Abstract
Glioblastoma (GBM) is the most common primary malignant brain tumor that nearly always results in a bad prognosis. Temozolomide plus radiotherapy (TEM+RAD) is the most common treatment for newly diagnosed GBM. With the development of molecularly targeted drugs, several clinical trials were reported; however, the efficacy of the treatment remains controversial. So we attempted to measure the dose of the molecularly targeted drug that could improve the prognosis of those patients. The appropriate electronic databases (PubMed, MEDLINE, EMBASE, and the Cochrane Library) were searched for relevant studies. A meta-analysis was performed after determining which studies met the inclusion criteria. Six randomized, controlled trials (RCTs) were identified for this meta-analysis, comprising 2,637 GBM patients. The benefit of overall survival (OS) was hazard ratio (HZ), 0.936 [95% confidence interval (CI), 0.852–1.028]. The benefit with respect to progression-free survival (PFS) rate was HZ of 0.796 (95% CI, 0.701–0.903). OS benefit of cilengitide was HZ of 0.792 (95% CI, 0.642–0.977). The adverse effects higher than grade 3 were 57.7% in the experimental group and 44.1% in the placebo group (odds ratio, 1.679; 95% CI, 1.434–1.967). The addition of molecularly targeted drugs to TEM + RAD did not improve the OS of patients with GBM; however, it did improve PFS in patients treated by cilengitide who could not get improvement in OS. The rate of adverse effects was higher in the experimental group than in the placebo group.
Key words: Molecularly targeted drugs, Radiotherapy and temozolomide, Treatment, Glioblastoma (GBM), Newly diagnosed, Overall survival (OS)
INTRODUCTION
Glioblastoma (GBM) is the most common and most lethal primary malignant brain tumor (1) that affects about 3 in 100,000 individuals each year with a median overall survival (OS) of <1.0 year (2). This disease usually develops in the cerebral hemisphere (3). Because the neoplasms are very aggressive, patients usually have a poor quality of life, and between 1973 and 1998 no new treatment had been developed (4). In 1999, the U.S. Food and Drug Administration (FDA) approved the use of temozolomide for treatment of GBM (5), and the standard treatment for newly diagnosed GBM is now maximal safe resection followed by radiotherapy plus chemotherapy with temozolomide (TEM + RAD) (5–7), which has improved OS from less than 1 year to 15 months (8). Because GBM remains the most dangerous brain tumor, research and development of new drug treatments continue.
Molecularly targeted drugs block the growth of cancer cells by interfering with specifically targeted molecules that are necessary for tumor growth (9). This therapy is widely used for several kinds of malignant tumor, and the prognosis has improved. Angiogenesis has emerged as the primary target of drug development (10), and bevacizumab, a humanized immunoglobulin G1 monoclonal antibody that inhibits vascular endothelial growth factor (VEGF), plays an important role in angiogenesis (11). Bevacizumab was the first FDA-approved antiangiogenic agent that proved effective against metastatic colorectal, breast, and lung cancers (12–15). After it was proven effective in recurrent GBM, it was used in the treatment of GBM (16,17). It was approved for newly diagnosed GBM in 2005 after the Stupp trial. In phase II clinical trials, bevacizumab proved effective against newly diagnosed GBM combined with TEM + RAD (2,10,18–23). Cilengitide is an inhibitor of αvβ3 and αvβ5 integrins, which are important proteins in angiogenesis (24). In phase I and II clinical trials, cilengitide showed antitumor activity. Nimotuzumab is a monoclonal antibody that blocks against epidermal growth factor receptor (EGFR) (25) and binds more specifically to EGFR-overexpressing cells. It has shown promising efficacy against GBM in phase II trials. In 2014, the research of Wang et al. showed that nimotuzumab combined with TEM + RAD could improve progression-free survival (PFS) and OS to 10.0 and 15.9 months (26). In 2010, the phase I/II clinical trial of Stupp et al. (7) showed that the addition of cilengitide could improve the median PFS and OS to 8 and 16.1 months. The same results were reported in an experiment with bevacizumab by Mayer (27) in 2015 and Hicks et al. (28) in 2015. But in 2014, two randomized clinical trials, AVAglio and RTOG-0825, showed no improvement in OS by combining a molecularly targeted drug with TEM + RAD. So we decided to measure the dose of the molecularly targeted drug that could improve the prognosis of those patients.
MATERIALS AND METHODS
Search Strategy
A literature search of PubMed, MEDLINE, EMBASE, and the Cochrane Library databases was performed by two reviewers (S.J.H. and H.H.Y.) independently from November 20, 2015 to February 20, 2016 to identify all studies that related to the molecularly targeted drugs and newly diagnosed GBM. Any disagreement was resolved unanimously by discussion. The following terms were used in the database searches: [(glioblastoma) OR (glioblastoma multiforme)] AND (newly diagnosed) AND (temozolomide) AND (radiotherapy) AND [(molecularly targeted) OR (anti-angiogenesis)]. In addition, the references cited in the articles and included in the analyses were reviewed for other citations.
Inclusion Criteria
The inclusion criteria for the clinical trials for this meta-analysis were as follows: (1) designed to compare the efficacy of molecularly targeted drugs combined with TEM + RAD and with TEM + RAD alone; (2) randomly controlled; (3) adult patients (≥18 years old) with a Karnofsky Performance Status score ≥60 and normal hematologic, renal, and hepatic function; (4) >100 GBM patients enrolled; and (5) data were available for PFS or OS. Studies containing molecularly targeted drugs combined with other treatments other than TEM + RAD and published in other languages with an English version were excluded.
Methodological Quality
Critical appraisal of the randomized controlled trials (RCTs) was based on the Jadad score, which reviews randomization, allocation concealment, blinding, and dropouts (withdrawals) in the trials to assess their methodological quality. A score of 1–3 is considered a low-quality trial, while a score of 4–7 is considered a high-quality trial. The results are shown in Table 1.
Table 1.
The Quality of Included Studies Using Jadad Scores
Studies Identified
The electronic searches resulted in the identification of 1,074 publications. The titles and abstract were examined to exclude irrelevant studies, resulting in 114 potentially eligible articles. The full text of those remaining articles was then examined, and seven studies (10,24,25,29–32) remained, of which one [Peters et al. (31)] was excluded for lack of data. Eventually, six RCTs comprising 2,637 newly diagnosed GBM patients were included in this meta-analysis (Fig. 1). The sample size ranged from 921 to 120 patients. All studies were published in English, and three were international. Three studies were on bevacizumab, two were on cilengitide, and one was on nimotuzumab (Table 1).
Figure 1.
Study selection flow chart.
Statistical Analyses
Specific parameters of the included studies [author, number of patients, treatments, hazard ratio (HZ) of OS, HZ of PFS, O 6-methylguanine-DNA-methyltransferase (MGMT)-methylated patients, recursive-partitioning analysis (RPA) class, and adverse effects] were collected (Table 2). A meta-analysis was performed using Comprehensive Meta-Analysis ver. 2.0 (Biostat, Englewood, NJ, USA). The HZ with a 95% confidence interval (CI) was used to assess the outcomes of the studies, and a value of p < 0.05 indicated statistical significance. The odds ratio (OR) was used to assess the outcomes of any adverse effects. I 2 statistics were performed to evaluate the heterogeneity among the studies, which was defined as low (0–25%), moderate (25–50%), high (50–75%), and extreme (>75%). Begg’s tests were performed to create funnel plots to assess publication bias. A sensitivity analysis was performed for each study, and the impacts of different interventions were evaluated.
Table 2.
Information on the Included Studies
| Study/No. of Patients | Treatment | OS HRs and 95% CIs | PFS HRs and 95% CIs | Subgroup | |||||
|---|---|---|---|---|---|---|---|---|---|
| MGMT-Methylated HZs | MGMT-Nonmethylated HZs | RPA Class | Adverse Events Grades 3 and 4 | ||||||
| III | IV | V | |||||||
| Chinot et al. (29) | 0.88 (0.76–1.02) | 0.64 (0.55–0.74) | PFS 0.61 (0.46–0.82) | PFS 0.56 (0.46–0.68) | PFS 0.64 (0.44–0.93) | PFS 0.62 (0.51–0.74) | PFS 0.72 (0.54–0.96) | ||
| 458 | Bev + Rad + Tem | 306/458 | |||||||
| 463 | Rad + Tem | 238/463 | |||||||
| Gilbert et al. (30) | 1.13 (0.93–1.37) | 0.79 (0.66–0.94) | OS 2.27 (0.91–5.68) | OS 1.02 (0.66–1.57) | OS 0.98 (0.54–1.81) | OS 1.14 (0.90–1.44) | OS 1.01 (0.66–1.56) | ||
| 320 | Bev + Rad + Tem | PFS 1.39 (0.67–2.89) | PFS 0.72 (0.48–1.07) | PFS 0.74 (0.43–1.25) | PFS 0.78 (0.63–0.96) | PFS 0.70 (0.46–1.06) | 126/303 | ||
| 317 | Rad + Tem | 93/300 | |||||||
| Stupp et al. (24) | 1.02 (0.81–1.29) | 0.93 (0.76–1.13) | PFS 1.02 (0.81–1.29) | N/A | OS 0.63 (0.31–1.31) | OS 1.08 (0.84–1.39) | |||
| 272 | Cil + Rad + Tem | OS 0.93 (0.76–1.13) | 169/272 | ||||||
| 273 | Rad + Tem | 158/273 | |||||||
| Westphal et al. (25) | 0.862 (0.568–1.308) | 0.953 (0.669–1.359) | OS 0.864 (0.273–2.734) | OS 0.807 (0.457–1.425) | N/A | N/A | |||
| 75 | Nim + Rad + Tem | 23/75 | |||||||
| 74 | Rad + Tem | 8/74 | |||||||
| Nabors et al. (32) | 0.686 (0.484–0.972) | 0.822 (0.595–1.134) | N/A | PFS 0.822 (0.595–1.134) | N/A | N/A | N/A | ||
| 88 | Stand Cil + Rad + Tem | 0.858 (0.612–1.204) | 0.794 (0.575–1.096) | N/A | OS 0.794 (0.575–1.096) | N/A | N/A | N/A | 47/88 |
| 88 | Intensive Cil + Rad + Tem | 36/88 | |||||||
| 89 | Rad + Tem | 30/89 | |||||||
| Chauffert et al. (10) | N/A | 0.82 (0.56–1.19) | N/A | N/A | N/A | N/A | N/A | ||
| 60 | Bev + Iri + Rad + Tem | 37/57 | |||||||
| 60 | Rad + Tem | 26/56 | |||||||
OS, overall survival; PFS, progression-free survival; HZ, hazard ratio; CI, confidence interval; RPA, recursive-partitioning analysis; Bev, bevacizumab; Rad, radiotherapy; Tem, temozolomide; N/A, not available.
RESULTS
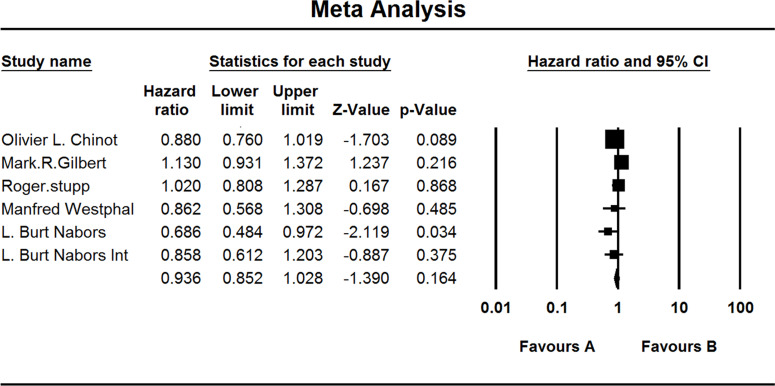
Five included studies investigated the OS of GBM patients. When the test for heterogeneity showed no significant differences among the studies (I 2 = 13.871), the fixed effects model was used (HZ, 0.936; 95% CI, 0.852–1.028; p = 0.164) (Fig. 2).
Figure 2.
Comparison of OS between the (A) experimental group and the (B) TEM + RAD group.
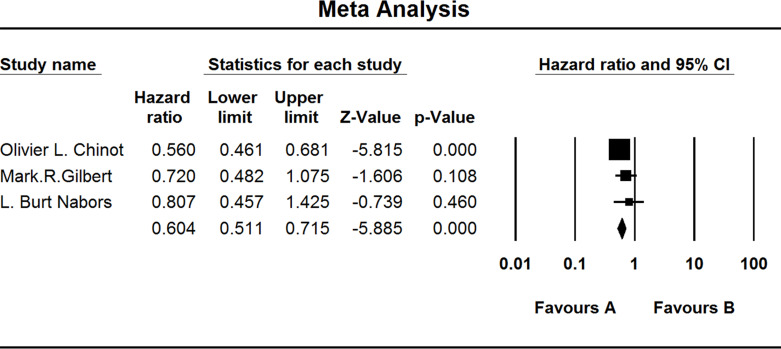
All included studies investigated the PFS of GBM patients. When the test for heterogeneity showed high significant differences among the studies (I 2 = 46.513), the random effects model was used (HZ, 0.796; 95% CI, 0.701–0.903); p < 0.001) (Fig. 3).
Figure 3.
Comparison of PFS between the (A) experiment group and the (B) TEM + RAD group.
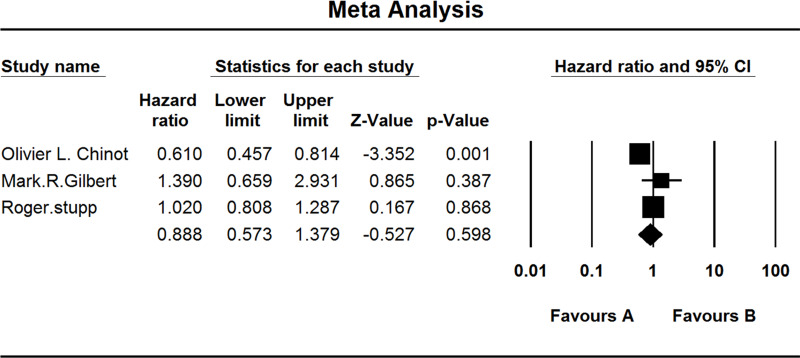
Three studies investigated the PFS of MGMT-methylated GBM patients. When the test for heterogeneity showed high significant differences among the studies (I 2 = 77.972), the random effects model was used (HZ, 0.888; 95% CI, 0.573–1.379; p = 0.598) (Fig. 4).
Figure 4.
Comparison of PFS of MGMT-methylated patients. (A) Experimental group and (B) TEM + RAD group.
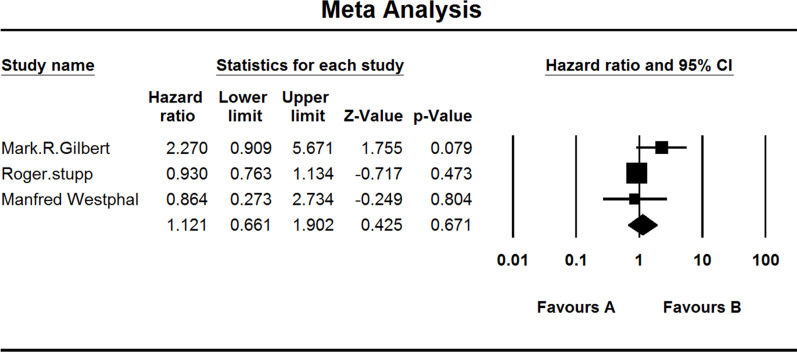
Three studies investigated the OS of MGMT-methylated GBM patients. When the test for heterogeneity showed middle significant differences among the studies (I 2 = 43.205), the random effects model was used (HZ, 1.121; 95% CI, 0.661–1.902; p = 0.671) (Fig. 5).
Figure 5.
Comparison of OS of MGMT-methylated patients. (A) Experimental group and (B) TEM+RAD group.
Three studies investigated the PFS of MGMT-nonmethylated GBM patients. When the test for heterogeneity showed no significant differences among the studies (I 2 = 13.451), the fixed effects model was used (HZ, 0.604; 95% CI, 0.511–0.715; p = 0.000) (Fig. 6).
Figure 6.
Comparison of PFS of MGMT-nonmethylated patients. (A) Experimental group and (B) TEM + RAD group.
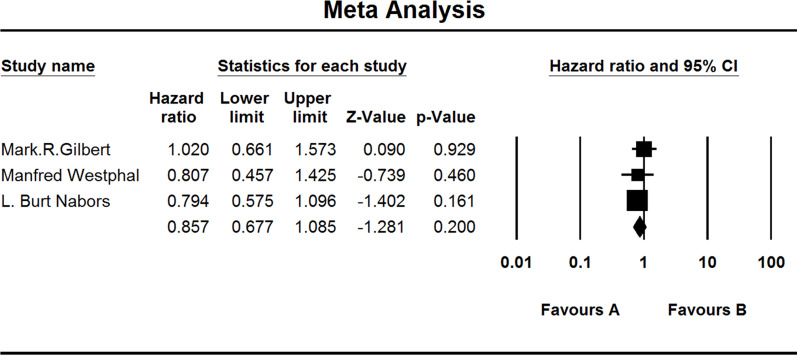
Three studies investigated the OS of MGMT-nonmethylated GBM patients. When the test for heterogeneity showed no significant differences among the studies (I 2 = 0.000), the fixed effects model was used (HZ, 0.857; 95% CI, 0.677–1.085; p = 0.200) (Fig. 7).
Figure 7.
Comparison of OS of MGMT-nonmethylated patients. (A) Experimental group and (B) TEM + RAD group.
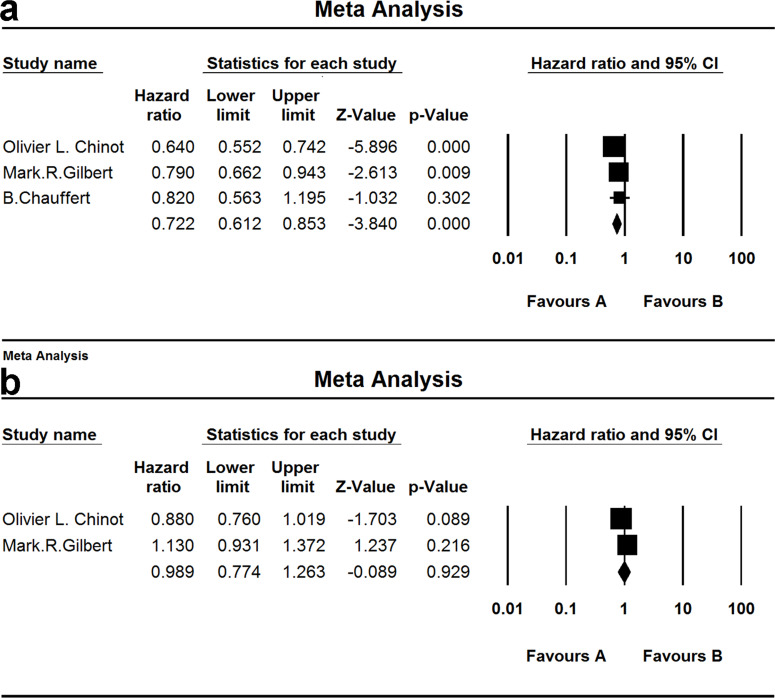
Three studies investigated PFS and two for the OS of patients treated by bevacizumab. When the test for heterogeneity showed no significant differences among the studies (I 2 = 47.891 and I 2 = 75.369), the random effects model was used [(HZ, 0.989; 95% CI, 0.774–1.263; p = 0.929) and (HZ, 0.722; 95% CI, 0.612–0.853; p = 0.000)] (Fig. 8).
Figure 8.
Comparison of OS and PFS of patients treated by bevacizumab. (a) Comparison of PFS; (b) comparison of OS. (A) Experimental group and (B) TEM+RAD group.
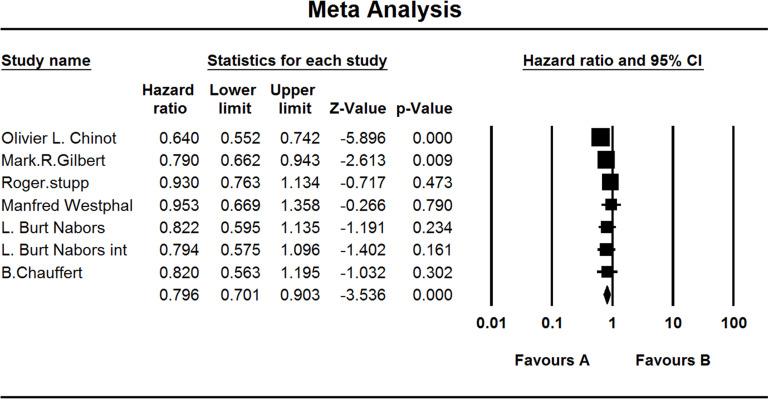
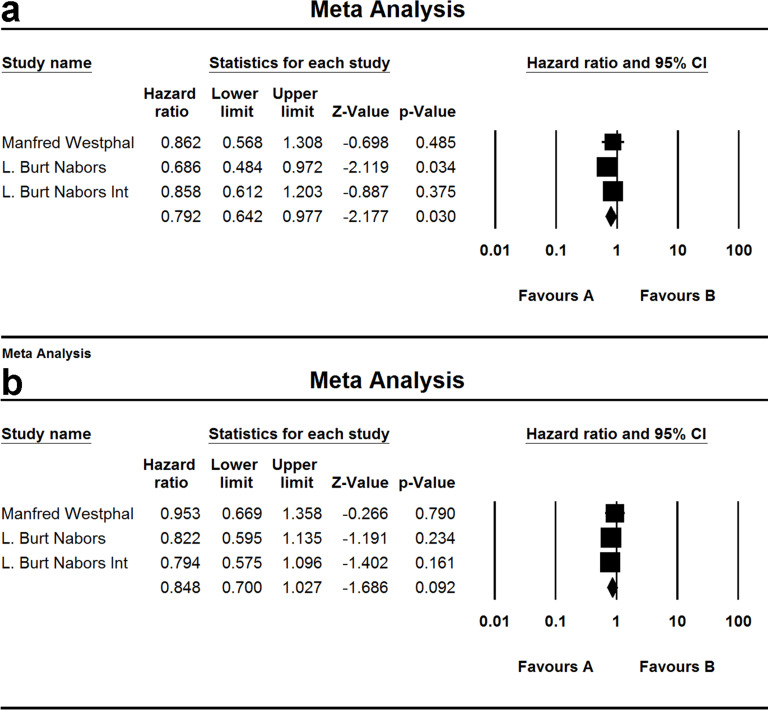
Two studies and three groups investigated PFS and two for the OS of patients treated by cilengitide. When the test for heterogeneity showed no significant difference among the studies (I 2 = 0.000 and I 2 = 0.000), the fixed effect model was used [(HZ, 0.792; 95% CI, 0.642–0.977; p = 0.030) and (HZ, 0.848; 95% CI, 0.700–1.027; p = 0.092)] (Fig. 9).
Figure 9.
Comparison of OS and PFS of patients treated by cilengitide. (a) Comparison of PFS; (b) comparison of OS. (A) Experimental group and (B) TEM + RAD group.
Three studies investigated the RPA classes of the patients. The comparison is shown in Table 3.
Table 3.
Analysis of the RPA Classes
| I 2 | HZ | 95% CI | p | |
|---|---|---|---|---|
| OS of RPA III | <0.001 | 0.816 | 0.514–1.298 | 0.391 |
| PFS of RPA III | <0.001 | 0.671 | 0.494–0.912 | 0.011 |
| PFS of RPA IV | 59.664 | 0.688 | 0.597–0.794 | 0.000 |
| PFS of RPA V | 39.149 | 0.799 | 0.629–1.015 | 0.066 |
OS, overall survival; PFS, progression-free survival; HZ, hazard ratio; CI, confidence interval; RPA, recursive-partitioning analysis.
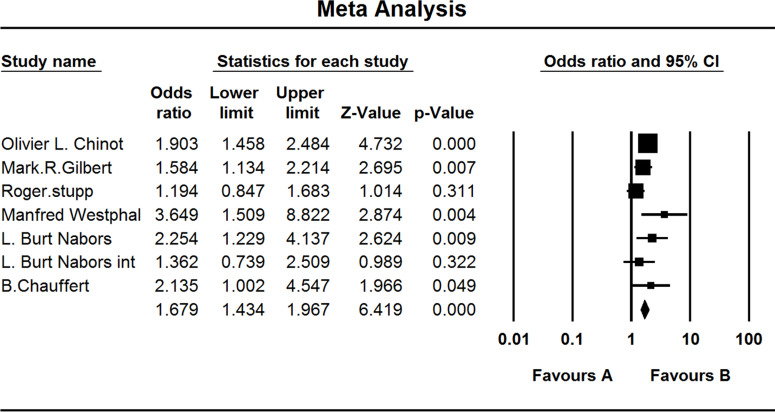
All studies investigated the adverse effects experienced by the GBM patients. When the test for heterogeneity showed no significant differences among the studies (I 2 = 36.615), the fixed effects model was used (OR, 1.679; 95% CI, 1.434–1.967; p < 0.001) (Fig. 10).
Figure 10.
Comparison of adverse effects. (A) Experimental group and (B) TEM + RAD group.
Qualitative Assessment and Publication Bias
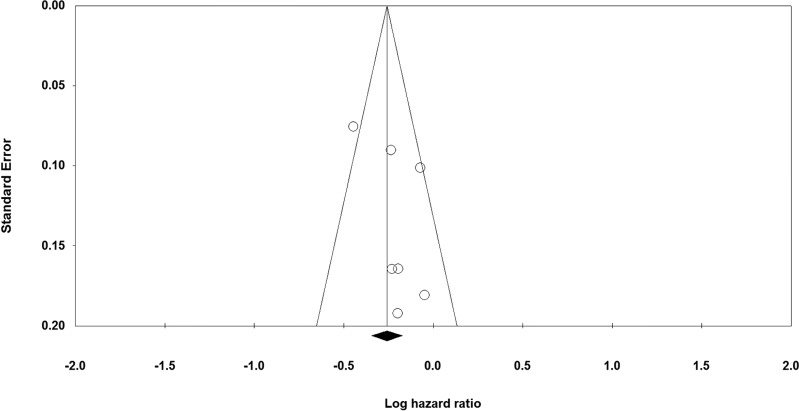
The quality of the studies included in this meta-analysis is shown in Table 1. It can be seen from the funnel plot that publication bias was low in all the comparisons (Fig. 11).
Figure 11.
Funnel plot of PFS between the experimental group and the TEM + RAD group.
DISCUSSION
The treatment of GBM is of utmost importance in the fields of neuroscience and oncology (11,13,33,34). With the discovery of temozolomide, OS and PFS have significantly improved (21), and the TEM + RAD treatment has been used worldwide (3,21,35,36). However, GBM is still the worst primary tumor of the central nervous system (5). Molecularly targeted drugs are widely used in the treatment of many kinds of malignant tumors and are highly specific and effective; they are believed to be the future of malignant tumor treatment (4,5,37–39). In this meta-analysis, the efficacy of new molecularly targeted drugs plus TEM + RAD treatment was measured for newly diagnosed GBM.
The results of this study showed that the new molecularly targeted drugs do not improve the OS of newly diagnosed GBM but can improve PFS of these patients (40). OS was most widely used in the studies for gauging the new treatments for cancer. In this meta-analysis, HZ of OS (0.936) did not show a significant difference (95% CI, 0.852–1.028). However, in the phase II clinical trials, there was improvement in the experimental group (10,23,41,42). This could have been because the RCTs included in this meta-analysis had larger populations. However, our research showed that patients could not get the benefit of OS from a combination of cilengitide with TEM + RAD. After the research of Stupp et al. (24), cilengitide was thought to be useless in the treatment of GBM. But further studies may still be needed for deep analysis to find out which group of patients is fit for treatment with cilengitide. This meta-analysis showed improved PFS (0.769; 95% CI, 0.704–0.840; p < 0.001); the median PFS in the experimental group was 3–4 months longer than that of the placebo group. Although OS was widely used for gauging the efficacy of cancer treatments, a recent study (40) found that PFS might be an appropriate surrogate for OS in GBM; however, our meta-analysis showed that PFS and OS are poorly correlated (R 2 = 0.002). When molecularly targeted drug treatments did not improve OS but did improve PFS, we determined that the new molecularly targeted drugs cannot improve the prognosis of newly diagnosed GBM even though PFS is correlated with the quality of life in cancer patients. In our meta-analysis, two studies provided data on the quality of life using different scales (13,14,43,44). In one study (45), the median time of the Karnofsky scale was 70 or higher, much higher than that in the placebo group. In another study (25), the functional scales (physical, roles, emotional, cognitive, and social) favored the experimental arm but without statistical significance. In the 2015 analysis by Taphoorn et al., health-related quality of life (HRQoL) in AVAglio was studied, and the result showed that the addition of bevacizumab to TEM + RAD gave no improvement in HRQoL during the PFS (46). In our study, the addition of molecularly targeted drugs to the TEM + RAD standard treatment showed no improvement in OS or the quality of life.
Correlative Factors
Many studies highlighted the MGMT promoter status as a predictive tool and indicated that biomarker testing could be helpful in deciding on individualized treatment (10,12,14,18) because the MGMT-methylated GBM patients are usually more sensitive to chemical therapy (20). However, in our meta-analysis, the molecularly targeted drug plus TEM + RAD treatment gave no difference on OS in either the MGMT-methylated patients or the MGMT-nonmethylated patients, but it did improve PFS in both. Thus, in our meta-analysis, we considered that MGMT status cannot be used as a biomarker. But in 2015, Sandmann et al. did a further study of the AVAglio and RTOG-0825 trials (47). This multivariable analysis found that the proneural subtype of GBM may derive OS benefit from the addition of bevacizumab to TEM + RAD therapy. The RPA classes are as follows in GBM: class III, <50 years old and a Karnofsky performance score of ≥90 (on a scale of 0 to 100, with higher scores indicating better function); class IV, <50 years old and a Karnofsky performance score of <90 (or ≥50 years old, a Karnofsky performance score of ≥70, a gross total or partial tumor resection, and an ability to work); and class V, ≥50 years old, a Karnofsky performance score of ≥70, a gross total or partial tumor resection, and an inability to work (or ≥50 years old, a Karnofsky performance score of ≥70, and tumor biopsy specimen only; or ≥50 years old and a Karnofsky performance score of <70) (14,25,29,48–50). In each class, OS and PFS were analyzed. The results showed that in RPA class III, OS HZ showed no difference, and in RPA classes III and IV, PFS HZ showed that molecularly targeted drugs could improve PFS in newly diagnosed GBM patients. In RPA class V, there was no difference in PFS HZ. But since there are just two to three studies included in our analysis, the bias was large. Our results showed that patients who got a higher RPA class got more benefit from the molecularly targeted drug. The same thing was found by Hawkins-Daarud et al. in 2015. That study showed that the subpopulation of patients who would receive the greatest benefit from bevacizumab and radiation therapy is those with large, aggressive tumors and who are not eligible for GTR (51). The addition of bevacizumab has been proven useful for the patients with recurrent disease that is more aggressive after recurrence. So the molecularly targeted drug may bring a benefit for the higher subgroup, although more studies are needed.
Safety Considerations
The adverse effect of treatment is very important to patients. In our meta-analysis, the adverse effects at grade 3 or higher were measured. We found that molecularly targeted drugs resulted in more adverse effects (OR, 1.661; 95% CI, 1.413–1.953; p < 0.001). As reported by these studies, the most common adverse effects were lymphopenia and thromboembolic events. These studies also showed that most of these effects were connected with molecularly targeted drugs (24,25,29,30).
Study Limitations
In this meta-analysis, molecularly targeted drugs were measured for newly diagnosed GBM using six RCTs. As shown in the funnel plot in Figure 11, there is publication bias in the comparisons, and this could have influenced the results of our meta-analysis. The MGMT biomarker was also measured in this study, and no difference was indicated; however, there are more biomarkers that were not mentioned in these studies and might also be connected to the treatments. The standard treatment for newly diagnosed GBM was surgery before TEM + RAD therapy; however, we did not compare this protocol because of the lack of data.
The present meta-analysis and systematic review confirmed that molecularly targeted drugs could improve PFS but could not improve OS or prognoses and, in fact, could result in more adverse effects. The development of new drugs and discovery of new biomarkers are still of utmost importance.
ACKNOWLEDGMENT
This work was supported by the Natural Science Foundation of Guangdong Province (2014A030310408; 2014A030313189), the Fundamental Research Funds for the Central University (20120171110083), and Science and Technology Project of Guangdong Province (2015A020212016; 2016A020214007).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Kawano H.; Hirano H.; Yonezawa H.; Yunoue S.; Yatsushiro K.; Ogita M.; Hiraki Y.; Uchida H.; Habu M.; Fujio S.; Oyoshi T.; Bakhtiar Y.; Sugata S.; Yamahata H.; Hanaya R.; Tokimura H.; Arita K. Improvement in treatment results of glioblastoma over the last three decades and beneficial factors. Br. J. Neurosurg. 29:206–212; 2015. [DOI] [PubMed] [Google Scholar]
- 2. Pope W. B.; Lai A.; Mehta R.; Kim H. J.; Qiao J.; Young J. R.; Xue X.; Goldin J.; Brown M. S.; Nghiemphu P. L.; Tran A.; Cloughesy T. F. Apparent diffusion coefficient histogram analysis stratifies progression-free survival in newly diagnosed bevacizumab-treated glioblastoma. Am. J. Neuroradiol. 32:882–889; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chamberlain M. C. Treatment of newly diagnosed malignant glioma in the elderly people: New trials that impact therapy. Int. J. Clin. Pract. 67:1225–1227; 2013. [DOI] [PubMed] [Google Scholar]
- 4. Nanegrungsunk D.; Onchan W.; Chattipakorn N.; Chattipakorn S. C. Current evidence of temozolomide and bevacizumab in treatment of gliomas. Neurol. Res. 37:167–183; 2015. [DOI] [PubMed] [Google Scholar]
- 5. Rizzo D.; Scalzone M.; Ruggiero A.; Maurizi P.; Attina G.; Mastrangelo S.; Lazzareschi I.; Ridola V.; Colosimo C.; Caldarelli M.; Balducci M.; Riccardi R. Temozolomide in the treatment of newly diagnosed diffuse brainstem glioma in children: A broken promise? J. Chemother. 27:106–110; 2015. [DOI] [PubMed] [Google Scholar]
- 6. Narayana A.; Gruber D.; Kunnakkat S.; Golfinos J. G.; Parker E.; Raza S.; Zagzag D.; Eagan P.; Gruber M. L. A clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastoma. J. Neurosurg. 116:341–345; 2012. [DOI] [PubMed] [Google Scholar]
- 7. Stupp R.; Hegi M. E.; Neyns B.; Goldbrunner R.; Schlegel U.; Clement P. M.; Grabenbauer G. G.; Ochsenbein A. F.; Simon M.; Dietrich P. Y.; Pietsch T.; Hicking C.; Tonn J. C.; Diserens A. C.; Pica A.; Hermisson M.; Krueger S.; Picard M.; Weller M. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 28:2712–2718; 2010. [DOI] [PubMed] [Google Scholar]
- 8. Vredenburgh J. J.; Desjardins A.; Kirkpatrick J. P.; Reardon D. A.; Peters K. B.; Herndon J. E. 2nd; Marcello J.; Bailey L.; Threatt S.; Sampson J.; Friedman A.; Friedman H. S. Addition of bevacizumab to standard radiation therapy and daily temozolomide is associated with minimal toxicity in newly diagnosed glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 82:58–66; 2012. [DOI] [PubMed] [Google Scholar]
- 9. Armstrong T. S.; Wefel J. S.; Wang M.; Gilbert M. R.; Won M.; Bottomley A.; Mendoza T. R.; Coens C.; Werner-Wasik M.; Brachman D. G.; Choucair A. K.; Mehta M. Net clinical benefit analysis of radiation therapy oncology group 0525: A phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 31:4076–4084; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chauffert B.; Feuvret L.; Bonnetain F.; Taillandier L.; Frappaz D.; Taillia H.; Schott R.; Honnorat J.; Fabbro M.; Tennevet I.; Ghiringhelli F.; Guillamo J. S.; Durando X.; Castera D.; Frenay M.; Campello C.; Dalban C.; Skrzypski J.; Chinot O. Randomized phase II trial of irinotecan and bevacizumab as neo-adjuvant and adjuvant to temozolomide-based chemoradiation compared with temozolomide-chemoradiation for unresectable glioblastoma: Final results of the TEMAVIR study from ANOCEFdagger. Ann. Oncol. 25:1442–1447; 2014. [DOI] [PubMed] [Google Scholar]
- 11. Goey A. K.; Figg W. D. Potential novel role of bevacizumab in glioblastoma and cervical cancer. Cancer Biol. Ther. 15:1296–1298; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mason W. P. Bevacizumab in recurrent glioblastoma: Five informative patient scenarios. Can. J. Neurol. Sci. 42:149–156; 2015. [DOI] [PubMed] [Google Scholar]
- 13. Field K. M.; Jordan J. T.; Wen P. Y.; Rosenthal M. A.; Reardon D. A. Bevacizumab and glioblastoma: Scientific review, newly reported updates, and ongoing controversies. Cancer 121:997–1007; 2015. [DOI] [PubMed] [Google Scholar]
- 14. Zhong J.; Ali A. N.; Voloschin A. D.; Liu Y.; Curran W. J. Jr.; Crocker I. R.; Shu H. K. Bevacizumab-induced hypertension is a predictive marker for improved outcomes in patients with recurrent glioblastoma treated with bevacizumab. Cancer 121:1456–1462; 2015. [DOI] [PubMed] [Google Scholar]
- 15. Leu K.; Enzmann D. R.; Woodworth D. C.; Harris R. J.; Tran A. N.; Lai A.; Nghiemphu P. L.; Pope W. B.; Cloughesy T. F.; Ellingson B. M. Hypervascular tumor volume estimated by comparison to a large-scale cerebral blood volume radiographic atlas predicts survival in recurrent glioblastoma treated with bevacizumab. Cancer Imaging 14:31; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galanis E.; Anderson S. K.; Lafky J. M.; Uhm J. H.; Giannini C.; Kumar S. K.; Kimlinger T. K.; Northfelt D. W.; Flynn P. J.; Jaeckle K. A.; Kaufmann T. J.; Buckner J. C. Phase II study of bevacizumab in combination with sorafenib in recurrent glioblastoma (N0776): A north central cancer treatment group trial. Clin. Cancer Res. 19:4816–4823; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schiff D.; Purow B. Bevacizumab in combination with irinotecan for patients with recurrent glioblastoma multiforme. Nat. Clin. Pract. Oncol. 5:186–187; 2008. [DOI] [PubMed] [Google Scholar]
- 18. Hofland K. F.; Hansen S.; Sorensen M.; Engelholm S.; Schultz H. P.; Muhic A.; Grunnet K.; Ask A.; Costa J. C.; Kristiansen C.; Thomsen C.; Poulsen H. S.; Lassen U. Neoadjuvant bevacizumab and irinotecan versus bevacizumab and temozolomide followed by concomitant chemoradiotherapy in newly diagnosed glioblastoma multiforme: A randomized phase II study. Acta Oncol. 53:939–944; 2014. [DOI] [PubMed] [Google Scholar]
- 19. Chinot O. L.; de La Motte Rouge T.; Moore N.; Zeaiter A.; Das A.; Phillips H.; Modrusan Z.; Cloughesy T. AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv. Ther. 28:334–340; 2011. [DOI] [PubMed] [Google Scholar]
- 20. Kunnakkat S.; Narayana A. Bevacizumab in the treatment of high-grade gliomas: An overview. Angiogenesis 14:423–430; 2011. [DOI] [PubMed] [Google Scholar]
- 21. Liu R.; Wang X.; Ma B.; Yang K.; Zhang Q.; Tian J. Concomitant or adjuvant temozolomide with whole-brain irradiation for brain metastases: A meta-analysis. Anticancer Drugs 21:120–128; 2010. [DOI] [PubMed] [Google Scholar]
- 22. Lou E.; Peters K. B.; Sumrall A. L.; Desjardins A.; Reardon D. A.; Lipp E. S.; Herndon J. E. 2nd; Coan A.; Bailey L.; Turner S.; Friedman H. S.; Vredenburgh J. J. Phase II trial of upfront bevacizumab and temozolomide for unresectable or multifocal glioblastoma. Cancer Med. 2:185–195; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Omuro A.; Beal K.; Gutin P.; Karimi S.; Correa D. D.; Kaley T. J.; DeAngelis L. M.; Chan T. A.; Gavrilovic I. T.; Nolan C.; Hormigo A.; Lassman A. B.; Mellinghoff I.; Grommes C.; Reiner A. S.; Panageas K. S.; Baser R. E.; Tabar V.; Pentsova E.; Sanchez J.; Barradas-Panchal R.; Zhang J.; Faivre G.; Brennan C. W.; Abrey L. E.; Huse J. T. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res. 20:5023–5031; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stupp R.; Hegi M. E.; Gorlia T.; Erridge S. C.; Perry J.; Hong Y. K.; Aldape K. D.; Lhermitte B.; Pietsch T.; Grujicic D.; Steinbach J. P.; Wick W.; Tarnawski R.; Nam D. H.; Hau P.; Weyerbrock A.; Taphoorn M. J.; Shen C. C.; Rao N.; Thurzo L.; Herrlinger U.; Gupta T.; Kortmann R. D.; Adamska K.; McBain C.; Brandes A. A.; Tonn J. C.; Schnell O.; Wiegel T.; Kim C. Y.; Nabors L. B.; Reardon D. A.; van den Bent M. J.; Hicking C.; Markivskyy A.; Picard M.; Weller M. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 15:1100–1108; 2014. [DOI] [PubMed] [Google Scholar]
- 25. Westphal M.; Heese O.; Steinbach J. P.; Schnell O.; Schackert G.; Mehdorn M.; Schulz D.; Simon M.; Schlegel U.; Senft C.; Geletneky K.; Braun C.; Hartung J. G.; Reuter D.; Metz M. W.; Bach F.; Pietsch T. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur. J. Cancer. 51:522–532; 2015. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y.; Pan L.; Sheng X. F.; Chen S.; Dai J. Z. Nimotuzumab, a humanized monoclonal antibody specific for the EGFR, in combination with temozolomide and radiation therapy for newly diagnosed glioblastoma multiforme: First results in Chinese patients. Asia Pac. J. Clin. Oncol. 12:e23–e29; 2014. [DOI] [PubMed] [Google Scholar]
- 27. Mayer T. M. Can we predict bevacizumab responders in patients with glioblastoma. J. Clin. Oncol. 33:2721–2722; 2015. [DOI] [PubMed] [Google Scholar]
- 28. Hicks M. J.; Funato K.; Wang L.; Aronowitz E.; Dyke J. P.; Ballon D. J.; Havlicek D. F.; Frenk E. Z.; De B. P.; Chiuchiolo M. J.; Sondhi D.; Hackett N. R.; Kaminsky S. M.; Tabar V.; Crystal R. G. Genetic modification of neurons to express bevacizumab for local anti-angiogenesis treatment of glioblastoma. Cancer Gene Ther. 22:1–8; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chinot O. L.; Wick W.; Cloughesy T. Bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 370:2049; 2014. [DOI] [PubMed] [Google Scholar]
- 30. Gilbert M. R.; Sulman E. P.; Mehta M. P. Bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 370:2048–2049; 2014. [DOI] [PubMed] [Google Scholar]
- 31. Peters K. B.; Lou E.; Desjardins A.; Reardon D. A.; Lipp E. S.; Miller E.; Herndon J. E. 2nd; McSherry F.; Friedman H. S.; Vredenburgh J. J. Phase II trial of upfront bevacizumab, irinotecan, and temozolomide for unresectable glioblastoma. Oncologist 20:727–728; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nabors L. B.; Fink K. L.; Mikkelsen T.; Grujicic D.; Tarnawski V.; Nam D. H.; Mazurkiewicz M.; Salacz M. E.; Ashby L.; Zagonel V.; Depenni R.; Perry J. R.; Hicking C.; Picard M.; Hegi M. E.; Lhermitte B.; Reardon D. A. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: Results of the open-label, controlled, randomized phase II CORE study. Neuro. Oncol. 17:708–717; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poulsen H. S.; Urup T.; Michaelsen S. R.; Staberg M.; Villingshoj M.; Lassen U. The impact of bevacizumab treatment on survival and quality of life in newly diagnosed glioblastoma patients. Cancer Manag. Res. 6:373–387; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nagane M.; Nishikawa R. Bevacizumab for glioblastoma—A promising drug or not? Cancers (Basel) 5:1456–1468; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen C.; Xu T.; Lu Y.; Chen J.; Wu S. The efficacy of temozolomide for recurrent glioblastoma multiforme. Eur. J. Neurol. 20:223–230; 2013. [DOI] [PubMed] [Google Scholar]
- 36. Khasraw M.; Ameratunga M.; Grommes C. Bevacizumab for the treatment of high-grade glioma: An update after phase III trials. Expert. Opin. Biol. Ther. 14:729–740; 2014. [DOI] [PubMed] [Google Scholar]
- 37. Cloughesy T.; Mason W.; Henriksson R.; Saran F.; Nishikawa R.; Hilton M.; Garcia J.; Pallaud C.; Chinot O. Efficacy and biomarker findings from AVAglio, a phase III trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma. J. Neurol. Sci. 33:e654; 2013. [Google Scholar]
- 38. Penas-Prado M.; Hess K. R.; Fisch M. J.; Lagrone L. W.; Groves M. D.; Levin V. A.; De Groot J. F.; Puduvalli V. K.; Colman H.; Volas-Redd G.; Giglio P.; Conrad C. A.; Salacz M. E.; Floyd J. D.; Loghin M. E.; Hsu S. H.; Gonzalez J.; Chang E. L.; Woo S. Y.; Mahajan A.; Aldape K. D.; Yung W. K.; Gilbert M. R. Randomized phase II adjuvant factorial study of dose-dense temozolomide alone and in combination with isotretinoin, celecoxib, and/or thalidomide for glioblastoma. Neuro. Oncol. 17:266–273; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Linde M. E.; Verhoeff J. J.; Richel D. J.; van Furth W. R.; Reijneveld J. C.; Verheul H. M.; Stalpers L. J. Bevacizumab in combination with radiotherapy and temozolomide for patients with newly diagnosed glioblastoma multiforme. Oncologist 20:107–108; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han K.; Ren M.; Wick W.; Abrey L.; Das A.; Jin J.; Reardon D. A. Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: A literature-based meta-analysis from 91 trials. Neuro. Oncol. 16:696–706; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sepulveda J. M.; Belda-Iniesta C.; Gil-Gil M.; Perez-Segura P.; Berrocal A.; Reynes G.; Gallego O.; Capellades J.; Ordonez J. M.; La Orden B.; Balana C. A phase II study of feasibility and toxicity of bevacizumab in combination with temozolomide in patients with recurrent glioblastoma. Clin. Transl. Oncol. 17:743–750; 2015. [DOI] [PubMed] [Google Scholar]
- 42. Field K. M.; Simes J.; Nowak A. K.; Cher L.; Wheeler H.; Hovey E. J.; Brown C. S.; Barnes E. H.; Sawkins K.; Livingstone A.; Freilich R.; Phal P. M.; Fitt G.; Rosenthal M. A. Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro. Oncol. 17:1504–1513; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Z.; Zhang G.; Zhu L.; Wang J.; Liu D.; Lian L.; Liu J.; Lai T.; Zhuang X. Retrospective analysis of bevacizumab in combination with fotemustine in Chinese patients with recurrent glioblastoma multiforme. Biomed. Res. Int. 2015:723612; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beije N.; Kraan J.; Taal W.; van der Holt B.; Oosterkamp H. M.; Walenkamp A. M.; Beerepoot L.; Hanse M.; van Linde M. E.; Otten A.; Vernhout R. M.; de Vos F. Y.; Gratama J. W.; Sleijfer S.; van den Bent M. J. Prognostic value and kinetics of circulating endothelial cells in patients with recurrent glioblastoma randomised to bevacizumab plus lomustine, bevacizumab single agent or lomustine single agent. A report from the Dutch Neuro-Oncology Group BELOB trial. Br. J. Cancer. 113:226–231; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chinot O. L.; Wick W.; Mason W.; Henriksson R.; Saran F.; Nishikawa R.; Carpentier A. F.; Hoang-Xuan K.; Kavan P.; Cernea D.; Brandes A. A.; Hilton M.; Abrey L.; Cloughesy T. Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 370:709–722; 2014. [DOI] [PubMed] [Google Scholar]
- 46. Taphoorn M. J.; Henriksson R.; Bottomley A.; Cloughesy T.; Wick W.; Mason W. P.; Saran F.; Nishikawa R.; Hilton M.; Theodore-Oklota C.; Ravelo A.; Chinot O. L. Health-related quality of life in a randomized phase III study of bevacizumab, temozolomide, and radiotherapy in newly diagnosed glioblastoma. J. Clin. Oncol. 33:2166–2175; 2015. [DOI] [PubMed] [Google Scholar]
- 47. Sandmann T.; Bourgon R.; Garcia J.; Li C.; cloughesy T.; Chinot O. L.; Wick W.; Nishikawa R.; Mason W.; Henriksson R.; Saran F.; Lai A.; Moore N.; Kharbanda S.; Peale F.; Hegde P.; Abrey L. E.; Phillips H. S.; Bais C. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: Retrospective analysis of the AVAglio trial. J. Clin. Oncol.; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ney D. E.; Carlson J. A.; Damek D. M.; Gaspar L. E.; Kavanagh B. D.; Kleinschmidt-DeMasters B. K.; Waziri A. E.; Lillehei K. O.; Reddy K.; Chen C. Phase II trial of hypofractionated intensity-modulated radiation therapy combined with temozolomide and bevacizumab for patients with newly diagnosed glioblastoma. J. Neurooncol. 122:135–143; 2015. [DOI] [PubMed] [Google Scholar]
- 49. Kickingereder P.; Wiestler B.; Graf M.; Heiland S.; Schlemmer H. P.; Wick W.; Wick A.; Bendszus M.; Radbruch A. Evaluation of dynamic contrast-enhanced MRI derived microvascular permeability in recurrent glioblastoma treated with bevacizumab. J. Neurooncol. 121:373–380; 2015. [DOI] [PubMed] [Google Scholar]
- 50. Niranjan A.; Kano H.; Iyer A.; Kondziolka D.; Flickinger J. C.; Lunsford L. D. Role of adjuvant or salvage radiosurgery in the management of unresected residual or progressive glioblastoma multiforme in the pre-bevacizumab era. J. Neurosurg. 122:757–765; 2015. [DOI] [PubMed] [Google Scholar]
- 51. Hawkins-Daarud A.; Rockne R.; Corwin D.; Anderson A. R.; Kinahan P.; Swanson K. R. In silico analysis suggests differential response to bevacizumab and radiation combination therapy in newly diagnosed glioblastoma. J. R. Soc. Interface 12:20150388; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]