Abstract
PFTK1 (PFTAIRE protein kinase 1), also named CDK14 (cyclin-dependent kinase 14), is a member of the cell division cycle 2 (CDC2)-related protein kinase family. It is highly expressed in several malignant tumors. However, the role of PFTK1 in the progression of non-small cell lung cancer (NSCLC) is still elusive. In this study, we aimed to explore the expression and function of PFTK1 in NSCLC cells. Our results showed that PFTK1 was significantly upregulated in human NSCLC cell lines. Silencing the expression of PFTK1 inhibited the proliferation of NSCLC cells. In addition, silencing the expression of PFTK1 endowed NSCLC cells with decreased migration and invasion abilities, as well as epithelial–mesenchymal transition (EMT) progress in A549 cells. A mechanistic study showed that knockdown of PFTK1 inhibited the expression of β-catenin, cyclin D1, and c-Myc in A549 cells. In summary, we report that small interfering RNA (siRNA)-PFTK1 might inhibit the proliferation and invasion of NSCLC cells by suppressing the Wnt/β-catenin signaling pathway. Therefore, PFTK1 may represent a novel therapeutic target for the treatment of NSCLC.
Key words: PFTK1, Non-small cell lung cancer (NSCLC), Invasion, Epithelial–mesenchymal transition (EMT)
INTRODUCTION
Lung cancer is the most common reason for cancer-related deaths in the world (1). Approximately 85% of lung cancer cases belong to the non-small cell lung cancer (NSCLC) category (2). Despite recent advances in clinical and experimental oncology, NSCLC patients often have a dismal outcome, with a 5-year survival rate at less than 11% (3,4). Therefore, identification of the molecular mechanism during NSCLC progression and metastasis may provide patients with novel diagnostic and therapeutic strategies.
Cell division cycle 2 (CDC2) was identified as the first cyclin-dependent kinase (CDK) that was essential for G1/S and G2/M transitions in Schizosaccharomyces pombe (5). PFTAIRE protein kinase 1 (PFTK1), also named CDK14, is a member of the CDC2-related protein kinase family. It is highly expressed in the brain, pancreas, kidney, heart, testis, and ovary, and minimally expressed in the spleen and thymus (6). Recent studies demonstrated that the expression of PFTK1 is upregulated in several malignant tumors such as pancreatic cancer, gastric cancer, esophageal cancer, and breast cancer (7–10). For example, Zhang et al. confirmed that PFTK1 expression was decreased in serum-starved ovarian cancer cells, and knocking down PFTK1 by small interfering RNA (siRNA) significantly inhibited ovarian cancer cell proliferation, migration, and invasion (11). However, the role of PFTK1 in the progression of NSCLC is still elusive. In this study, we aimed to explore the expression and function of PFTK1 in NSCLC cells. Our study showed that knockdown of PFTK1 inhibited the migration, invasion, and epithelial–mesenchymal transition (EMT) process in NSCLC cells.
MATERIALS AND METHODS
Cell Culture
Three human NSCLC cell lines (A549, H1299, and 95-D) and a normal human bronchial epithelial (HBE) cell line were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS; Sijiqing Biochemical, Hangzhou, China) at 37°C in a humidified incubator containing 5% CO2.
Real-Time Quantitative Polymerase Chain Reaction Analysis
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Total RNA (5 µg) was then subjected to TaqMan one-step reverse transcription (Applied Biosystems, Foster City, CA, USA), followed by real-time polymerase chain reaction (PCR; ABI PRISM 7700 Sequence Detection System; Applied Biosystems) according to the manufacturer’s instructions. The following primers were used: PFTK1, 5′-CCAAGGAGTTGCTGCTTTTC-3′ (sense) and 5′-GAATGAACTCCAGGCCATGT-3′ (antisense); β-actin, 5′-CCGTGAAAAGATGACCCAGATC-3′ (sense), 5′-CACAGCCTGGATGGCTACGT-3′ (antisense). The PCR procedure was as follows: 94°C for 3 min, 94°C for 20 s, 55°C for 30 s, and 72°C for 20 s; 2 s for plate reading for 40 cycles; and melting curve from 65°C to 95°C. β-Actin was used as the internal standard for normalizing gene expression. The amplified products were analyzed by 1.5% agarose gel electrophoresis. For relative quantification, the levels of individual gene mRNA transcripts were calculated using the 2−ΔΔCt method.
Western Blot Analysis
Cells were homogenized and lysed in radioimmunoprecipitation assay (RIPA) lysis buffer (100 mM NaCl; 50 mM Tris-HCl, pH 7.5; 1% Triton X-100; 1 mM EDTA; 10 mM β-glycerophosphate; 2 mM sodium vanadate; and protease inhibitor). Protein concentrations were measured by using the Bradford method. Equal amounts of protein (30 µg protein each lane) were separated by 12% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Boston, MA, USA). After blocking with 5% skim milk in Tris-buffered saline (TBS)/Tween 20 (0.05%, v/v) for 1 h, the membranes were incubated with primary antibodies overnight at 4°C. The following primary antibodies were used: anti-PFTK1, anti-E-cadherin, anti-vimentin, anti-fibronectin, anti-N-cadherin, anti-β-catenin, anti-cyclin D1, anti-c-Myc, and anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After being washed three times, the membranes were reacted with horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The bound antibodies were visualized by chemiluminescence using an ECL kit (Amersham Bioscience, UK).
Silencing of Gene Expression of siRNA and Cell Transfection
For the siRNA-knockout experiment, A549 cells were transfected with negative control siRNA (si-Mock) or siRNA-PFTK1 (si-PFTK1) using Lipofectamine 2000 Transfection Reagent (Invitrogen) according to the manufacturer’s instructions. The oligonucleotides for the human PFTK1-specific siRNA were 5′-GTTCATTCTTTACCACATT-3′ (sense) and 5′-AGGTTGCATCTTTGTTGAA-3′ (antisense).
Cell Proliferation Assay
Cell proliferation was measured using the MTT assay. In brief, A549 cells (1 × 104 cells/well) were seeded in 96-well plates and transfected with si-Mock or si-PFTK1, respectively. After transfection for 24, 48, 72, and 96 h, the MTT solution (0.2 mg/ml; Sigma-Aldrich) was added to each well and incubated for an additional 4 h. Afterward, 150 µl of dimethyl sulfoxide (DMSO) (Sigma-Aldrich) was added to dissolve the formazan crystals, and the absorbance was measured at 490 nm using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA). The data were presented as optical density (OD) value.
Cell Migration and Invasion Assays
Cell migration and invasion were performed in a 24-well plate using Transwell chambers (BD Biosciences, San Jose, CA, USA). For the invasion assay, the chamber was precoated with 100 ml of Matrigel (BD Biosciences) at a 1:4 dilution in DMEM to form a genuine reconstituted basement membrane. In brief, 2 × 105 cells resuspended in 100 µl of serum-free medium were added to the upper chamber, while medium containing 10% FBS was added to the lower chamber. The noninvading cells were removed by scraping 12 h (for migration assay) or 24 h (for invasion assay) after incubation at 37°C in 5% CO2. The invasive cells attached on the lower chamber were fixed with 4% formaldehyde in phosphate-buffered saline (PBS) and were stained with 1% crystal violet in 2% ethanol. Subsequently, the invasive cells were counted in randomly selected fields under a light microscope (×100 magnification; Olympus, Tokyo, Japan).
Statistical Analysis
All results are expressed as means ± standard deviation (SD). All data were evaluated using SPSS 13.0 software. Statistical comparisons between groups were performed using a one-way analysis of variance followed by a Student’s t-test. A value of p < 0.05 was considered to indicate a statistically significant difference.
RESULTS
PFTK1 Is Highly Expressed in NSCLC Cell Lines
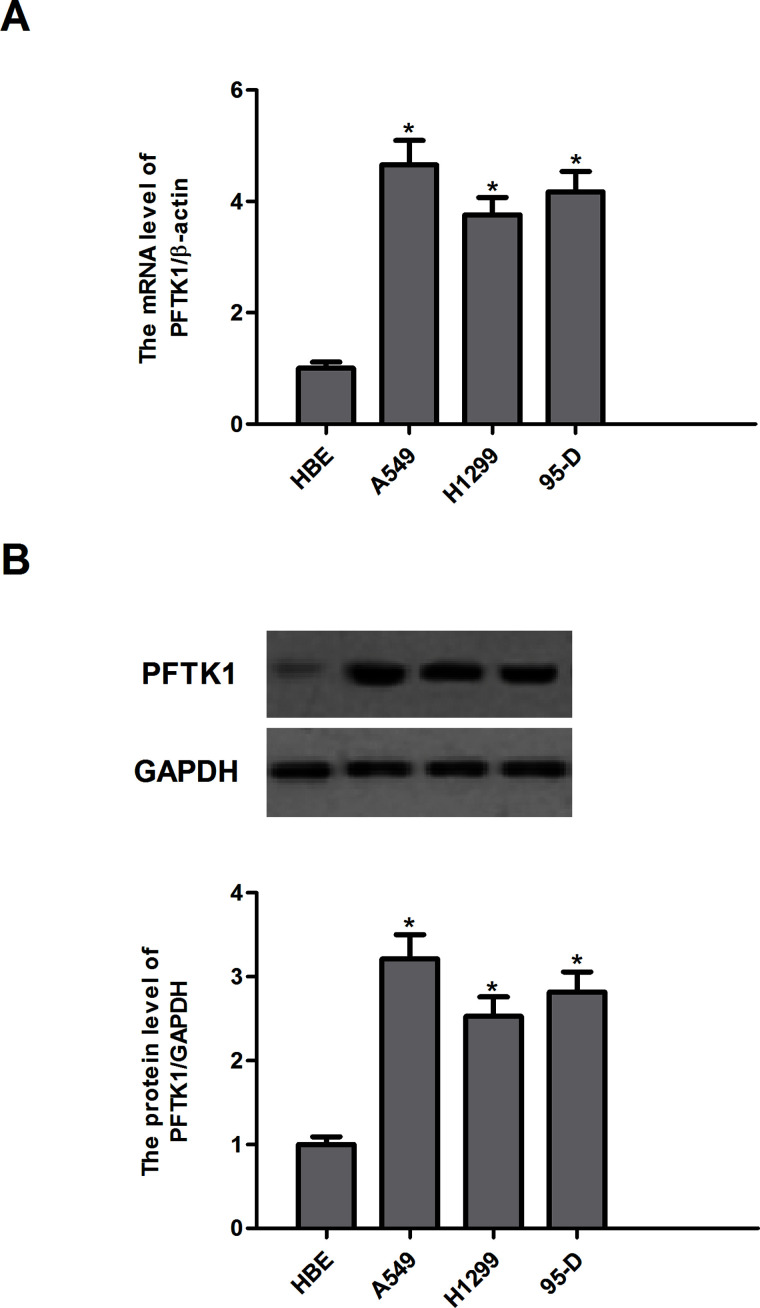
PFTK1 mRNA and protein levels were detected in three human NSCLC cell lines (A549, H1299, and 95-D) and one normal HBE cells. The results demonstrated that the expression of PFTK1 mRNA was higher in A549, H1299, and 95-D cells, as compared with HBE cells (Fig. 1A). Western blotting showed similar results (Fig. 1B).
Figure 1.
PFTK1 is highly expressed in NSCLC cell lines. (A) The mRNA expression of PFTK1 in human NSCLC cell lines (A549, H1299, and 95-D) was detected by qRT-PCR. (B) Protein expression of PFTK1 in human NSCLC cell lines (A549, H1299, and 95-D) was detected by Western blotting. Data are means ± SD in duplicate of at least three independent experiments. *p < 0.05 compared with the control group.
Knockdown of PFTK1 Inhibits the Proliferation of NSCLC Cells
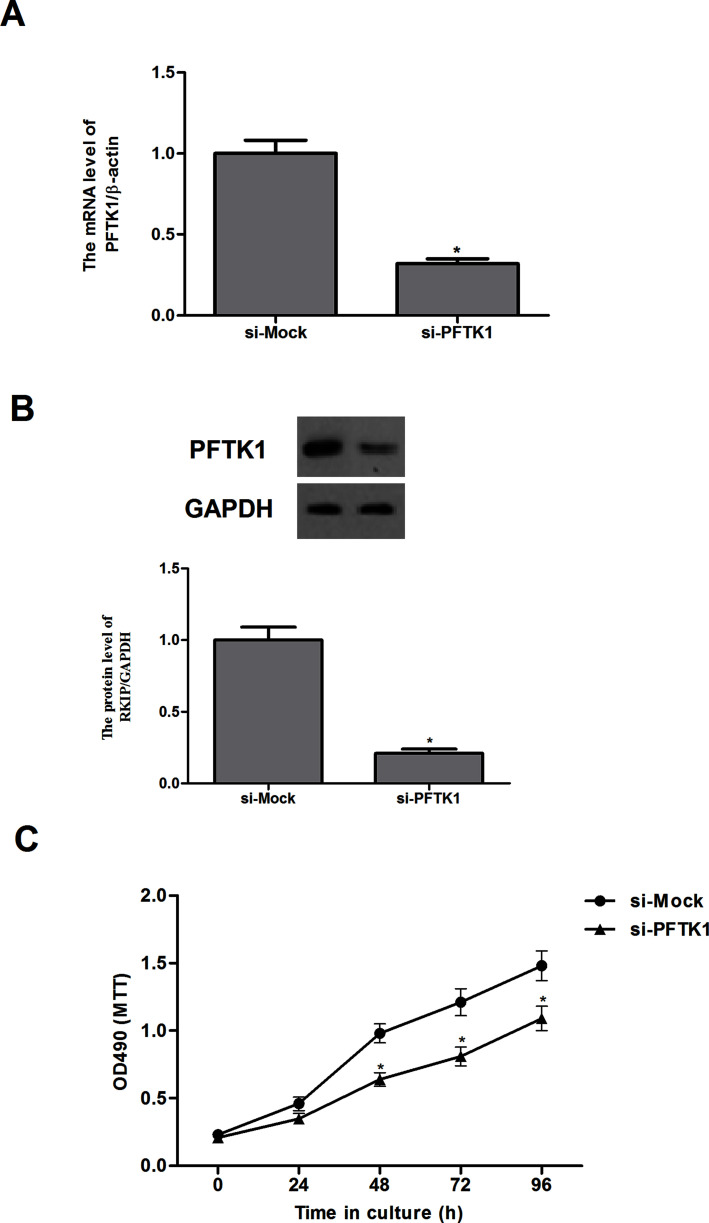
To ascertain the role of PFTK1 in NSCLC, A549 cells were transfected with si-Mock or si-PFTK1 for 24 h, and the transfection efficiency was tested by reverse transcription (RT)-PCR and Western blotting assays. The results of quantitative RT-PCR (qRT-PCR) indicated that PFTK1 silencing significantly reduced the expression of PFTK1 mRNA in A549 cells (Fig. 2A). The Western blot results showed the PFTK1 expression was evidently decreased by si-PFTK1 compared with the si-Mock in A549 cells (Fig. 2B). Then the effect of PFTK1 on cell growth was observed. The MTT assay demonstrated that knockdown of PFTK1 significantly inhibited cell proliferation of A549 cells in a time-dependent manner (Fig. 2C).
Figure 2.
Knockdown of PFTK1 inhibits the proliferation of NSCLC cells. A549 cells were transfected with si-PFTK1 or si-Mock, respectively. The corresponding transfection efficiency was detected by qRT-PCR (A) and Western blot (B). (C) The effect of PFTK1 inhibition on NSCLC cell proliferation was measured by MTT. Data are means ± SD in duplicate of at least three independent experiments. *p < 0.05 compared with the si-Mock group.
Knockdown of PFTK1 Inhibits the Migration and Invasion of NSCLC Cells
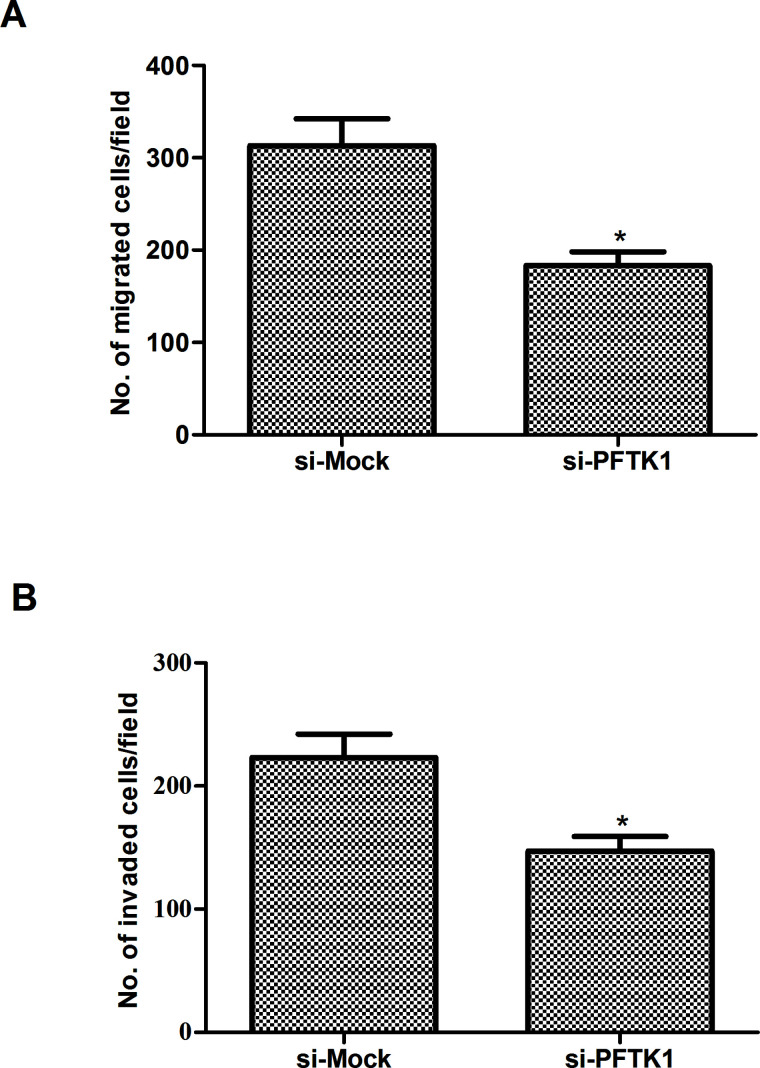
We then investigated the effect of PFTK1 on the migration and invasion of NSCLC cells. As indicated in Figure 3A, PFTK1 knockdown significantly inhibited migration of A549 cells as assessed by Transwell migration assay. Similarly, PFTK1 knockdown could also significantly prevent A549 cells from invading through Matrigel-coated polycarbonate filter in the Transwell chamber (Fig. 3B).
Figure 3.
Knockdown of PFTK1 inhibits the migration and invasion of NSCLC cells. A549 cells were transfected with si-PFTK1 or si-Mock for 24 h, respectively. (A) Cell migration was examined in a Transwell chamber. (B) Cell invasion was examined in a Boyden chamber precoated with Matrigel. Data are means ± SD in duplicate of at least three independent experiments. *p < 0.05 compared with the si-Mock group.
Knockdown of PFTK1 Suppresses the EMT Progression in NSCLC Cells
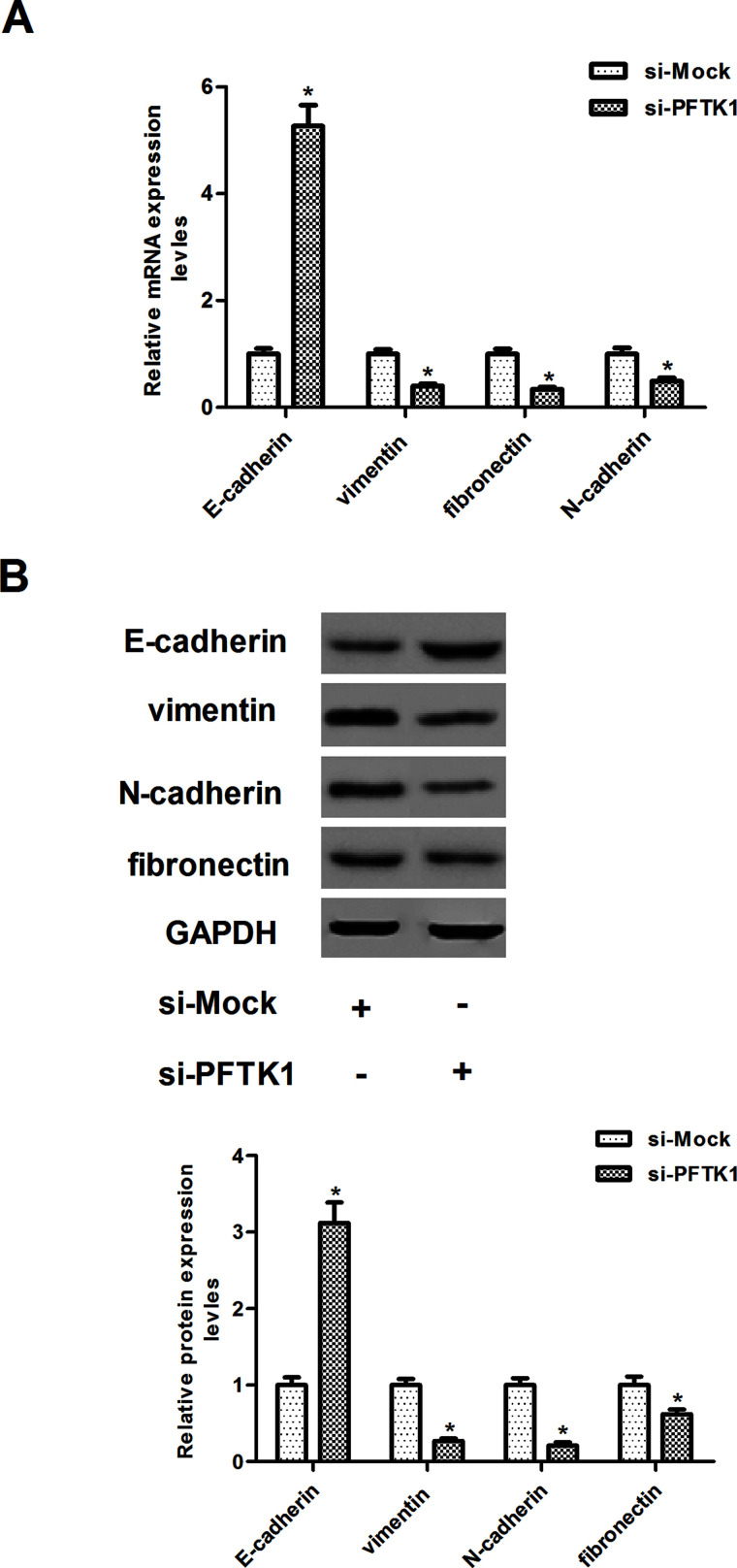
EMT was reported to play an important role in inducing tumor invasion and metastasis. To investigate whether PFTK1 silencing decreased cancer invasion by inhibiting EMT, we analyzed the mRNA level of several EMT markers in PFTK1-silencing and control A549 cells. As shown in Figure 4A, compared with the si-Mock-transfected A549 cells, PFTK1 knockdown A549 cells demonstrated a higher mRNA expression level of epithelial marker E-cadherin, while lower mRNA expression levels of mesenchymal markers vimentin, fibronectin, and N-cadherin. Consistent with mRNA expression, Western blot analysis demonstrated that knockdown of PFTK1 increased E-cadherin and decreased vimentin, fibronectin, and N-cadherin protein levels in A549 cells (Fig. 4B).
Figure 4.
Knockdown of PFTK1 suppresses the EMT progression in NSCLC cells. A549 cells were transfected with si-PFTK1 or si-Mock for 24 h, respectively. (A) The mRNA expression levels of E-cadherin, vimentin, fibronectin, and N-cadherin were measured using qRT-PCR. (B) The protein expression levels of E-cadherin, vimentin, fibronectin, and N-cadherin were examined by SDS-PAGE and Western blotting. Data are means ± SD in duplicate of at least three independent experiments. *p < 0.05 compared with the si-Mock group.
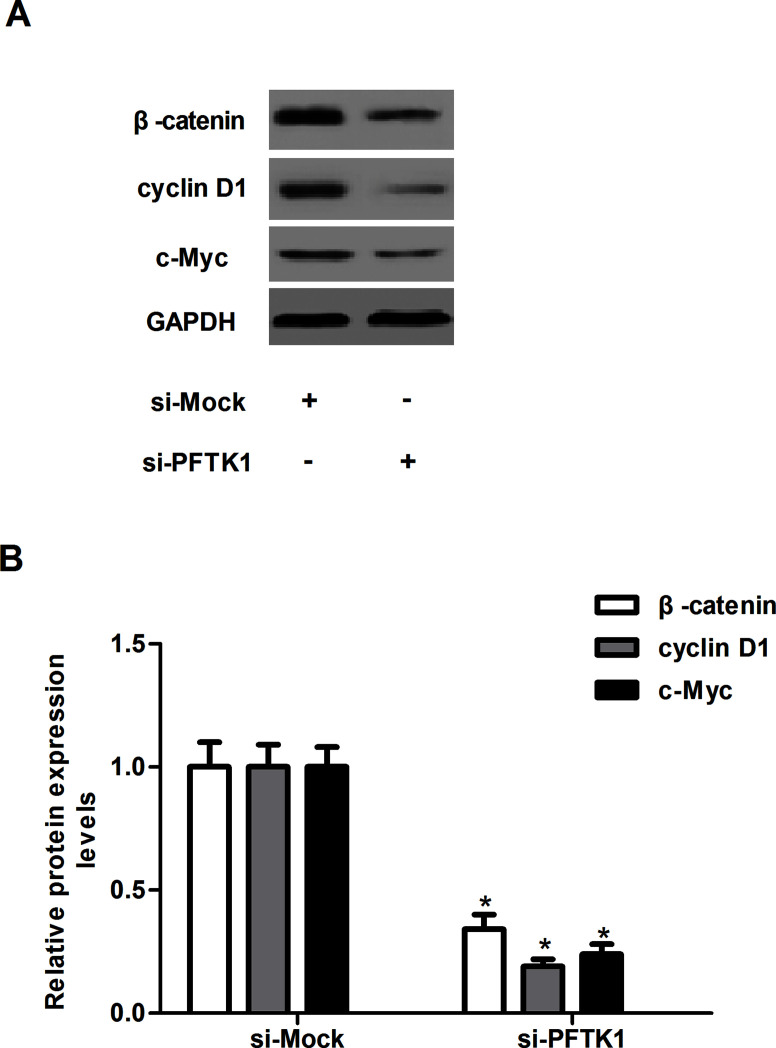
Knockdown of PFTK1 Regulates Activation of Wnt/β-Catenin Signaling in NSCLC Cells
The Wnt/β-catenin signaling pathway is involved in the proliferation and invasion of NSCLC. To further explore a potential mechanism of PFTK1-regulated NSCLC cell proliferation and invasion, we investigated the effect of PFTK1 on the expression of β-catenin, cyclin D1, and c-Myc in A549 cells. As shown in Figure 5, PFTK1 knockdown markedly downregulated the expression of β-catenin, cyclin D1, and c-Myc in A549 cells, as compared with the si-Mock-transfected A549 cells.
Figure 5.
Knockdown of PFTK1 regulates activation of Wnt/β-catenin signaling in NSCLC cells. A549 cells were transfected with si-PFTK1 or si-Mock for 24 h, respectively. (A) The protein expression levels of β-catenin, cyclin D1, and c-Myc were examined by SDS-PAGE and Western blotting. (B) The relative protein expression levels of β-catenin, cyclin D1, and c-Myc were quantified using Image-Pro Plus 6.0 software and normalized to GAPDH. Data are means ± SD in duplicate of at least three independent experiments. *p < 0.05 compared with the si-Mock group.
DISCUSSION
The key finding of this study is that PFTK1 was significantly upregulated in human NSCLC cell lines. Silencing the expression of PFTK1 inhibited the proliferation of NSCLC cells. In addition, silencing the expression of PFTK1 endowed NSCLC cells with decreased migration and invasion, as well as the EMT progress in A549 cells. A mechanistic study showed that knockdown of PFTK1 inhibited the expression of β-catenin, cyclin D1, and c-Myc in A549 cells.
PFTK1 has been shown to be overexpressed in several types of cancer and has been associated with tumor progression and development. In their study, Fan et al. used Western blot and immunohistochemistry experiments to demonstrate that PFTK1 expression was higher in glioma tissues compared with normal brain tissues, and its level was associated with the tumor grade (12). Another study showed that PFTK1 is upregulated in human esophageal cancer and is associated with tumor growth (9). Consistent with these results, in the current study we observed that the expression of PFTK1 was obviously upregulated in human NSCLC cell lines, suggesting that PFTK1 may function as an oncogenic protein in the development and progression of human NSCLC.
Metastasis is the main cause of cancer-related mortality, and it involves a series of steps including invasion and migration (13). A recent study showed that PFTK1 downregulation inhibited the migration and invasion of pancreatic cancer cells (7). Similar results were observed in epithelial ovarian cancer cells (11). Similarly, in the current study, we found that PFTK1 knockdown significantly inhibited migration and invasion of A549 cells, suggesting that PFTK1 may play an important role in these processes of NSCLC.
EMT is a cellular process during which epithelial polarized cells become motile mesenchymal-appearing cells, which, in turn, promotes the metastatic potential of carcinoma (14). Reduction or a loss of E-cadherin expression has a crucial role in tumor progression to invasive cancer and is also one of the well-established hallmarks of EMT (15). In the current study, we found that knockdown of PFTK1 increased E-cadherin and decreased vimentin, fibronectin, and N-cadherin levels in A549 cells. These data suggest that PFTK1 silencing blocks the migration and invasion of NSCLC cells by repressing the EMT process.
Increasing evidence has reported that the Wnt/β-catenin signaling pathway plays an important role in regulating NSCLC progression, cell cycle, and invasion (16–18). Activation of the Wnt pathway increases the expression of EMT regulator Snail and vimentin in NSCLC cells (19–21). β-Catenin is a central factor in canonical Wnt signaling and induces the transcription of several target genes, which are implicated in cell survival, proliferation, and metastasis (22). Moreover, it was reported that β-catenin enhances cell–cell adhesion by associating with cadherin complexes in adherent junctions of cell membrane (23). A growing number of studies have shown that β-catenin expression is frequently lost in NSCLC, and loss of β-catenin was correlated with lymph node metastasis and high TNM stages in multiple cancers and was associated with poor prognosis in NSCLC (24–26). Herein, we found that PFTK1 silencing significantly inhibited the expression of β-catenin, cyclin D1, and c-Myc in A549 cells. On the basis of these findings, we concluded that PFTK1 participated in Wnt/β-catenin signaling in human NSCLC cells.
In summary, we report that PFTK1 downregulation might inhibit the proliferation and invasion of NSCLC cells by suppressing the Wnt/β-catenin signaling pathway. Therefore, PFTK1 may represent a novel therapeutic target for the treatment of NSCLC.
ACKNOWLEDGMENTS
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Siegel R.; Naishadham D.; Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 63:11–30; 2013. [DOI] [PubMed] [Google Scholar]
- 2. Sandri A. B. A.; Rzyman W.; Hubbard R. B.; Wang X. F.; Fraser S.; Routledge T.; Scarci M. Pathology of non-small cell lung cancer and small cell lung cancer. Asian Pac. J. Surg. Oncol. 1:113–124; 2015. [Google Scholar]
- 3. Apostolakis E. D. D. Adjuvant therapy in completely resected non-small cell lung cancer. Asian Pac. J. Surg. Oncol. 1:191–200; 2015. [Google Scholar]
- 4. Bruera S. S. E.; McDevitt J.; Kelly M.; Wang L. K.; Hong T. M.; Chen H. Y. Surgery for non-small cell lung cancer. Asian Pac. J. Surg. Oncol. 1:157–170; 2015. [Google Scholar]
- 5. Sherr C. J.; Roberts J. M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes. Dev. 13:1501–1512; 1999. [DOI] [PubMed] [Google Scholar]
- 6. Yang T.; Chen J. Y. Identification and cellular localization of human PFTAIRE1. Gene 267:165–172; 2001. [DOI] [PubMed] [Google Scholar]
- 7. Zheng L.; Zhou Z.; He Z. Knockdown of PFTK1 inhibits tumor cell proliferation, invasion and epithelial-to-mesenchymal transition in pancreatic cancer. Int. J. Clin. Exp. Pathol. 8:14005; 2015. [PMC free article] [PubMed] [Google Scholar]
- 8. Yang L.; Zhu J., Huang H.; Yang Q.; Cai J.; Wang Q.; Zhu J.; Shao M.; Xiao J.; Cao J. PFTK1 promotes gastric cancer progression by regulating proliferation, migration and invasion. PLoS One 10:e0140451; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyagaki H.; Yamasaki M.; Miyata H.; Takahashi T.; Kurokawa Y.; Nakajima K.; Takiguchi S.; Fujiwara Y.; Ishii H.; Tanaka F. Overexpression of PFTK1 predicts resistance to chemotherapy in patients with oesophageal squamous cell carcinoma. Br. J. Cancer 106:947–954; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gu X.; Wang Y.; Wang H.; Ni Q.; Zhang C.; Zhu J.; Huang W.; Xu P.; Mao G.; Yang S. Upregulated PFTK1 promotes tumor cell proliferation, migration, and invasion in breast cancer. Med. Oncol. 32:1–13; 2015. [DOI] [PubMed] [Google Scholar]
- 11. Zhang W.; Liu R.; Tang C.; Xi Q.; Lu S.; Chen W.; Zhu L.; Cheng J.; Chen Y.; Wang W. PFTK1 regulates cell proliferation, migration and invasion in epithelial ovarian cancer. Int. J. Biol. Macromol. 85:405–416; 2016. [DOI] [PubMed] [Google Scholar]
- 12. Fan S.; Zhao C.; Zhang L.; Dai S.; Ren J.; Zhang X.; Ban N.; He X.; Yang L.; Bao Z. Knockdown of PFTK1 inhibits the migration of glioma cells. J. Mol. Neurosci. 57:257–264; 2015. [DOI] [PubMed] [Google Scholar]
- 13. Leber M. F.; Efferth T. Molecular principles of cancer invasion and metastasis (review). Int. J. Oncol. 34:881–895; 2009. [DOI] [PubMed] [Google Scholar]
- 14. Thiery J. P.; Acloque H.; Huang R. Y.; Nieto M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890; 2009. [DOI] [PubMed] [Google Scholar]
- 15. Xiao D.; He J. Epithelial mesenchymal transition and lung cancer. J. Thorac. Dis. 2:154–159; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang L.; Chen Y.; Cui T.; Knösel T.; Zhang Q.; Albring K. F.; Huber O.; Petersen I. Desmoplakin acts as a tumor suppressor by inhibition of the Wnt/β-catenin signaling pathway in human lung cancer. Carcinogenesis 33:1863–1870; 2012. [DOI] [PubMed] [Google Scholar]
- 17. Chen Z.; Li J.; Li Q.; Fan J.; Dong X.; Xu J.; Wang X.; Yang G.; Yan P.; Wen G. Suppression of PPN/MG61 attenuates Wnt/β-catenin signaling pathway and induces apoptosis in human lung cancer. Oncogene 27:3483–3488; 2008. [DOI] [PubMed] [Google Scholar]
- 18. Gao Y.; Song C.; Hui L.; Li C. Y.; Wang J.; Tian Y.; Han X.; Chen Y.; Tian D. L.; Qiu X. Overexpression of RNF146 in non-small cell lung cancer enhances proliferation and invasion of tumors through the Wnt/β-catenin signaling pathway. PLoS One 9:e85377; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu C. W.; Li C. H.; Peng Y. J.; Cheng Y. W.; Chen H. W.; Liao P. L.; Kang J. J.; Yeng M. H. Snail regulates Nanog status during the epithelial-mesenchymal transition via the Smad1/Akt/GSK3β signaling pathway in non-small-cell lung cancer. Oncotarget 5:3880–3894; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Argast G. M.; Krueger J. S.; Thomson S.; Sujka-Kwok I.; Carey K.; Silva S.; O’Connor M.; Mercado P.; Mulford I. J.; Young G. D. Inducible expression of TGFβ, Snail and Zeb1 recapitulates EMT in vitro and in vivo in a NSCLC model. Clin. Exp. Met. 28:593–614; 2011. [DOI] [PubMed] [Google Scholar]
- 21. He W.; He S.; Wang Z.; Shen H.; Fang W.; Zhang Y.; Qian W.; Lin M.; Yuan J.; Wang J. Astrocyte elevated gene- (AEG-1) induces epithelial-mesenchymal transition in lung cancer through activating Wnt/β-catenin signaling. BMC Cancer 15:107; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cadigan K. M.; Nusse R. Wnt signaling: A common theme in animal development. Genes Dev. 11:3286–3305; 1997. [DOI] [PubMed] [Google Scholar]
- 23. Polette M.; Mestdagt M.; Bindels S.; Nawrocki-Raby B.; Hunziker W.; Foidart J. M.; Birembaut P.; Gilles C. eta-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs 185:61–65; 2007. [DOI] [PubMed] [Google Scholar]
- 24. Miao Y.; Wang L.; Zhang X.; Xu X.; Jiang G.; Fan C.; Liu Y.; Lin X.; Yu J.; Zhang Y. Promoter methylation-mediated silencing of β-catenin enhances invasiveness of non-small cell lung cancer and predicts adverse prognosis. PLoS One 9:e112258; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Retera J.; Leers M.; Sulzer M. A.; Theunissen P. The expression of beta-catenin in non-small-cell lung cancer: A clinicopathological study. J. Clin. Pathol. 51:891–894; 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu H.; Wang L.; Lin D.; Liu Y.; Liu N.; Yuan X.; Wang E. Abnormal beta-catenin and reduced axin expression are associated with poor differentiation and progression in non-small cell lung cancer. Am. J. Clin. Pathol. 125:534–541; 2006. [DOI] [PubMed] [Google Scholar]