Abstract
Dyslipidemia related diseases such as hyperlipidemia and atherosclerosis are the leading cause of death in humans. While cellular and molecular basis on the pathophysiology of dyslipidemia has been extensively investigated over decades, we still lack comprehensive understanding on the etiology of dyslipidemia due to the complexity and the innate multimodality of the diseases. While mouse has been the model organism of choice to investigate the pathophysiology of human dyslipidemia, zebrafish, a small freshwater fish which has traditionally used to study vertebrate development, has recently emerged as an alternative model organism. In this review, we will provide comprehensive perspective on zebrafish as a model organism for human dyslipidemia; we will discuss the attributes of zebrafish as a model, and compare the lipid metabolism in zebrafish and humans. In addition, we will summarize current landscape of zebrafish-based dyslipidemia research.
Keywords: Zebrafish, Dyslipidemia, Atherosclerosis, Lipid metabolism
INTRODUCTION
Hyperlipidemia, which shows elevated levels of blood lipids and associated lipoproteins, is one of the key contributing factor for cardiovascular diseases (CVDs) which are the leading causes of death worldwide.1 Elevated circulating lipids in blood is not only the cause of atherosclerosis, but also predisposes to a spectrum of human diseases such as steatohepatitis, renal disorders, diabetes, and even neurodegenerative disorders.2 Based on the underlying cause, dyslipidemia could be further categorized as primary and secondary dyslipidemia. Primary dyslipidemia is caused by genetic factors; for instance, mutations in genes involving apolipoproteins (apo) and low-density lipoprotein (LDL) receptors and proteins related to lipid metabolism has been implicated familial hyperlipidemias including hypercholesterolemia and hypertriglyceridemia.3,4,5 In contrast, secondary dyslipidemia is caused by lifestyle factors such as continuous consumption of high fat and high calorie diets, or chronic diseases which adversely affect the homeostasis of blood lipid levels, including obesity and diabetes.6,7 In most cases, however, the etiology of dyslipidemia appears to be multifactorial, with both genetic predispositions and environmental factors contributing to the onset of dyslipidemia.
Due to its clinical importance and its immense socioeconomic impacts, the underlying causes as well as diverse clinical interventive therapies have been extensively investigated. For in vivo studies, mammalian models, in particular, mice (Mus musculus), has been predominantly used. However, experiments using rodent models require infrastructural support and considerable staff, and are relatively expensive. Furthermore, pathogenic events in mice model can only be analyzed after autopsy, thus preventing real-time observation on the progression of the diseases. Therefore, there has been unmet needs for an alternative experimental model. Recently, zebrafish (Danio rerio), a small tropical freshwater fish which has been widely used as a vertebrate model to study embryogenesis, has gained popularity as a valuable model to study lipid metabolism and associated disorders.8,9,10,11,12,13
In this review, we will introduce zebrafish as an emerging model to investigate fundamental cellular and molecular mechanisms underlying the pathophysiology of dyslipidemia and associated diseases. We will briefly summarize the advantages and potential disadvantages of zebrafish as a model organism, and describe our current understanding on the lipid metabolism in zebrafish, and compare differences and similarities of zebrafish and humans. Lastly, we will highlight critical findings in zebrafish pertinent to dyslipidemia research to provide a comprehensive perspective on how zebrafish model could be applied to better understand the pathophysiology of human dyslipidemia.
ZEBRAFISH AS A MODEL ORGANISM FOR DYSLIPIDEMIA RESEARCH
Zebrafish, D. rerio, is a small tropical freshwater fish, which has been extensively used as a model organism to delineate underlying cellular and molecular mechanisms underlying the development of vertebrates, in particular, the early embryogenesis.14,15 Recently, the use of zebrafish as a model organism has been extended beyond early development, and more recently, it is gaining popularity as a model organism of choice to investigate diverse physiological processes, due to its many attributes. Compared to mice model, zebrafish model offers a number of unique advantages for biomedical research. Zebrafish requires relatively less husbandry and low cost of housing and maintenance. Zebrafish generates large numbers of embryos; an average, a pair of zebrafish could provide up to 500 embryos per week. Since zebrafish produces externally fertilized embryos, a female zebrafish could be spared in the process of obtaining embryos, and therefore, theoretically, a single zebrafish pair could produce thousands of embryos with identical genetic background. In addition, zebrafish embryos are optically transparent, which allows easy access to developing embryos and easy access to perform experimental manipulations. In combination with a plethora of available transgenic lines and vital dyes, the accessibility to a developing embryo enables in vivo monitoring of developmental processes without perturbation of the embryos. Furthermore, zebrafish embryos undergo extremely rapid development; by 24 hours post-fertilization, the majority of organogenesis is completed in zebrafish embryos, which allows real time imaging of the entire organogenesis without any interruption.16,17,18,19
As a vertebrate, zebrafish possess major organs and tissues found in humans, of which developmental ontogeny is similarly regulated as humans. Moreover, zebrafish have a similar genomic structure to humans. Genome sequencing has shown that over 70 percent of zebrafish genes have orthologous genes in human genome. Combined together, these attributes make zebrafish the only vertebrate model which is amenable to large scale forward genetic approach to date.14 With a fully sequenced genome combined with a high fecundity, an array of modern genome editing tools has been applied to zebrafish research, including very promising knock-in technologies.20,21,22,23,24 For instance, it has been recently demonstrated that CRISPR/Cas9 technology could be applied to effectively generate mutations, but also be utilized to edit in zebrafish genome, which has precipitated the generation of genetic model to investigate the function of genes which are implicated in various human diseases.25
Lipid metabolism and dyslipidemia research in zebrafish has been pioneered by Drs. Steven Farber and Marnie Halpern, who demonstrated digestive physiology of lipids in zebrafish using fluorescent phospholipid sensor.26 By taking advantage of transparent and externally fertilized embryos, they were able to trace the process of lipid metabolism in live embryos. While zebrafish digestive tract appears to be anatomically distinct from mammals, they do share fundamental digestive physiology to their mammalian relatives. It has been shown that element-controlled regimen without genetic manipulation is enough to induce hyperlipidemia in zebrafish.27 Moreover, zebrafish retains remarkable similarities with humans in molecular mechanisms associated with hyperlipidemia and atherosclerosis, including lipid metabolism and lipoprotein oxidation.28 Combined with lipid metabolism is regulated by evolutionarily conserved molecular mechanisms, these features allow zebrafish model to serve as a surrogate to understand the underlying causes of hyperlipidemia and associated diseases in humans and to identify new therapeutic targets for these chronic diseases.
However, there are a number of limitation of zebrafish as a model system to understand human physiology. For instance, as a poikilotherm, zebrafish has a distinct metabolic process compared to homeotherm.29 Therefore, while zebrafish retains key characteristic feature of metabolic regulations found in mammals, zebrafish might not utilize similar cellular and molecular mechanisms to respond to metabolic changes.30 In addition, emergence and expansion of adipose tissue during development appear to be distinctively modulated in zebrafish and humans,13 hinting that lipid storage in postnatal stage might occur differently in zebrafish and humans. Consistent with this idea, we and others have recently shown that genes of which expression is regulated by lipid metabolism in zebrafish may be distinct from humans. Therefore, results obtained from zebrafish research could provide invaluable insight to understand pathophysiology of dyslipidemia and associated diseases in humans, in particular, for teasing apart the contribution of genetic factors and etiology of the symptoms. However, extra cautions need to be taken when translating the outcomes of zebrafish research to directly to human diseases to interpret physiological responses and alterations.
LIPID METABOLISM IN ZEBRAFISH AND HUMANS
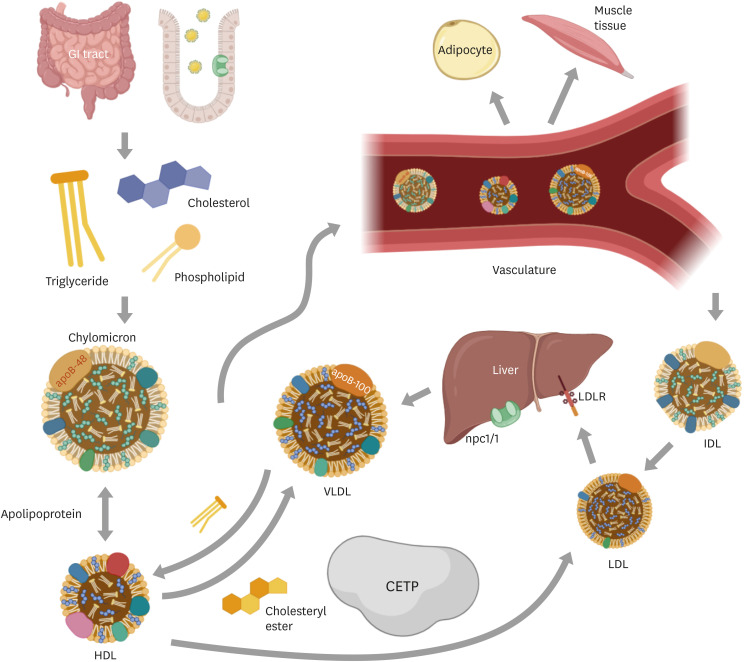
Lipids serve as the primary source of energy and are utilized as essential components of plasma membrane. In addition, they have been shown to function as signaling molecules which could elicit diverse cellular responses.31 However, excessive lipids in the body can be detrimental and could predispose to diverse CVDs including atherosclerosis. In humans as well as other mammalian models, high-fat diet (HFD) and high-cholesterol diet (HCD) not only induce lipid accumulation but also trigger expansion and proliferation of adipocytes.32 Normally, digestion of ingested fat occurs from mouth to gastrointestinal tract, especially in duodenum, by lipase and colipase. Fatty acids and monoglycerides, products by lipolysis, are transferred into enterocytes along with phospholipids and cholesterol, and re-esterified into triglycerides (TGs) (Fig. 1).32 TGs are combined with cholesterol, fat-soluble vitamins, and proteins to form chylomicrons, which are the transport vehicle for dietary fat. The major protein component of chylomicrons is truncated apoB-48, which has only 48% length of full-length apoB-100. Chylomicrons also contain other apo families including apoA-I, apoA-II and apoA-IV, and apoC.33 Chylomicrons acquire apoC and apoE by interacting with circulating high-density lipoproteins (HDLs). While chylomicrons are made in the intestine, very low-density lipoproteins (VLDLs), other TG-rich lipoproteins, are formed in the liver. VLDLs are synthesized through a different pathway and the major structural protein in full-length apoB-100.34 Both chylomicrons and VLDLs transport TG between supplying organs, liver and intestine, and storage adipose tissue and consuming tissues such as muscles, where they are hydrolyzed into fatty acids to produce energy. VLDL remnants, known as intermediate-density lipoproteins, can be further converted to LDLs, and circulating LDL particles in bloods are cleared by liver via LDL receptors. Transference of cholesterol esters and TGs between VLDLs, LDLs and HDLs is controlled by cholesteryl ester transfer protein (CETP), which modulates the balance between LDLs and VLDLs. Cholesterol absorption in liver and intestine occurs mainly through Niemann-Pick C1 like-1 (NPC1L1).35
Fig. 1. Lipid ingestion and metabolism. Lipids from foods are digested in GI tract. Ingested lipids are hydrolyzed into small, absorbable molecules such as fatty acids and monoglycerides and products by lipolysis are subsequently re-esterified into TGs in the enterocytes. Cholesterol is absorbed through the transport protein npc1l1, a critical mediator of cholesterol absorption. TGs with cholesterol, fat-soluble vitamins, cholesteryl ester and phospholipids forms chylomicrons in the intestine. Chylomicron acquires apolipoproteins from HDLs in vasculature. VLDLs are synthesized in liver, and they possess apoB-100, in contrast, chylomicrons possess apoB-48, although both deliver TGs to peripheral tissue. LDLs, converted particles from IDLs, are cleared in liver via LDL receptors. CETP mediates exchange of cholesteryl ester and TGs between VLDLs, LDLs and HDLs.
GI, gastrointestinal; TG, triglyceride; HDL, high-density lipoprotein; VLDL, very low-density lipoprotein; apo, apolipoprotein; LDL, low-density lipoprotein; IDL, intermediate density lipoprotein; CETP, cholesteryl ester transfer protein.
Zebrafish also possesses similar gastrointestinal organ system to humans, which contains cell types responsible for lipid absorption and processing.9 During embryonic development, zebrafish obtains lipid from yolk sac and starts to uptake external food at 5dpf, which eventually replace yolk as the primary energy source. At early developmental stages, the yolk syncytial later strongly expresses apo such as apoe, apoa1, apoc2, to catabolize the maternally deposited yolk. In addition, zebrafish produces VLDLs from yolk lipids at these stages.33,34 Similar to mammals, microsomal TG transfer protein play an indispensable role in packaging triacylglycerol and betalipoproteins into lipoprotein particles, and therefore, facilitate the transport of lipids in zebrafish.33,34 Most importantly, zebrafish does possess CETP, and is prone to atherosclerosis.36 Since other model organisms, including both rodents and non-rodent mammalian species, are generally resistant to atherosclerosis due to the composition of their lipoproteins, this feature renders zebrafish as a unique model to investigate lipid metabolism in humans.
Consistent with their physiological similarities, it has been reported that zebrafish genome harbors orthologues of human genes important for lipid metabolism, including microsomal TG transfer protein (mttp), fatty acid transport protein (slc27a) and acyl-CoA synthetase (acsl) gene families, as well as the LDL receptor (ldlr). In addition, the expression pattern of these genes appear to be comparable to humans.37,38,39,40,41 One important differences in lipid metabolism between zebrafish and humans is that zebrafish only produces full-length apoB (apoB-100) and lack truncated apoB (apoB-48). Therefore, despite their structural similarity to humans, zebrafish chylomicrons are coated with apoB-100 which renders them less likely to undergo rapid clearance by the liver and mature as LDL. In addition, zebrafish contains 2 additional apob paralogues in the genome, apobb1 and apobb2, which are expressed in a spatiotemporally distinct manner.34 Therefore, it is possible that these 2 genes may retain a unique role in modulating lipid metabolism in zebrafish. Overall, lipid metabolism in zebrafish and human shares striking similarities.
HYPERLIPIDEMIA AND ATHEROSCLEROSIS
Hyperlipidemia is the most prevalent dyslipidemia in humans, of which characteristic symptoms include abnormally high levels of lipids in the blood plasma. Hyperlipidemia can be caused by diverse factors such as constituent of ingested-nutrition, metabolic syndrome including diabetes, obesity and inherited genetic mutations.42,43 Those risk factors are closely associated with disturbances of lipid physiology, which eventually induce an elevated level of lipids, most notably the TG. It has been shown that a combination of environmental factors and genetic abnormalities triggers the onset of hyperlipidemia in humans. Secondary hyperlipidemia, also known as acquired hyperlipidemia, is derived from altered underlying environmental factors including unhealthy diet, metabolic disorder such as diabetes mellitus, disturbed endocrine system such as hypothyroidism, side effects of glucocorticoid and estrogen medication, and complications related to renal and liver diseases.44 Primary (familial) hyperlipidemia can be caused by single or multiple genetic abnormalities. For instance, deficiency of lipoprotein lipase and apoC-II results in increased chylomicrons,45 and mutations of LDL receptor and apoB gene can lead to increased levels of LDL in plasma due to lack of uptake of LDL particles.46
Chronic hyperlipidemia and hypercholesterolemia have been shown to predispose to atherosclerosis; persistent elevation of TG and cholesterol level in blood plasma accelerates the development of atheroma, an accumulation of fatty materials which adhere to arteries. The atheromatous plaque on the luminal side of the arteries substantially increases the likelihood of developing atherosclerosis. Atherosclerosis is a progressive inflammatory disorder by nature. It has been shown that immune cells, in particular, macrophages, facilitate the progression of atherosclerosis.47 Macrophages which are attracted to the site of atheromas uptake fatty deposits on the luminal side of the arteries, and eventually differentiate as foam cells which are highly inflammatory and exacerbate lesion formation. Zebrafish forms atheromas as a response to the diet-induced hypercholesterolemia.48 Using a fluorescent cholesterol ester, it has been shown that the HCD in zebrafish could initiate alterations in vasculature, which are reminiscent of the early stage atherosclerosis in humans.49 While it is apparent that zebrafish could undergo initial phase of atherosclerosis under HFD and HCD, however, to date, it remains unclear whether zebrafish develops full blown atherosclerosis beyond the initial phase.
ZEBRAFISH MODEL FOR DYSLIPIDEMIA RESEARCH
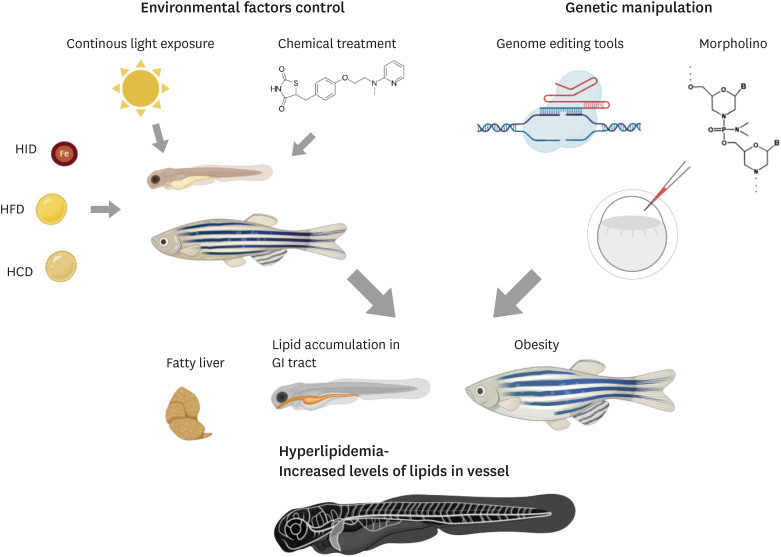
A number of zebrafish models to study obesity and hyperlipidemia have been established (Fig. 2). In this section, we will summarize the use of zebrafish as a model for dyslipidemia research. For convenience's sake, we will introduce prevailing approaches utilizing zebrafish as a model to investigate human dyslipidemia into 2 categories.
Fig. 2. Zebrafish hyperlipidemia model. As shown in humans, dyslipidemia in zebrafish is similarly induced by either environmental or genetic factors. Environmental factors such as continuous light exposure, chemical treatment, and diet regimens can influence lipid metabolism and predispose to dyslipidemia in zebrafish. In addition, abrogation of genes associated with lipid metabolism such as nr1h3 and apoc2 using genome editing tools or morpholino results in dysregulated lipid metabolism, leading to fatty liver, obesity and lipid accumulation in vasculature and other organs.
HID, high-iron diet; HFD, high-fat diet; HCD, high-cholesterol diet.
1. Inducer based approaches
Inducer-based approaches have been successfully used in other model organisms to emulate human dyslipidemia conditions. Briefly, animals are fed with a HFD or HCD and subsequent physiological alterations are assessed. In zebrafish, feeding regimen, environmental conditions, as well as chemical inducers have been successfully applied to induce hyperlipidemia in both adult and larvae. It has been shown that alterations in diet regimen such as Artemia overfeeding, high iron diet, as well as classical HFD and HCD have shown to precipitate the onset of dyslipidemia in zebrafish by altering lipid metabolism. For instance, it has been shown that overfeeding zebrafish with Artemia significantly increased the body mass and plasma TG level in zebrafish, emulating the obesity induced hyperlipidemia in humans.50 Indeed, a number of well-characterized regulators of lipid metabolism such as APOH, IL-6 and IL-1β in the coagulation cascade, and SREBF1, PPARα/γ, NR1H3 and LEP in lipid metabolism has been identified by transcriptomic analysis using zebrafish overfed with Artemia.50 In addition, long-term application of high iron diet regimen in zebrafish also elevates lipid level in blood plasma as shown in rodent models.51
It has been shown that zebrafish also demonstrate robust responses toward classic diet regimen used to induce hyperlipidemia and hypercholesterolemia. As shown in other dyslipidemia animal models, HFD and a HCD could promote obesity, hyperglycemia and hyperlipidemia rapidly in adult and larvae zebrafish. At 5 days after the start of HFD or HCD regimen, lipid accumulation within the vasculature and other organs such as liver and intestine is markedly increased in larva zebrafish; within the vasculature, fatty streak formation in the arteries and vascular processes characteristic of early development of human atherosclerosis could be found in HFD or HCD fed zebrafish.29,33 Consistent with this observation, the level of oxidized LDL, which known to contribute to initiate the formation of atherosclerotic lesions, was increased in HCD fed zebrafish.33 Moreover, proportion of VLDL and LDL fraction within total lipoprotein was substantially elevated at the expense of HDL in HCD zebrafish model.33 The HCD fed zebrafish also showed the thickening of the endothelial cell layer and increased vascular permeability, suggesting that dysregulation of lipoprotein metabolism not only causes hyperlipidemia and hypercholesterolemia, but also could impede vascular development and angiogenesis-dependent vascular remodeling.
In addition to aforementioned approaches which directly interfere with zebrafish metabolism to create dyslipidemia conditions, alternative approaches which indirectly modulate zebrafish metabolism have been evaluated. For instance, it has been shown that zebrafish larvae exposed to continuous light for 6 days had a 7-fold higher prevalence of adipocytes compared with control fish.52 Under the continuous light exposure, changes in the expression of circadian rhythm genes, including period2 and rev-erbα, adversely affect lipid metabolism.52 In addition, treatment of chemical mutagens for one day such as rosiglitazone, phenylephrine, and T0070907, along with HFD or HCD regimen, successfully altered lipid metabolism in zebrafish.53,54
2. Genetic approach
It has been proposed that lipid metabolism disorders in humans are not only associated with environmental factors, but are heavily influenced by genetic makeup.42,55 In humans, a number of large-scale genome-wide association study analyses have convincingly demonstrated the association of specific single nucleotide polymorphisms with dyslipidemia and helped to identify the region of the chromosome which may harbor the candidate genes for the hereditary dyslipidemia.56 Rodent models have been traditionally used to validate candidate genes associated with human dyslipidemia. For instance, rodents which have mutations in the loci linked to lipid metabolism such as apoB, apoE and LDL receptor, phenocopy human hyperlipidemia symptoms and have been used for studying various CVDs. However, rodent models have innate limitations as a gene discovery model since these models are not suitable for forward genetic screen, a frequently used tool to identify novel genes associated with a specific phenotype.
As the only vertebrate model organism amenable to forward genetic screen, zebrafish has recently garnered attention as an alternative model to rodents. Indeed, several zebrafish mutants which defective lipid processing and metabolism have been identified from mutagenesis forward genetic screens using chemical mutagens or pseudotyped retrovirus.26,57,58,59 These mutants invariably develop symptoms reminiscent of human metabolic diseases such as hepatic steatosis and non-alcoholic fatty liver disease, and therefore, could serve as critical resource to better delineate molecular underpinning of human pathophysiology. For instance, zebrafish harvest moon (hmn) mutation, which affects glutamine--fructose-6-phosphate transaminase 1 (gfpt1) locus, a key enzyme for hexosamine biosynthetic pathway exhibits excessive lipid accumulation in the liver and an elevated level of TG, similar to clinical hallmarks of metabolic syndrome in human.60 The phenotype found in zebrafish hmn mutants is consistent with the idea that hexosamine biosynthetic pathway could predispose to the onset of diverse metabolism-related diseases including diabetes and atherosclerosis.61,62 In addition, forward genetic screens in zebrafish have identified mutations in a set of novel genes which have not been implicated in hyperlipidemia and atherosclerosis, suggesting that zebrafish model could serve as an effective gene discovery platform to identify candidates for novel risk factors for human hyperlipidemia and atherosclerosis.57
By using gene editing technologies, zebrafish genome can be efficiently manipulated to generate a specific mutation, which enables us to decipher the precise function of potential disease-causing genes. For instance, CRISPR/Cas9 has shown to introduce mutations in vivo with up to 50% targeting efficiency and generate biallelic mutations in zebrafish.25 Moreover, in vivo gene knockdown could be achieved in zebrafish with a relative ease using morpholino, a modified anti-sense oligonucleotide.63 Since zebrafish genome harbors orthologs of key factors regulating lipid metabolism in mammals and major classes of apo, as well as CETP, genetic manipulation of these genes could help us to uncover novel molecular underpinning of dyslipidemia and to identify novel therapeutic targets for dyslipidemia-associated symptoms.8 To date, a number of zebrafish mutants affecting key regulators of lipid metabolism has been generated. For instance, deletion of zebrafish Lxr gene nr1h3, an orthologue of liver X receptors (Lxrs) which functions as master regulators of cholesterol catabolism and neutral lipids transport, leads to hypercholesterolemia and hepatic steatosis.64 In addition, abrogation of zebrafish apoc2, an orthologue of APOC2 which activates lipoprotein lipase, induces clear symptoms of atherosclerotic plaques such as lipid deposition in vasculature and organs and lipid-loaded macrophages, reminiscent of human pathophysiology.28 Moreover, it has been reported that attenuating the function of genes associated with lipid metabolism by morpholino could provide quick and easy assessment on the function of a specific gene in lipid metabolism, without generating genetic mutations. For instance, knock-down of genes associated with cholesterol transport such as abca1 and abcg1 result in increased level of free cholesterol.65 Taken together, these findings suggest that zebrafish offers an unparalleled versatility as an in vivo model to dissect the underlying genetic contribution to dyslipidemia.
PHARMACOLOGICAL HIGH THROUGHPUT SCREENING USING ZEBRAFISH MODEL
Zebrafish genome contains orthologues of more than 80% of human disease-associated genes are present in zebrafish genome, although zebrafish are phylogenetically more distant from humans than rodents and utilizes distinct metabolic regulation.24 Based on the similarity in genetic composition, combined with its attributes as a model organism, zebrafish has been extensively used for pharmacological screens.66,67,68 Since the advent of the chemical biology, zebrafish has been extensively used as a vertebrate model to identify proteins which could be targeted by chemical modulators.69 Pioneered by Peterson and Schreiber,67 chemical screens using zebrafish have identified an arrays of novel compounds with various biological effects. For instance, widely-used small molecule antagonists of Bone Morphogenetic Protein signaling, dorsomorphin and its derivatives, were first identified by chemical screening using zebrafish.70 By utilizing available zebrafish mutants, chemical screens could be targeted to identify a selected group of chemical modulators affecting a specific genetic mutation. GS4012, a novel chemical agonist of vascular endothelial growth factor-A signaling, has been identified to selectively impact angiogenesis and could ameliorate the phenotype of hey2 mutation.71 More recently, zebrafish-based chemical screen has successfully identified prostaglandin as key factors which modulates the proliferation of the hematopoietic stem cells,72 which could revolutionize the hematopoietic stem cell transplantation practice.
For hyperlipidemia and atherosclerosis, pharmacological screen using zebrafish could be similarly applied to identify novel therapeutic agents (Fig. 3). For instance, Clifton and others73 have used zebrafish to identify novel inhibitors for lipid absorption which could be used to modulate diet-induced hyperlipidemia, suggesting that zebrafish-based chemical screen could be used to identify novel first-in-class drugs. In addition, pharmacological screen using hyperlipidemic zebrafish model could identify novel chemicals which could complement with currently available therapeutic options in a high-throughput, timely and cost-effective manner. For instance, many drugs currently used for treating hyperlipidemia in human such as atorvastatin (Lipitor), a competitive inhibitor of HMG-CoA reductase, have shown to exert similar effects in dyslipidemic zebrafish,74 which opens up the opportunity to utilize zebrafish to delineate pharmacokinetics. Since statins which are widely used for treatment of hyperlipidemia could cause side effects including muscle pain, headache, dizziness, sleep problems, rarely, inflammation of organs and even myopathy in patients,75 there remains an unmet need to identify alternatives to these medications.
Fig. 3. High-throughput drug screen using zebrafish hyperlipidemia models. A pair of zebrafish provides hundreds of eggs per week, which enables production of genetically identical individuals for high throughput screen. In addition, zebrafish larvae are small enough to be raised in a small volume of aqueous media within a single well of multi-well plate, which increases the efficacy and reproducibility of the high throughput screen. Efficacy of candidates can be verified by lipid staining such as Oil Red O, Nile Red and BODIPY staining. Combined with phenotypic resemblance between hyperlipidemic zebrafish and humans, these aforementioned attributes as a model organism have propelled zebrafish as an important alternative model to investigate pathophysiology of dyslipidemia in humans.
Moreover, zebrafish offers a competitive edge over cell culture based screen, since potential side effects of candidates could be assessed within developing embryos. Using zebrafish, most of results can be assessed by uncomplicated assay like Nile Red staining visualizing lipid accumulation. With ever-increasing number of hyperlipidemia and CVD associated mutations in humans, it is increasingly important to provide a custom-tailored therapy. Since zebrafish can be easily manipulated genetically using genome editing tools, which allows to screen for a target specific antagonists and agonists, zebrafish model is likely to thrive as a model for drug discovery targeting dyslipidemia in humans.
CONCLUSION
Zebrafish which has been traditionally utilized to investigate diverse aspects of vertebrate development, emerges as a unique model for studying human dyslipidemia. Here, we provide a brief yet comprehensive review on unique features of zebrafish as a dyslipidemia model. It is apparent that zebrafish offers a unique alternative model to provide novel insights on related human diseases. Therefore, it is certain that dyslipidemia-related research in zebrafish will continue to advance our current understanding on the pathophysiology of dyslipidemia, but also will help us to identify novel therapeutic intervention for lipid metabolism and related diseases.
ACKNOWLEDGEMENTS
We thank the member of Jin laboratory for helpful discussion and critical reading of the manuscript. All figures are created with BioRender.com.
Footnotes
Funding: This work was supported by grants from the Korean Ministry of Trade, Industry, and Energy (2016-10063396), and the National Research Foundation of Korea (NRF-2016R1A5A1007318, NRF-2017R1A2B2007211, and NRF-2019R1A2C2088125) to Suk-Won Jin.
Conflict of Interest: The authors have no conflicts of interest to declare.
- Writing - original draft: Ka J, Jin SW.
- Writing - review & editing: Jin SW.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phan J, Ng V, Sheinbaum A, French S, Choi G, El Kabany M, et al. Hyperlipidemia and nonalcoholic steatohepatitis predispose to hepatocellular carcinoma development without cirrhosis. J Clin Gastroenterol. 2019;53:309–313. doi: 10.1097/MCG.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 3.Ishibashi S, Herz J, Maeda N, Goldstein JL, Brown MS. The two-receptor model of lipoprotein clearance: tests of the hypothesis in “knockout” mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc Natl Acad Sci U S A. 1994;91:4431–4435. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havel RJ, Kotite L, Vigne JL, Kane JP, Tun P, Phillips N, et al. Radioimmunoassay of human arginine-rich apolipoprotein, apoprotein E. Concentration in blood plasma and lipoproteins as affected by apoprotein E-3 deficiency. J Clin Invest. 1980;66:1351–1362. doi: 10.1172/JCI109988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolini S, Cantafora A, Averna M, Cortese C, Motti C, Martini S, et al. Clinical expression of familial hypercholesterolemia in clusters of mutations of the LDL receptor gene that cause a receptor-defective or receptor-negative phenotype. Arterioscler Thromb Vasc Biol. 2000;20:E41–E52. doi: 10.1161/01.atv.20.9.e41. [DOI] [PubMed] [Google Scholar]
- 6.LaBrecque DR, Abbas Z, Anania F, Ferenci P, Khan AG, Goh KL, et al. World Gastroenterology Organisation global guidelines: nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467–473. doi: 10.1097/MCG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 7.Farzadfar F, Finucane MM, Danaei G, Pelizzari PM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3·0 million participants. Lancet. 2011;377:578–586. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 8.Fang L, Liu C, Miller YI. Zebrafish models of dyslipidemia: relevance to atherosclerosis and angiogenesis. Transl Res. 2014;163:99–108. doi: 10.1016/j.trsl.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlegel A. Zebrafish models for dyslipidemia and atherosclerosis research. Front Endocrinol (Lausanne) 2016;7:159. doi: 10.3389/fendo.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sæle Ø, Rød KE, Quinlivan VH, Li S, Farber SA. A novel system to quantify intestinal lipid digestion and transport. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:948–957. doi: 10.1016/j.bbalip.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson JL, Carten JD, Farber SA. Zebrafish lipid metabolism: from mediating early patterning to the metabolism of dietary fat and cholesterol. Methods Cell Biol. 2011;101:111–141. doi: 10.1016/B978-0-12-387036-0.00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minchin JE, Rawls JF. In vivo imaging and quantification of regional adiposity in zebrafish. Methods Cell Biol. 2017;138:3–27. doi: 10.1016/bs.mcb.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minchin JE, Rawls JF. A classification system for zebrafish adipose tissues. Dis Model Mech. 2017;10:797–809. doi: 10.1242/dmm.025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nüsslein-Volhard C. The zebrafish issue of development. Development. 2012;139:4099–4103. doi: 10.1242/dev.085217. [DOI] [PubMed] [Google Scholar]
- 15.Langdon YG, Mullins MC. Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu Rev Genet. 2011;45:357–377. doi: 10.1146/annurev-genet-110410-132517. [DOI] [PubMed] [Google Scholar]
- 16.Thisse C, Zon LI. Organogenesis--heart and blood formation from the zebrafish point of view. Science. 2002;295:457–462. doi: 10.1126/science.1063654. [DOI] [PubMed] [Google Scholar]
- 17.Sehnert AJ, Stainier DY. A window to the heart: can zebrafish mutants help us understand heart disease in humans? Trends Genet. 2002;18:491–494. doi: 10.1016/s0168-9525(02)02766-x. [DOI] [PubMed] [Google Scholar]
- 18.Stainier DY. A glimpse into the molecular entrails of endoderm formation. Genes Dev. 2002;16:893–907. doi: 10.1101/gad.974902. [DOI] [PubMed] [Google Scholar]
- 19.Warga RM, Stainier DY. The guts of endoderm formation. Results Probl Cell Differ. 2002;40:28–47. doi: 10.1007/978-3-540-46041-1_3. [DOI] [PubMed] [Google Scholar]
- 20.Sung YH, Kim JM, Kim HT, Lee J, Jeon J, Jin Y, et al. Highly efficient gene knockout in mice and zebrafish with RNA-guided endonucleases. Genome Res. 2014;24:125–131. doi: 10.1101/gr.163394.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, et al. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat Biotechnol. 2011;29:697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sander JD, Dahlborg EJ, Goodwin MJ, Cade L, Zhang F, Cifuentes D, et al. Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA) Nat Methods. 2011;8:67–69. doi: 10.1038/nmeth.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu C, Smith T, McNulty J, Rayla AL, Lakshmanan A, Siekmann AF, et al. Evaluation and application of modularly assembled zinc-finger nucleases in zebrafish. Development. 2011;138:4555–4564. doi: 10.1242/dev.066779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu K, Petree C, Requena T, Varshney P, Varshney GK. Expanding the CRISPR toolbox in zebrafish for studying development and disease. Front Cell Dev Biol. 2019;7:13. doi: 10.3389/fcell.2019.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farber SA, Pack M, Ho SY, Johnson ID, Wagner DS, Dosch R, et al. Genetic analysis of digestive physiology using fluorescent phospholipid reporters. Science. 2001;292:1385–1388. doi: 10.1126/science.1060418. [DOI] [PubMed] [Google Scholar]
- 27.Landgraf K, Schuster S, Meusel A, Garten A, Riemer T, Schleinitz D, et al. Short-term overfeeding of zebrafish with normal or high-fat diet as a model for the development of metabolically healthy versus unhealthy obesity. BMC Physiol. 2017;17:4. doi: 10.1186/s12899-017-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Gates KP, Fang L, Amar MJ, Schneider DA, Geng H, et al. Apoc2 loss-of-function zebrafish mutant as a genetic model of hyperlipidemia. Dis Model Mech. 2015;8:989–998. doi: 10.1242/dmm.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ka J, Pak B, Han O, Lee S, Jin SW. Comparison of transcriptomic changes between zebrafish and mice upon high fat diet reveals evolutionary convergence in lipid metabolism. Biochem Biophys Res Commun. 2020;530:638–643. doi: 10.1016/j.bbrc.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 30.Babin PJ, Vernier JM. Plasma lipoproteins in fish. J Lipid Res. 1989;30:467–489. [PubMed] [Google Scholar]
- 31.Osborne N, Stainier DY. Lipid receptors in cardiovascular development. Annu Rev Physiol. 2003;65:23–43. doi: 10.1146/annurev.physiol.65.092101.142235. [DOI] [PubMed] [Google Scholar]
- 32.Stoletov K, Fang L, Choi SH, Hartvigsen K, Hansen LF, Hall C, et al. Vascular lipid accumulation, lipoprotein oxidation, and macrophage lipid uptake in hypercholesterolemic zebrafish. Circ Res. 2009;104:952–960. doi: 10.1161/CIRCRESAHA.108.189803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang L, Harkewicz R, Hartvigsen K, Wiesner P, Choi SH, Almazan F, et al. Oxidized cholesteryl esters and phospholipids in zebrafish larvae fed a high cholesterol diet: macrophage binding and activation. J Biol Chem. 2010;285:32343–32351. doi: 10.1074/jbc.M110.137257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otis JP, Zeituni EM, Thierer JH, Anderson JL, Brown AC, Boehm ED, et al. Zebrafish as a model for apolipoprotein biology: comprehensive expression analysis and a role for apoA-IV in regulating food intake. Dis Model Mech. 2015;8:295–309. doi: 10.1242/dmm.018754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tseng WC, Loeb HE, Pei W, Tsai-Morris CH, Xu L, Cluzeau CV, et al. Modeling Niemann-Pick disease type C1 in zebrafish: a robust platform for in vivo screening of candidate therapeutic compounds. Dis Model Mech. 2018;11:dmm034165. doi: 10.1242/dmm.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C, Gaudet D, Miller YI. Deficient cholesterol esterification in plasma of apoc2 knockout zebrafish and familial chylomicronemia patients. PLoS One. 2017;12:e0169939. doi: 10.1371/journal.pone.0169939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlegel A, Stainier DY. Microsomal triglyceride transfer protein is required for yolk lipid utilization and absorption of dietary lipids in zebrafish larvae. Biochemistry. 2006;45:15179–15187. doi: 10.1021/bi0619268. [DOI] [PubMed] [Google Scholar]
- 38.Avraham-Davidi I, Ely Y, Pham VN, Castranova D, Grunspan M, Malkinson G, et al. ApoB-containing lipoproteins regulate angiogenesis by modulating expression of VEGF receptor 1. Nat Med. 2012;18:967–973. doi: 10.1038/nm.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quinlivan VH, Farber SA. Lipid uptake, metabolism, and transport in the larval zebrafish. Front Endocrinol (Lausanne) 2017;8:319. doi: 10.3389/fendo.2017.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nath AK, Ma J, Chen ZZ, Li Z, Vitery MD, Kelley ML, et al. Genetic deletion of gpr27 alters acylcarnitine metabolism, insulin sensitivity, and glucose homeostasis in zebrafish. FASEB J. 2020;34:1546–1557. doi: 10.1096/fj.201901466R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Hare EA, Wang X, Montasser ME, Chang YP, Mitchell BD, Zaghloul NA. Disruption of ldlr causes increased LDL-C and vascular lipid accumulation in a zebrafish model of hypercholesterolemia. J Lipid Res. 2014;55:2242–2253. doi: 10.1194/jlr.M046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allayee H, Aouizerat BE, Cantor RM, Dallinga-Thie GM, Krauss RM, Lanning CD, et al. Families with familial combined hyperlipidemia and families enriched for coronary artery disease share genetic determinants for the atherogenic lipoprotein phenotype. Am J Hum Genet. 1998;63:577–585. doi: 10.1086/301983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allayee H, Castellani LW, Cantor RM, de Bruin TW, Lusis AJ. Biochemical and genetic association of plasma apolipoprotein A-II levels with familial combined hyperlipidemia. Circ Res. 2003;92:1262–1267. doi: 10.1161/01.RES.0000075600.87675.16. [DOI] [PubMed] [Google Scholar]
- 44.Stone NJ. Secondary causes of hyperlipidemia. Med Clin North Am. 1994;78:117–141. doi: 10.1016/s0025-7125(16)30179-1. [DOI] [PubMed] [Google Scholar]
- 45.Baggio G, Manzato E, Gabelli C, Fellin R, Martini S, Enzi GB, et al. Apolipoprotein C-II deficiency syndrome. Clinical features, lipoprotein characterization, lipase activity, and correction of hypertriglyceridemia after apolipoprotein C-II administration in two affected patients. J Clin Invest. 1986;77:520–527. doi: 10.1172/JCI112332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Millar JS, Maugeais C, Ikewaki K, Kolansky DM, Barrett PH, Budreck EC, et al. Complete deficiency of the low-density lipoprotein receptor is associated with increased apolipoprotein B-100 production. Arterioscler Thromb Vasc Biol. 2005;25:560–565. doi: 10.1161/01.ATV.0000155323.18856.a2. [DOI] [PubMed] [Google Scholar]
- 47.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13:709–721. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SJ, Choi W, Seo E, Yeom E. Association of early atherosclerosis with vascular wall shear stress in hypercholesterolemic zebrafish. PLoS One. 2015;10:e0142945. doi: 10.1371/journal.pone.0142945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thierer JH, Ekker SC, Farber SA. The LipoGlo reporter system for sensitive and specific monitoring of atherogenic lipoproteins. Nat Commun. 2019;10:3426. doi: 10.1038/s41467-019-11259-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oka T, Nishimura Y, Zang L, Hirano M, Shimada Y, Wang Z, et al. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010;10:21. doi: 10.1186/1472-6793-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SH, Yadav D, Kim SJ, Kim JR, Cho KH. High consumption of iron exacerbates hyperlipidemia, atherosclerosis, and female sterility in zebrafish via acceleration of glycation and degradation of serum lipoproteins. Nutrients. 2017;9:690. doi: 10.3390/nu9070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kopp R, Billecke N, Legradi J, den Broeder M, Parekh SH, Legler J. Bringing obesity to light: rev-erbα, a central player in light-induced adipogenesis in the zebrafish? Int J Obes. 2016;40:824–832. doi: 10.1038/ijo.2015.240. [DOI] [PubMed] [Google Scholar]
- 53.Den Broeder MJ, Kopylova VA, Kamminga LM, Legler J. Zebrafish as a model to study the role of peroxisome proliferating-activated receptors in adipogenesis and obesity. PPAR Res. 2015;2015:358029. doi: 10.1155/2015/358029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Z, Li Q, Maddison R, Ni Mhurchu C, Jiang Y, Wei DM, et al. A school-based comprehensive intervention for childhood obesity in china: a cluster randomized controlled trial. Child Obes. 2019;15:105–115. doi: 10.1089/chi.2018.0251. [DOI] [PubMed] [Google Scholar]
- 55.Connelly PW, Maguire GF, Little JA. Apolipoprotein CIISt. Michael. Familial apolipoprotein CII deficiency associated with premature vascular disease. J Clin Invest. 1987;80:1597–1606. doi: 10.1172/JCI113246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Zhou D, Zhang Z, Song Y, Zhang D, Zhao T, et al. Effects of genetic variants on lipid parameters and dyslipidemia in a Chinese population. J Lipid Res. 2011;52:354–360. doi: 10.1194/jlr.P007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SH, Wu SY, Baek JI, Choi SY, Su Y, Flynn CR, et al. A post-developmental genetic screen for zebrafish models of inherited liver disease. PLoS One. 2015;10:e0125980. doi: 10.1371/journal.pone.0125980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hugo SE, Schlegel A. A genetic screen for zebrafish mutants with hepatic steatosis identifies a locus required for larval growth. J Anat. 2017;230:407–413. doi: 10.1111/joa.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadler KC, Amsterdam A, Soroka C, Boyer J, Hopkins N. A genetic screen in zebrafish identifies the mutants vps18, nf2 and foie gras as models of liver disease. Development. 2005;132:3561–3572. doi: 10.1242/dev.01918. [DOI] [PubMed] [Google Scholar]
- 60.Hugo SE, Schlegel A. A genetic model to study increased hexosamine biosynthetic flux. Endocrinology. 2017;158:2420–2426. doi: 10.1210/en.2017-00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Largo R, Martínez-Calatrava MJ, Sánchez-Pernaute O, Marcos ME, Moreno-Rubio J, Aparicio C, et al. Effect of a high dose of glucosamine on systemic and tissue inflammation in an experimental model of atherosclerosis aggravated by chronic arthritis. Am J Physiol Heart Circ Physiol. 2009;297:H268–H276. doi: 10.1152/ajpheart.00142.2009. [DOI] [PubMed] [Google Scholar]
- 62.Stender S, Astrup P. Glucosamine and experimental atherosclerosis. Increased wet weight and changed composition of cholesterol fatty acids in aorta of rabbits fed a cholesterol-enriched diet with added glucosamine. Atherosclerosis. 1977;26:205–213. doi: 10.1016/0021-9150(77)90103-4. [DOI] [PubMed] [Google Scholar]
- 63.Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- 64.Pinto CL, Kalasekar SM, McCollum CW, Riu A, Jonsson P, Lopez J, et al. Lxr regulates lipid metabolic and visual perception pathways during zebrafish development. Mol Cell Endocrinol. 2016;419:29–43. doi: 10.1016/j.mce.2015.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fang L, Choi SH, Baek JS, Liu C, Almazan F, Ulrich F, et al. Control of angiogenesis by AIBP-mediated cholesterol efflux. Nature. 2013;498:118–122. doi: 10.1038/nature12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cassar S, Adatto I, Freeman JL, Gamse JT, Iturria I, Lawrence C, et al. Use of zebrafish in drug discovery toxicology. Chem Res Toxicol. 2020;33:95–118. doi: 10.1021/acs.chemrestox.9b00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci U S A. 2000;97:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nat Rev Drug Discov. 2015;14:721–731. doi: 10.1038/nrd4627. [DOI] [PubMed] [Google Scholar]
- 69.Wiley DS, Redfield SE, Zon LI. Chemical screening in zebrafish for novel biological and therapeutic discovery. Methods Cell Biol. 2017;138:651–679. doi: 10.1016/bs.mcb.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, et al. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peterson RT, Shaw SY, Peterson TA, Milan DJ, Zhong TP, Schreiber SL, et al. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol. 2004;22:595–599. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- 72.North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clifton JD, Lucumi E, Myers MC, Napper A, Hama K, Farber SA, et al. Identification of novel inhibitors of dietary lipid absorption using zebrafish. PLoS One. 2010;5:e12386. doi: 10.1371/journal.pone.0012386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Al-Habsi AA, Massarsky A, Moon TW. Exposure to gemfibrozil and atorvastatin affects cholesterol metabolism and steroid production in zebrafish (Danio rerio) Comp Biochem Physiol B Biochem Mol Biol. 2016;199:87–96. doi: 10.1016/j.cbpb.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 75.Golomb BA, Evans MA. Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs. 2008;8:373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]