Abstract
Hypoxia-induced epithelial-to-mesenchymal transition (EMT) could facilitate tumor progression. TIPE2, the tumor necrosis factor-α (TNF-α)-induced protein 8-like 2 (also known as TNFAIP8L2), is a member of the TNF-α-induced protein 8 (TNFAIP8, TIPE) family and has been involved in the development and progression of several tumors. However, the effects of TIPE2 on the EMT process in glioma cells and the underlying mechanisms of these effects have not been previously reported. In our study, we assessed the roles of TIPE2 in the EMT process in glioma cells in response to hypoxia. Our results indicated that TIPE2 expression was significantly decreased in human glioma cell lines. TIPE2 overexpression significantly inhibited hypoxia-induced migration and invasion, as well as suppressed the EMT process in glioma cells. Furthermore, TIPE2 overexpression prevented hypoxia-induced expression of β-catenin, cyclin D1, and c-myc in human glioma cells. In summary, these data suggest that TIPE2 overexpression inhibited hypoxia-induced Wnt/β-catenin pathway activation and EMT in glioma cells.
Key words: TIPE2, Hypoxia, Glioma, Epithelial-to-mesenchymal transition (EMT)
INTRODUCTION
Malignant glioma is the most devastating and aggressive tumor in the brain, and its incidence has increased rapidly across the world in recent decades (1). Despite considerable progress in treatment strategies, including surgery and a combination of chemotherapy and radiation, the prognosis for high-grade gliomas remains poor (2,3). This disappointing outcome suggests that new effective treatments are needed to improve outcomes for patients with malignant gliomas.
Epithelial-to-mesenchymal transition (EMT) is a critical developmental process implicated in cancer progression, invasion, and metastasis. During EMT progress, tumor cells lose epithelial E-cadherin expression and cellular adhesion and acquire increased potential for local invasion and the ability to evade to distant organs (4). Solid tumors often contain regions with insufficient oxygen delivery, a condition known as hypoxia (5). An important part of hypoxia response is the stimulation of EMT, resulting in a formation of mesenchymal-like cell phenotype and increase in cell motility (6).
TIPE2, the tumor necrosis factor-α (TNF-α)-induced protein 8-like 2 (also known as TNFAIP8L2), is a member of the TNF-α-induced protein 8 (TNFAIP8, TIPE) family (7). It plays an essential role in maintaining immune homeostasis (8). Recently, several studies showed that TIPE2 is involved in the development and progression of tumors. Cao et al. reported that overexpression of TIPE2 in hepatocellular carcinoma-derived cell lines inhibits tumor cell growth, migration, and invasion in vitro and suppresses growth and metastasis of hepatocellular carcinoma in vivo (9). Another study demonstrated that TIPE2 was lowly expressed in human lung cancer cell lines, and TIPE2 overexpression inhibited lung cancer cell proliferation, colony formation, and cell invasion, and reduced mesenchymal marker N-cadherin expression in vitro (10). However, to the best of our knowledge, the effects of TIPE2 on the EMT process in glioma cells and the underlying mechanisms of these effects have not been previously reported. In our study, we assessed the roles of TIPE2 in the EMT process in glioma cells in response to hypoxia.
MATERIALS AND METHODS
Cell Culture and Hypoxia Treatment
Human glioma cell lines (U87, U251, and U373MG) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco, Rockville, MD, USA) and 100 U ml streptomycin and penicillin (Gibco) in a humidified atmosphere containing 5% CO2 incubator at 37°C. Human microglia cells (ScienCell, Carlsbad, CA, USA) were used as control.
For hypoxic treatment, U87 and U251 cells were challenged by 99% N2 + 1% O2 for 0, 6, 12, or 24 h and then harvested for further analysis.
Construction of TIPE2 Overexpressing Cell Lines
Full-length human TIPE2 was generated from human peripheral blood mononuclear cell (PBMC) cDNA by polymerase chain reaction (PCR) and cloned into pcDNA3.1 vector. For in vitro transfection, U251 cells at a density of 1 × 105 cells/well were seeded in 24-well culture plates. After the cells were 70% confluent, the recombinant plasmid pcDNA3.1-TIPE2 and mock plasmid were transferred into U251 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The transfection efficiency was confirmed using Western blotting.
Quantitative RT-PCR
Total RNA was isolated from human glioma cells using the RNA Plus Kit (Invitrogen). Five micrograms of total RNA was converted to cDNA by SuperScript reverse transcriptase and then amplified by Platinum Taq Polymerase using the SuperScript One-Step RT-PCR kit (Invitrogen). The specific primers for TIPE2 were 5′-ACTGAGTAAGATGGCGGGTCG-3′ (sense) and 5′-TTCTGGCGAAAGCGGGTAG-3′ (antisense), and for β-actin were 5′-AAATCGTGCGTGACATCAAAGA-3′ (sense) and 5′-GGCCATCTCCTGCTCGAA-3′ (antisense). The PCR procedure was as follows: 94°C for 4 min; 94°C for 20 s, 55°C for 30 s, and 72°C for 20 s; 2 s for plate reading for 35 cycles; and melt curve from 65°C to 95°C. The relative quantification of gene expression level was compared with the internal referee β-actin using the 2−ΔΔCt method.
Western Blot
Cells were washed twice with phosphate-buffered saline (PBS) and lysed in lysis buffer. The concentration of protein was measured by the BCA kit (Takara Biotechnology, Dalian, China). Forty micrograms of protein per lane was separated on 10% SDS-PAGE gel and transferred to the nitrocellulose membranes (Pierce, Rockford, IL, USA). Nonspecific binding was blocked by incubating with 5% nonfat milk in TBST (Tris-buffered saline with Tween 20) buffer at room temperature for 1 h followed by incubation in the primary antibodies (anti-TIPE2, anti-E-cadherin, anti-N-cadherin, anti-vimenin, anti-β-catenin, anti-cyclin D1, anti-c-myc, and anti-GAPDH) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. After three washes, the membranes were incubated in peroxidase-conjugated secondary antibody immunoglobulin G (IgG) for 1 h. The immune-reactive protein bands on the membrane were visualized using an enhanced chemiluminescence detection system (Amersham, Little Chalfont, UK). The developed films were digitized and densitometrically analyzed using the Gel-Pro Analyzer version 4.0 software (Media Cybernetics, Silver Spring, MD, USA).
Cell Migration and Invasion Assay
Cell invasion assay was performed using a Transwell chamber (8.0-μm pore size; Costar, Cambridge, MA, USA). In brief, cells at a density of 1 × 105 cells/well were plated in the upper chamber, and 500 µl of medium containing chemotactic factor was placed in the lower chamber. After incubation at 37°C for 24 h, the tumor cells remaining inside the upper chamber were removed with cotton swabs. The cells on the lower surface of the membrane were fixed with paraformaldehyde, stained with crystal violet, and counted under a microscope.
The invasion assay was done using the same procedure, except that the membrane was coated with Matrigel to form a matrix barrier.
Statistical Analysis
Data are expressed as mean ± standard deviation (SD) of triplicate samples. Statistical evaluation was performed using a Student’s t-test or one-way analysis of variance (ANOVA) followed by Dunnett’s test. A value of p < 0.05 was considered statistically significant.
RESULTS
TIPE2 Is Lowly Expressed in Human Glioma Tissues and Cell Lines
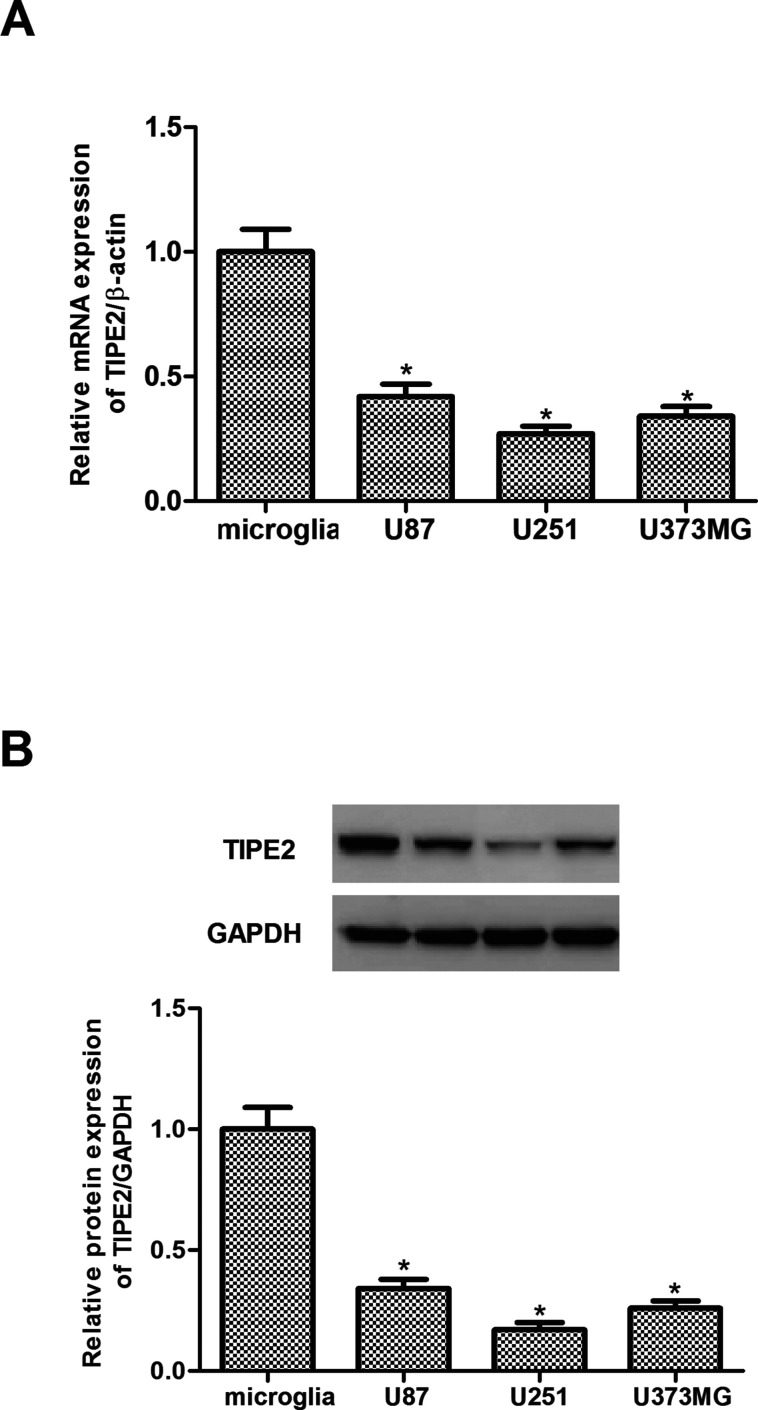
We first evaluated the expression of TIPE2 in three human glioma cells. As indicated in Figure 1A, the expression of TIPE2 mRNA in the three human glioma cell lines was significantly lower than those in normal human astrocytes. Consistent with the results of real-time quantitative PCR (RT-qPCR), Western blotting analysis showed that the expression of TIPE2 protein was significantly decreased in the three human glioma cell lines (Fig. 1B).
Figure 1.
TIPE2 was lowly expressed in human glioma cell lines. (A) Expression of TIPE2 mRNA in human glioma cell lines was analyzed by real-time quantitative polymerase chain reaction (RT-qPCR). (B) Expression of TIPE2 protein in human glioma cell lines was analyzed by Western blot. The values shown represent the mean ± standard deviation (SD) and n = 3 per group. *p < 0.05 compared with normal human microglia cells.
TIPE2 Is Involved in Hypoxia-Induced EMT of Glioma Cells
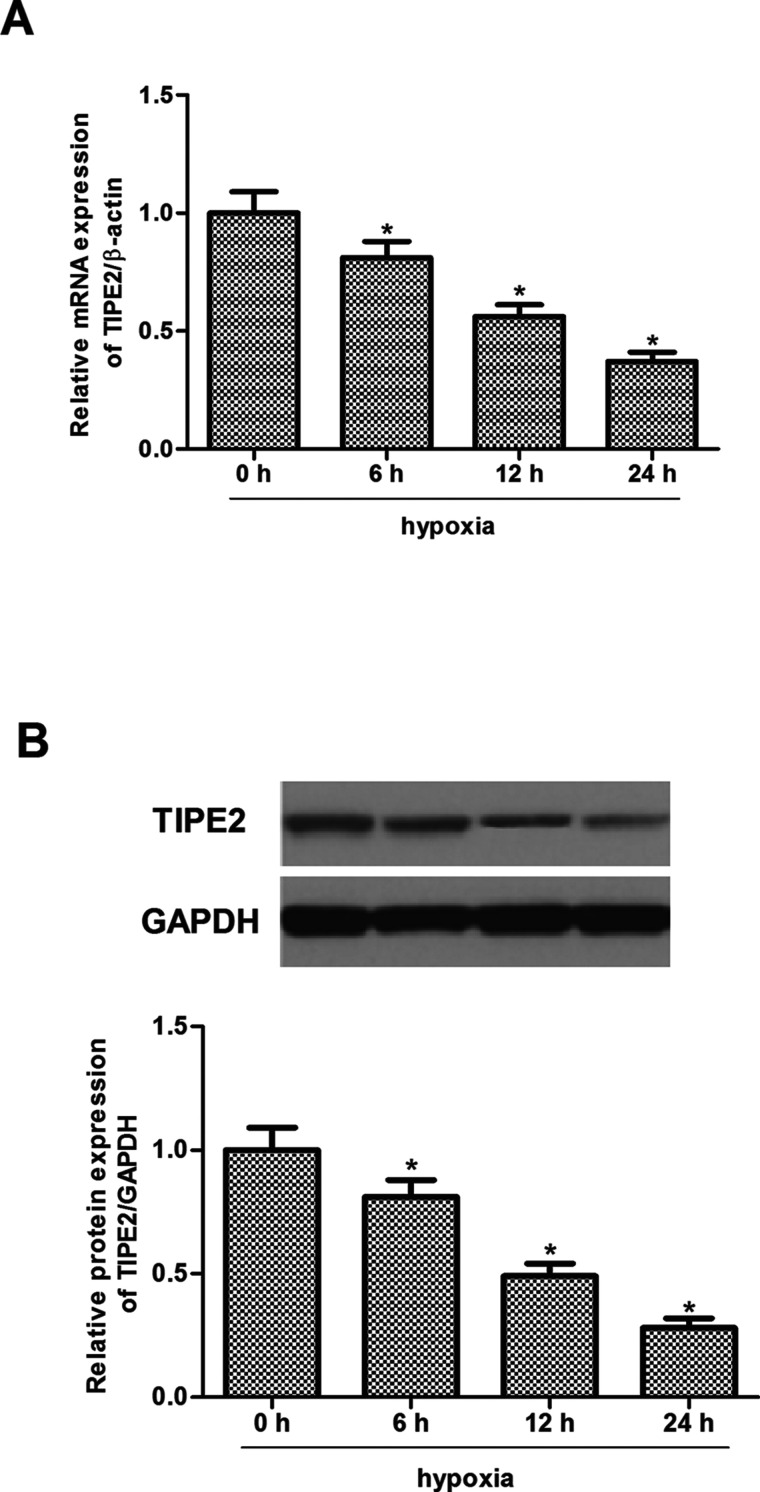
Thus, we detected the modulation of TIPE2 expression by hypoxia in human glioma cells. U251 cells were exposed to hypoxia for 0, 6, 12, or 24 h. After the treatment, TIPE2 mRNA and protein expression was analyzed by RT-qPCR and Western blot. The results indicated that the expression levels of TIPE2 at both mRNA (Fig. 2A) and protein (Fig. 2B) were significantly reduced by hypoxia in U251 cells.
Figure 2.
Hypoxia decreased TIPE2 expression in U251 cells. U251 cells were treated with 100 µM CoCl2 and then incubated for 0, 6, 12, or 24 h. (A) mRNA expression of TIPE2 in U251 cells. (B) Protein expression of TIPE2 in U251 cells. The values shown represent the mean ± SD and n = 3 per group. *p < 0.05 compared with the normoxia group.
TIPE2 Inhibits Hypoxia-Induced Migration and Invasion in U251 Cells
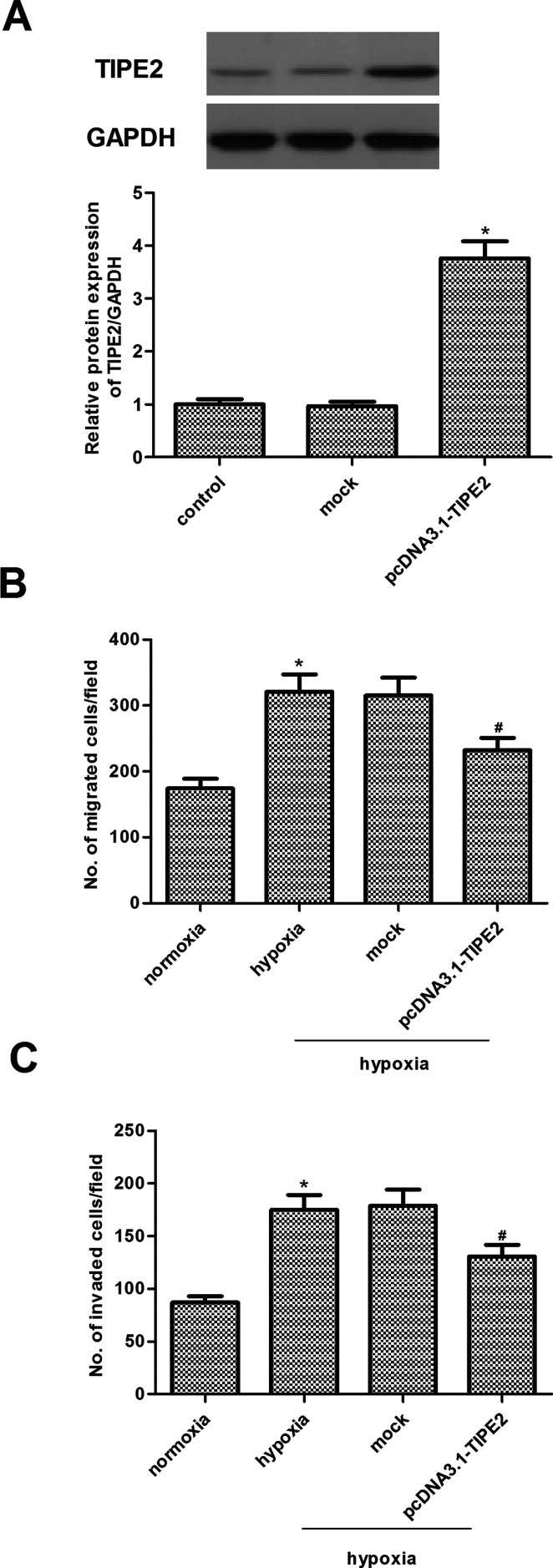
Given that TIPE2 expression was decreased by hypoxia, we asked whether ectopic expression of TIPE2 could reverse hypoxia-induced migration and invasion in U251 cells. To restore TIPE2 expression, we overexpressed TIPE2 in U251 cells by the transfection of recombinant adenovirus vector, and the results indicated that TIPE2 expression was significantly increased by pcDNA3.1-TIPE2 (Fig. 3A). We then performed Transwell migration and Matrigel invasion assays to investigate the effects of TIPE2 overexpression on the hypoxia-induced migration and invasion of U251 cells. As shown in Figure 3B and C, following hypoxia exposure the migration and invasion of U251 cells were significantly increased, as compared with the normoxia group. At the same time, TIPE2 overexpression significantly inhibited the transforming growth factor-β1 (TGF-β1)-induced migration and invasion of U251 cells.
Figure 3.
TIPE2 overexpression inhibits hypoxia-induced migration and invasion in U251 cells. (A) The transfection efficiency was confirmed by Western blot. (B) Transwell migration assay of cell migration in U251 cells under hypoxic condition. (C) Matrigel invasion assay of cell invasion in U251 cells under hypoxic condition. The values shown represent the mean ± SD and n = 3 per group. *p < 0.05 compared with the normoxia group. #p < 0.05 compared with the hypoxia group.
TIPE2 Suppresses Hypoxia-Induced EMT in U251 Cells
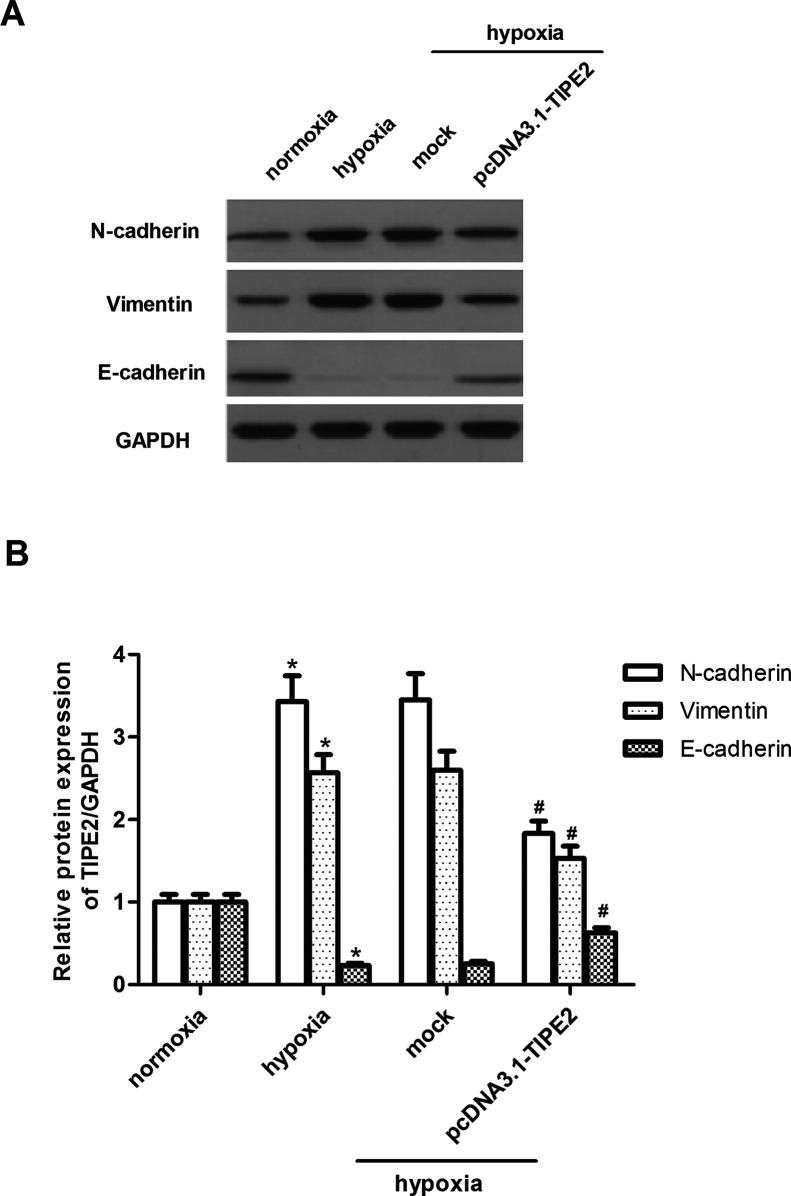
We next asked whether ectopic expression of TIPE2 could influence the EMT process in glioma cells induced by hypoxia. As indicated in Figure 4A, we observed that the mesenchymal markers N-cadherin and vimentin were upregulated in hypoxia-stimulated U251 cells, while the epithelial marker E-cadherin was significantly downregulated. However, TIPE2 transfection significantly reduced N-cadherin and vimentin protein levels and increased E-cadherin protein level compared to mock cells (Fig. 4B).
Figure 4.
TIPE2 overexpression suppresses hypoxia-induced EMT in U251 cells. U251 cells were treated with 100 µM CoCl2 and then incubated for 24 h. (A) The protein expression levels of N-cadherin, vimentin, and E-cadherin were determined by Western blot. (B) Quantification analysis was performed using Gel-Pro Analyzer version 4.0 software. The values shown represent the mean ± SD and n = 3 per group. *p < 0.05 compared with the normoxia group. #p < 0.05 compared with the hypoxia group.
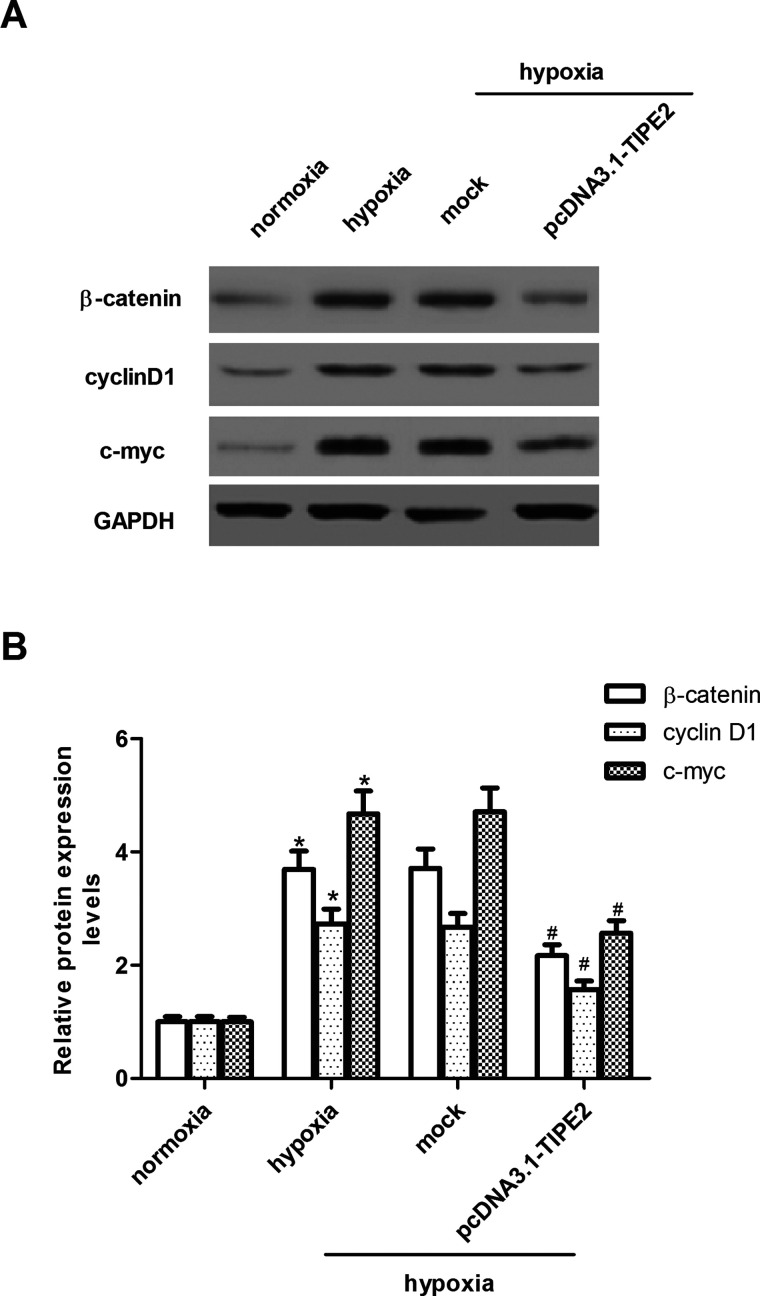
TIPE2 Suppresses Hypoxia-Induced Wnt/β-Catenin Pathway Activation in U251 Cells
The Wnt/β-catenin pathway plays a critical role in tumor initiation, progression, and metastasis. So we examined the effects of TIPE2 on the key components of the Wnt signaling pathway in U251 cells. As shown in the Western blot analysis in Figure 5A, hypoxia significantly increased protein levels of β-catenin, cyclin D1, and c-myc in U251 cells, as compared to the normoxia group. However, TIPE2 overexpression suppressed the expression of β-catenin, cyclin D1, and c-myc under the same hypoxic condition (Fig. 5B).
Figure 5.
TIPE2 overexpression suppresses hypoxia-induced Wnt pathway activation in U251 cells. U251 cells were treated with 100 µM CoCl2 and then incubated for 24 h. (A) The protein expression levels of β-catenin, cyclin D1, and c-myc were determined by Western blot. (B) Quantification analysis was performed using Gel-Pro Analyzer version 4.0 software. The values shown represent the mean ± SD and n = 3 per group. *p < 0.05 compared with the normoxia group. #p < 0.05 compared with the hypoxia group.
DISCUSSION
In this study, we found that TIPE2 expression was lowly expressed in human glioma cells. Hypoxia treatment obviously decreased the expression of TIPE2 in U251 cells. In addition, TIPE2 overexpression inhibited hypoxia-induced EMT process, as well as suppressed the migration and invasion in U251 cells. Furthermore, TIPE2 overexpression inhibits the expression of β-catenin, cyclin D1, and c-myc under hypoxic conditions.
Emerging evidence is increasingly showing that aberrant expression of TIPE2 may contribute to the development and progression of various cancers (11–13). One study demonstrated that TIPE2 expression is completely lost or significantly downregulated in human primary HCC but is high in all the paired adjacent nontumor tissues (14). TIPE2 was also expressed in GES-1 gastric mucous epithelial cells but lost in all three types of gastric cancer cells (15). Consistent with the previous reports, in this study we observed that TIPE2 expression was lowly expressed in human glioma cells. Hypoxia treatment obviously decreased the expression of TIPE2 in U251 cells. These results suggest that TIPE2 may function as a tumor suppressor involved in the development and progression of glioma.
Reduction or a loss of E-cadherin expression has a crucial role in the progression of tumors to invasive cancer and is also one of the well-established hallmarks of EMT (16). Previous studies reported that increased motility and invasion are positively correlated with EMT (17,18). In addition, hypoxia is one of the most important environmental factors that induce the EMT process in glioma (19,20). Most recently, one study showed that TIPE2 was able to suppress TNF-α-induced hepatocellular carcinoma metastasis (11). In this study, we found that TIPE2 overexpression inhibited hypoxia-induced EMT process, as well as suppressed the migration and invasion in U251 cells. These data suggest that TIPE2 negatively regulates the EMT process, consequently affecting cell migration and invasion in response to hypoxia.
Several studies suggest that the Wnt/β-catenin signaling pathway plays an important role in EMT (21). Activation of the Wnt pathway increases the expression of EMT regulator Snail and vimentin in glioma cells (22). Once in the nucleus, β-catenin regulates expression of the genes involved in the activation of the Wnt/β-catenin signaling pathway, including cyclin D1 and c-myc. β-Catenin was found to regulate expression of the genes involved in the activation of the Wnt/β-catenin signaling pathway, including cyclin D1 and c-myc, which are involved in cell survival, proliferation, and metastasis. Previous studies found that hypoxia plays an important role in activating the Wnt pathway in the development of tumors (23,24). Zhao et al. reported that knockdown of β-catenin prevented the EMT phenotype and metastasis in LNCaP cells induced by HIF-1α (25). Moreover, a previous study demonstrated that TIPE2 reduced the total levels of p-Akt, pGSK3β, and β-catenin as well as the nuclear level of β-catenin in gastric cancer cells, and the antimetastatic effect of TIPE2 was related with inhibition of Akt signaling and enhancement of GSK3β activity followed by the degradation and decreased translocation to the nucleus of β-catenin in gastric cancer (15). In this study, we showed that hypoxia significantly increased the expression of β-catenin, cyclin D1, and c-myc; however, TIPE2 overexpression inhibits the expression of β-catenin, cyclin D1, and c-myc under hypoxic condition in U251 cells.
In summary, our study is the first to show that TIPE2 inhibits hypoxia-induced Wnt/β-catenin pathway activation and EMT in glioma cells. Thus, these findings reveal that TIPE2 might potentially become a novel strategy for targeting glioma.
ACKNOWLEDGMENTS
This research was funded by the key scientific and technological project of Department of Science and Technology of Henan Province (Grant No. 162102310162).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Schwartzbaum J. A.; Fisher J. L.; Aldape K. D.; Wrensch M. Epidemiology and molecular pathology of glioma. Nat. Clin. Pract. Neurol. 2:494–503; 2006. [DOI] [PubMed] [Google Scholar]
- 2. Stupp R.; Mason W. P.; Van Den Bent M. J.; Weller M.; Fisher B.; Taphoorn M. J.; Belanger K.; Brandes A. A.; Marosi C., Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352:987–996; 2005. [DOI] [PubMed] [Google Scholar]
- 3. Minniti G.; De Sanctis V.; Muni R.; Filippone F.; Bozzao A.; Valeriani M.; Osti M.; De Paula U.; Lanzetta G.; Tombolini V. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J. Neurooncol. 88:97–103; 2008. [DOI] [PubMed] [Google Scholar]
- 4. Radisky D. C. Epithelial-mesenchymal transition. J. Cell Sci. 118:4325–4326; 2005. [DOI] [PubMed] [Google Scholar]
- 5. Jiang J.; Tang Y.-L.; Liang X.-H. EMT: A new vision of hypoxia promoting cancer progression. Cancer Biol. Ther. 11:714–723; 2011. [DOI] [PubMed] [Google Scholar]
- 6. Lu X.; Kang Y. Hypoxia and hypoxia-inducible factors: Master regulators of metastasis. Clin. Cancer Res. 16:5928–5935; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang G.; Hao C.; Lou Y.; Xi W.; Wang X.; Wang Y.; Qu Z.; Guo C.; Chen Y.; Zhang Y. Tissue-specific expression of tipe2 provides insights into its function. Mol. Immunol. 47:2435–2442; 2010. [DOI] [PubMed] [Google Scholar]
- 8. Sun H.; Gong S.; Carmody R. J.; Hilliard A.; Li L.; Sun J.; Kong L.; Xu L.; Hilliard B.; Hu S. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell 133:415–426; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao X.; Zhang L.; Shi Y.; Sun Y.; Dai S.; Guo C.; Zhu F.; Wang J.; Wang X.; Chen Y. H. Human tumor necrosis factor (TNF)-alpha-induced protein 8-like 2 suppresses hepatocellular carcinoma metastasis through inhibiting rac1. Mol. Cancer 12:149–158; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y.; Li X.; Liu G.; Sun R.; Wang L.; Wang J.; Wang H. Downregulated TIPE2 is associated with poor prognosis and promotes cell proliferation in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 457:43–49; 2015. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y. H.; Yan H. Q.; Wang F.; Wang Y. Y.; Jiang Y. N.; Wang Y. N.; Gao F. G. TIPE2 inhibits TNF-α-induced hepatocellular carcinoma cell metastasis via Erk1/2 downregulation and NF-κB activation. Int. J. Oncol. 46:254–264; 2015. [DOI] [PubMed] [Google Scholar]
- 12. Zhao Q.; Zhao M.; Dong T.; Zhou C.; Peng Y.; Zhou X.; Fan B.; Ma W.; Han M.; Liu S. Tumor necrosis factor-α-induced protein-8 like-2 (tipe2) upregulates p27 to decrease gastic cancer cell proliferation. J. Cell Biochem. 116:1121–1129; 2015. [DOI] [PubMed] [Google Scholar]
- 13. Li X. TIPE2 regulates tumor-associated macrophages in skin squamous cell carcinoma. Tumor Biol. 37:5585–5590; 2016. [DOI] [PubMed] [Google Scholar]
- 14. Gus-Brautbar Y.; Johnson D.; Zhang L.; Sun H.; Wang P.; Zhang S.; Zhang L.; Chen Y. H. The anti-inflammatory TIPE2 is an inhibitor of the oncogenic Ras. Mol. Cell 45:610–618; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu J.; Zhang H.; Xu C.; Xu H.; Zhou X.; Xie Y.; Tao M. TIPE2 functions as a metastasis suppressor via negatively regulating β-catenin through activating GSK3β in gastric cancer. Int. J. Oncol. 48:199–206; 2016. [DOI] [PubMed] [Google Scholar]
- 16. Yilmaz M.; Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metast. Rev. 28:15–33; 2009. [DOI] [PubMed] [Google Scholar]
- 17. Kudo-Saito C.; Shirako H.; Takeuchi T.; Kawakami Y. Cancer metastasis is accelerated through immunosuppression during snail-induced EMT of cancer cells. Cancer Cell 15:195–206; 2009. [DOI] [PubMed] [Google Scholar]
- 18. Kang Y.; Massagué J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell 118:277–279; 2004. [DOI] [PubMed] [Google Scholar]
- 19. Gupta R.; Chetty C.; Bhoopathi P.; Lakka S.; Mohanam S.; Rao J. S.; Dinh D. H. Downregulation of uPA/uPAR inhibits intermittent hypoxia-induced epithelial-mesenchymal transition (EMT) in DAOY and D283 medulloblastoma cells. Int. J. Oncol. 38:733–744; 2011. [DOI] [PubMed] [Google Scholar]
- 20. Zhang X.; Song Q.; Wei C.; Qu J. LRIG1 inhibits hypoxia-induced vasculogenic mimicry formation via suppression of the EGFR/PI3K/AKT pathway and epithelial-to-mesenchymal transition in human glioma SHG-44 cells. Cell Stress Chaperon 20:631–641; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang Y. G.; Luo Y.; He D. l.; Li X.; Zhang L. l.; Peng T.; Li M.C.; Lin Y. H. Role of Wnt/β-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1α. Int. J. Urol. 14:1034–1039; 2007. [DOI] [PubMed] [Google Scholar]
- 22. Kahlert U. D.; Maciaczyk D.; Doostkam S.; Orr B. A.; Simons B.; Bogiel T.; Reithmeier T.; Prinz M.; Schubert J.; Niedermann G. Activation of canonical WNT/β-catenin signaling enhances in vitro motility of glioblastoma cells by activation of ZEB1 and other activators of epithelial-to-mesenchymal transition. Cancer Lett. 325:42–53; 2012. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Q.; Bai X.; Chen W.; Ma T.; Hu Q.; Liang C.; Xie S.; Chen C.; Hu L.; Xu S. Wnt/β-catenin signaling enhances hypoxia-induced epithelial–mesenchymal transition in hepatocellular carcinoma via crosstalk with HIF-1α signaling. Carcinogenesis 34:962–973; 2013. [DOI] [PubMed] [Google Scholar]
- 24. Liu L.; Zhu X.-D.; Wang W.-Q.; Shen Y.; Qin Y.; Ren Z.-G.; Sun H.-C.; Tang Z.-Y. Activation of β-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin. Cancer Res. 16:2740–2750; 2010. [DOI] [PubMed] [Google Scholar]
- 25. Zhao J.-H.; Luo Y.; Jiang Y.-G.; He D.-L.; Wu C.-T. Knockdown of β-catenin through shRNA cause a reversal of emt and metastatic phenotypes induced by HIF-1α. Cancer Invest. 29:377–382; 2011. [DOI] [PubMed] [Google Scholar]