Abstract
Ubiquitin-conjugating enzyme E2T (UBE2T), a member of the E2 family, was found to be overexpressed in a great many cancers such as bladder cancer, lung cancer, and prostate cancer. However, there have been no reports on the role of UBE2T in osteosarcoma. In this study, we tried to make the effects of UBE2T on osteosarcoma clear. The study results showed that UBE2T was overexpressed in osteosarcoma tissues and cell lines. Moreover, UBE2T knockdown inhibited osteosarcoma cell proliferation, migration, and invasion. We also observed that UBE2T downregulation could suppress the activity of the PI3K/Akt signaling pathway. Therefore, we concluded that UBE2T exerted its inhibitory effects on osteosarcoma cells via suppressing the PI3K/Akt signaling pathway. These findings indicated that UBE2T may be a potential therapeutic target for osteosarcoma treatment.
Key words: Ubiquitin-conjugating enzyme E2T (UBE2T), Osteosarcoma, Proliferation, Invasion, PI3K/Akt signaling pathway
INTRODUCTION
Osteosarcoma, a common malignant bone tumor often occurring in children and adolescents, possesses a highly metastatic potential (1–3). About 20% of osteosarcoma patients, at initial diagnosis, suffer from clinically detectable metastatic disease; about 40% of the patients, at the advanced stage, suffer from metastases mainly developing in the lungs (4). With more and more advanced treatment, the survival rate for osteosarcoma patients without metastasis has been greatly increased to 60–70% (5). However, the situation is still not optimistic for patients with metastatic tumors, who have a survival rate of only 30% (6,7). Therefore, it is desirable to discover a novel strategy for a better therapeutic outcome for osteosarcoma patients.
Ubiquitin-conjugating enzyme E2T (UBE2T), a member of the E2 family, was first discovered in a case of Fanconi anemia (FA) (8–10). It is part of the ubiquitin–proteasome degradation system and plays its role in combination with specific E3 ubiquitin ligase to achieve degradation or functional changes of relevant substrates (11,12). This process governs the turnover of regulatory proteins involving primary cellular processes such as differentiation, angiogenesis, apoptosis, cell cycle progression, and cellular signaling pathways (11). For example, UBE2T has been reported to be independently employed in regulating FANCD2 monoubiquitination and to take part in the FA pathway together with FANCD2 (9,13). Recent studies have indicated that disrupted UBE2T expression, by affecting the DNA damage–repair response, could directly result in FA and increase the sensitivity of tumor cells to crosslink agents (10,14). What is interesting is the UBE2T location, 1q32.1, which is amplified in a variety of cancers (15). In addition, UBE2T overexpression has been found in a number of cancers such as bladder cancer, lung cancer, and prostate cancer (16–19). However, there have been no reports on the role of UBE2T in osteosarcoma.
In this study, we concentrated on making the role of UBE2T in osteosarcoma clear. The results showed that UBE2T was overexpressed in osteosarcoma tissues and cell lines. Moreover, UBE2T downregulation inhibited osteosarcoma cell proliferation, migration, and invasion via suppressing the PI3K/Akt signaling pathway. These findings indicated that UBE2T may be a potential therapeutic target for osteosarcoma.
MATERIALS AND METHODS
Patients and Osteosarcoma Samples
Osteosarcoma tissues and matching noncancerous bone tissues were obtained from osteosarcoma patients at Nanfang Hospital (Guangzhou, China). All the tissue samples were collected and frozen in liquid nitrogen for future experiments. All the patients agreed to participate in the study and gave written consent. The use of tissue samples in the study was approved by the Ethics Committee of Nanfang Hospital.
Cell Lines and Cell Culture
Human osteosarcoma cell lines (HOS, MG63, and SaOS2) and human osteoblast cell line (HCO) were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). All the cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Tauranga, New Zealand) supplemented with 10% fetal bovine serum (FBS; Gibco, Rockville, MD, USA), 100 µg/ml streptomycin (Sigma-Aldrich, St. Louis, MO, USA), and 100 U/ml penicillin (Sigma-Aldrich), followed by incubation in a humidified atmosphere with 5% CO2 at room temperature.
Quantitative Real-Time PCR
Total RNA was isolated from the tissues or the cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The cDNA synthesis was conducted with the PrimeScript RT Reagent kit (BD Biosciences, Franklin Lakes, NJ, USA) following the manufacturer’s instructions. The PCR was performed under the following conditions: 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 15 s, and 72°C for 15 s. The following primers were used: UBE2T, 5′-CAAATATTAGGTGGAGCCAACAC-3′ (forward) and 5′-TAGATCACCTTGGCAAAGAACC-3′ (reverse); β-actin, 5′-AGAAAATCTGGCACCACACC-3′ (forward) and 5′-TAGCACAGCCTGGATAGCAA-3′ (reverse). β-Actin was used as an internal control. The relative mRNA expression levels were calculated using the comparative CT method (2−ΔΔCt) (20).
Western Blot
The proteins were extracted from the tissues or the cells in RIPA lysis buffer (Beyotime, Jiangsu, China). After centrifugation at 12,000 × g for 10 min at 4°C, the supernatants were separated using 10% SDS-PAGE (Bioworld Technology, St. Louis Park, MN, USA), and then the separated proteins were transferred to a PVDF membrane (Life Technologies, Gaithersburg, MD, USA). The membrane was blocked in PBS and 5% skim milk for 1 h and then incubated overnight at 4°C with the primary antibodies against UBE2T, PI3K, p-PI3K, Akt, p-Akt, or β-actin (Cell Signaling Technology, San Jose, CA, USA). The membrane was incubated at room temperature for 1 h with secondary HRP-conjugated antibodies (Cell Signaling Technology). The relative protein expression levels were evaluated using the Quantity One software (Life Technologies).
Plasmids and Transfection Assay
UBE2T siRNA (siUBE2T) and scrambled siRNA (siNC) plasmids were purchased from Life Technologies. The sequences of siUBE2T and siNC were GCUGACAUAUCCUCAGAAUTT and UUCUCCGAACGUGUCACGUTT, respectively. HOS and MG63 cells were transfected with siUBE2T or siNC plasmid using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer’s instruction. After 24 h of transfection, the cells were collected for the following studies. Western blot assays were performed to determine the transfection efficiency.
Proliferation Assay In Vitro
MTT assays were performed to measure the rate of cell proliferation in vitro. Briefly, the cells transfected with siUBE2T or siNC were planted into 96-well plates at a density of 1 × 105 cells/well and then cultured for 24, 48, or 72 h, respectively. The transfected cells were incubated with 25 µl of MTT (Sigma-Aldrich) for 4 h at 37°C, followed by removing of supernatants and adding of 150 µl of DMSO (Sigma-Aldrich). The absorbance value was measured at 450 nm with a microplate reader (BioTek Instruments, Winooski, VT, USA).
Transwell Assay
To measure the effects of UBE2T on invasion capacities of osteosarcoma cells, the matrix-coated Transwell assay was conducted. In brief, transfected cells (1 × 105 cells/well) were suspended in serum-free medium and then added to the upper chamber coated with matrix. The lower chamber was filled with normal cell culture medium. After 24 h of incubation at 37°C, cells on the membrane were clipped off, and those below the membrane were fixed and stained. The number of invaded cells was counted under a microscope (200×). For migration assay, common Transwell chambers were applied instead of the matrix-coated ones.
Tumor Xenograft Growth Assay In Vivo
Male BALB/c nude mice that were 4 to 6 weeks old were purchased from Shanghai Slac Laboratory Animal Co. Ltd. (Shanghai, China). All animal studies were approved by the Institutional Animal Care and Use Committee of Nanfang Hospital. For the tumor growth assay, 5 × 103 cells transfected with siUBE2T or siNC were resuspended in PBS medium and then inoculated subcutaneously into nude mice. The treatment time was 35 days. The tumors were measured every 7 days since their appearance, and the tumor volume (cm3) was calculated by the following formula: length × width2/2.
Statistical Analysis
Statistical analysis was performed using the SPSS 13.0 software (IBM, Armonk, NY, USA). Student’s t-tests were carried out to make a comparison between different groups. Data were expressed as mean ± SD. A value of p < 0.05 was considered statistically significant.
RESULTS
UBE2T Was Overexpressed in Osteosarcoma Tissues and Cell Lines
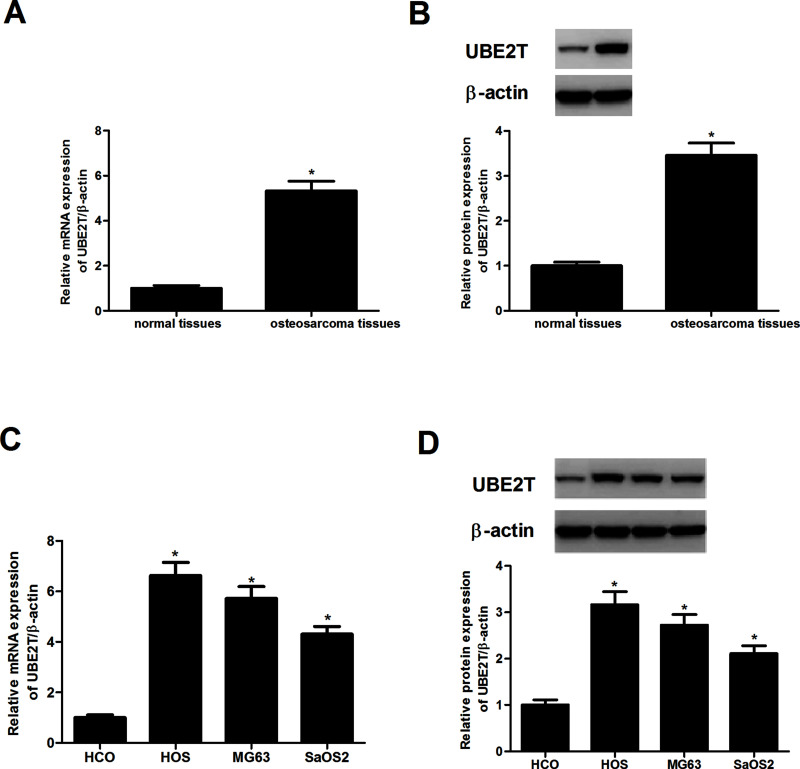
UBE2T expression in human osteosarcoma tissues and corresponding normal tissues was measured by RT-PCR and Western blot assays. As shown in Figure 1A and B, UBE2T was overexpressed in osteosarcoma tissues but was hardly detected in the matching normal tissues. Additionally, as shown in Figure 1C and D, a higher expression level of UBE2T was observed in three osteosarcoma cell lines (HOS, MG63, and SaOS2) when compared to the osteoblast cell line HCO.
Figure 1.
Ubiquitin-conjugating enzyme E2T (UBE2T) was overexpressed in osteosarcoma tissues and cell lines. (A, B) RT-PCR and Western blot analysis were conducted to evaluate the expression levels of UBE2T in osteosarcoma tissues and matching normal tissues. (C, D) mRNA and protein expression levels of UBE2T in three osteosarcoma cell lines (HOS, MG63, and SaOS2) and the osteoblast cell line HCO. *p < 0.05.
UBE2T Knockdown Inhibited Osteosarcoma Cell Proliferation In Vitro and Tumor Growth In Vivo
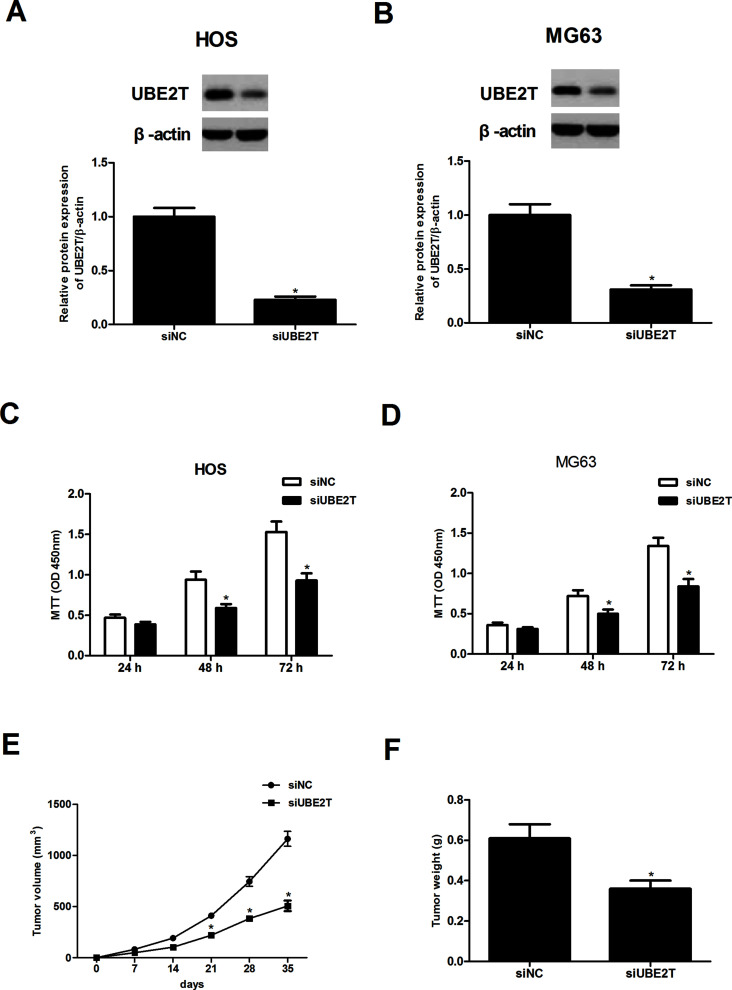
UBE2T knockdown was achieved by transfection of siRNA plasmids. The transfection efficiency was verified by the Western blot assay. As shown in Figure 2A, the protein expression levels of UBE2T were significantly decreased in HOS-siUBE2T cells in comparison with the HOS-siNC group. Similar results were found in MG63-siUBE2T cells (Fig. 2B).
Figure 2.
UBE2T knockdown inhibited osteosarcoma cell proliferation in vitro and tumor growth in vivo. (A, B) The Western blot assay was performed to detect the transfection efficiency of HOS-siUBE2T and MG63-siUBE2T cells. (C, D) A lower proliferation rate was found in HOS-siUBE2T and MG63-siUBE2T cells, compared to corresponding control cells. (E, F) A significant decrease in tumor volume and tumor weight was observed in MG63-siUBE2T cells, compared to the MG63-siNC group (n = 5). *p < 0.05.
MTT assays were conducted to investigate the effects of UBE2T knockdown on proliferation of osteosarcoma cells. The results revealed that UBE2T knockdown dramatically inhibited the growth of HOS-siUBE2T (Fig. 2C) and MG63-siUBE2T (Fig. 2D) cells. To confirm whether the growth-inhibiting effect of UBE2T knockdown on osteosarcoma cells was associated with the tumor growth in vivo, MG63 cells transfected with siUBE2T or siNC were subcutaneously inoculated into mice. The results showed that knockdown of UBE2T remarkably decreased the tumor volume (Fig. 2E) and the tumor weight (Fig. 2F).
UBE2T Knockdown Inhibited Osteosarcoma Cell Migration and Invasion In Vitro
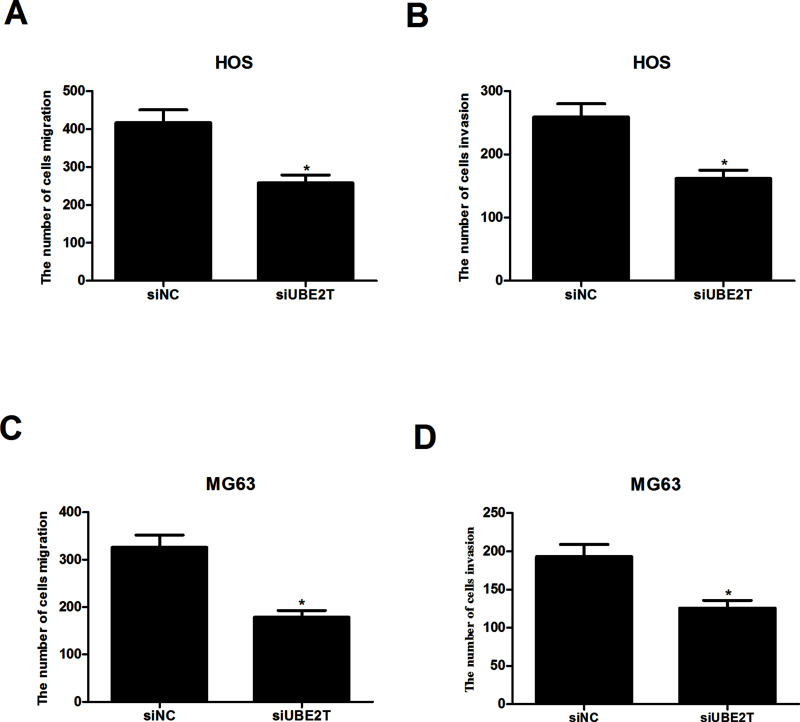
We performed Transwell assays to evaluate the effects of UBE2T knockdown on migration and invasion of osteosarcoma cells. We used matrix-coated Transwell chambers to measure the invasive capability and matrix-free ones to measure the migratory capability. As shown in Figure 3A and B, UBE2T knockdown greatly reduced the number of migrated and invaded HOS-siUBE2T cells, compared to the HOS-siNC group. In addition, we found that UBE2T knockdown similarly affected migration and invasion of MG63-siUBE2T cells (Fig. 3C and D).
Figure 3.
UBE2T knockdown inhibited osteosarcoma cell migration and invasion in vitro. (A, B) As indicated by Transwell assays, UBE2T knockdown obviously weakened the migratory and invasive capabilities of HOS-siUBE2T cells, in comparison with corresponding control cells. (C, D) UBE2T knockdown exerted a similar effect on the migration and invasion of MG63-siUBE2T cells. *p < 0.05.
UBE2T Knockdown Inhibited the Activity of PI3K/Akt Signaling Pathway
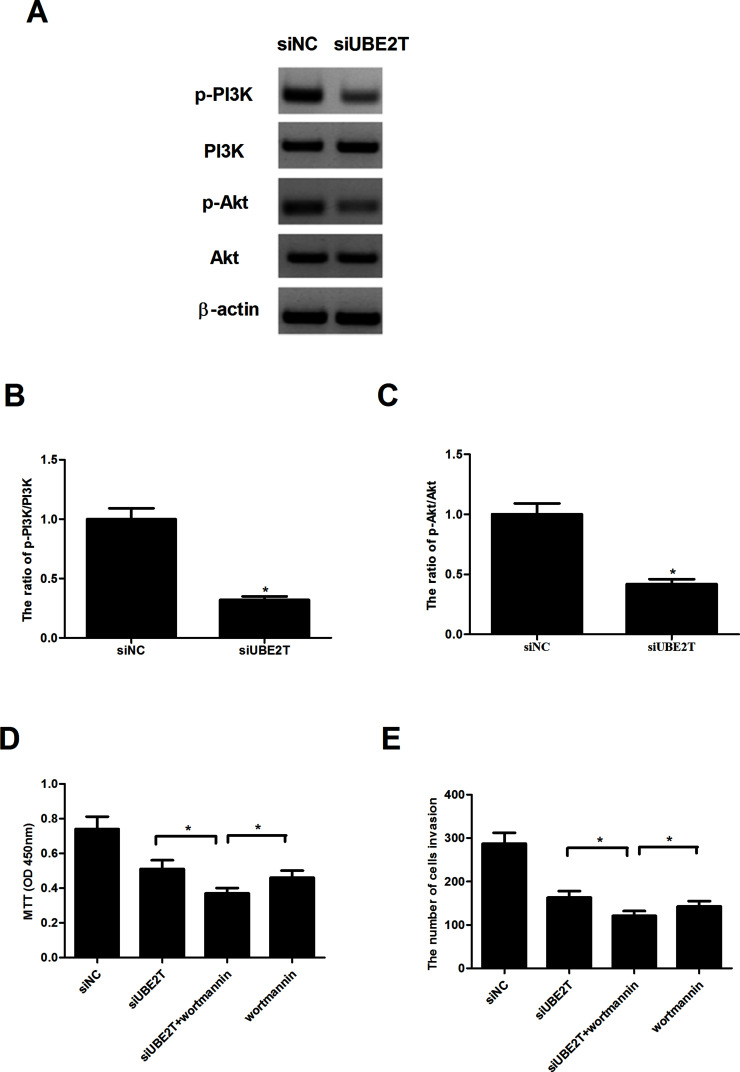
We investigated the effects of UBE2T knockdown on the activity of the PI3K/Akt signaling pathway. As indicated by the Western blot assay, a significant decrease in the protein levels of p-PI3K and p-Akt was observed in MG63-siUBE2T cells (Fig. 4A–C) in comparison with corresponding control cells. However, the total protein levels of PI3K and Akt were little affected. We also tested the effects of an Akt inhibitor (wortmannin) on osteosarcoma cell proliferation and invasion mediated by knockdown of UBE2T. As assessed by the cell proliferation assay, wortmannin dramatically potentiated siUBE2T-inhibited MG63 cell proliferation (Fig. 4D). In addition, wortmannin similarly enhanced the suppressive effect of siUBE2T on MG63 cell invasion (Fig. 4E).
Figure 4.
UBE2T knockdown inhibited the activity of the PI3K/Akt signaling pathway. (A) Western blot assays were performed to measure the protein levels of p-PI3K, PI3K, p-Akt, and Akt, with β-actin as the control. A significant decrease in the protein levels of p-PI3K (B) and p-Akt (C) was observed in MG63-siUBE2T cells, compared to the MG63-siNC group. However, the total protein levels of PI3K and Akt were unaffected. (D) MG63 cells were transfected with siUBE2T or siNC in the presence or absence of wortmannin (100 nM) for 48 h. Cell proliferation was measured by the MTT assay. (E) Cell invasion was detected by the Transwell assay. *p < 0.05.
DISCUSSION
Osteosarcoma is a kind of malignant bone tumor mainly occurring in children and adolescents. Although the survival rate of osteosarcoma patients has been greatly increased by chemotherapy, the overall outcome of osteosarcoma patients is still poor because of drug resistance, especially multidrug resistance. Therefore, it is desperately necessary to find a new therapeutic strategy for a more effective osteosarcoma treatment.
UBE2T, a novel therapeutic target, has been found to be overexpressed in various cancers such as bladder, lung, and prostate cancers (10,17–19). Furthermore, recent studies have shown that UBE2T downregulation inhibited proliferation, migration, and invasion of nasopharyngeal carcinoma cells, and its depletion significantly suppressed tumor formation and metastasis of prostate cancer cells (19,21). Consistent with all these findings, the results of our in vitro and in vivo studies indicated that UBE2T was overexpressed in osteosarcoma tissues and cell lines and that UBE2T knockdown inhibited osteosarcoma cell proliferation, migration, and invasion.
Proliferation, migration, and invasion are all essential cellular processes and important for tumor formation and development. In the present study, we performed corresponding experiments to investigate the effects of UBE2T knockdown on these processes. First, we carried out RT-PCR and Western blot assays to detect the mRNA and protein expression levels of UBE2T in osteosarcoma tissues and cell lines. As revealed by the study results, UBE2T was upregulated in osteosarcoma tissues and cell lines. After we downregulated UBE2T in two osteosarcoma cell lines, HOS and MG63, via transfection of siRNA plasmids, we performed both in vitro and in vivo assays to evaluate the role of UBE2T knockdown in proliferation, migration, and invasion of osteosarcoma cell lines. As expected, we observed that UBE2T knockdown exerted a significant inhibitory effect on these processes. However, the underlying mechanisms remained unclear.
The PI3K/Akt signaling pathway is of great importance in the malignant development of various tumors (22,23). This signaling pathway acts as a mediator of proliferation, migration, and invasion of cancer cells (24,25). It is reported that PI3K is often activated by oncogenes, and its activation can facilitate cancer cell proliferation (26). In carcinogenesis of PI3K, Akt plays a significant role as a major downstream effector of PI3K (27). Akt is capable of regulating many cellular processes, and its deregulation is essential in activating tumorigenic characteristics such as unbounded cell proliferation, invasion, and metastasis (28). In addition, Akt is found to be downregulated in a variety of cancers including osteosarcoma (29). In view of the crucial role of the PI3K/Akt signaling pathway in tumor progression, in our study we investigated whether UBE2T exerted any influence on this signaling pathway in osteosarcoma. We performed Western blot to examine the effects of UBE2T knockdown on the protein expression levels of PI3K, p-PI3K, Akt, and p-Akt. The assay results showed that knockdown of UBE2T remarkably decreased the protein levels of pPI3K and p-Akt without affecting the total protein levels of PI3K and Akt. We also examined the effects of an Akt inhibitor (wortmannin) on siUBE2T-mediated osteosarcoma cell proliferation and invasion. The results showed that wortmannin remarkably potentiated siUBE2T-inhibited MG63 cell proliferation and invasion. Taken together, we concluded that UBE2T knockdown inhibited osteosarcoma cell proliferation, migration, and invasion via suppressing the PI3K/Akt signaling pathway. Considering that the development of osteosarcoma is a multistep process involving diverse cellular pathways, more studies will be needed to make clear the molecular mechanisms involved in osteosarcoma initiation and progression (30).
In conclusion, we demonstrated that UBE2T was overexpressed in osteosarcoma tissues and cell lines. Furthermore, UBE2T knockdown inhibited osteosarcoma cell proliferation, migration, and invasion via suppressing the PI3K/Akt signaling pathway. These findings may be used as evidence supporting the role of UBE2T, a potential therapeutic target for osteosarcoma treatment.
ACKNOWLEDGMENT
The authors declare no conflicts of interest.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Ottaviani G.; Robert R. S.; Huh W. W.; Palla S.; Jaffe N. Sociooccupational and physical outcomes more than 20 years after the diagnosis of osteosarcoma in children and adolescents. Cancer 119(20):3727–3736; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ottaviani G.; Jaffe N. The etiology of osteosarcoma. Cancer Treat. Res. 152(152):15–32; 2009. [DOI] [PubMed] [Google Scholar]
- 3. Damron T. A.; Ward W. G.; Stewart A. Osteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base Report. Clin. Orthop. Relat. Res. 459(459):40–47; 2007. [DOI] [PubMed] [Google Scholar]
- 4. Wang F.; Ke Z. F.; Wang R.; Wang Y. F.; Huang L. L.; Wang L. T. Astrocyte elevated gene-1 (AEG-1) promotes osteosarcoma cell invasion through the JNK/c-Jun/MMP-2 pathway. Biochem. Biophys. Res. Commun. 452(4):933–939; 2014. [DOI] [PubMed] [Google Scholar]
- 5. Schwartz C. L.; Gorlick R.; Teot L.; Krailo M.; Chen Z.; Goorin A.; Grier H. E.; Bernstein M. L.; Meyers P. Multiple drug resistance in osteogenic sarcoma: INT0133 from the Children’s Oncology Group. J. Clin. Oncol. 25(15):2057–2062; 2007. [DOI] [PubMed] [Google Scholar]
- 6. Ferrari S.; Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr. Opin. Oncol. 19(4):341–346; 2007. [DOI] [PubMed] [Google Scholar]
- 7. Zhang P.; Yang Y.; Zweidlermckay P. A.; Hughes D. P. M. Critical role of notch signaling in osteosarcoma invasion and metastasis. Clin Cancer Res 14(10):2962–2969; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8. Joenje H.; Patel K. J. The emerging genetic and molecular basis of Fanconi anaemia. Nat. Rev. Genet. 2(6):446–457; 2001. [DOI] [PubMed] [Google Scholar]
- 9. Alpi A.; Langevin F.; Mosedale G.; Machida Y. J.; Dutta A.; Patel K. J. UBE2T, the Fanconi anemia core complex, and FANCD2 are recruited independently to chromatin: A basis for the regulation of FANCD2 monoubiquitination. Mol. Cell. Biol. 27(24):8421–8430; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rickman K.; Lach F.; Abhyankar A.; Donovan F.; Sanborn E.; Kennedy J.; Sougnez C.; Gabriel S.; Elemento O.; Chandrasekharappa S. Deficiency of UBE2T, the E2 ubiquitin ligase necessary for FANCD2 and FANCI ubiquitination, causes FA-T subtype of Fanconi anemia. Cell Rep. 12(1):35–41; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu H.; Xiang P.; Pan Q.; Huang Y.; Xie N.; Zhu W. Ubiquitin-conjugating enzyme E2T is an independent prognostic factor and promotes gastric cancer progression. Tumor Biol; 2016. [DOI] [PubMed] [Google Scholar]
- 12. Zhou M. J.; Chen F. Z.; Chen H. C. Ubiquitination involved enzymes and cancer. Med. Oncol. 31(8):1–7; 2014. [DOI] [PubMed] [Google Scholar]
- 13. Machida Y. J.; Machida Y.; Chen Y.; Gurtan A. M.; Kupfer G. M.; D’Andrea A. D.; Dutta A. UBE2T is the E2 in the Fanconi anemia pathway and undergoes negative autoregulation. Mol. Cell 23(4):589–596; 2006. [DOI] [PubMed] [Google Scholar]
- 14. Brand T. M.; Iida M.; Luthar N.; Starr M. M.; Huppert E. J.; Wheeler D. L. Nuclear EGFR as a molecular target in cancer. Radiother. Oncol. 108(3):370–377; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 15. Corson T. W.; Huang A. M.; Gallie B. L. KIF14 is a candidate oncogene in the 1q minimal region of genomic gain in multiple cancers. Oncogene 24(30):4741–4753; 2005. [DOI] [PubMed] [Google Scholar]
- 16. Takat R.; Katagiri T.; Kanehir M.; Tsunod T.; Shuin T.; Miki T.; Namiki M.; Kohri K.; Matsushit Y.; Fujiok T. Predicting response to methotrexate, vinblastine, doxorubicin, and cisplatin neoadjuvant chemotherapy for bladder cancers through genome-wide gene expression profiling. Clin. Cancer Res. 11(7):2625–2636; 2005. [DOI] [PubMed] [Google Scholar]
- 17. Hao J.; Xu A.; Xie X.; Tian T.; Gao S.; Xiao X.; He D. Elevated expression of UBE2T in lung cancer tumors and cell lines. Tumour Biol. 29(3):195–203; 2008. [DOI] [PubMed] [Google Scholar]
- 18. Kikuchi T.; Daigo Y.; Katagiri T.; Tsunoda T.; Okada K.; Kakiuchi S.; Zembutsu H.; Furukawa Y.; Kawamura M.; Kobayashi K. Expression profiles of non-small cell lung cancers on cDNA microarrays: Identification of genes for prediction of lymph-node metastasis and sensitivity to anti-cancer drugs. Oncogene 22(14):2192–2205; 2003. [DOI] [PubMed] [Google Scholar]
- 19. Wen M.; Yongwon K.; Wang Y.; Mao J. H.; Wei G. Elevated expression of UBE2T exhibits oncogenic properties in human prostate cancer. Oncotarget 6(28):25226–25239; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livak K. J.; Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ C T method. Methods 25(4):402–408; 2001. [DOI] [PubMed] [Google Scholar]
- 21. Hu W.; Xiao L.; Cao C.; Hua S.; Wu D. UBE2T promotes nasopharyngeal carcinoma cell proliferation, invasion, and metastasis by activating the AKT/GSK3β/β-catenin pathway. Oncotarget 7(12):15161–15172; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ren W.; Liu Y.; Wan S.; Fei C.; Wang W.; Chen Y.; Zhang Z.; Wang T.; Wang J.; Zhou L. BMP9 inhibits proliferation and metastasis of HER2-positive SK-BR-3 breast cancer cells through ERK1/2 and PI3K/AKT pathways. PLoS One 9(5):e96816; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23. Brader S.; Eccles S. A. Phosphoinositide 3-kinase signalling pathways in tumor progression, invasion and angiogenesis. Tumori 90(1):2–8; 2004. [DOI] [PubMed] [Google Scholar]
- 24. Qiao M.; Chang X.; Jing F.; Rojanasakul Y.; Jiang B. H. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 18(12):2262–2271; 2007. [DOI] [PubMed] [Google Scholar]
- 25. Vivanco I.; Sawyers C. L. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat. Rev. Cancer 2(7):489–501; 2002. [DOI] [PubMed] [Google Scholar]
- 26. Wong K. K.; Engelman J. A.; Cantley L. C. Targeting the PI3K signaling pathway in cancer. Curr. Opin. Genet. Dev. 20(1): 87–90; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manning B. D.; Cantley L. C. AKT/PKB signaling: Navigating downstream. Cell 129(7):1261–1274; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheung A. K. L.; Ip J. C. Y.; Chu A. C. H.; Yue C.; Leong M. M. L.; Ko J. M. Y.; Shuen W. H.; Hong L. L.; Lung M. L. PTPRG suppresses tumor growth and invasion via inhibition of Akt signaling in nasopharyngeal carcinoma. Oncotarget 6(15):13434–13447; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang B.; Shi Z. Enhanced anticancer effect of gemcitabine by genistein in osteosarcoma: The role of Akt and nuclear factor-kappaB. Anticancer Drugs 21(3):288–296; 2010. [DOI] [PubMed] [Google Scholar]
- 30. Luetke A.; Meyers P. A.; Lewis I.; Juergens H. Osteosarcoma treatment—Where do we stand? A state of the art review. Cancer Treat. Rev. 40(4):523–532; 2014. [DOI] [PubMed] [Google Scholar]