Abstract
Osteosarcoma (OS) is the most common malignant primary bone tumor disease. HIF-1α was predicted to be the target gene of microRNA-20b (miR-20b). The present study was designed to illustrate the effect of miR-20b in regulating osteosarcoma via targeting HIF-1α. In this study, we found that the expression of HIF-1α was significantly increased, while miR-20b obviously decreased in OS patients and OS cell lines compared with healthy controls. Moreover, the luciferase report confirmed the targeting reaction between miR-20b and HIF-1α. Additionally, the overexpression of miR-20b suppressed the invasion and growth of both MG63 and U2OS cells, and inhibited the expressions of HIF-1α and VEGF pathway proteins, while the inhibition of miR-20b led to the reverse results. Furthermore, the overexpression of HIF-1α affected the suppression effect of miR-20b in MG63 cells, indicating that miR-20b suppresses the tumor cell process via inhibiting the expression of HIF-1α. Taken together, our results suggest that the upregulation of miR-20b affects the expression of HIF-1α, downregulates the VEGF pathway proteins, and suppresses cell invasion and proliferation rate. These results provide a potential therapeutic strategy for osteosarcoma.
Key words: Osteosarcoma (OS), Hypoxia-inducible transcription factor-1α (HIF-1α), microRNA-20b (miR-20b), Vascular endothelial growth factor (VEGF) pathway
INTRODUCTION
Osteosarcoma (OS) is the most frequent primary malignant bone tumor, which is commonly found in children and young adolescents with a male predominance (1). OS is highly aggressive and primarily metastasizes to lung (2). Considerable progress has been made by combination chemotherapy and aggressive surgical resection in the treatment of OS. However, the survival rate of OS patients has not increased in recent decades (3). Thus, it is important to develop novel therapeutic strategies against OS that are more effective by understanding the molecular mechanisms underlying its development and progression.
Previous studies have indicated that hypoxia-inducible transcription factor-1 (HIF-1) mediates chemotherapy and radiation resistance (4). Thus, the HIF-1 pathway has become an important area for OS therapy research. HIF-1 is an important element in the cellular response to hypoxia; it is a heterodimer that consists of α and β subunits (5). Under normoxic conditions, HIF-1α is degraded by the proteasome, preventing it from forming a complex with the HIF-1β subunit (6,7). However, HIF-1α is stabilized and activated under physiologic hypoxia (8). It has been reported that HIF-1α plays a critical role in normal skeletal development and bone repair (9). The expression and stability of HIF-1α was enhanced in OS cells compared to normal, nontransformed osteoblast (OB) bone cells (10). These studies suggested that HIF-1α was a promising molecular target for the prevention and treatment of OS.
The activation of HIF-1α regulates various hypoxia response genes, which include vascular endothelial growth factor (VEGF). VEGF specifically binds to two transmembrane VEGF receptor tyrosine kinases in endothelial cells to initiate intracellular signal transduction pathways that mediate angiogenesis and vascular permeability. VEGF is one of the most prominent angiogenic factors in promoting tumor progression (11,12). It has been reported that the VEGF promoter contains a potential HIF-1α binding site (13,14). Increased VEGF expression is associated with tumor growth and metastasis in osteosarcoma cell lines and other primary tumor samples (15). A previous study demonstrated that hypoxia is associated with increased HIF-1α protein expression and subsequent upregulation of VEGF expression, which affected the angiogenesis in osteosarcoma (16). These reports suggested that HIF-1α may affect the expression of VEGF and regulate the proliferation and metastasis of OS cells.
MicroRNAs (miRNAs) are a group of noncoding regulatory RNAs, 20–25 nucleotides in length, which are known to regulate different cellular processes such as proliferation, differentiation, apoptosis, cell metabolism, and angiogenesis (17,18). miRNA-20b (miR-20b) belongs to the miRNA 106a-363 cluster, which, together with miR 17-92 and miR 106b-25 clusters, form a large family of highly similar miRNAs called the miR-17 family (19). HIF-1α was reported as a target of the miR-17-92 microRNA cluster in lung cancer cells (20). It has been reported that miR-20b reduced VEGF protein expression, and the VEGF expression in breast cancer cells is mediated by HIF-1 in a miR-20b-dependent manner (21).
In this study, we identified the function for miR-20b in the context of OS. We found that the expression of miR-20b was significantly decreased in OS cell lines and OS patients compared with healthy controls, leading to overexpression of the target gene HIF-1α, and regulating the VEGF pathway. Taken together, our results suggest that miR-20b downregulates HIF-1α and may provide novel insight into the metastasis of OS.
MATERIALS AND METHODS
Patients
Forty patients (14 males and 26 females) with osteosarcoma and 35 healthy volunteers (15 males and 20 females) were consecutively included in this study. Fasting venous peripheral blood samples were drawn from each patient and healthy controls and were preserved with heparin. Patients with OS were recruited from the Department of Orthopedics, First Affiliated Hospital of Zhengzhou University. Healthy volunteers were recruited from students at Zhengzhou University. The study was approved by the Ethics Committee of our institution. Informed consent was signed by the participants.
Cell Culture
Human osteoblast (OB) cells lines were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and were grown in modified Eagle’s medium (MEM; Invitrogen, Carlsbad, CA, USA) supplemented with 20% fetal bovine serum (FBS; Invitrogen) and 1% antibiotic–antimycotic (Invitrogen), while the osteosarcoma cell lines MG63 and U2OS (ATCC) were grown in MEM supplemented with 10% FBS and 1% antibiotic–antimycotic. All cells were incubated at 37°C in a humidified 21% O2, 5% CO2 atmosphere.
Quantitative RT-PCR (qRT-PCR)
Total cellular RNA was isolated using the miRNeasy Mini Kit (Qiagen Inc., Valencia, CA, USA) and quantified through 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Ten nanograms of total RNA were reverse transcribed using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The obtained cDNA was amplified using the TaqMan miR-20b MicroRNA assay (Applied Biosystems). A 67-bp cDNA product was amplified by PCR with the primers: miR-20b, 5′-CCCAAAGTGCTCATAGTG-3′ (sense); common antisense primer, 5′-GACTGTTCCTCTCTTCCTC-3′ (22). For real-time PCR, the above primers and the TaqMan probe [6-FAM]TTGCGACTACACACACACACACA [BHQ1a-6FAM] were mixed with TaqManH Universal PCR Master Mix (Applied Biosystems). The reaction mixtures were incubated at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min, according to the Stratagene qRT-PCR instrument. miR-20b expression was normalized to U6 RNA. Gene mRNA expression was normalized to β-actin. Relative gene expression was quantified by the ΔCt method.
Western Blot Analyses
Cell lysates (30 mg of total protein) and prestained molecular weight markers were separated by SDS-PAGE followed by transfer onto nitrocellulose membranes. The membranes were blocked in TBST (Tris-buffered saline with 0.5% of Triton X-100) containing 5% nonfat milk, and probed with the primary antibodies against HIF-1α, VEGF, Cdc42, P38, HSP27, and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation with the secondary antibody, membranes were extensively washed. A fluorescent Western blotting detection system was used, and the band density of each gene was normalized to the corresponding density of β-actin.
Transfection Assay
The transfection assay was performed as described in a previous study (23). miRNA mimics and inhibitors specific for miR-20b (Invitrogen) were used to increase and silence the expression of miR-20b, respectively, in OS cell lines. The inhibitors or mimics (30 nM) were transfected into indicated cells using Lipofectamine™ 2000 (Invitrogen), together with the corresponding control molecules (30 nM), 48 h prior to subsequent experiments. For HIF-1α overexpression experiments, the overexpression of HIF-1α was achieved by PCR amplification using HIF-1α cDNA as a template, and the HIF-1α-expressing vector was constructed by inserting HIF-1α cDNA into the pcDNA3.1 vector. Then 2 mg of recombinant plasmid and 200 pmol of miR-20b mimics, miR-20b mimic control, miR-20b inhibitor, and inhibitor control (Ambion, Austin, TX, USA) were transfected into 3 × 106 MG63 cells for 48 h by electroporation using a Nucleofector instrument. After transfection, the cells were allowed to recover by incubating for 4 h at 37°C. The experiment was replicated three times for data calculations.
Cell Proliferation Assay
Cell viability was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, cells were seeded in 96-well plates infected with AAV vectors and subjected to OGD treatments for the indicated times. Thereafter, the old media were discarded, and fresh medium containing MTT (5 mg/ml MTT in PBS; Sangon, Shanghai, China) was added and incubated for additional 4 h. Dimethyl sulfoxide was used to dissolve the formazan, and the absorbance at 490 nm was measured using an ELISA reader once every 24 h (BioTek, Winooski, VT, USA).
Invasion Assay
The Transwell invasion chamber was washed with serum-free medium, and 20 µl Matrigel (1 mg/ml) was added to evenly cover the surface of the polycarbonate membrane (8 µm pore size) to create the Matrigel membrane. The chamber was divided into upper and lower chambers. For invasion assays, MG63 and U2OS cells (4 × 105) were serum starved overnight and seeded in starvation medium on the top chamber. The bottom chamber contained 10% FBS in RPMI-1640 medium, which acted as a chemoattractant. After 48 h of incubation, cells from the top chamber were removed by cotton swab, and invading cells were fixed with 4% formaldehyde M for 15 min and then stained with a crystal violet solution for 10 min. Images of the invading cells were photographed using an inverted microscope, and total cell numbers were counted and quantified by ImageJ software. The results are presented as the mean ± SD, and the experiment was repeated three times for each group.
Dual-Luciferase Reporter Assay
The target gene was predicted by TargetScan (http://www.targetscan.org/). A 450-bp fragment of the 3′-untranslated region (3′-UTR) of HIF-1α mRNA containing the target sequence (GCACTTT) of miR-20b was amplified by RT-PCR (HIF-1α, sense 5′-CTCTGAGCTCTATCTGGAAGGTATGTG-3′, antisense 5′-CCTCAAGCTTCAGTTAGTGTTAGACCC-3′). The fragment was designated HIF-1α 3′-UTR and inserted into the pMIR-REPORT™ luciferase reporter vector (SacI and HindIII restriction enzyme sites; Ambion). Another expressing vector was also constructed by the insertion of a mutated HIF-1α 3′-UTR in which the target sequence of miR-20b was mutated into GCAATTT using the QuikChangeH Site-Directed Mutagenesis Kit (Stratagene, Santa Clara, CA, USA) (22). The recombinant reporter vectors with normal and or mutated HIF-1α 3′-UTR were cotransfected with miR-20b mimic, miR-20b mimic control, miR-20b inhibitor, or miR-20b inhibitor control into MG63 using TransMessenger™ Transfection Reagent (Qiagen, Germany). The luciferase assay was performed according to the manufacturer’s instructions. The relative luciferase activities were normalized to that of the control cells.
Statistical Analysis
The quantitative data were expressed as mean ± SD. Statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Bonferroni test. Differences with a value of p < 0.05 were regarded as statistically significant.
RESULTS
Inverse Level of miR-20b and HIF-1α in OB and OS Cells
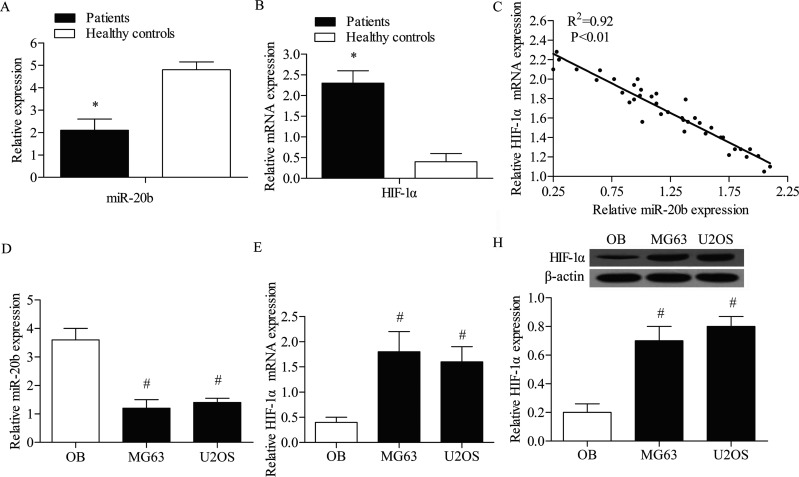
The expression levels of miR-20b and HIF-1α were detected in OS patients by qPCR; the results indicated that the expression level of miR-20b was significantly lower in OS patients compared with healthy controls (p < 0.05) (Fig. 1A). The HIF-1α mRNA expression level was increased in OS patients compared with healthy controls (p < 0.05) (Fig. 1B), and there was a strong negative correlation between the expression level of miR-20b and the mRNA expression level of HIF-1α in OS patients (Fig. 1C). The expression level of miR-20b and HIF-1α was also detected in OB, MG63, and U2OS cell lines by qPCR. The results indicated that the level of miR-20b was significantly lower in OS cells (MG63 and U2OS) compared with OB cells (p < 0.05) (Fig. 1D). In addition, the mRNA and protein expression levels of HIF-1α were examined by qRT-PCR and Western blot analysis, respectively (Fig. 1E, F). The results showed that both the mRNA and protein levels of HIF-1α were significantly elevated in OS cells compared with OB cells.
Figure 1.
Inverse level of miR-20b and HIF-1α in OS patients and healthy controls. (A) The expression levels of miR-20b in OS patients and healthy controls were measured by quantitative real-time PCR (qRT-PCR). (B) The mRNA expression of HIF-1α in OS patients and healthy controls was assessed by qRT-PCR assays. (C) The correlation between miR-20b expression levels and HIF-1α mRNA expression levels in OS patients. (D) The expression levels of miR-20b in OB cell lines and OS cell lines were measured by quantitative real-time PCR (qRT-PCR). (E) The mRNA expression of HIF-1α in OB cell line and OS cell lines was assessed by qRT-PCR assay. (F) Western blotting assay was used to detect the expression profile of HIF-1α in OB and OS cell lines. Relative protein expression was quantified using Image-Pro Plus 6.0 software and normalized to β-actin. Data are represented as the mean ± SD of three experiments. *p < 0.05 versus healthy controls group, #p < 0.05 versus OB group.
miR-20b Suppresses the Invasion and Proliferation of OS Cells
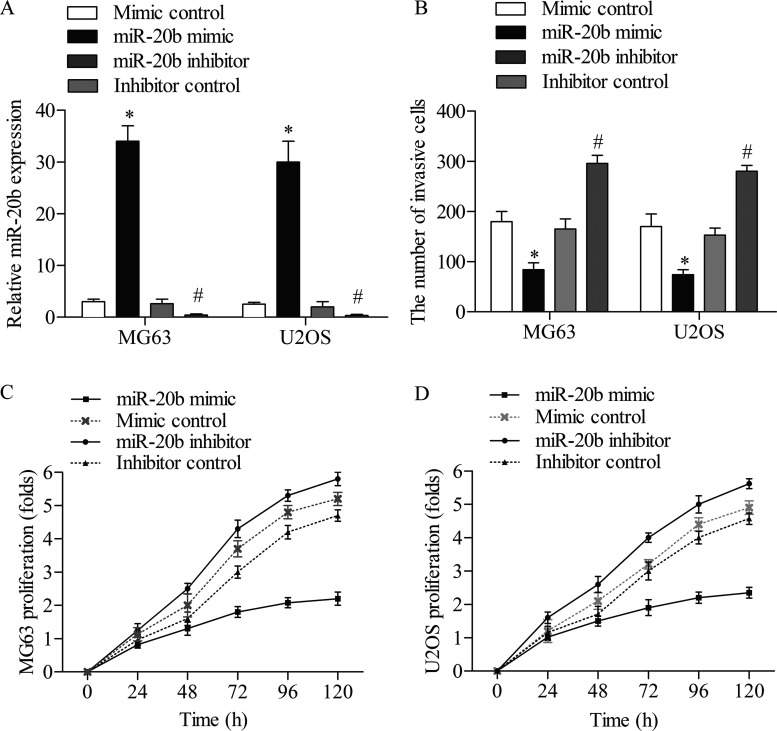
Given that the level of miR-20b was decreased in OS cell lines (MG63 and U2OS), it was of interest to investigate its role in the biology of OS cells. miR-20b mimic was taken to amplify the expression of miR-20b, whereas a synthetic inhibitor specific for miR-20b was employed to suppress the expression of endogenous miR-20b in OS cell lines. The efficiency of this miR-20b mimic or inhibitor was confirmed by qPCR assay (Fig. 2A). Treatment of the miR-20b mimic significantly decreased the invasive cells of MG63 and U2OS compared with the mimic control group (p < 0.05), while treatment of the miR-20b inhibitor strongly increased the cell invasion ability of MG63 and U2OS compared with the inhibitor control group (p < 0.05) (Fig. 2B). In addition, overexpression of miR-20b decreased the proliferation rate of MG63 cells compared with the mimic control group, and the inhibition of miR-20b strongly promoted the growth of MG63 cells compared with the inhibitor control group (Fig. 2C). Similar MTT results were obtained in U2OS cells; the cell growth was restrained by the overexpression of miR-20b. Moreover, the U2OS cells were multiply increased after the treatment of miR-20b inhibitor (Fig. 2D). These findings suggested that miR-20b suppressed the invasion and proliferation of OS cells.
Figure 2.
Effect of miR-20b on the invasion and proliferation of MG63 and U2OS cells. MG63 and U2OS cells were transfected with miR-20b mimic, mimic control, miR-20b inhibitor, or inhibitor control, respectively. (A) The relative miR-20b levels in MG63 and U2OS cells were measured by qRT-PCR. (B) The number of invading MG63 and U2OS cells. The proliferation of MG63 (C) and U2OS (D) cells was determined at the indicated time points by MTT assays. All experiments were repeated three times with three replicates. *p < 0.05 compared to mimic control group; #p < 0.05 compared to inhibitor control group.
HIF-1α Is Targeted by miR-20b
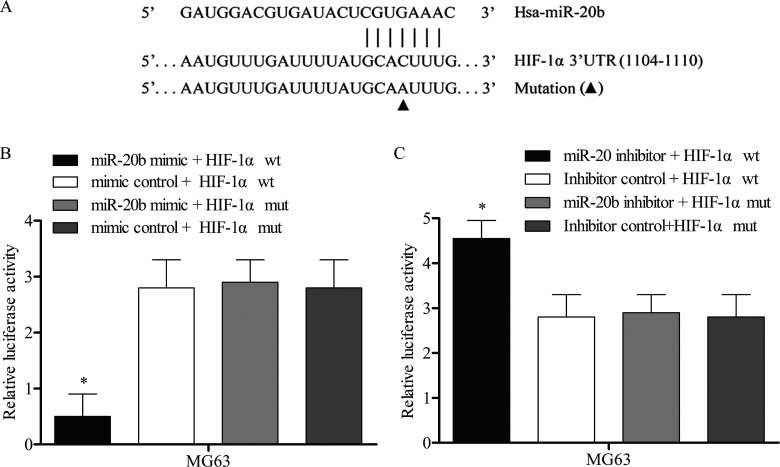
The bioinformatics software program TargetScan was used to predict the HIF-1α targeted miRNA. According to the results, the potential binding target sites of miR-20b were found in the 3′-UTR of HIF-1α gene (Fig. 3A). To experimentally confirm HIF-1α as an authentic target of miR-20b in MG63 cells, the plasmid pMIR-REPORT- HIF-1α wt or pMIR-REPORT- HIF-1α mut was transfected into MG63 cells together with miR-20b mimics or mimic control. After 48 h of transfection, the results showed that the luciferase activity in the HIF-1α-wt with miR-20b mimics group was significantly reduced compared with the other three groups (Fig. 3B). Consistently, the luciferase reporter vectors of HIF-1α-wt and HIF-1α-mut were cotransfected with miR-20b inhibitors or inhibitor controls into MG63 cells. The results showed that miR-20b inhibitor reversed the reduction in the expression level of luciferase with wild-type HIF-1α 3′-UTR in MG63 cells (Fig. 3C). The above data demonstrated that HIF-1α was a genuine target of miR-20b.
Figure 3.
The target relationship between miR-20b and HIF-1α. The target gene was predicted by TargetScan database and identified by luciferase activity report. (A) The wild-type and the mutant HIF-1α 3′-UTR contained the target sequence of miR-20b. (B) MG63 cells were cotransfected with miR-20b mimic or mimic control luciferase reporter vectors containing wild-type (wt) or mutant (mut) HIF-1α 3′-UTR. (C) A similar luciferase assay was performed in MG63 cell lines treated with miR-20b inhibitor or inhibitor control. Luciferase activity was represented as firefly luciferase normalized to renilla luciferase. Data are represented as the mean ± SD of three experiments. *p < 0.05 versus other three groups.
miR-20b Inhibits VEGF Pathway by Downregulating HIF-1α
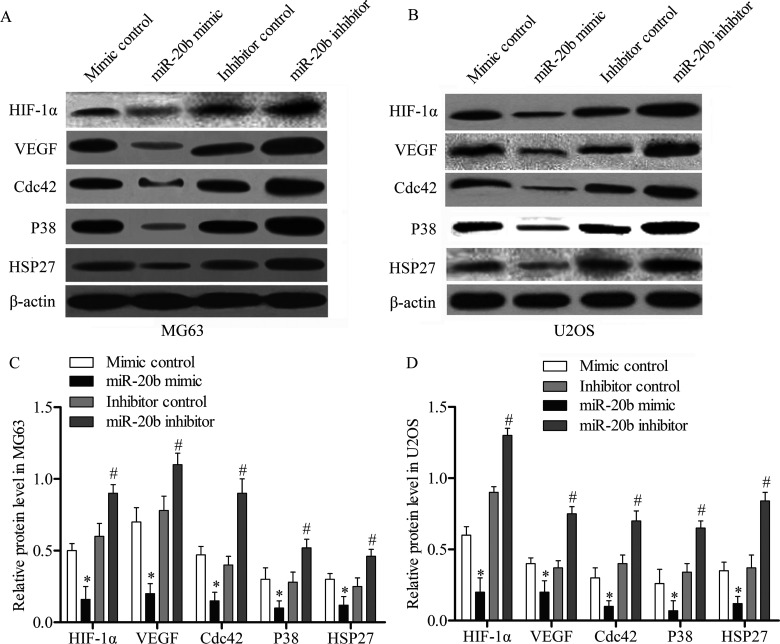
VEGF is highly correlated with metastatic progression and survival of osteosarcoma patients (24). HIF-1α has been reported to activate VEGF pathway genes (25). Thus, we detected the effect of miR-20b overexpression and suppression on expression of the HIF-1α and VEGF pathways. The expression levels of HIF-1α/VEGF pathway-related genes, including HIF-1α, VEGF, Cdc42, P38, and HSP27, were detected using Western blotting analysis. The results revealed that miR-20b mimic effectively decreased the expression of the five above proteins compared with cells transfected with mimic control, while the miR-20b inhibitor was able to increase the protein expression in MG63 cells compared with inhibitor control (p < 0.05) (Fig. 4A, C). The Western blotting assay was also performed in U2OS cells, and low expression levels were detected among the VEGF pathway-related proteins under miR-20b mimic-treated samples compared with the mimic control. Subsequently, the miR-20b inhibitor intensively promotes expression of the five proteins compared to the inhibitor control (p < 0.05) (Fig. 4B, D). The above data suggested that the activation of VEGF pathway was suppressed by miR-20b overexpression in OS cells.
Figure 4.
miR-20b regulates VEGF pathway via targeting HIF-1α. The MG63 and U2OS cells were transfected with the miR-20b mimic, mimic control, miR-20b inhibitor, or inhibitor control, separately. (A) The protein expression levels of HIF-1α and VEGF pathway genes in MG63 cells were measured by Western blotting. (B) A similar Western blotting assay was performed in U2OS cells. (C) Relative protein expression levels in MG63 cells were quantified using Image-Pro Plus 6.0 software and normalized to β-actin. (D) Relative protein expression in U2OS cells. Data are represented as the mean ± SD of three experiments. *p < 0.05 versus mimic control, #p < 0.05 versus inhibitor control.
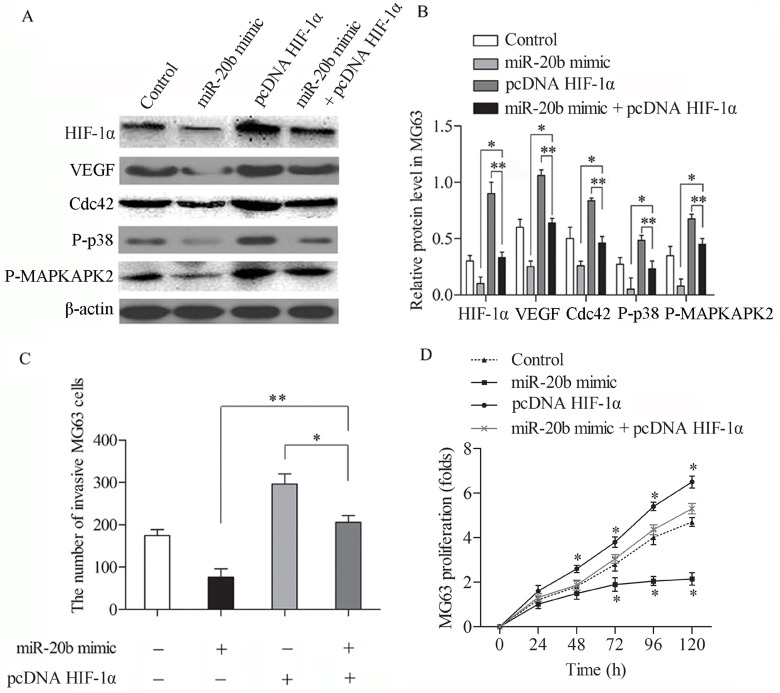
Overexpression of HIF-1α Restores the Inhibition Effect of miR-20b in MG63 Cells
To determine whether overexpression of HIF-1α counteracts the effect of miR-20b in OS cells, we cotransfected miR-20b mimic or mimic control with or without the HIF-1α overexpression vector into MG63 cells. Western blotting was taken to measure the expression level of HIF-1α, VEGF, Cdc42, P38, and HSP27 (Fig. 5A, B). In the miR-20b inhibitor plus overexp HIF-1α group, the protein expressions of HIF-1α, VEGF, Cdc42, P38, and HSP27 were increased compared with the miR-20b mimic group (p < 0.05) and were strongly decreased compared with the overexp HIF-1α group (p < 0.05) (Fig. 5B). The results illustrated that the overexpression of HIF-1α restored the miR-20b inhibition on the protein expression of VEGF pathway in MG63 cells. To further confirm the offset on miR-20b inhibition by HIF-1α overexpression, we examined the invading number of MG63 cells treated with miR-20b mimic or mimic control with or without HIF-1α overexpression vector. The overexpression of HIF-1α reversed the inhibition effect of miR-20b on cell invasion ability in MG63 cells (Fig. 5C). The MTT assay was employed to examine the proliferation rate of MG63 cells under similar treatments. The proliferation rate was strongly promoted when MG63 cells were cotransfected with overexpression of HIF-1α and the miR-20b mimic compared with the miR-20b mimic transfection group (Fig. 5D). These findings confirmed that the overexpression of HIF-1α restored the miR-20b inhibition effect in MG63 cells.
Figure 5.
Overexpression of HIF-1α offsets the suppression effect of miR-20b in MG63 cells. Cells were treated with miR-20b mimic or mimic control with or without HIF-1α overexpression vector. (A) HIF-1α, VEGF, Cdc42, P38, and HSP27 expressions were detected by Western blotting. (B) Relative protein expressions in MG63 cells were quantified using Image-Pro Plus 6.0 software and normalized to β-actin. (C) The number of invading MG63 cells. (D) MTT assay was employed to examine proliferation rates of MG63 cells under similar treatments. The data are shown as mean ± SD of three independent experiments. *p < 0.05, **p < 0.01 indicates significantly different.
DISCUSSION
OS is the most common malignant bone tumor. Accumulated evidences show that HIF-1α is a susceptibility gene for OS (26,27). Consistently, we found that the expression of HIF-1α was significantly increased in OS patients and OS cell lines compared with healthy control cells in the present study. To investigate the role of HIF-1α in OS, the noncoding region, the 3′-UTR, of HIF-1α was studied in this research. miRNAs are noncoding RNAs that can suppress the expression of protein-coding genes by binding to the target sequence at the 3′-UTR of the target gene (28). In this study, we predicted miR-20b to be the target miRNA for HIF-1α, which may be involved in the pathogenesis of OS.
The strikingly decreased expression level of miR-20b has been reported in many cancer cell lines, including liver cancer H22, breast cancer 4T1, prostate cancer RM1, and melanoma B16 (22,29). miR-20b has also been reported to regulate VEGF expression by targeting HIF-1α and STAT3 in the breast cancer cell line MCF-7 (21). The target connection between HIF-1α and miR-20b was predicted by the bioinformatics software program TargetScan. Thus, we chose miR-20b to be the target miRNA for HIF-1α. In our study, the results showed that miR-20b was decreased significantly in OS patients compared with healthy controls, which was consistent with a former study in breast cancer cells (21). Furthermore, we detected a strong negative correlation between the expression level of miR-20b and the protein expression level of HIF-1α in OS patients. To verify the targeting reaction between miR-20b and HIF-1α, the luciferase reporter vectors of wild-type and mutant HIF-1α were constructed. The results showed that the overexpression of miR-20b inhibited luciferase expression when cells were transfected with HIF-1α-wt luciferase reporter vector, but not in HIF-1α-mut groups. Moreover, the inhibition of miR-20b increased luciferase activity in HIF-1α-wt transfection group compared with HIF-1α-mut groups. These results demonstrated that HIF-1α is a target gene for miR-20b.
HIF-1α was previously shown to be overexpressed in the OS cell lines U2OS and MG63 (30). Also, it was overexpressed in metastatic OS tumors (31). El Naggar et al. confirmed that OS cells displayed enhanced expression and stability of HIF-1α compared to normal, nontransformed OB bone cells, which rendered OS cells more resistant to hypoxia, leading to the survival of OS cells (10). From the above aspect, targeting HIF-1α with certain small RNAs provides a novel strategy to prevent the abnormal growth of OS cells. It has been reported that the inhibition of HIF-1α transcripts using small hairpin RNAs in the SaOS-2 human OS cell line efficiently inhibits cell growth (32). Another report has suggested that the silencing of HIF-1α together with β-elemene could induce the apoptosis of OS cells (26). Thus, to investigate the role of miR-20b in OS cell growth and invasion via targeting HIF-1α, we detected the effect of miR-20b inhibition and miR-20b overexpression on the proliferation and invasion ability of MG63 and U2OS cells. The results showed that the miR-20b mimic strongly inhibited the expression of HIF-1α and the cell proliferation and invasion. However, under the inhibition of miR-20b, HIF-1α was significantly increased, which was similar to the physiological feature in OS, and the cell growth and invasion were strongly promoted in both MG63 and U2OS cells. These results confirmed that miR-20b acted as a negative control in the expression of HIF-1α and the cell proliferation and invasion in OS cells.
VEGF is a key regulatory factor related to angiopoiesis, carcinogenesis, and metastasis. It has been reported that mesenchymal stem cells (MSCs) promote chemokines and chemokine receptor 4 (CXCR4) to mediate OS growth and pulmonary metastasis (33). Predicted by the KEGG pathway database, the VEGF pathway is located downstream of HIF-1α. Through VEGF, the HIF/VEGF/VEGF receptor pathway was upregulated by approximately 40% in multiple myeloma (MM) cases, leading to increased angiogenesis (34). Our study revealed that the inhibition of VEGF and its pathway proteins (Cdc42, P38, HSP27, and β-actin) was detected with miR-20b mimic transfection compared with mimic control group. The results indicated that the overexpression of miR-20b inhibits the expressions of VEGF pathway proteins by decreasing HIF-1α expression, which may reduce the proliferation and invasion of OS cells. In order to further confirm the inhibition effect of miR-20b on the expression of VEGF pathway proteins and on the proliferation and invasion of OS cells, HIF-1α was overexpressed in MG63 and U2OS cells. The results showed that the overexpression of HIF-1α restored the miR-20b inhibition effect on the protein expression of VEGF pathway in MG63 cells, and the overexpression of HIF-1α exhibited the most invasive cell numbers and faster growth rate compared to the other three groups. These results suggest that miR-20b is a newly identified miRNA that suppresses the expression of HIF-1α in OS cells. These findings can contribute to our understanding of the regulatory network of VEGF pathway in human cancers. HIF-1α is shown as a new OS-associated tumor-promoting gene in this study. It is interesting to develop a therapeutic strategy targeting HIF-1α for OS treatment in further studies.
In conclusion, our results demonstrate that the overexpression of miR-20b affects the expression of HIF-1α, downregulates the VEGF pathway proteins, and suppresses invasion and proliferation rates of OS cells. This study provides an important clue to help to elucidate the pathogenesis of OS and implicates miR-20b as a potential therapeutic target for OS.
ACKNOWLEDGMENTS
The authors would like to thank the members of the Department of Orthopaedics, The First Affiliated Hospital of Zhengzhou University, for providing helpful discussions concerning the present study.
REFERENCES
- 1. Hung G. Y.; Horng J. L.; Yen H. J.; Yen C. C.; Chen W. M.; Chen P. C. H.; Wu H. T. H.; Chiou H.-J. Incidence patterns of primary bone cancer in Taiwan (2003–2010): A population-based study. Ann. Surg. Oncol. 21(8):2490–2498; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He H.; Ni J.; Huang J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol. Lett. 7(5):1352–1362; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chou A. J.; Merola P. R.; Wexler L. H.; Gorlick R. G.; Vyas Y. M.; Healey J. H.; LaQuaglia M. P.; Huvos A. G.; Meyers P. A. Treatment of osteosarcoma at first recurrence after contemporary therapy. Cancer 104(10):2214–2221; 2005. [DOI] [PubMed] [Google Scholar]
- 4. Höckel M.; Vaupel P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 93(4):266–276; 2001. [DOI] [PubMed] [Google Scholar]
- 5. Wang G. L.; Jiang B. H.; Rue E. A.; Semenza G. L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92(12):5510–5514; 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maxwell P. H.; Wiesener M. S.; Chang G. W.; Clifford S. C.; Vaux E. C.; Cockman M. E.; Wykoff C. C.; Pugh C. W.; Maher E. R.; Ratcliffe P. J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399(6733):271–275; 1999. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y. V.; Semenza G. L. RACK1 vs. HSP90: Competition for HIF-1α degradation vs. stabilization. Cell Cycle 6(6):656–659; 2007. [DOI] [PubMed] [Google Scholar]
- 8. Huang L. E.; Arany Z.; Livingston D. M.; Bunn H. F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its α subunit. J. Biol. Chem. 271(50):32253–32259; 1996. [DOI] [PubMed] [Google Scholar]
- 9. Wan C.; Gilbert S. R.; Wang Y.; Cao X.; Shen X.; Ramaswamy G.; Jacobsen K. A.; Alaql Z. S.; Eberhardt A. W.; Gerstenfeld L. C. Activation of the hypoxia-inducible factor-1α pathway accelerates bone regeneration. Proc. Natl. Acad. Sci. USA 105(2):686–691; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El Naggar A.; Clarkson P.; Zhang F.; Mathers J.; Tognon C.; Sorensen P. H. Expression and stability of hypoxia inducible factor 1α in osteosarcoma. Pediatr. Blood Cancer 59(7):1215–1222; 2012. [DOI] [PubMed] [Google Scholar]
- 11. Ferrara N.; Kerbel R. S. Angiogenesis as a therapeutic target. Nature 438(7070):967–974; 2005. [DOI] [PubMed] [Google Scholar]
- 12. Gilbert M. R.; Dignam J. J.; Armstrong T. S.; Wefel J. S.; Blumenthal D. T.; Vogelbaum M. A.; Colman H.; Chakravarti A.; Pugh S.; Won M. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 370(8):699–708; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forsythe J. A.; Jiang B. H.; Iyer N. V.; Agani F.; Leung S. W.; Koos R. D.; Semenza G. L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16(9):4604–4613; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Unwith S.; Zhao H.; Hennah L.; Ma D. The potential role of HIF on tumour progression and dissemination. Int. J. Cancer 136(11):2491–2503; 2015. [DOI] [PubMed] [Google Scholar]
- 15. Daft P. G.; Yang Y.; Napierala D.; Zayzafoon M. The growth and aggressive behavior of human osteosarcoma is regulated by a CaMKII-controlled autocrine VEGF signaling mechanism. PloS One 10(4):e0121568; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Q.; Yang S.; Wang R.; Ye S.; Xia T.; Ma D. Effect of silencing HIF-1alpha by RNA interference on expression of vascular endothelial growth factor in osteosarcoma cell line SaOS-2 under hypoxia. Chin. J. Cancer 24(5):531–535; 2005. [PubMed] [Google Scholar]
- 17. Paw I.; Carpenter R. C.; Watabe K.; Debinski W.; Lo H.-W. Mechanisms regulating glioma invasion. Cancer Lett. 362(1):1–7; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sinha M.; Ghatak S.; Roy S.; Sen C. K. microRNA–200b as a switch for inducible adult angiogenesis. Antioxid. Redox Sign. 22(14):1257–1272; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanzer A.; Stadler P. F. Molecular evolution of a microRNA cluster. J. Mol. Biol. 339(2):327–335; 2004. [DOI] [PubMed] [Google Scholar]
- 20. Taguchi A.; Yanagisawa K.; Tanaka M.; Cao K.; Matsuyama Y.; Goto H.; Takahashi T. Identification of hypoxia-inducible factor-1α as a novel target for miR-17-92 microRNA cluster. Cancer Res. 68(14):5540–5545; 2008. [DOI] [PubMed] [Google Scholar]
- 21. Cascio S.; D’Andrea A.; Ferla R.; Surmacz E.; Gulotta E.; Amodeo V.; Bazan V.; Gebbia N.; Russo A. miR-20b modulates VEGF expression by targeting HIF-1α and STAT3 in MCF-7 breast cancer cells. J. Cell. Physiol. 224(1):242–249; 2010. [DOI] [PubMed] [Google Scholar]
- 22. Lei Z.; Li B.; Yang Z.; Fang H.; Zhang G. M.; Feng Z. H.; Huang B. Regulation of HIF-1alpha and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS One 4(10):e7629; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu F. J.; Kaur P.; Karolina D. S.; Sepramaniam S.; Armugam A.; Wong P. T.; Jeyaseelan K. MiR-335 regulates Hif-1α to reduce cell death in both mouse cell line and rat ischemic models. PLoS One 10(6):e0128432; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin F.; Zheng S. E.; Shen Z.; Tang L. N.; Chen P.; Sun Y. J.; Zhao H.; Yao Y. Relationships between levels of CXCR4 and VEGF and blood-borne metastasis and survival in patients with osteosarcoma. Med. Oncol. 28(2):649–653; 2011. [DOI] [PubMed] [Google Scholar]
- 25. Yang J.; Yang D.; Sun Y.; Sun B.; Wang G.; Trent J. C.; Araujo D. M.; Chen K.; Zhang W. Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer 117(21):4925–4938; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liang D.; Yang M.; Guo B.; Yang L.; Cao J.; Zhang X. HIF-1α induced by β-elemene protects human osteosarcoma cells from undergoing apoptosis. J. Cancer Res. Clin. 138(11):1865–1877; 2012. [DOI] [PubMed] [Google Scholar]
- 27. Matsubara T.; DiResta G. R.; Kakunaga S.; Li D.; Healey J. H. Additive influence of extracellular pH, oxygen tension, and pressure on invasiveness and survival of human osteosarcoma cells. Front. Oncol. 3; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ambros V.; Lee R. C.; Lavanway A.; Williams P. T.; Jewell D. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr. Biol. 13(10):807–818; 2003. [DOI] [PubMed] [Google Scholar]
- 29. ARDI C. Vitamin D manipulates miR-181c, miR-20b and miR-15a in human umbilical vein endothelial cells exposed to a diabetic-like environment. Cardiovasc. Diabetol. 13:8; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng D. D.; Zhao H. G.; Yang Y. S.; Hu T.; Yang Q. C. GSK3β negatively regulates HIF1α mRNA stability via nucleolin in the MG63 osteosarcoma cell line. Biochem. Biophys. Res. Commun. 443(2):598–603; 2014. [DOI] [PubMed] [Google Scholar]
- 31. Mizobuchi H.; García-Castellano J. M.; Philip S.; Healey J. H.; Gorlick R. Hypoxia markers in human osteosarcoma: An exploratory study. Clin. Orthop. Relat. Res. 466(9):2052–2059; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu Q.; Yang S.; Ye S.; Wang R. Therapeutic effects of RNA interference targeting HIF-1 alpha gene on human osteosarcoma. Zhonghua Yi Xue Za Zhi 85(6):409–413; 2005. [PubMed] [Google Scholar]
- 33. Zhang P.; Dong L.; Yan K.; Long H.; Yang T. T.; Dong M. Q.; Zhou Y.; Fan Q. Y.; Ma B. A. CXCR4-mediated osteosarcoma growth and pulmonary metastasis is promoted by mesenchymal stem cells through VEGF. Oncol. Rep. 30(4):1753–1761; 2013. [DOI] [PubMed] [Google Scholar]
- 34. Giatromanolaki A.; Bai M.; Margaritis D.; Bourantas K. L.; Koukourakis M. I.; Sivridis E.; Gatter K. C. Hypoxia and activated VEGF/receptor pathway in multiple myeloma. Anticancer Res. 30(7):2831–2836; 2010. [PubMed] [Google Scholar]