUNCTION
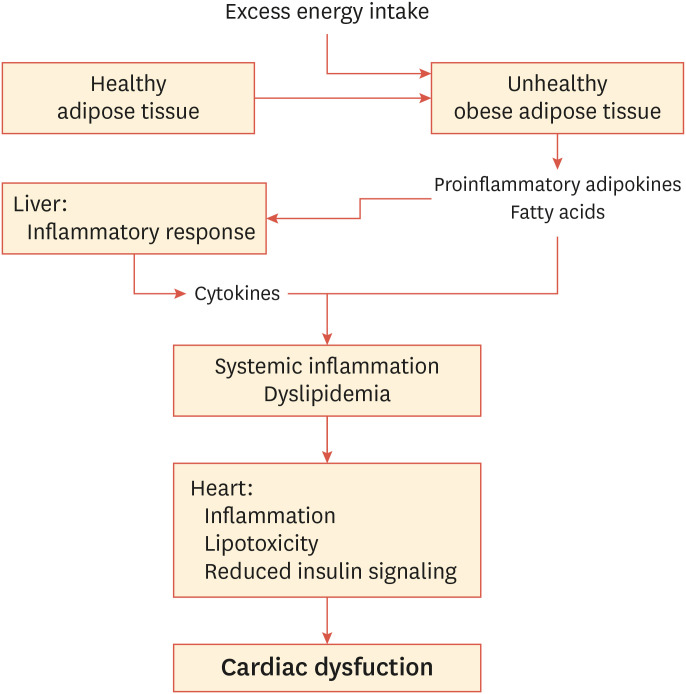
Low-grade inflammation in abdominal adipose tissue also contributes to hepatic inflammation due to portal delivery of abdominal fat—derived cytokines and lipids.11,16 Thus, TNF-α and IL-6 originating from adipocytes, as well as from macrophages, in adipose tissue and the liver22 create systemic inflammation and subsequent dysregulation of insulin action in peripheral tissues, such as skeletal and cardiac muscle24 (Fig. 1).
Fig. 1. Increasing visceral obesity causes inflammatory responses and metabolic dysregulation in fat and liver tissue. This condition involves infiltration of monocytes and macrophages and subsequent secretion of pro-inflammatory adipokines and elevated release of free fatty acids, leading to systemic inflammation, which promotes insulin resistance in several organs, including the heart. In addition, an elevated supply of lipids (free and esterified fatty acids) exceeds the fatty acid oxidation capacity and causes lipotoxicity in the myocardium, eventually leading to cardiac dysfunction.
Although the role of inflammation in the etiology of myocardial insulin resistance is limited, Ko et al.25 reported that high-fat feeding of rats caused increased macrophage infiltration in myocardial tissue from these animals, as well as increased cytokine and suppressor of cytokine signaling proteins levels in cardiomyocytes. These observations were associated with reduced myocardial insulin sensitivity and glucose metabolism. It was proposed that cytokines from macrophages and cardiomyocytes activate their receptors and associated signaling pathways to increase serine phosphorylation of insulin receptor substrate 1 (IRS-1). This eventually leads to insulin resistance via inhibition of protein kinase B/Akt and reduced glucose transporter type 4 (GLUT4) translocation.26
Increased uptake of fatty acids (FAs) also plays a central role in the development of cardiac insulin resistance in obesity. Increased FA uptake is catalyzed, in part, by the translocation of FA transporters (FAT/CD36) to the sarcolemma.27,28,29 However, not all FAs entering the cell are utilized for oxidative purposes, and long-chain FAs in the form of acyl-CoA provide substrates for nonoxidative processes such as triglyceride, diacylglycerol, and ceramide synthesis.30,31 The accumulation of these substances is known to activate kinases, including JNK, IKK, and protein kinase C, which down-regulate insulin signaling32,33 via serine phosphorylation of IRS-1.27,34 Besides its adverse effects on insulin signaling and glucose metabolism, excessive lipid accumulation may also have direct lipotoxic effects on cardiomyocytes.30,35
The mismatch between FA uptake and oxidation by cardiomyocytes27,28 and the consequent myocardial lipid accumulation and insulin resistance may have serious cardiac consequences that ultimately lead to compromised cardiac mechanical function.35,36 Thus, reduced left ventricular (LV) systolic function has also been demonstrated in several animal models of obesity,37,38,39 except for some studies in diet-induced obese rats that showed unchanged or mildly reduced systolic function.40,41 FA binding protein 4, an intracellular lipid-binding protein involved in the transportation of FAs, has been suggested to be strongly associated with inflammation, obesity, diabetes, and cardiovascular diseases (CVD).42 Cardiac-specific overexpression of this protein in mice resulted in greater cardiac hypertrophy following transverse aorta constriction than in wild-type controls.43 Furthermore, transgenic mice expressing mutated lipoprotein lipase (GPI-anchored human LPL) in cardiomyocytes developed dilated cardiomyopathy with lipid accumulation within myocytes.44 Mice with cardiomyocyte-restricted knockout of the insulin receptor also exhibited reduced heart size and mildly impaired contractile function, indicating that insulin signaling is an important physiological regulator of growth and function.45,46
Many studies have demonstrated that obesity (isolated or co-existing with hypertension) in humans is associated with abnormal diastolic function,47,48,49 whereas impairment of systolic function is not consistently observed.50 Obesity-related dysfunction includes left heart remodeling (i.e., left atrial dilatation and LV hypertrophy) as well as abnormalities in LV contractile and relaxation functions (i.e., LV stiffness and impaired relaxation).47,51,52,53 This condition can ultimately progress to cardiac hypertrophy and/or systolic dysfunction when lipotoxicity and/or local perfusion heterogeneities result in cell death and fibrosis.36,54,55,56
OBESITY-INDUCED ALTERATIONS IN MYOCARDIAL SUBSTRATE UTILIZATION: LOSS OF METABOLIC FLEXIBILITY
Approximately 50%–70% of the energy (ATP) requirement of the healthy heart is produced by oxidation of long-chain FAs, which are bound to albumin or esterified in circulating triglycerides, whereas carbohydrates, lactate, and to some extent also ketone bodies and amino acids account for the rest of overall ATP production.57,58 Although the normal heart seems to prefer FAs for the production of energy, it has the ability to change to other substrates for the generation of ATP to ensure that its energy demands are met. The contribution of individual substrates to ATP production depends on substrate availability, hormonal status, and energy demand, and the capacity of the heart to switch between the different energy substrates is referred to as “metabolic flexibility.” In the 1960s, Sir Philip Randle performed landmark studies showing how metabolic products of increased FA oxidation can inhibit glucose uptake in muscle.59 This mechanism, subsequently known as the Randle cycle, is the basis of metabolic flexibility in healthy individuals, which allows energy-requiring organs such as heart and skeletal muscle to switch between fuels, depending on nutrient composition and intake, as well as variations in insulin signaling. As mentioned above, the substrate transporters GLUT4 (for glucose) and CD36 (for FAs), play a central role in this dynamic balance of substrate utilization.60 CD36 plays a central role in facilitating cellular long-chain FA uptake across the plasma membrane, acting in concert with other membrane proteins, such as FA-binding protein.61 With the development of insulin resistance, however, the metabolic flexibility of the heart (as well as skeletal muscle) deteriorates,55 so that myocardial energy production becomes primarily dependent on FA oxidation. As a consequence, accumulation of the intermediates of FA metabolism in cardiomyocytes results in a state of lipotoxicity (as discussed above),30,56 causing cellular oxidative stress, impaired cytosolic and mitochondrial calcium homeostasis, and mitochondrial dysfunction.
ACUTE AND SUSTAINED ELEVATIONS OF THE FA SUPPLY LEAD TO INCREASED MYOCARDIAL OXYGEN CONSUMPTION (MVO2) AND IMPAIRED ENERGETICS
A study conducted in the beginning of the 1970s62 using a canine model reported that MVO2 increased markedly in response to acute elevations in the plasma concentration of FA. In addition, higher FA oxidation and MVO2 were reported in obese relative to non-obese young women.63 It has been suggested that uncoupling of oxidative phosphorylation and induction of energy-wasting triglyceride-FA64,65 and Ca2+ cycling66 could contribute to this elevation in MVO2. Moreover, it was proposed that an excess substrate supply might result in impaired transcriptional regulation of proteins involved in the pathways of cardiac energy metabolism.67 Thus, it was reported that patients undergoing coronary artery bypass graft surgery exhibited elevated plasma FA concentrations, which were associated with higher expression of cardiac mitochondrial uncoupling proteins.67 Moreover, an impaired cardiac energy reserve in patients with type 2 diabetes mellitus (as reflected by a lower myocardial phosphocreatine [PCr]/ATP ratio) was correlated with fasting plasma FA concentration,68 a finding that is also in line with increased mitochondrial uncoupling. Cardiac PCr/ATP ratios have also been documented during catecholamine stress69 or exercise70 in people with obesity and insulin resistance, although this response is not always observed.55 Whether a lower myocardial PCr/ATP ratio in diabetic cardiomyopathy is a cause or effect of the progression to heart failure is currently unknown.71
Cardiac efficiency is characterized by the relationship between the mechanical performance and energy consumption of the heart in the form of ATP utilization or oxygen consumption. The development of the pressure-volume conductance catheter enabled calculation of the total work performed by the heart during the cardiac cycle as the pressure-volume area (PVA), and the relationship between MVO2 and PVA can be used to calculate the oxygen used for mechanical activity versus the oxygen consumed for basal metabolism and excitation-contraction coupling. Oxygen consumption for the latter 2 processes is achieved by extrapolating the MVO2-PVA relationship to zero work and is referred to as unloaded MVO2.72 Around the turn of the 21st century, Korvald et al.73 showed, for the first time, that the MVO2-PVA relationship was significantly influenced by changes in myocardial substrate metabolism in pigs. Thus, a change in myocardial metabolism from glucose towards higher FA oxidation shifted the in vivo MVO2-PVA relationship upward in a parallel manner, reflecting that hearts exposed to high levels of FAs used more energy, independent of the workload. This elevation in MVO2 was ascribed to a higher unloaded MVO2 (i.e., the use of more oxygen for basal metabolism and excitation-contraction coupling), and the increased ratio between MVO2 and work was translated into decreased cardiac efficiency. Similar observations were reported by How et al.74 using isolated perfused working mouse hearts exposed to different workloads. In the same manner as in pigs, elevation of the FA concentration in the perfusion buffer shifted the MVO2-PVA relationship upward, producing a near 30% increase in unloaded MVO2. It should be noted that the FA-induced elevation in MVO2 can by no means be explained by the switch in metabolism from glucose to FAs, since the difference in the phosphorylation-to-oxidation (P/O) ratios between FA and glucose oxidation (2.33 vs. 2.58, respectively) could account for a maximum increase in oxygen consumption of 11%. Other mechanisms, such as uncoupling of oxidative phosphorylation in the mitochondria and induction of futile cycles, as discussed above, could explain the high MVO2 during predominant FA utilization. In line with this notion, Cole et al.75 reported a lower mitochondrial maximal respiratory capacity and efficiency (P/O ratio) in high-fat–fed rats and suggested that decreased respiratory coupling can contribute to the impaired cardiac efficiency observed following obesity.
CHANGES IN CARDIAC METABOLISM AND FUNCTION IN OBESE AND DIABETIC ANIMALS RESULT IN REDUCED CARDIAC EFFICIENCY
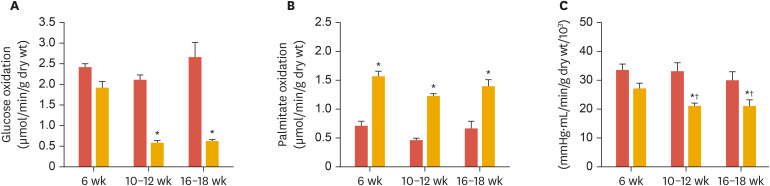
Over the years, our laboratory has studied energy metabolism and cardiac performance of ex vivo perfused hearts from type 2 diabetic (db/db) as well as diet-induced obese mice. In accordance with other researchers,76,77 we have demonstrated repeatedly that hearts from these mice exhibit altered substrate metabolism, characterized by an over-reliance on FAs for cardiac energy production and low contribution of glucose.51,52,78 Aasum et al.51 made the important observation that changes in cardiac metabolism in db/db mice preceded the development of cardiac dysfunction (Fig. 2) (including increased susceptibility to ischemia-reperfusion), indicating a causal relationship between altered cardiac metabolism and the development of ventricular dysfunction in diabetes.
Fig. 2. Age-dependent changes in myocardial substrate oxidation and ventricular function in control (db/+, red columns) and type 2 diabetic (db/db, yellow columns) mice. (A) Reduction of glucose oxidation in db/db hearts after 10–12 weeks, while fatty acid oxidation had already significantly increased at 6 weeks (B), preceding the decline of left ventricular function (C), measured as PSP times CO. Modified from Aasum et al.51.
PSP, peak systolic pressure; CO, cardiac output.
*p<0.05 vs. db/+; †p<0.05 vs. 6 week.
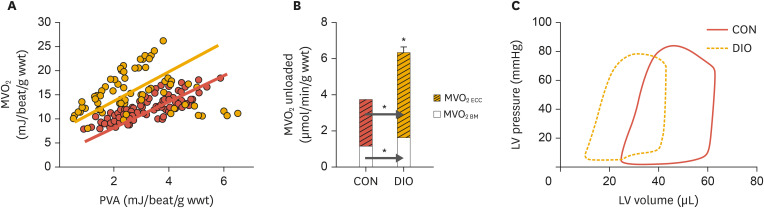
Later studies demonstrated ventricular dysfunction, not only in db/db hearts, but also in hearts from diet-induced obese mice.51,52,78,79,80 As mentioned above, these hearts show metabolic shifts towards predominant FA utilization, and the MVO2-PVA relationships obtained from these hearts were also lifted upward relative to those of normal mouse hearts53,79 (Fig. 3A). These results therefore demonstrate that not only acute elevation in myocardial FA oxidation (as discussed above), but also chronic elevation of FA oxidation, results in decreased cardiac efficiency (i.e. the ratio between MVO2 and cardiac work). Furthermore, by unloading and chemically arresting hearts, it was shown that the increased oxygen consumption of hearts in diet-induced obese mice was due to increases in both excitation-contraction coupling and basal metabolism (Fig. 3B).53
Fig. 3. Increased myocardial oxygen consumption and ventricular dysfunction in DIO mice. (A) Relationship between MVO2 and total cardiac work (measured as PVA) in isolated perfused hearts from lean CON (red line) and DIO mice (yellow line). (B) The increased oxygen consumption of the DIO hearts is explained by increased oxygen cost for excitation-contraction coupling as well as for basal metabolism. (C) Leftward shift of the pressure-volume loop of DIO heart relative to control, indicating concentric remodeling and ventricular stiffness. Modified from Hafstad et al.53.
MVO2, myocardial oxygen consumption; PVA, pressure-volume area; CON, control; DIO, diet-induced obese; LV, left ventricular.
*p<0.05 vs. CON.
Further examination of ventricular function in hearts from both diabetic and obese mice by pressure-volume analysis clearly revealed diastolic dysfunction, both in hearts from db/db mice74 and in hearts from diet-induced obese mice53 This change in LV function was reflected in a marked leftward shift in the pressure-volume loop (Fig. 3C), indicative of the development of concentric remodeling.79,81,82 In accordance with previous studies on diabetes-induced cardiac remodeling, the hearts exhibited increased fibrosis, impaired metalloproteinase expression, and elevated oxidative stress.83,84 Park et al.85 also reported that chronic high-fat feeding and obesity in mice impaired myocardial glucose metabolism, which was associated with ventricular hypertrophy and cardiac dysfunction. The same group reported that diet-induced obesity in mice led to increased macrophage and cytokine levels in the heart, which was associated with significant reductions in AMPK phosphorylation and down-regulation of glucose metabolism.25
In summary, the healthy heart is characterized by a high degree of metabolic flexibility, allowing optimal matching of metabolic supply and demand. During conditions of insulin resistance and diabetes, the cardiac muscle is not able to switch effectively from FAs to glucose metabolism in the post-prandial state. As a consequence, the heart becomes metabolically less flexible and ineffective in adapting its fuel preferences to altered energy supply and demand. When relying primarily on FA oxidation for energy production, the heart uses more oxygen for a given workload, compared with a heart oxidizing a mixture of FAs and glucose. The FA-induced elevation in MVO2 is due to increased oxygen use for non-contractile processes (i.e., basal metabolism and excitation-contraction coupling). The development of both ventricular dysfunction and mechanoenergetic impairments in diabetes/obesity is clearly multifactorial and complex and, in addition to alterations in myocardial substrate utilization, the involvement of Ca2+ handling, oxidative stress, mitochondrial dysfunction, and structural remodeling has been proposed.49,50,65,86,87,88 Diabetes is also associated with impaired myocardial Ca2+ handling, including increased ryanodine receptor 2 Ca2+ leak,88,89 which most likely contributes to the increased oxygen consumption demonstrated herein and in previous studies.75,79,80,90,91
TREATMENT STRATEGIES
The obvious solution to prevent adipose tissue inflammation and the accompanying metabolic and cardiovascular complications is to apply strategies for the targeted reduction of this particular fat store in obese individuals. Lifestyle interventions, including changes in diet and physical activity, remain the cornerstone of treatment for obesity and insulin resistance. Both reduced calorie intake and increased calorie expenditure via daily exercise should result in weight loss, but these interventions have not been effective in achieving lasting weight loss. A major part of lost weight is regained within 1 year following the end of treatment, and almost all weight is regained within 5 years.92,93 The pharmaceutical industry has therefore developed a number of anti-obesogenic medications, including some developed for maintenance of insulin sensitivity. However, several of these agents have been withdrawn from the market due to safety concerns.93 A new class of anti-diabetic drugs, sodium-glucose cotransporter-2 inhibitors,94 could hold promise for combatting the obesity epidemic in the future. Although their main effects are to inhibit glucose reabsorption in the renal proximal tubular cells and to reduce blood glucose levels through increased glycosuria, some of these drugs (dapagliflozin and canagliflozin) have been shown to reduce body weight through reductions in fat mass, including both visceral fat and subcutaneous fat.36,95 Liraglutide (Saxenda) is a glucagon-like peptide-1 receptor agonist that was developed for the treatment of type 2 diabetes. It turned out, however, that liraglutide is also an effective treatment for obesity,96 in part through its actions in the limbic system of the brain,97 regulating appetite and calorie intake. Pharmacotherapies to prevent obesity will not be further discussed in this review, however, and readers should refer to sources such as the comprehensive review by Van Gaal and Dirinck.93
In the final section, we will briefly focus on the use of marine omega-3 FAs in the control of energy homeostasis and their potential role in weight management due to their anti-inflammatory and insulin-sensitizing effects. Long-chain omega-3 polyunsaturated FAs (PUFAs) from fish oil are considered to have beneficial health effects.98 Thus, treatment of severely obese non-diabetic patients with eicosapentaenoic acid and docosahexaenoic acid was shown to reduce adipose tissue mass and systemic inflammation.99 A recent meta-analysis of 13 randomized controlled trials, which included over 120,000 participants, confirmed that PUFA supplementation reduces the risk for CHD and CVD, myocardial infarction, and death due to CHD and CVD.100,101 A systematic review and meta-analysis by Natto et al.102 also concluded that PUFA consumption may be associated with lower plasma levels of inflammatory biomarkers in patients with diabetes. However, results regarding the effects of PUFAs on glucose metabolism, insulin resistance, and type 2 diabetes are less clear,103 most likely due to differences in the choice of PUFA preparation, dosage, and intervention.104 Although the benefits of PUFA intake remain controversial for some diseases and conditions, the anti-inflammatory effects of these compounds are well accepted.105
We have previously reported that dietary supplementation with a small amount of oil from the marine crustacean, Calanus finmarchicus, reduced both intra-abdominal and hepatic fat deposition, while simultaneously exerting a strong anti-inflammatory action in adipose tissue during high-fat feeding in male C57bl/6J mice.13 Recently, we also reported106 that dietary supplementation with Calanus oil was able to prevent the obesity-induced decline in myocardial glucose utilization in hearts from high-fat–fed mice. More importantly, post-ischemic recovery of these hearts was significantly better than that of hearts from mice on a non-supplemented high-fat diet, indicating the cardioprotective properties of the Calanus oil in obesity. Of note, this effect was achieved with a much lower dose (2%, w/w) than was used in similar experiments in the past.107 It should be emphasized that the above study included female mice, and in contrast to results obtained with male mice that obesity impaired the recovery of cardiac function after an ischemic insult,53,78,80 we observed that the post-ischemic recovery of ventricular function in hearts from high-fat—fed female mice was not impaired relative to hearts from mice receiving normal chow. This result confirms previous observations by Edland et al.,108 who reported that cardioprotection was afforded by long-term feeding of an obesogenic high-fat diet in hearts from female mice. In addition to other possible sex differences, mRNA expression of TNF-α and IL-6 in adipose tissue was hardly detectable in response to high-fat feeding in the female mice, in contrast to previous results with male mice.12,13 The low inflammatory status could probably be explained by the finding that high-fat feeding induced only a relatively mild degree of adiposity in the female mice, so that the signal for adipokine secretion17 was missing. In addition, it has been reported that the genes involved in inflammation are more highly up-regulated in males than in females.109 Still, dietary Calanus oil resulted in less deposition of intra-abdominal fat than in untreated high-fat–diet mice. The underlying mechanism is not clear, but increased adipose tissue lipolysis and/or decreased lipogenesis, as well as increased hepatic drainage of FAs from the abdominal fat stores, are possibilities that could be further investigated. Although clinical studies are sparse, recent studies in elderly untrained overweight participants110 suggested that a combination of moderate exercise and intake of oil from C. finmarchicus may promote fat loss. It was also shown that wax ester—bound PUFAs from Calanus oil were significantly incorporated into the membranes of red blood cells, thereby increasing the omega-3-index.111
CONCLUSION
Adipose tissue appears to act as a priming tissue that initiates inflammation in obesity in response to excess energy intake. Thus, obesity-induced dysfunction of visceral and ectopic adipose tissue, including the release of proinflammatory cytokines and FA, is a major contributor to potential pathogenic mechanisms leading to insulin resistance and type 2 diabetes. Preclinical studies have demonstrated that these conditions are associated with a marked shift in myocardial metabolism towards predominant FA utilization for energy production. Over time, this switch in myocardial metabolism leads to a lipotoxic milieu and subsequent metabolic and functional derangements in the heart. Prevention of obesity-related cardiometabolic disease should therefore focus on anti-obesogenic strategies to restore normal adipose tissue metabolism, and understanding the inflammatory responses in adipose tissues of obese individuals is therefore of clear clinical importance.
Footnotes
Funding: This work was supported by UiT The Arctic University of Norway.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Jansen KM.
- Writing - original draft: Larsen TS.
- Writing - review & editing: Larsen TS.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. World health statistics 2016: monitoring health for the SDGs. Geneva: World Health Organization; 2016. [Google Scholar]
- 3.Zhang X, Zhang M, Zhao Z, Huang Z, Deng Q, Li Y, et al. Geographic variation in prevalence of adult obesity in China: results from the 2013–2014 national chronic disease and risk factor surveillance. Ann Intern Med. 2020;172:291–293. doi: 10.7326/M19-0477. [DOI] [PubMed] [Google Scholar]
- 4.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 5.Larsen TS, Nilsson NO, Blix AS. Seasonal changes in lipogenesis and lipolysis in isolated adipocytes from Svalbard and Norwegian reindeer. Acta Physiol Scand. 1985;123:97–104. doi: 10.1111/j.1748-1716.1985.tb07566.x. [DOI] [PubMed] [Google Scholar]
- 6.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Item F, Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes Rev. 2012;13(Suppl 2):30–39. doi: 10.1111/j.1467-789X.2012.01035.x. [DOI] [PubMed] [Google Scholar]
- 8.Knebel B, Goeddeke S, Poschmann G, Markgraf DF, Jacob S, Nitzgen U, et al. Novel insights into the adipokinome of obese and obese/diabetic mouse models. Int J Mol Sci. 2017;18:1928. doi: 10.3390/ijms18091928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20:2358. doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Höper AC, Salma W, Khalid AM, Hafstad AD, Sollie SJ, Raa J, et al. Oil from the marine zooplankton Calanus finmarchicus improves the cardiometabolic phenotype of diet-induced obese mice. Br J Nutr. 2013;110:2186–2193. doi: 10.1017/S0007114513001839. [DOI] [PubMed] [Google Scholar]
- 13.Höper AC, Salma W, Sollie SJ, Hafstad AD, Lund J, Khalid AM, et al. Wax esters from the marine copepod Calanus finmarchicus reduce diet-induced obesity and obesity-related metabolic disorders in mice. J Nutr. 2014;144:164–169. doi: 10.3945/jn.113.182501. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 15.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim KH, Lee MS. Autophagy as a crosstalk mediator of metabolic organs in regulation of energy metabolism. Rev Endocr Metab Disord. 2014;15:11–20. doi: 10.1007/s11154-013-9272-6. [DOI] [PubMed] [Google Scholar]
- 19.Panee J. Monocyte chemoattractant protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012;60:1–12. doi: 10.1016/j.cyto.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solinas G, Karin M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB J. 2010;24:2596–2611. doi: 10.1096/fj.09-151340. [DOI] [PubMed] [Google Scholar]
- 23.Marcus Y, Shefer G, Stern N. Adipose tissue renin-angiotensin-aldosterone system (RAAS) and progression of insulin resistance. Mol Cell Endocrinol. 2013;378:1–14. doi: 10.1016/j.mce.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko HJ, Zhang Z, Jung DY, Jun JY, Ma Z, Jones KE, et al. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in the heart. Diabetes. 2009;58:2536–2546. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.du Toit EF, Donner DG. Myocardial insulin resistance: an overview of its causes, effects, and potential therapy. In: Arora S, editor. Insulin resistance. London: IntechOpen; 2012. p. 189. [Google Scholar]
- 27.Koonen DP, Glatz JF, Bonen A, Luiken JJ. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim Biophys Acta. 2005;1736:163–180. doi: 10.1016/j.bbalip.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Luiken JJ, Coort SL, Koonen DP, van der Horst DJ, Bonen A, Zorzano A, et al. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch. 2004;448:1–15. doi: 10.1007/s00424-003-1199-4. [DOI] [PubMed] [Google Scholar]
- 29.Bonen A, Luiken JJ, Glatz JF. Regulation of fatty acid transport and membrane transporters in health and disease. Mol Cell Biochem. 2002;239:181–192. [PubMed] [Google Scholar]
- 30.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 31.Chess DJ, Stanley WC. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res. 2008;79:269–278. doi: 10.1093/cvr/cvn074. [DOI] [PubMed] [Google Scholar]
- 32.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. 2006;147:4160–4168. doi: 10.1210/en.2006-0251. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Keung W, Samokhvalov V, Wang W, Lopaschuk GD. Role of fatty acid uptake and fatty acid beta-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim Biophys Acta. 2010;1801:1–22. doi: 10.1016/j.bbalip.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 35.van Herpen NA, Schrauwen-Hinderling VB. Lipid accumulation in non-adipose tissue and lipotoxicity. Physiol Behav. 2008;94:231–241. doi: 10.1016/j.physbeh.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 36.van den Brom CE, Huisman MC, Vlasblom R, Boontje NM, Duijst S, Lubberink M, et al. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography. Cardiovasc Diabetol. 2009;8:39. doi: 10.1186/1475-2840-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glenn DJ, Wang F, Nishimoto M, Cruz MC, Uchida Y, Holleran WM, et al. A murine model of isolated cardiac steatosis leads to cardiomyopathy. Hypertension. 2011;57:216–222. doi: 10.1161/HYPERTENSIONAHA.110.160655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 40.Carroll JF, Zenebe WJ, Strange TB. Cardiovascular function in a rat model of diet-induced obesity. Hypertension. 2006;48:65–72. doi: 10.1161/01.HYP.0000224147.01024.77. [DOI] [PubMed] [Google Scholar]
- 41.Sun X, Pan H, Tan H, Yu Y. High free fatty acids level related with cardiac dysfunction in obese rats. Diabetes Res Clin Pract. 2012;95:251–259. doi: 10.1016/j.diabres.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 42.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Qiao C, Chang L, Guo Y, Fan Y, Villacorta L, et al. Cardiomyocyte overexpression of FABP4 aggravates pressure overload-induced heart hypertrophy. PLoS One. 2016;11:e0157372. doi: 10.1371/journal.pone.0157372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J, Wende AR, Sena S, Theobald HA, Soto J, Sloan C, et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol. 2008;22:2531–2543. doi: 10.1210/me.2008-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, et al. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277:37670–37677. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- 47.Leopold JA. Obesity-related cardiomyopathy is an adipocyte-mediated paracrine disease. Trends Cardiovasc Med. 2015;25:127–128. doi: 10.1016/j.tcm.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blomstrand P, Sjöblom P, Nilsson M, Wijkman M, Engvall M, Länne T, et al. Overweight and obesity impair left ventricular systolic function as measured by left ventricular ejection fraction and global longitudinal strain. Cardiovasc Diabetol. 2018;17:113. doi: 10.1186/s12933-018-0756-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mouton AJ, Li X, Hall ME, Hall JE. Obesity, hypertension, and cardiac dysfunction: novel roles of immunometabolism in macrophage activation and inflammation. Circ Res. 2020;126:789–806. doi: 10.1161/CIRCRESAHA.119.312321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pascual M, Pascual DA, Soria F, Vicente T, Hernández AM, Tébar FJ, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–1156. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–441. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 52.Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- 53.Hafstad AD, Lund J, Hadler-Olsen E, Höper AC, Larsen TS, Aasum E. High- and moderate-intensity training normalizes ventricular function and mechanoenergetics in mice with diet-induced obesity. Diabetes. 2013;62:2287–2294. doi: 10.2337/db12-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study) Am J Cardiol. 1991;68:85–89. doi: 10.1016/0002-9149(91)90716-x. [DOI] [PubMed] [Google Scholar]
- 55.Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54:1524–1532. doi: 10.1016/j.jacc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 56.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 57.Opie LH. Metabolism of the heart in health and disease. II. Am Heart J. 1969;77:100–122. doi: 10.1016/0002-8703(69)90135-5. [DOI] [PubMed] [Google Scholar]
- 58.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 60.Chanda D, Luiken JJ, Glatz JF. Signaling pathways involved in cardiac energy metabolism. FEBS Lett. 2016;590:2364–2374. doi: 10.1002/1873-3468.12297. [DOI] [PubMed] [Google Scholar]
- 61.Glatz JF, Luiken JJ. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J Lipid Res. 2018;59:1084–1093. doi: 10.1194/jlr.R082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mjos OD. Effect of free fatty acids on myocardial function and oxygen consumption in intact dogs. J Clin Invest. 1971;50:1386–1389. doi: 10.1172/JCI106621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peterson LR, Soto PF, Herrero P, Mohammed BS, Avidan MS, Schechtman KB, et al. Impact of gender on the myocardial metabolic response to obesity. JACC Cardiovasc Imaging. 2008;1:424–433. doi: 10.1016/j.jcmg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borst P, Loos JA, Christ EJ, Slater EC. Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta. 1962;62:509–518. doi: 10.1016/0006-3002(62)90232-9. [DOI] [PubMed] [Google Scholar]
- 65.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 66.Tada M, Katz AM. Phosphorylation of the sarcoplasmic reticulum and sarcolemma. Annu Rev Physiol. 1982;44:401–423. doi: 10.1146/annurev.ph.44.030182.002153. [DOI] [PubMed] [Google Scholar]
- 67.Taegtmeyer H, Golfman L, Sharma S, Razeghi P, van Arsdall M. Linking gene expression to function: metabolic flexibility in the normal and diseased heart. Ann N Y Acad Sci. 2004;1015:202–213. doi: 10.1196/annals.1302.017. [DOI] [PubMed] [Google Scholar]
- 68.Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107:3040–3046. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- 69.Rider OJ, Francis JM, Ali MK, Holloway C, Pegg T, Robson MD, et al. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation. 2012;125:1511–1519. doi: 10.1161/CIRCULATIONAHA.111.069518. [DOI] [PubMed] [Google Scholar]
- 70.Levelt E, Rodgers CT, Clarke WT, Mahmod M, Ariga R, Francis JM, et al. Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J. 2016;37:3461–3469. doi: 10.1093/eurheartj/ehv442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bakermans AJ, Bazil JN, Nederveen AJ, Strijkers GJ, Boekholdt SM, Beard DA, et al. Human cardiac 31P-MR spectroscopy at 3 Tesla cannot detect failing myocardial energy homeostasis during exercise. Front Physiol. 2017;8:939. doi: 10.3389/fphys.2017.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suga H. Total mechanical energy of a ventricle model and cardiac oxygen consumption. Am J Physiol. 1979;236:H498–H505. doi: 10.1152/ajpheart.1979.236.3.H498. [DOI] [PubMed] [Google Scholar]
- 73.Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influences left ventricular energetics in vivo . Am J Physiol Heart Circ Physiol. 2000;278:H1345–H1351. doi: 10.1152/ajpheart.2000.278.4.H1345. [DOI] [PubMed] [Google Scholar]
- 74.How OJ, Aasum E, Kunnathu S, Severson DL, Myhre ES, Larsen TS. Influence of substrate supply on cardiac efficiency, as measured by pressure-volume analysis in ex vivo mouse hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2979–H2985. doi: 10.1152/ajpheart.00084.2005. [DOI] [PubMed] [Google Scholar]
- 75.Cole MA, Murray AJ, Cochlin LE, Heather LC, McAleese S, Knight NS, et al. A high fat diet increases mitochondrial fatty acid oxidation and uncoupling to decrease efficiency in rat heart. Basic Res Cardiol. 2011;106:447–457. doi: 10.1007/s00395-011-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carley AN, Atkinson LL, Bonen A, Harper ME, Kunnathu S, Lopaschuk GD, et al. Mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice. Arch Physiol Biochem. 2007;113:65–75. doi: 10.1080/13813450701422617. [DOI] [PubMed] [Google Scholar]
- 77.Neitzel AS, Carley AN, Severson DL. Chylomicron and palmitate metabolism by perfused hearts from diabetic mice. Am J Physiol Endocrinol Metab. 2003;284:E357–E365. doi: 10.1152/ajpendo.00380.2002. [DOI] [PubMed] [Google Scholar]
- 78.Aasum E, Belke DD, Severson DL, Riemersma RA, Cooper M, Andreassen M, et al. Cardiac function and metabolism in type 2 diabetic mice after treatment with BM 17.0744, a novel PPAR-α activator. Am J Physiol Heart Circ Physiol. 2002;283:H949–H957. doi: 10.1152/ajpheart.00226.2001. [DOI] [PubMed] [Google Scholar]
- 79.How OJ, Aasum E, Severson DL, Chan WY, Essop MF, Larsen TS. Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes. 2006;55:466–473. doi: 10.2337/diabetes.55.02.06.db05-1164. [DOI] [PubMed] [Google Scholar]
- 80.Hafstad AD, Khalid AM, How OJ, Larsen TS, Aasum E. Glucose and insulin improve cardiac efficiency and postischemic functional recovery in perfused hearts from type 2 diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2007;292:E1288–E1294. doi: 10.1152/ajpendo.00504.2006. [DOI] [PubMed] [Google Scholar]
- 81.Devereux RB, Roman MJ, Paranicas M, O'Grady MJ, Lee ET, Welty TK, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–2276. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 82.Zhang HS, Wang SQ. Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-alpha (TNF-alpha)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J Mol Cell Cardiol. 2006;41:138–148. doi: 10.1016/j.yjmcc.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 83.Fukuda M, Nakamura T, Kataoka K, Nako H, Tokutomi Y, Dong YF, et al. Potentiation by candesartan of protective effects of pioglitazone against type 2 diabetic cardiovascular and renal complications in obese mice. J Hypertens. 2010;28:340–352. doi: 10.1097/HJH.0b013e32833366cd. [DOI] [PubMed] [Google Scholar]
- 84.Van Linthout S, Seeland U, Riad A, Eckhardt O, Hohl M, Dhayat N, et al. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2008;103:319–327. doi: 10.1007/s00395-008-0715-2. [DOI] [PubMed] [Google Scholar]
- 85.Park SY, Cho YR, Finck BN, Kim HJ, Higashimori T, Hong EG, et al. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-alpha causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–2524. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 86.Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, et al. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55:608–615. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 87.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 88.Belke DD, Dillmann WH. Altered cardiac calcium handling in diabetes. Curr Hypertens Rep. 2004;6:424–429. doi: 10.1007/s11906-004-0035-3. [DOI] [PubMed] [Google Scholar]
- 89.Stølen TO, Høydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, et al. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res. 2009;105:527–536. doi: 10.1161/CIRCRESAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- 90.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109:2191–2196. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 91.Boardman N, Hafstad AD, Larsen TS, Severson DL, Aasum E. Increased O2 cost of basal metabolism and excitation-contraction coupling in hearts from type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1373–H1379. doi: 10.1152/ajpheart.01264.2008. [DOI] [PubMed] [Google Scholar]
- 92.Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 2014;22:5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Gaal L, Dirinck E. Pharmacological approaches in the treatment and maintenance of weight loss. Diabetes Care. 2016;39(Suppl 2):S260–S267. doi: 10.2337/dcS15-3016. [DOI] [PubMed] [Google Scholar]
- 94.Devenny JJ, Godonis HE, Harvey SJ, Rooney S, Cullen MJ, Pelleymounter MA. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity (Silver Spring) 2012;20:1645–1652. doi: 10.1038/oby.2012.59. [DOI] [PubMed] [Google Scholar]
- 95.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crane J, McGowan B. The GLP-1 agonist, liraglutide, as a pharmacotherapy for obesity. Ther Adv Chronic Dis. 2016;7:92–107. doi: 10.1177/2040622315620180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mancini MC, de Melo ME. The burden of obesity in the current world and the new treatments available: focus on liraglutide 3.0 mg. Diabetol Metab Syndr. 2017;9:44. doi: 10.1186/s13098-017-0242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 99.Itariu BK, Zeyda M, Hochbrugger EE, Neuhofer A, Prager G, Schindler K, et al. Long-chain n-3 PUFAs reduce adipose tissue and systemic inflammation in severely obese nondiabetic patients: a randomized controlled trial. Am J Clin Nutr. 2012;96:1137–1149. doi: 10.3945/ajcn.112.037432. [DOI] [PubMed] [Google Scholar]
- 100.Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. 2019;8:e013543. doi: 10.1161/JAHA.119.013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rimm EB, Appel LJ, Chiuve SE, Djoussé L, Engler MB, Kris-Etherton PM, et al. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2018;138:e35–e47. doi: 10.1161/CIR.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Natto ZS, Yaghmoor W, Alshaeri HK, Van Dyke TE. Omega-3 fatty acids effects on inflammatory biomarkers and lipid profiles among diabetic and cardiovascular disease patients: a systematic review and meta-analysis. Sci Rep. 2019;9:18867–18867. doi: 10.1038/s41598-019-54535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buoite Stella A, Gortan Cappellari G, Barazzoni R, Zanetti M. Update on the impact of omega 3 fatty acids on inflammation, insulin resistance and sarcopenia: a review. Int J Mol Sci. 2018;19:218. doi: 10.3390/ijms19010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.El-Bayoumy K, Manni A. Customized prevention trials could resolve the controversy of the effects of omega-3 fatty acids on cancer. Nutr Cancer. 2020;72:183–186. doi: 10.1080/01635581.2019.1651348. [DOI] [PubMed] [Google Scholar]
- 105.Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 106.Jansen KM, Moreno S, Garcia-Roves PM, Larsen TS. Dietary Calanus oil recovers metabolic flexibility and rescues postischemic cardiac function in obese female mice. Am J Physiol Heart Circ Physiol. 2019;317:H290–H299. doi: 10.1152/ajpheart.00191.2019. [DOI] [PubMed] [Google Scholar]
- 107.Rustan AC, Hustvedt BE, Drevon CA. Dietary supplementation of very long-chain n-3 fatty acids decreases whole body lipid utilization in the rat. J Lipid Res. 1993;34:1299–1309. [PubMed] [Google Scholar]
- 108.Edland F, Wergeland A, Kopperud R, Åsrud KS, Hoivik EA, Witsø SL, et al. Long-term consumption of an obesogenic high fat diet prior to ischemia-reperfusion mediates cardioprotection via Epac1-dependent signaling. Nutr Metab (Lond) 2016;13:87. doi: 10.1186/s12986-016-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes. 2010;34:989–1000. doi: 10.1038/ijo.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wasserfurth P, Nebl J, Schuchardt JP, Müller M, Boßlau TK, Krüger K, et al. Effects of exercise combined with a healthy diet or Calanus finmarchicus oil supplementation on body composition and metabolic markers-a pilot study. Nutrients. 2020;12:2139. doi: 10.3390/nu12072139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wasserfurth P, Nebl J, Boßlau TK, Krüger K, Hahn A, Schuchardt JP. Intake of Calanus finmarchicus oil for 12 weeks improves omega-3 index in healthy older subjects engaging in an exercise programme. Br J Nutr. 2020:1–8. doi: 10.1017/S0007114520002809. [DOI] [PMC free article] [PubMed] [Google Scholar]